SESN2 Knockdown Increases Betulinic Acid-Induced Radiosensitivity of Hypoxic Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Conditions and Treatment of Cells
2.2. Microarray Analysis
2.3. siRNA Transfection and BA Treatment
2.4. Analysis of RNA Expression
2.5. Analysis of Protein Expression
2.6. Cytotoxicity
2.7. Clonogenic Survival and Radiosensitivity
2.8. Analysis of Autophagy: Bafilomycin Clamp Assay
2.9. Analysis of ROS
2.10. Quantification of γH2AX Foci Formation
2.11. Statistical Analysis
3. Results
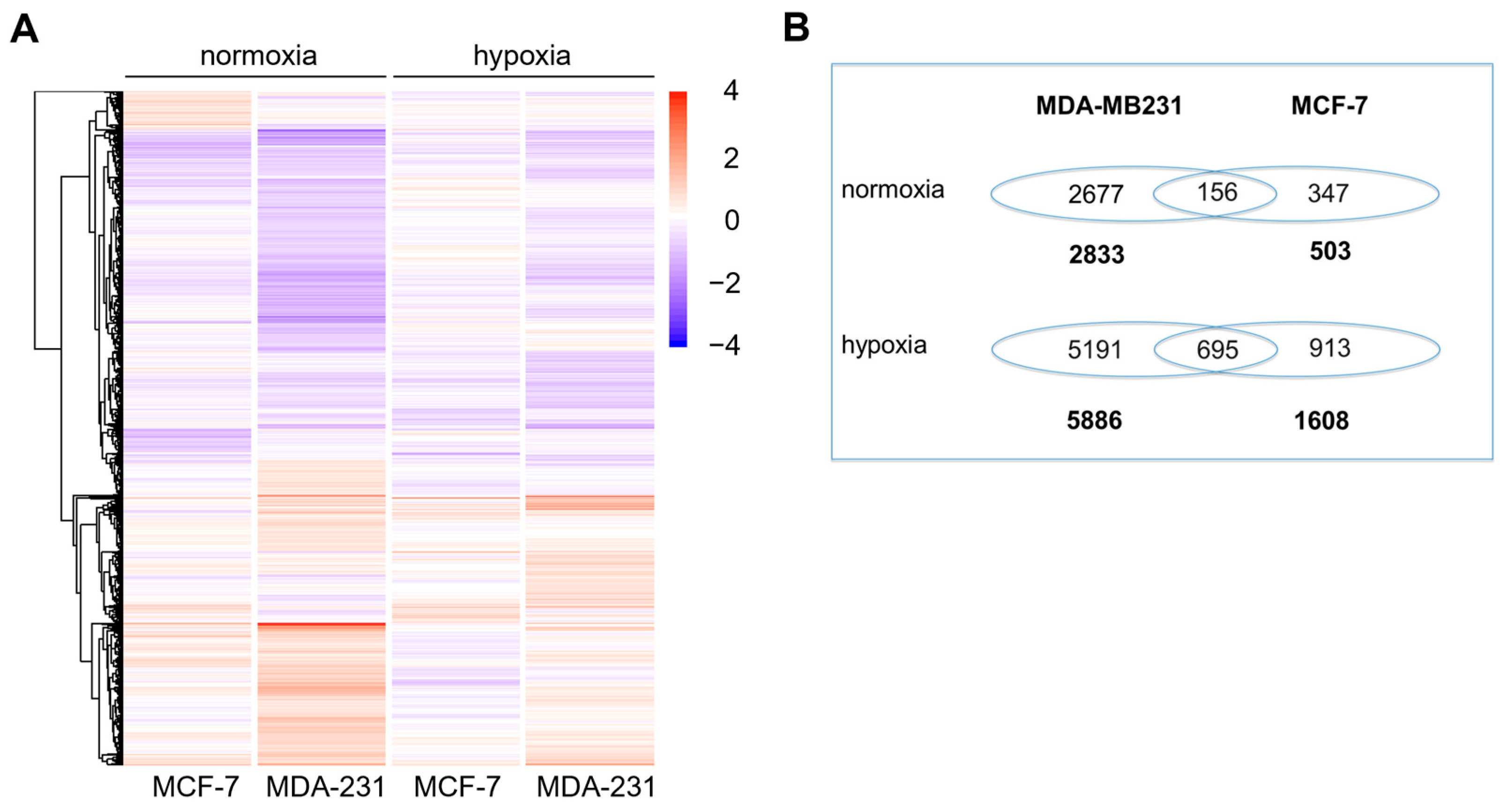
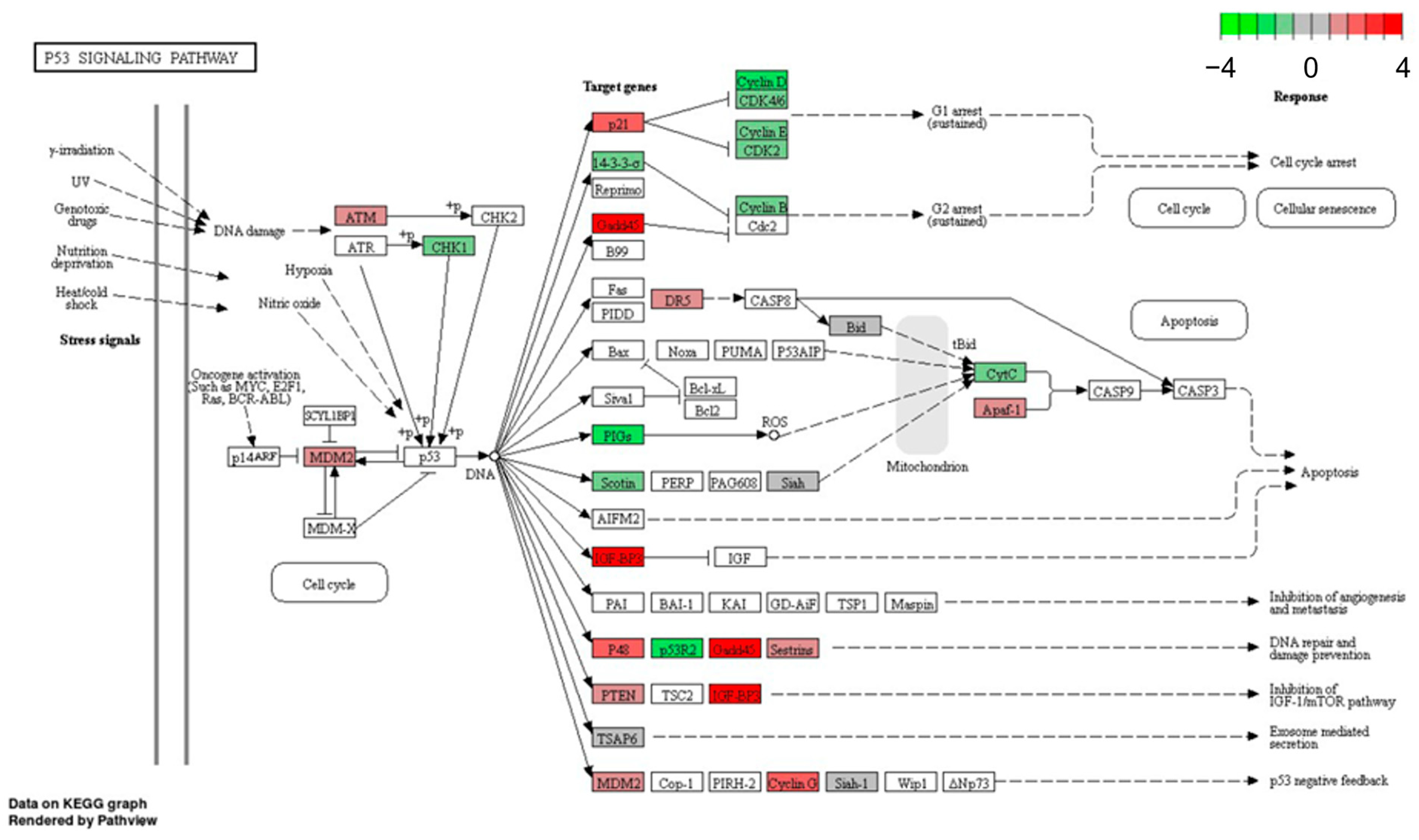
3.1. Microarray Analysis
3.2. Validation of Microarray Data
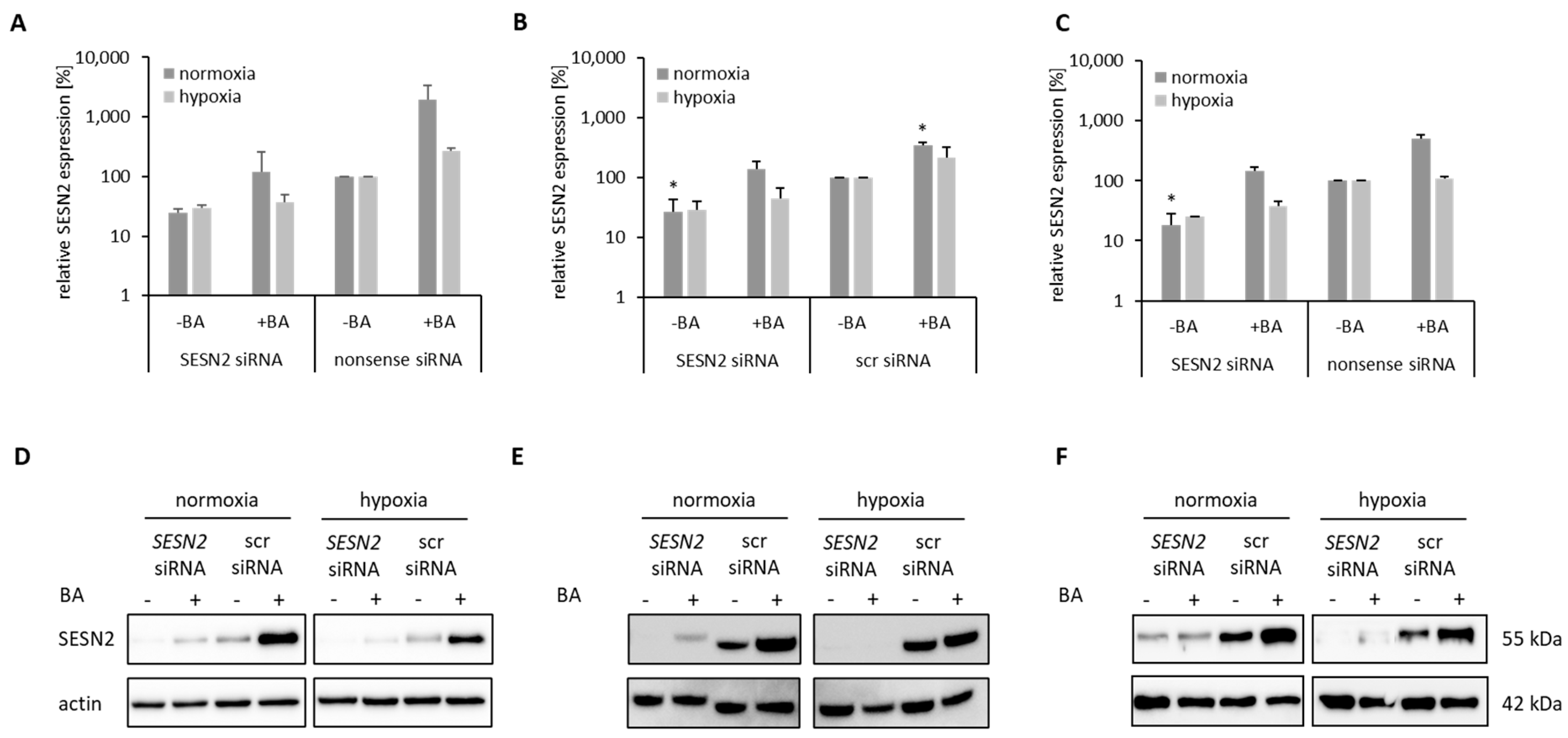
3.3. Knockdown of BA-Induced SESN2 Expression
3.4. Cytotoxicity of SESN2-Silenced Breast Cancer Cells
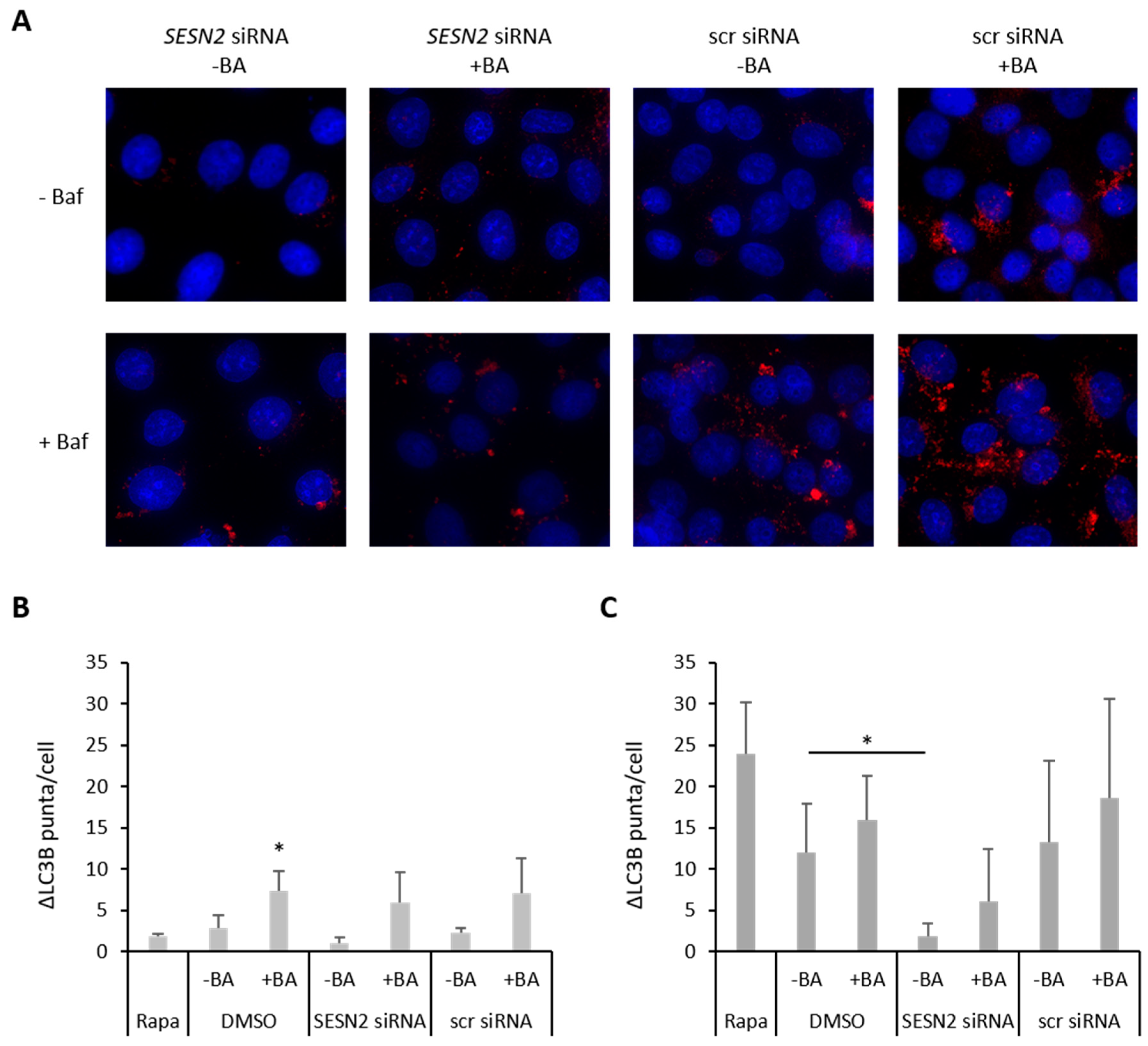
3.5. Effect of SESN2 Silencing on Autophagy Induction in BA-Treated Breast Cancer Cells
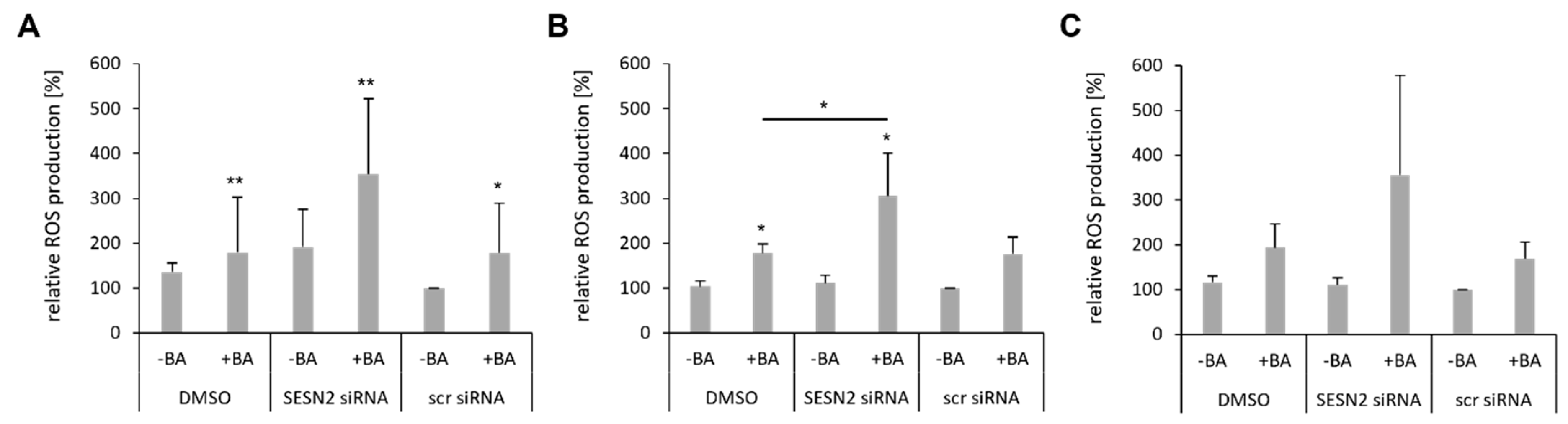
3.6. SESN2 Silencing Enhances ROS Production of BA-Treated Breast Cancer Cells
3.7. SESN2 Silencing Enhances DNA Damage of BA-Treated Breast Cancer Cells
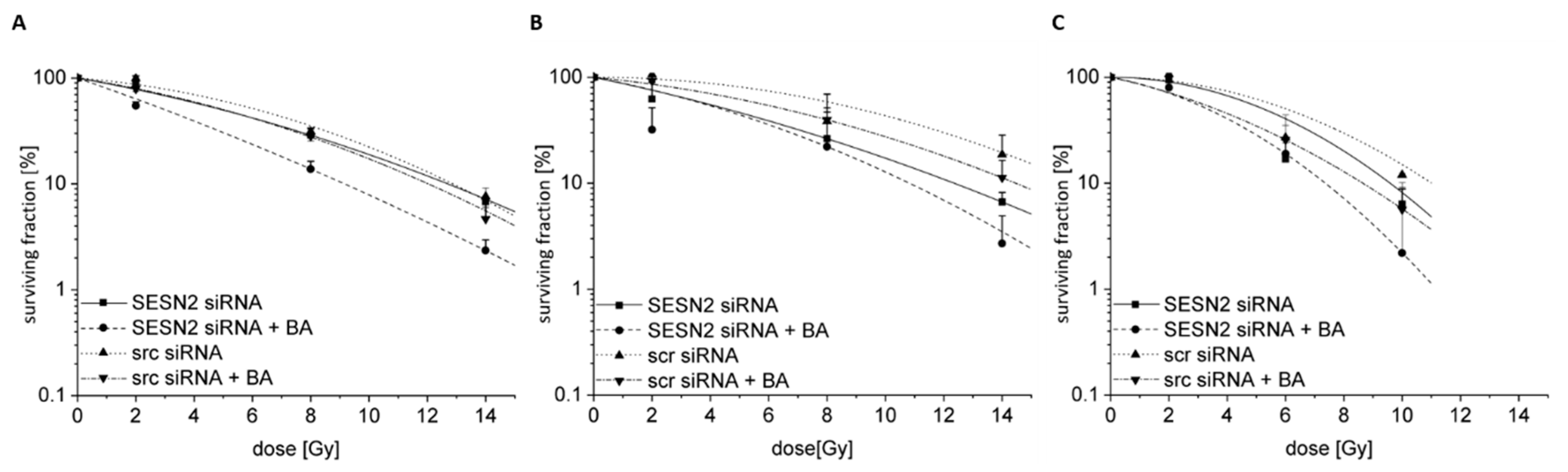
3.8. Radiosensitization of BA-Treated Breast Cancer Cells after SESN2 Silencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Vanderplas, A.; Hughes, M.E.; Theriault, R.L.; Edge, S.B.; Wong, Y.-N.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; Weeks, J.C. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012, 118, 5463–5472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Wang, M.; Schmid, T.; Xin, Z.; Kozhuharova, L.; Yu, W.-K.; Huang, Y.; Cai, F.; Biskup, E. Hypoxia in Breast Cancer-Scientific Translation to Therapeutic and Diagnostic Clinical Applications. Front. Oncol. 2021, 11, 652266. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochim. Biophys. Acta 2016, 1863, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, X.; Dong, S.; Zhou, W. Betulinic acid in the treatment of tumour diseases: Application and research progress. Biomed. Pharmacother. 2021, 142, 111990. [Google Scholar] [CrossRef] [PubMed]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A. Betulin and betulinic acid in cancer research. J. Pre-Clin. Clin. Res. 2018, 12, 72–75. [Google Scholar] [CrossRef]
