The Consequences of GBA Deficiency in the Autophagy–Lysosome System in Parkinson’s Disease Associated with GBA
Abstract
1. Introduction
2. Parkinson’s Disease and GBA
2.1. Parkinson’s Disease and the Lysosomal System
2.2. Parkinson’s Disease Associated with GBA
3. The GBA Gene Encodes the β-Glucocerebrosidase Enzyme
4. Lipid Metabolism Alterations Associated with GBA Deficiency
4.1. Sphingolipid Alterations
4.2. GBA and Lysosomal Cholesterol Metabolism
5. α-Synuclein Metabolism
5.1. α-Synuclein Protein
5.2. α-Synuclein Interaction with Lipid Membranes
5.3. Direct GBA–α-Synuclein Interaction
6. GBA Deficiency and Macroautophagy Dysfunction
6.1. Macroautophagy in Parkinson’s Disease
6.2. Macroautophagy in Parkinson’s Disease Associated with GBA
6.2.1. GBA Inhibition by Conduritol-β-Epoxide (CBE)
6.2.2. Inhibition of GBA Expression
6.2.3. GBA-Knockout Models
6.2.4. Mutant GBA Models
6.2.5. Other Pathways Related to Macroautophagy Dysfunction in PD-GBA
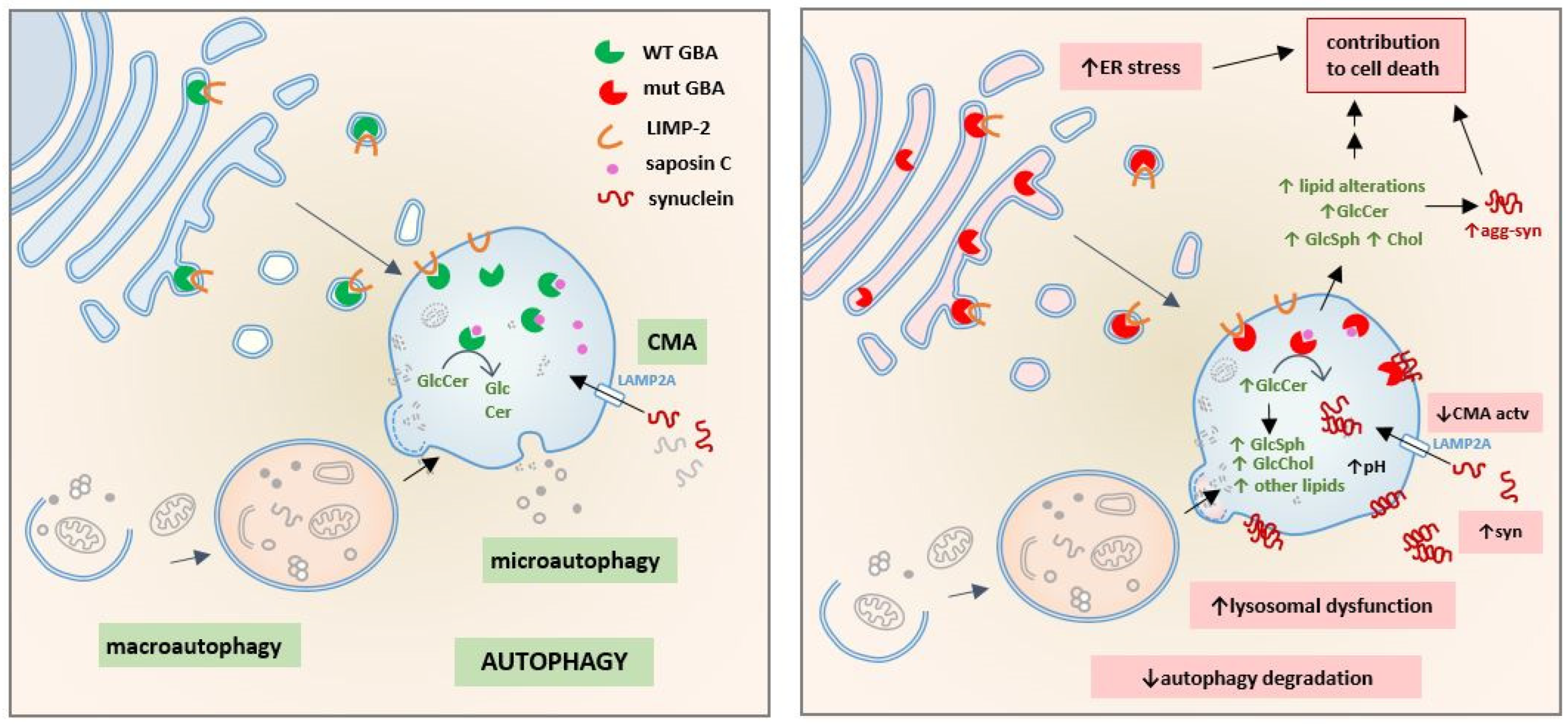
7. Chaperone-Mediated Autophagy Impairment Related to GBA Dysfunction
7.1. CMA Pathway
7.2. CMA in Parkinson’s Disease: CMA-Dependent Degradation of α-Synuclein
7.3. CMA in Parkinson’s Disease Associated with GBA
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Levine, B.; Klionsky, D.J. Autophagy Wins the 2016 Nobel Prize in Physiology or Medicine: Breakthroughs in Baker’s Yeast Fuel Advances in Biomedical Research. Proc. Natl. Acad. Sci. USA 2017, 114, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Tasset, I.; Arias, E.; Pampliega, O.; Wong, E.; Martinez-Vicente, M.; Cuervo, A.M. Autophagy and the Hallmarks of Aging. Ageing Res. Rev. 2021, 72, 101468. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cadwell, K.; Cecconi, F.; Choi, A.M.K.; et al. Autophagy in Major Human Diseases. EMBO J. 2021, 40, 1–63. [Google Scholar] [CrossRef]
- Hara, T.; Nakamura, K.; Matsui, M.; Yamamoto, A.; Nakahara, Y.; Suzuki-Migishima, R.; Yokoyama, M.; Mishima, K.; Saito, I.; Okano, H.; et al. Suppression of Basal Autophagy in Neural Cells Causes Neurodegenerative Disease in Mice. Nature 2006, 441, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Waguri, S.; Chiba, T.; Murata, S.; Iwata, J.I.; Tanida, I.; Ueno, T.; Koike, M.; Uchiyama, Y.; Kominami, E.; et al. Loss of Autophagy in the Central Nervous System Causes Neurodegeneration in Mice. Nature 2006, 441, 880–884. [Google Scholar] [CrossRef]
- Ahmed, I.; Liang, Y.; Schools, S.; Dawson, V.L.; Dawson, T.M.; Savitt, J.M. Development and Characterization of a New Parkinson’s Disease Model Resulting from Impaired Autophagy. J. Neurosci. 2012, 32, 16503–16509. [Google Scholar] [CrossRef]
