Intraovarian, Isoform-Specific Transcriptional Roles of Progesterone Receptor in Ovulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Superovulation
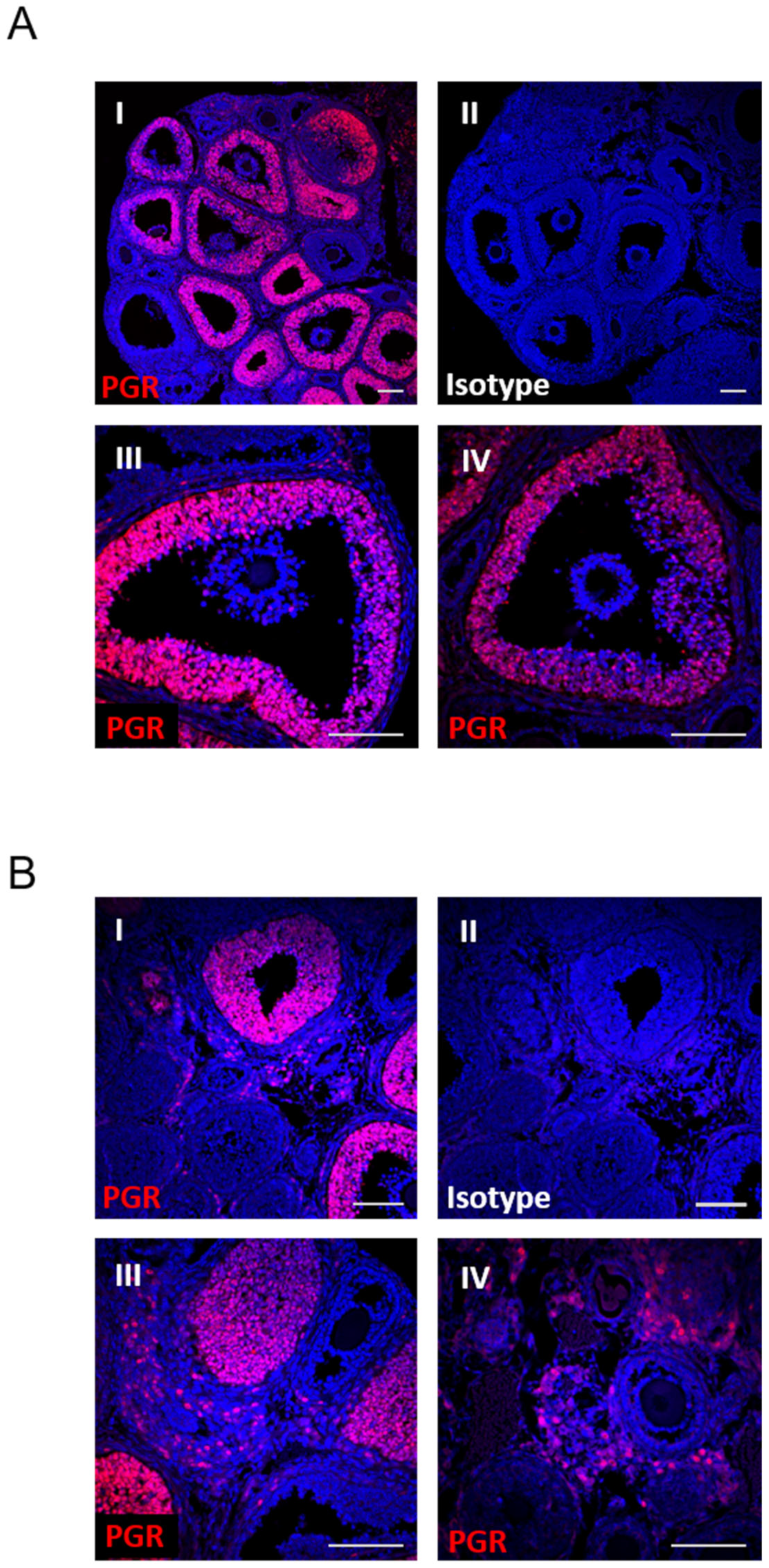
2.2. Histology and Immunofluorescence
2.3. Ovarian Transplant
2.4. Oocyte JC-1 Staining
2.5. COC Microarray
2.6. Seahorse Extracellular Flux Assay
2.7. RNA-seq
2.8. Quantitative Real-Time PCR
2.9. Western Blot
2.10. Statistical Analysis
3. Results
3.1. PGR Specifically Regulates Rupture of the Follicle at Ovulation
3.2. Ovarian Progesterone Receptor Is Essential to Ovulation and Fertility
3.3. Ovulatory Cumulus–Oocyte Complexes Are Subtly Altered in PRKO Mice
3.4. Mitochondrial Metabolism Is Not Altered in PRKO Granulosa Cells at Ovulation
3.5. Key PGR Downstream Pathways Include a Transcription Factor Network and Actin Fiber Gene Regulation
3.6. PGR Regulation of the Ovarian Stromal Transcriptome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, J.Y.; Su, Y.Q.; Ariga, M.; Law, E.; Jin, S.L.; Conti, M. Egf-like growth factors as mediators of lh action in the ovulatory follicle. Science 2004, 303, 682–684. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, H.; Cao, X.; Motola, S.; Popliker, M.; Conti, M.; Tsafriri, A. Epidermal growth factor family members: Endogenous mediators of the ovulatory response. Endocrinology 2005, 146, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.; Thao, K.; Conti, M. Genetic dissection of epidermal growth factor receptor signaling during luteinizing hormone-induced oocyte maturation. PLoS ONE 2011, 6, e21574. [Google Scholar] [CrossRef] [PubMed]
- Hizaki, H.; Segi, E.; Sugimoto, Y.; Hirose, M.; Saji, T.; Ushikubi, F.; Matsuoka, T.; Noda, Y.; Tanaka, T.; Yoshida, N.; et al. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin e receptor subtype ep(2). Proc. Natl. Acad. Sci. USA 1999, 96, 10501–10506. [Google Scholar] [CrossRef] [PubMed]
- Akison, L.K.; Alvino, E.R.; Dunning, K.R.; Robker, R.L.; Russell, D.L. Transient invasive migration in mouse cumulus oocyte complexes induced at ovulation by luteinizing hormone. Biol. Reprod. 2012, 86, 125. [Google Scholar] [CrossRef]
- Ochsner, S.A.; Day, A.J.; Rugg, M.S.; Breyer, R.M.; Gomer, R.H.; Richards, J.S. Disrupted function of tumor necrosis factor-alpha-stimulated gene 6 blocks cumulus cell-oocyte complex expansion. Endocrinology 2003, 144, 4376–4384. [Google Scholar] [CrossRef]
- Hess, K.A.; Chen, L.; Larsen, W.J. Inter-alpha-inhibitor binding to hyaluronan in the cumulus extracellular matrix is required for optimal ovulation and development of mouse oocytes. Biol. Reprod. 1999, 61, 436–443. [Google Scholar] [CrossRef]
- Fulop, C.; Szanto, S.; Mukhopadhyay, D.; Bardos, T.; Kamath, R.V.; Rugg, M.S.; Day, A.J.; Salustri, A.; Hascall, V.C.; Glant, T.T.; et al. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development 2003, 130, 2253–2261. [Google Scholar] [CrossRef]