- Bache, M.; Münch, C.; Güttler, A.; Wichmann, H.; Theuerkorn, K.; Emmerich, D.; Paschke, R.; Vordermark, D. Betulinyl Sulfamates as Anticancer Agents and Radiosensitizers in Human Breast Cancer Cells. Int. J. Mol. Sci. 2015, 16, 26249–26262. [Google Scholar] [CrossRef]
- Bache, M.; Zschornak, M.P.; Passin, S.; Kessler, J.; Wichmann, H.; Kappler, M.; Paschke, R.; Kaluđerović, G.N.; Kommera, H.; Taubert, H.; et al. Increased betulinic acid induced cytotoxicity and radiosensitivity in glioma cells under hypoxic conditions. Radiat. Oncol. 2011, 6, 111. [Google Scholar] [CrossRef]
- Saeed, M.E.M.; Mahmoud, N.; Sugimoto, Y.; Efferth, T.; Abdel-Aziz, H. Betulinic Acid Exerts Cytotoxic Activity Against Multidrug-Resistant Tumor Cells via Targeting Autocrine Motility Factor Receptor (AMFR). Front. Pharmacol. 2018, 9, 481. [Google Scholar] [CrossRef]
- Shin, J.; Lee, H.-J.; Jung, D.-B.; Jung, J.H.; Lee, H.-J.; Lee, E.-O.; Lee, S.G.; Shim, B.S.; Choi, S.H.; Ko, S.G.; et al. Suppression of STAT3 and HIF-1 alpha mediates anti-angiogenic activity of betulinic acid in hypoxic PC-3 prostate cancer cells. PLoS ONE 2011, 6, e21492. [Google Scholar] [CrossRef]
- Shen, H.; Liu, L.; Yang, Y.; Xun, W.; Wei, K.; Zeng, G. Betulinic Acid Inhibits Cell Proliferation in Human Oral Squamous Cell Carcinoma via Modulating ROS-Regulated p53 Signaling. Oncol. Res. 2017, 25, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Shankar, E.; Zhang, A.; Franco, D.; Gupta, S. Betulinic Acid-Mediated Apoptosis in Human Prostate Cancer Cells Involves p53 and Nuclear Factor-Kappa B (NF-κB) Pathways. Molecules 2017, 22, 264. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.-R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef] [PubMed]
- Ala, M. Sestrin2 in cancer: A foe or a friend? Biomark. Res. 2022, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Álvarez, M.; Strippoli, R.; Donadelli, M.; Bazhin, A.V.; Cordani, M. Sestrins as a Therapeutic Bridge between ROS and Autophagy in Cancer. Cancers 2019, 11, 1415. [Google Scholar] [CrossRef] [PubMed]