- Sato, S.; Uchihara, T.; Fukuda, T.; Noda, S.; Kondo, H.; Saiki, S.; Komatsu, M.; Uchiyama, Y.; Tanaka, K.; Hattori, N. Loss of Autophagy in Dopaminergic Neurons Causes Lewy Pathology and Motor Dysfunction in Aged Mice. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Bourdenx, M.; Martín-Segura, A.; Scrivo, A.; Rodriguez-Navarro, J.A.; Kaushik, S.; Tasset, I.; Diaz, A.; Storm, N.J.; Xin, Q.; Juste, Y.R.; et al. Chaperone-Mediated Autophagy Prevents Collapse of the Neuronal Metastable Proteome. Cell 2021, 184, 2696–2714.e25. [Google Scholar] [CrossRef]
- Martinez-Vicente, M. Autophagy in Neurodegenerative Diseases: From Pathogenic Dysfunction to Therapeutic Modulation. Semin. Cell Dev. Biol. 2015, 40, 115–126. [Google Scholar] [CrossRef]
- Stavoe, A.K.H.; Holzbaur, E.L.F.; Stavoe, A.K.H.; Holzbaur, E.L.F.; Holzbaur, E.L.F. Neuronal Autophagy Declines Substantially with Age and Is Rescued by Overexpression of WIPI2 Overexpression of WIPI2. Autophagy 2019, 16, 1–2. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.M. Epidemiology of Parkinson’s Disease. Neurol. Clin. 1992, 10, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective Neuronal Vulnerability in Parkinson Disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K.; Bratzke, H.; Hamm-Clement, J.; Sandmann-Keil, D.; Rüb, U. Staging of the Intracerebral Inclusion Body Pathology Associated with Idiopathic Parkinson’s Disease (Preclinical and Clinical Stages). J. Neurol. 2002, 249, iii1–iii5. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-Synuclein in Lewy Bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Mahul-Mellier, A.-L.; Burtscher, J.; Maharjan, N.; Weerens, L.; Croisier, M.; Kuttler, F.; Leleu, M.; Knott, G.W.; Lashuel, H.A. The Process of Lewy Body Formation, Rather than Simply α-Synuclein Fibrillization, Is One of the Major Drivers of Neurodegeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 4971–4982. [Google Scholar] [CrossRef]
- Klein, A.D.; Mazzulli, J.R. Is Parkinson’s Disease a Lysosomal Disorder? Brain 2018, 141, 2255–2262. [Google Scholar] [CrossRef]
- Goedert, M.; Jakes, R.; Spillantini, M.G. The Synucleinopathies: Twenty Years On. J. Parkinsons. Dis. 2017, 7, S51–S69. [Google Scholar] [CrossRef] [PubMed]
- Bandres-Ciga, S.; Diez-Fairen, M.; Kim, J.J.; Singleton, A.B. Genetics of Parkinson’s Disease: An Introspection of Its Journey towards Precision Medicine. Neurobiol. Dis. 2020, 137, 104782. [Google Scholar] [CrossRef] [PubMed]
- Lesage, S.; Brice, A. Parkinson’s Disease: From Monogenic Forms to Genetic Susceptibility Factors. Hum. Mol. Genet. 2009, 18, R48–R59. [Google Scholar] [CrossRef] [PubMed]