- Robker, R.L.; Hennebold, J.D.; Russell, D.L. Coordination of ovulation and oocyte maturation: A good egg at the right time. Endocrinology 2018, 159, 3209–3218. [Google Scholar] [CrossRef]
- Rosewell, K.L.; Al-Alem, L.; Zakerkish, F.; McCord, L.; Akin, J.W.; Chaffin, C.L.; Brannstrom, M.; Curry, T.E., Jr. Induction of proteinases in the human preovulatory follicle of the menstrual cycle by human chorionic gonadotropin. Fertil. Steril. 2015, 103, 826–833. [Google Scholar] [CrossRef]
- Peluffo, M.C.; Murphy, M.J.; Baughman, S.T.; Stouffer, R.L.; Hennebold, J.D. Systematic analysis of protease gene expression in the rhesus macaque ovulatory follicle: Metalloproteinase involvement in follicle rupture. Endocrinology 2011, 152, 3963–3974. [Google Scholar] [CrossRef] [PubMed]
- Brannstrom, M.; Woessner, J.F., Jr.; Koos, R.D.; Sear, C.H.; LeMaire, W.J. Inhibitors of mammalian tissue collagenase and metalloproteinases suppress ovulation in the perfused rat ovary. Endocrinology 1988, 122, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.L.; Doyle, K.M.; Ochsner, S.A.; Sandy, J.D.; Richards, J.S. Processing and localization of adamts-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J. Biol. Chem. 2003, 278, 42330–42339. [Google Scholar] [CrossRef]
- Brown, H.M.; Dunning, K.R.; Robker, R.L.; Boerboom, D.; Pritchard, M.; Lane, M.; Russell, D.L. Adamts1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol. Reprod. 2010, 83, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.M.; Ko, C.; Jo, M.; Brannstrom, M.; Curry, T.E. Ovulation: Parallels with inflammatory processes. Endocr. Rev. 2019, 40, 369–416. [Google Scholar] [CrossRef]
- Brännström, M.; Bonello, N.; Norman, R.J.; Robertson, S.A. Reduction of ovulation rate in the rat by administration of a neutrophil-depleting monoclonal antibody. J. Reprod. Immunol. 1995, 29, 265–270. [Google Scholar] [CrossRef]
- Oakley, O.R.; Kim, H.; El-Amouri, I.; Lin, P.C.; Cho, J.; Bani-Ahmad, M.; Ko, C. Periovulatory leukocyte infiltration in the rat ovary. Endocrinology 2010, 151, 4551–4559. [Google Scholar] [CrossRef]
- Van der Hoek, K.H.; Maddocks, S.; Woodhouse, C.M.; van Rooijen, N.; Robertson, S.A.; Norman, R.J. Intrabursal injection of clodronate liposomes causes macrophage depletion and inhibits ovulation in the mouse ovary. Biol. Reprod. 2000, 62, 1059–1066. [Google Scholar] [CrossRef]
- Hellberg, P.; Thomsen, P.; Janson, P.O.; Brannstrom, M. Leukocyte supplementation increases the luteinizing hormone-induced ovulation rate in the in vitro-perfused rat ovary. Biol. Reprod. 1991, 44, 791–797. [Google Scholar] [CrossRef]