- Budanov, A.V.; Karin, M. The p53-regulated Sestrin gene products inhibit mTOR signaling. Cell 2008, 134, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-L.; Fu, Z.-X.; Fang, M.; Guo, J.-B.; Zhao, Q.-N.; Lu, W.-D.; Zhou, Q.-Y. Decreased expression of sestrin 2 predicts unfavorable outcome in colorectal cancer. Oncol. Rep. 2015, 33, 1349–1357. [Google Scholar] [CrossRef]
- Chen, K.-B.; Xuan, Y.; Shi, W.-J.; Chi, F.; Xing, R.; Zeng, Y.-C. Sestrin2 expression is a favorable prognostic factor in patients with non-small cell lung cancer. Am. J. Transl. Res. 2016, 8, 1903–1909. [Google Scholar]
- Chen, S.; Yan, W.; Lang, W.; Yu, J.; Xu, L.; Xu, X.; Liu, Y.; Bao, H. SESN2 correlates with advantageous prognosis in hepatocellular carcinoma. Diagn. Pathol. 2017, 12, 13. [Google Scholar] [CrossRef]
- Wei, J.-L.; Fang, M.; Fu, Z.-X.; Zhang, S.-R.; Guo, J.-B.; Wang, R.; Lv, Z.-B.; Xiong, Y.-F. Sestrin 2 suppresses cells proliferation through AMPK/mTORC1 pathway activation in colorectal cancer. Oncotarget 2017, 8, 49318–49328. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, J.; Huang, H.; Xiang, D.; Li, Y.; Zhang, D.; Li, J.; Wang, Y.; Jin, H.; Jiang, G.; et al. SESN2/sestrin 2 induction-mediated autophagy and inhibitory effect of isorhapontigenin (ISO) on human bladder cancers. Autophagy 2016, 12, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.S.; Gil, M.; Saha, S.K.; Kwak, H.J.; Park, H.-W.; Vellingiri, B.; Cho, S.-G. Sestrin2 Expression Has Regulatory Properties and Prognostic Value in Lung Cancer. J. Pers. Med. 2020, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Bae, J.; Park, S.; Kang, H.-G.; Shin, S.M.; Won, G.; Kim, J.-S.; Cho, S.-G.; Choi, Y.; Oh, S.-M.; et al. mTOR-Dependent Role of Sestrin2 in Regulating Tumor Progression of Human Endometrial Cancer. Cancers 2020, 12, 2515. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Huang, Q.; Niu, K.; Wang, B.; Li, Y.; Dai, C.; Chen, Z.; Tao, K.; Dai, J. Sestrin 2 confers primary resistance to sorafenib by simultaneously activating AKT and AMPK in hepatocellular carcinoma. Cancer Med. 2018, 7, 5691–5703. [Google Scholar] [CrossRef] [PubMed]