- Simón-Sánchez, J.; Schulte, C.; Bras, J.M.; Sharma, M.; Gibbs, J.R.; Berg, D.; Paisan-Ruiz, C.; Lichtner, P.; Scholz, S.W.; Hernandez, D.G.; et al. Genome-Wide Association Study Reveals Genetic Risk Underlying Parkinson’s Disease. Nat. Genet. 2009, 41, 1308–1312. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Nalls, M.A.; Hallgrímsdóttir, I.B.; Hunkapiller, J.; van der Brug, M.; Cai, F.; Kerchner, G.A.; Ayalon, G.; Bingol, B.; Sheng, M.; et al. A Meta-Analysis of Genome-Wide Association Studies Identifies 17 New Parkinson’s Disease Risk Loci. Nat. Genet. 2017, 49, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Identification of Novel Risk Loci, Causal Insights, and Heritable Risk for Parkinson’s Disease: A Meta-Analysis of Genome-Wide Association Studies. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Nalls, M.A.; Pankratz, N.; Lill, C.M.; Do, C.B.; Hernandez, D.G.; Saad, M.; DeStefano, A.L.; Kara, E.; Bras, J.; Sharma, M.; et al. Large-Scale Meta-Analysis of Genome-Wide Association Data Identifies Six New Risk Loci for Parkinson’s Disease. Nat. Genet. 2014, 46, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Romero, A.; Montpeyó, M.; Martinez-Vicente, M. The Emerging Role of the Lysosome in Parkinson’s Disease. Cells 2020, 9, 2399. [Google Scholar] [CrossRef]
- Sidransky, E.; Samaddar, T.; Tayebi, N. Mutations in GBA Are Associated with Familial Parkinson Disease Susceptibility and Age at Onset. Neurology 2009, 73, 1424–1425, author reply 1425-6. [Google Scholar] [CrossRef]
- Sidransky, E.; Nalls, M.A.A.; Aasly, J.O.O.; Aharon-Peretz, J.; Annesi, G.; Barbosa, E.R.R.; Bar-Shira, A.; Berg, D.; Bras, J.; Brice, A.; et al. Multicenter Analysis of Glucocerebrosidase Mutations in Parkinson’s Disease. N. Engl. J. Med. 2009, 361, 1651–1661. [Google Scholar] [CrossRef]
- McNeill, A.; Duran, R.; Hughes, D.A.; Mehta, A.; Schapira, A.H.V. A Clinical and Family History Study of Parkinson’s Disease in Heterozygous Glucocerebrosidase Mutation Carriers. J. Neurol. Neurosurg. Psychiatry 2012, 83, 853–854. [Google Scholar] [CrossRef]
- Vieira, S.R.L.; Schapira, A.H.V. Glucocerebrosidase Mutations and Parkinson Disease. J. Neural Transm. 2022, 129, 1105–1117. [Google Scholar] [CrossRef]
- Smith, L.; Schapira, A.H.V. V GBA Variants and Parkinson Disease: Mechanisms and Treatments. Cells 2022, 11, 1261. [Google Scholar] [CrossRef]