- Migone, F.F.; Cowan, R.G.; Williams, R.M.; Gorse, K.J.; Zipfel, W.R.; Quirk, S.M. In vivo imaging reveals an essential role of vasoconstriction in rupture of the ovarian follicle at ovulation. Proc. Natl. Acad. Sci. USA 2016, 113, 2294–2299. [Google Scholar] [CrossRef]
- Robinson, R.S.; Woad, K.J.; Hammond, A.J.; Laird, M.; Hunter, M.G.; Mann, G.E. Angiogenesis and vascular function in the ovary. Reproduction 2009, 138, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Kim, E.K.; Kim, K.H.; Lee, K.A.; Kang, D.W.; Kim, H.Y.; Bridges, P.; Ko, C. Expression pattern of endothelin system components and localization of smooth muscle cells in the human pre-ovulatory follicle. Hum. Reprod. 2011, 26, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, H.M.; Tomaszewski, C.E.; Chang, F.L.; Moravek, M.B.; Xu, M.; Padmanabhan, V.; Shikanov, A. The ovarian stroma as a new frontier. Reproduction 2020, 160, R25–R39. [Google Scholar] [CrossRef]
- Lydon, J.P.; DeMayo, F.J.; Funk, C.R.; Mani, S.K.; Hughes, A.R.; Montgomery, C.A., Jr.; Shyamala, G.; Conneely, O.M.; O’Malley, B.W. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995, 9, 2266–2278. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.V.; Hennebold, J.D.; Kahl, C.A.; Stouffer, R.L. Knockdown of progesterone receptor (pgr) in macaque granulosa cells disrupts ovulation and progesterone production. Biol. Reprod. 2016, 94, 109. [Google Scholar] [CrossRef]
- Natraj, U.; Richards, J.S. Hormonal regulation, localization, and functional activity of the progesterone receptor in granulosa cells of rat preovulatory follicles. Endocrinology 1993, 133, 761–769. [Google Scholar] [CrossRef]
- Schutt, B.; Schultze-Mosgau, M.H.; Draeger, C.; Chang, X.; Lowen, S.; Kaiser, A.; Rohde, B. Effect of the novel selective progesterone receptor modulator vilaprisan on ovarian activity in healthy women. J. Clin. Pharm. 2018, 58, 228–239. [Google Scholar] [CrossRef]
- Robker, R.L.; Russell, D.L.; Espey, L.L.; Lydon, J.P.; O’Malley, B.W.; Richards, J.S. Progesterone-regulated genes in the ovulation process: Adamts-1 and cathepsin l proteases. Proc. Natl. Acad. Sci. USA 2000, 97, 4689–4694. [Google Scholar] [CrossRef]
- Peavey, M.C.; Wu, S.P.; Li, R.; Liu, J.; Emery, O.M.; Wang, T.; Zhou, L.; Wetendorf, M.; Yallampalli, C.; Gibbons, W.E.; et al. Progesterone receptor isoform b regulates the oxtr-plcl2-trpc3 pathway to suppress uterine contractility. Proc. Natl. Acad. Sci. USA 2021, 118, e2011643118. [Google Scholar] [CrossRef]
- Gal, A.; Lin, P.C.; Cacioppo, J.A.; Hannon, P.R.; Mahoney, M.M.; Wolfe, A.; Fernandez-Valdivia, R.; Lydon, J.P.; Elias, C.F.; Ko, C. Loss of fertility in the absence of progesterone receptor expression in kisspeptin neurons of female mice. PLoS ONE 2016, 11, e0159534. [Google Scholar]
- Chappell, P.E.; Lydon, J.P.; Conneely, O.M.; O’Malley, B.W.; Levine, J.E. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology 1997, 138, 4147–4152. [Google Scholar] [CrossRef] [PubMed]