- Sanli, T.; Linher-Melville, K.; Tsakiridis, T.; Singh, G. Sestrin2 modulates AMPK subunit expression and its response to ionizing radiation in breast cancer cells. PLoS ONE 2012, 7, e32035. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Song, W.; Wang, Y.; Deng, W.; Tang, T.; Fan, W.; Qu, S. Radiosensitizing effects of Sestrin2 in PC3 prostate cancer cells. Iran. J. Basic Med. Sci. 2018, 21, 621–624. [Google Scholar] [CrossRef]
- Tang, Z.; Wei, X.; Li, T.; Wang, W.; Wu, H.; Dong, H.; Liu, Y.; Wei, F.; Shi, L.; Li, X.; et al. Sestrin2-Mediated Autophagy Contributes to Drug Resistance via Endoplasmic Reticulum Stress in Human Osteosarcoma. Front. Cell Dev. Biol. 2021, 9, 722960. [Google Scholar] [CrossRef]
- Weinholdt, C.; Wichmann, H.; Kotrba, J.; Ardell, D.H.; Kappler, M.; Eckert, A.W.; Vordermark, D.; Grosse, I. Prediction of regulatory targets of alternative isoforms of the epidermal growth factor receptor in a glioblastoma cell line. BMC Bioinformatics 2019, 20, 434. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Da Huang, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Güttler, A.; Theuerkorn, K.; Riemann, A.; Wichmann, H.; Kessler, J.; Thews, O.; Bache, M.; Vordermark, D. Cellular and radiobiological effects of carbonic anhydrase IX in human breast cancer cells. Oncol. Rep. 2019, 41, 2585–2594. [Google Scholar] [CrossRef]
- Güttler, A.; Eiselt, Y.; Funtan, A.; Thiel, A.; Petrenko, M.; Keßler, J.; Thondorf, I.; Paschke, R.; Vordermark, D.; Bache, M. Betulin Sulfonamides as Carbonic Anhydrase Inhibitors and Anticancer Agents in Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 8808. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Murugan, S.; Amaravadi, R.K. Methods for Studying Autophagy Within the Tumor Microenvironment. Adv. Exp. Med. Biol. 2016, 899, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.N.; Mowers, E.E.; Drake, L.E.; Macleod, K.F. Measuring autophagy in stressed cells. Methods Mol. Biol. 2015, 1292, 129–150. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, M.; Güttler, A.; Funtan, A.; Keßler, J.; Emmerich, D.; Paschke, R.; Vordermark, D.; Bache, M. Combined 3-O-acetylbetulin treatment and carbonic anhydrase IX inhibition results in additive effects on human breast cancer cells. Chem. Biol. Interact. 2021, 333, 109326. [Google Scholar] [CrossRef]