- Toufaily, C.; Schang, G.; Zhou, X.; Wartenberg, P.; Boehm, U.; Lydon, J.P.; Roelfsema, F.; Bernard, D.J. Impaired lh surge amplitude in gonadotrope-specific progesterone receptor knockout mice. J. Endocrinol. 2020, 244, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Mittaz, L.; Russell, D.L.; Wilson, T.; Brasted, M.; Tkalcevic, J.; Salamonsen, L.A.; Hertzog, P.J.; Pritchard, M.A. Adamts-1 is essential for the development and function of the urogenital system. Biol. Reprod. 2004, 70, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Dinh, D.T.; Breen, J.; Akison, L.K.; DeMayo, F.J.; Brown, H.M.; Robker, R.L.; Russell, D.L. Tissue-specific progesterone receptor-chromatin binding and the regulation of progesterone-dependent gene expression. Sci. Rep. 2019, 9, 11966. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, J.A.; Lin, P.P.; Hannon, P.R.; McDougle, D.R.; Gal, A.; Ko, C. Granulosa cell endothelin-2 expression is fundamental for ovulatory follicle rupture. Sci. Rep. 2017, 7, 817. [Google Scholar] [CrossRef]
- Palanisamy, G.S.; Cheon, Y.P.; Kim, J.; Kannan, A.; Li, Q.; Sato, M.; Mantena, S.R.; Sitruk-Ware, R.L.; Bagchi, M.K.; Bagchi, I.C. A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor b controls ovulation in mice. Mol. Endocrinol. 2006, 20, 2784–2795. [Google Scholar] [CrossRef]
- Kim, J.; Sato, M.; Li, Q.; Lydon, J.P.; Demayo, F.J.; Bagchi, I.C.; Bagchi, M.K. Peroxisome proliferator-activated receptor gamma is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol. Cell Biol. 2008, 28, 1770–1782. [Google Scholar] [CrossRef]
- Akison, L.K.; Robertson, S.A.; Gonzalez, M.B.; Richards, J.S.; Smith, C.W.; Russell, D.L.; Robker, R.L. Regulation of the ovarian inflammatory response at ovulation by nuclear progesterone receptor. Am. J. Reprod. Immunol. 2018, 79, e12835. [Google Scholar] [CrossRef]
- Dinh, D.T.; Breen, J.; Nicol, B.; Smith, K.M.; Nicholls, M.; Emery, A.; Wong, Y.Y.; Barry, S.C.; Yao, H.H.C.; Robker, R.L.; et al. Progesterone receptor-a cooperates with runx transcription factors to mediate ovulation. BioRxiv Prepr. 2021. [Google Scholar]
- Mulac-Jericevic, B.; Lydon, J.P.; DeMayo, F.J.; Conneely, O.M. Defective mammary gland morphogenesis in mice lacking the progesterone receptor b isoform. Proc. Natl. Acad. Sci. USA 2003, 100, 9744–9749. [Google Scholar] [CrossRef]
- Mulac-Jericevic, B.; Mullinax, R.A.; DeMayo, F.J.; Lydon, J.P.; Conneely, O.M. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-b isoform. Science 2000, 289, 1751–1754. [Google Scholar] [CrossRef] [PubMed]
- Ismail, P.M.; Li, J.; DeMayo, F.J.; O’Malley, B.W.; Lydon, J.P. A novel lacz reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol. Endocrinol. 2002, 16, 2475–2489. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.G.M.; Vintersten, K.; Behringer, R. Surgical procedures. Manipulating the Mouse Embryo: A Laboratory Manual, 3rd ed.; Cold Spring Harb. Lab. Press: New York, NY, USA, 2003. [Google Scholar]