- Noda, A. Radiation-induced unrepairable DSBs: Their role in the late effects of radiation and possible applications to biodosimetry. J. Radiat. Res. 2017, 59, ii114–ii120. [Google Scholar] [CrossRef]
- Kim, H.-J.; Cho, H.-S.; Ban, H.S.; Nakamura, H. Suppression of HIF-1α accumulation by betulinic acid through proteasome activation in hypoxic cervical cancer. Biochem. Biophys. Res. Commun. 2020, 523, 726–732. [Google Scholar] [CrossRef]
- Karna, E.; Szoka, L.; Palka, J.A. Betulinic acid inhibits the expression of hypoxia-inducible factor 1alpha and vascular endothelial growth factor in human endometrial adenocarcinoma cells. Mol. Cell. Biochem. 2010, 340, 15–20. [Google Scholar] [CrossRef]
- Wang, S.; Wang, K.; Zhang, C.; Zhang, W.; Xu, Q.; Wang, Y.; Zhang, Y.; Li, Y.; Zhang, Y.; Zhu, H.; et al. Overaccumulation of p53-mediated autophagy protects against betulinic acid-induced apoptotic cell death in colorectal cancer cells. Cell Death Dis. 2017, 8, e3087. [Google Scholar] [CrossRef]
- Liang, Z.; Pan, R.; Meng, X.; Su, J.; Guo, Y.; Wei, G.; Zhang, Z.; He, K. Transcriptome study of oleanolic acid in the inhibition of breast tumor growth based on high-throughput sequencing. Aging 2021, 13, 22883–22897. [Google Scholar] [CrossRef] [PubMed]
- Budanov, A.V.; Shoshani, T.; Faerman, A.; Zelin, E.; Kamer, I.; Kalinski, H.; Gorodin, S.; Fishman, A.; Chajut, A.; Einat, P.; et al. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene 2002, 21, 6017–6031. [Google Scholar] [CrossRef]
- Wang, J.-M.; Liu, B.-Q.; Li, C.; Du, Z.-X.; Sun, J.; Yan, J.; Jiang, J.-Y.; Wang, H.-Q. Sestrin 2 protects against metabolic stress in a p53-independent manner. Biochem. Biophys. Res. Commun. 2019, 513, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.T.; Lee, S.H.; Kim, J.I.; Kim, Y.M. Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a p53-independent manner. Int. J. Mol. Med. 2014, 33, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Luo, M.; Zhang, J.; Han, F.; Hou, N.; Pan, R.; Sun, X. A paradoxical role for sestrin 2 protein in tumor suppression and tumorigenesis. Cancer Cell Int. 2021, 21, 606. [Google Scholar] [CrossRef]
- Ben-Sahra, I.; Dirat, B.; Laurent, K.; Puissant, A.; Auberger, P.; Budanov, A.; Tanti, J.-F.; Bost, F. Sestrin2 integrates Akt and mTOR signaling to protect cells against energetic stress-induced death. Cell Death Differ. 2013, 20, 611–619. [Google Scholar] [CrossRef]