- Akison, L.K.; Boden, M.J.; Kennaway, D.J.; Russell, D.L.; Robker, R.L. Progesterone receptor-dependent regulation of genes in the oviducts of female mice. Physiol Genom. 2014, 46, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. Fastqc: A quality control tool for high throughput sequence data [online]. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 1 September 2021).
- Lindgreen, S. Adapterremoval: Easy cleaning of next-generation sequencing reads. BMC Res. Notes 2012, 5, 337. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for rna-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for rna-seq data with deseq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis. Available online: https://ggplot2.tidyverse.org (accessed on 1 April 2022).
- Kim, D.; Langmead, B.; Salzberg, S.L. Hisat: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Adhikari, D.; Lee, I.W.; Yuen, W.S.; Carroll, J. Oocyte mitochondria-key regulators of oocyte function and potential therapeutic targets for improving fertility. Biol. Reprod. 2022, 106, 366–377. [Google Scholar] [CrossRef]
- Robker, R.L.; Richards, J.S. Progesterone: Lessons from the progesterone receptor knockout. In Ovulation: Evolving Scientific and Clinical Concepts; Adashi, E.Y., Ed.; Spriger: New York, NY, USA, 2000; pp. 121–130. [Google Scholar]
- Behera, M.A.; Dai, Q.; Garde, R.; Saner, C.; Jungheim, E.; Price, T.M. Progesterone stimulates mitochondrial activity with subsequent inhibition of apoptosis in mcf-10a benign breast epithelial cells. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1089–E1096. [Google Scholar] [CrossRef]
- Sim, C.B.; Phipson, B.; Ziemann, M.; Rafehi, H.; Mills, R.J.; Watt, K.I.; Abu-Bonsrah, K.D.; Kalathur, R.K.R.; Voges, H.K.; Dinh, D.T.; et al. Sex-specific control of human heart maturation by the progesterone receptor. Circulation 2021, 143, 1614–1628. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Azuma, K.; Ikeda, K.; Inoue, S. Mechanisms underlying the regulation of mitochondrial respiratory chain complexes by nuclear steroid receptors. Int. J. Mol. Sci. 2020, 21, 6683. [Google Scholar] [CrossRef] [PubMed]
- Vallenius, T.; Scharm, B.; Vesikansa, A.; Luukko, K.; Schafer, R.; Makela, T.P. The pdz-lim protein ril modulates actin stress fiber turnover and enhances the association of alpha-actinin with f-actin. Exp. Cell Res. 2004, 293, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Tamura, N.; Ohno, K.; Katayama, T.; Kanayama, N.; Sato, K. The pdz-lim protein clp36 is required for actin stress fiber formation and focal adhesion assembly in bewo cells. Biochem. Biophys. Res. Commun. 2007, 364, 589–594. [Google Scholar] [CrossRef]