- Zhao, B.; Shah, P.; Budanov, A.V.; Qiang, L.; Ming, M.; Aplin, A.; Sims, D.M.; He, Y.-Y. Sestrin2 protein positively regulates AKT enzyme signaling and survival in human squamous cell carcinoma and melanoma cells. J. Biol. Chem. 2014, 289, 35806–35814. [Google Scholar] [CrossRef]
- Seo, K.; Ki, S.H.; Park, E.Y.; Shin, S.M. 5-Fluorouracil inhibits cell migration by induction of Sestrin2 in colon cancer cells. Arch. Pharm. Res. 2017, 40, 231–239. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Lee, Y.-J.; Lee, T.-C. Association of platelet-derived growth factor receptor β accumulation with increased oxidative stress and cellular injury in sestrin 2 silenced human glioblastoma cells. FEBS Lett. 2011, 585, 1853–1858. [Google Scholar] [CrossRef][Green Version]
- Yang, H.J.; Kim, N.; Seong, K.M.; Youn, H.; Youn, B. Investigation of radiation-induced transcriptome profile of radioresistant non-small cell lung cancer A549 cells using RNA-seq. PLoS ONE 2013, 8, e59319. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Chang, Y.-C.; Wang, S.-Y.; Yang, M.-H.; Chang, C.-H.; Hsiao, M.; Kitsis, R.N.; Lee, Y.-J. OncomiR miR-182-5p Enhances Radiosensitivity by Inhibiting the Radiation-Induced Antioxidant Effect through SESN2 in Head and Neck Cancer. Antioxidants 2021, 10, 1808. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zheng, H.; Cai, Q.; Wei, L. Autophagy and Tumour Radiotherapy. Adv. Exp. Med. Biol. 2020, 1207, 375–387. [Google Scholar] [CrossRef] [PubMed]



| Gene | Gene ID | Assay ID | Product Length |
|---|---|---|---|
| ATM | 472 | Hs00175892_m1 | 89 bp |
| CCND1 | 595 | Hs00765553_m1 | 57 bp |
| CCND2 | 894 | Hs00153380_m1 | 69 bp |
| CCNE1 | 898 | Hs01026536_m1 | 64 bp |
| CDK6 | 1021 | Hs01026371_m1 | 64 bp |
| CDKN1A | 1026 | Hs00355782_m1 | 66 bp |
| GADD45A | 1647 | Hs00169255_m1 | 123 bp |
| GADD45B | 4616 | Hs00169587_m1 | 74 bp |
| GADD45G | 10912 | Hs00198672_m1 | 62 bp |
| IGFBP3 | 3486 | Hs00426287_m1 | 66 bp |
| MMGT1 | 93380 | Hs00298045_m1 | 72 bp |
| PMAIP1 | 5366 | Hs00560402_m1 | 90 bp |
| SESN2 | 83667 | Hs00230241_m1 | 65 bp |
| STEAP3 | 55240 | Hs00217292_m1 | 61 bp |
| ZMAT3 | 64393 | Hs00536976_m1 | 62 bp |
| Gene | Position | Sequence | Product Length | |
|---|---|---|---|---|
| SESN2 (NM_031459.5) | sense | 1455-1475 | 5′-CAATACCATCGCCATGCACAG-3′ | 246 bp |
| antisense | 1700-1680 | 5′-AAGTTCACGTGGACCTTCTCT-3‘ | ||
| HPRT (NM_000194.3) | sense | 371-390 | 5’-TTGCTGACCTGCTGGATTAC-3’ | 262 bp |
| antisense | 632-613 | 5’-CTTGCGACCTTGACCATCTT-3’ |
| MCF-7 (wtp53) | MDA-MB-231 (mtp53) | |||||
|---|---|---|---|---|---|---|
| DMSO | DMSO vs. BA | DMSO | DMSO vs. BA | |||
| KEGG Pathway | Normoxia vs. Hypoxia | Normoxia | Hypoxia | Normoxia vs. Hypoxia | Normoxia | Hypoxia |
| hsa00010:Glycolysis/Gluconeogenesis | ||||||
| hsa00051:Fructose and mannose metabolism | ||||||
| hsa00230:Purine metabolism | ||||||
| hsa00240:Pyrimidine metabolism | ||||||
| hsa00280:Valine, leucine and isoleucine degradation | ||||||
| hsa00620:Pyruvate metabolism | ||||||
| hsa00640:Propanoate metabolism | ||||||
| hsa00670:One carbon pool by folate | ||||||
| hsa00970:Aminoacyl-tRNA biosynthesis | ||||||
| hsa03030:DNA replication | ||||||
| hsa03040:Spliceosome | ||||||
| hsa03410:Base excision repair | ||||||
| hsa03430:Mismatch repair | ||||||
| hsa04110:Cell cycle | ||||||
| hsa04115:p53 signaling pathway | ||||||
| hsa04120:Ubiquitin mediated proteolysis | ||||||
| hsa04142:Lysosome | ||||||
| hsa04510:Focal adhesion | ||||||
| hsa04621:NOD-like receptor signaling pathway | ||||||
| hsa04722:Neurotrophin signaling pathway | ||||||
| hsa05200:Pathways in cancer | ||||||
| hsa05222:Small cell lung cancer | ||||||
| MDA-MB-231 | HS578T | T47D | MCF-7 | Cal51 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Normoxia | Hypoxia | Normoxia | Hypoxia | Normoxia | Hypoxia | Normoxia | Hypoxia | Normoxia | Hypoxia | Gene |
| ATM | −0.2 | −0.4 | n.d. | n.d. | n.d. | n.d. | −0.1 | −0.5 | n.d. | n.d. | ATM |
| CCND1 | −0.1 | −0.7 ** | −0.1 | 0.6 ** | 0.3 * | 1.0 * | −0.3 ** | −0.3 | −0.1 | −0.1 | CCND1 |
| CCND2 | 1.4 ** | −0.3 * | n.v. | n.v. | n.v. | n.v. | 1.3 | −0.9 | −0.3 | −0.4 | CCND2 |
| CCNE1 | 0.1 | 1.1 *** | 0.3 | 0.4 | −0.3 ** | 1.5 ** | −0.3 | 0.9 * | −0.3 | −0.3 * | CCNE1 |