- Poulsen, L.C.; Botkjaer, J.A.; Ostrup, O.; Petersen, K.B.; Andersen, C.Y.; Grondahl, M.L.; Englund, A.L.M. Two waves of transcriptomic changes in periovulatory human granulosa cells. Hum. Reprod. 2020, 35, 1230–1245. [Google Scholar] [CrossRef]
- Zuo, J.; Wen, M.; Li, S.; Lv, X.; Wang, L.; Ai, X.; Lei, M. Overexpression of cxcr4 promotes invasion and migration of non-small cell lung cancer via egfr and mmp-9. Oncol. Lett. 2017, 14, 7513–7521. [Google Scholar] [CrossRef]
- Wu, X.; Xiao, J.; Zhao, C.; Zhao, C.; Han, Z.; Wang, F.; Yang, Y.; Jiang, Y.; Fang, F. Claudin1 promotes the proliferation, invasion and migration of nasopharyngeal carcinoma cells by upregulating the expression and nuclear entry of beta-catenin. Exp. Med. 2018, 16, 3445–3451. [Google Scholar]
- Kommagani, R.; Szwarc, M.M.; Vasquez, Y.M.; Peavey, M.C.; Mazur, E.C.; Gibbons, W.E.; Lanz, R.B.; DeMayo, F.J.; Lydon, J.P. The promyelocytic leukemia zinc finger transcription factor is critical for human endometrial stromal cell decidualization. PLoS Genet. 2016, 12, e1005937. [Google Scholar] [CrossRef]
- Hernandez-Quiles, M.; Broekema, M.F.; Kalkhoven, E. Ppargamma in metabolism, immunity, and cancer: Unified and diverse mechanisms of action. Front. Endocrinol. (Lausanne) 2021, 12, 624112. [Google Scholar] [CrossRef]
- Park, C.J.; Lin, P.C.; Zhou, S.; Barakat, R.; Bashir, S.T.; Choi, J.M.; Cacioppo, J.A.; Oakley, O.R.; Duffy, D.M.; Lydon, J.P.; et al. Progesterone receptor serves the ovary as a trigger of ovulation and a terminator of inflammation. Cell Rep. 2020, 31, 107496. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, K.M.; Dinh, D.T.; Akison, L.K.; Nicholls, M.; Dunning, K.R.; Morimoto, A.; Lydon, J.P.; Russell, D.L.; Robker, R.L. Intraovarian, Isoform-Specific Transcriptional Roles of Progesterone Receptor in Ovulation. Cells 2022, 11, 1563. https://doi.org/10.3390/cells11091563
Smith KM, Dinh DT, Akison LK, Nicholls M, Dunning KR, Morimoto A, Lydon JP, Russell DL, Robker RL. Intraovarian, Isoform-Specific Transcriptional Roles of Progesterone Receptor in Ovulation. Cells. 2022; 11(9):1563. https://doi.org/10.3390/cells11091563
Chicago/Turabian StyleSmith, Kirsten M., Doan T. Dinh, Lisa K. Akison, Matilda Nicholls, Kylie R. Dunning, Atsushi Morimoto, John P. Lydon, Darryl L. Russell, and Rebecca L. Robker. 2022. "Intraovarian, Isoform-Specific Transcriptional Roles of Progesterone Receptor in Ovulation" Cells 11, no. 9: 1563. https://doi.org/10.3390/cells11091563
APA StyleSmith, K. M., Dinh, D. T., Akison, L. K., Nicholls, M., Dunning, K. R., Morimoto, A., Lydon, J. P., Russell, D. L., & Robker, R. L. (2022). Intraovarian, Isoform-Specific Transcriptional Roles of Progesterone Receptor in Ovulation. Cells, 11(9), 1563. https://doi.org/10.3390/cells11091563