| CDK6 | 0.2 | 0 | 0.1 | 0.5 | 0.6 | 0.0 | 0.2 | −1.0 ** | −0.1 | 0.1 | CDK6 |
| CDKN1A | 1.3 * | 1.6 *** | 0.1 | 0.2 | 0.4 | 1.5 ** | 0.6 | 0.0 | −0.1 | 0.2 | CDKN1A |
| GADD45A | 2.1 * | 2.5 ** | 0.6 | 0.5 | 1.9 ** | 1.4 *** | 1.7 | 0.6 | 0.4 | 1.3 * | GADD45A |
| GADD45B | 0.2 ** | 1.3 *** | 0.2 | −0.4 | 0.3 * | 1.3 * | 0.1 | 0.1 | 0.0 | 0.6 * | GADD45B |
| GADD45G | 1,8 ** | 4.3 *** | n.d. | n.d. | 1.5 ** | 1.6 * | 0.8 ** | 0.4 | 0.5 | 0.3 | GADD45G |
| IGFBP3 | −0.2 | −0.8 *** | 0.8 * | −0.5 | 1.1 ** | 1.2 * | 0.5 | 0.6 | −0.2 | 0.2 | IGFBP3 |
| PMAIP1 | 1.4 ** | 1.3 *** | 0.4 * | 0.5 | 1.0 *** | 2.5 *** | 0.5 | 0.5 | 0.4 | 1.0 ** | PMAIP1 |
| SESN2 | 2.5 ** | 2.2 *** | 1.0 | 0.2 | 1.2 ** | 1.8 | 1.1 * | 1.8 * | 0.4 | 0.8 * | SESN2 |
| STEAP3 | −0.4 *** | −0.9 *** | 0.1 | −1.0 ** | 0.2 * | 0.2 | 0.0 | 0.0 | 0.0 | 0.1 | STEAP3 |
| ZMAT3 | 0.2 | 0.1 | n.d. | n.d. | n.d. | n.d. | 0.3 | -0.4 | n.d. | n.d. | ZMAT3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güttler, A.; Weinholdt, C.; Ruff, E.; Reidt, J.; Darnstaedt, E.; Wildemann, A.; Petrenko, M.; Keßler, J.; Kappler, M.; Grosse, I.; et al. SESN2 Knockdown Increases Betulinic Acid-Induced Radiosensitivity of Hypoxic Breast Cancer Cells. Cells 2023, 12, 177. https://doi.org/10.3390/cells12010177
Güttler A, Weinholdt C, Ruff E, Reidt J, Darnstaedt E, Wildemann A, Petrenko M, Keßler J, Kappler M, Grosse I, et al. SESN2 Knockdown Increases Betulinic Acid-Induced Radiosensitivity of Hypoxic Breast Cancer Cells. Cells. 2023; 12(1):177. https://doi.org/10.3390/cells12010177
Chicago/Turabian StyleGüttler, Antje, Claus Weinholdt, Elisabeth Ruff, Judith Reidt, Elisa Darnstaedt, Alicia Wildemann, Marina Petrenko, Jacqueline Keßler, Matthias Kappler, Ivo Grosse, and et al. 2023. "SESN2 Knockdown Increases Betulinic Acid-Induced Radiosensitivity of Hypoxic Breast Cancer Cells" Cells 12, no. 1: 177. https://doi.org/10.3390/cells12010177
APA StyleGüttler, A., Weinholdt, C., Ruff, E., Reidt, J., Darnstaedt, E., Wildemann, A., Petrenko, M., Keßler, J., Kappler, M., Grosse, I., Vordermark, D., & Bache, M. (2023). SESN2 Knockdown Increases Betulinic Acid-Induced Radiosensitivity of Hypoxic Breast Cancer Cells. Cells, 12(1), 177. https://doi.org/10.3390/cells12010177