- Schapira, A.H. V Glucocerebrosidase and Parkinson Disease: Recent Advances. Mol. Cell. Neurosci. 2015, 66, 37–42. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, G.; Desouza, R.M.; Balestrino, R.; Schapira, A.H.; O’ Regan, G.; Balestrino, R.; Schapira, A.H. Glucocerebrosidase Mutations in Parkinson Disease. J. Parkinsons. Dis. 2017, 7, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Höglinger, G.; Schulte, C.; Jost, W.H.; Storch, A.; Woitalla, D.; Krüger, R.; Falkenburger, B.; Brockmann, K.; Storch, A.; Woitalla, D.; et al. GBA-Associated PD: Chances and Obstacles for Targeted Treatment Strategies. J. Neural Transm. 2022, 129, 1219–1233. [Google Scholar] [CrossRef]
- Neumann, J.; Bras, J.; Deas, E.; O’sullivan, S.S.; Parkkinen, L.; Lachmann, R.H.; Li, A.; Holton, J.; Guerreiro, R.; Paudel, R.; et al. Glucocerebrosidase Mutations in Clinical and Pathologically Proven Parkinson’s Disease. Brain 2009, 132, 1783–1794. [Google Scholar] [CrossRef]
- Gan-Or, Z.; Giladi, N.; Rozovski, U.; Shifrin, C.; Rosner, S.; Gurevich, T.; Bar-Shira, A.; Orr-Urtreger, A. Genotype-Phenotype Correlations between GBA Mutations and Parkinson Disease Risk and Onset. Neurology 2008, 70, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Cilia, R.; Tunesi, S.; Marotta, G.; Cereda, E.; Siri, C.; Tesei, S.; Zecchinelli, A.L.; Canesi, M.; Mariani, C.B.; Meucci, N.; et al. Survival and Dementia in GBA-Associated Parkinson’s Disease: The Mutation Matters. Ann. Neurol. 2016, 80, 662–673. [Google Scholar] [CrossRef]
- Liu, G.; Boot, B.; Locascio, J.J.; Jansen, I.E.; Winder-Rhodes, S.; Eberly, S.; Elbaz, A.; Brice, A.; Ravina, B.; van Hilten, J.J.; et al. Specifically Neuropathic Gaucher’s Mutations Accelerate Cognitive Decline in Parkinson’s. Ann. Neurol. 2016, 80, 674–685. [Google Scholar] [CrossRef]
- Thaler, A.; Bregman, N.; Gurevich, T.; Shiner, T.; Dror, Y.; Zmira, O.; Gan-Or, Z.; Bar-Shira, A.; Gana-Weisz, M.; Orr-Urtreger, A.; et al. Parkinson’s Disease Phenotype Is Influenced by the Severity of the Mutations in the GBA Gene. Parkinsonism Relat. Disord. 2018, 55, 45–49. [Google Scholar] [CrossRef]
- Anheim, M.; Elbaz, A.; Lesage, S.; Durr, A.; Condroyer, C.; Viallet, F.; Pollak, P.; Bonaïti, B.; Bonaïti-Pellié, C.; Brice, A. Penetrance of Parkinson Disease in Glucocerebrosidase Gene Mutation Carriers. Neurology 2012, 78, 417–420. [Google Scholar] [CrossRef]
- Lerche, S.; Schulte, C.; Wurster, I.; Machetanz, G.; Roeben, B.; Zimmermann, M.; Deuschle, C.; Hauser, A.-K.K.; Böhringer, J.; Krägeloh-Mann, I.; et al. The Mutation Matters: CSF Profiles of GCase, Sphingolipids, α-Synuclein in PDGBA. Mov. Disord. 2021, 36, 1216–1228. [Google Scholar] [CrossRef]
- Lerche, S.; Wurster, I.; Roeben, B.; Zimmermann, M.; Riebenbauer, B.; Deuschle, C.; Hauser, A.; Schulte, C.; Berg, D.; Maetzler, W. Parkinson’s Disease: Glucocerebrosidase 1 Mutation Severity Is Associated with CSF Alpha-Synuclein Pro Fi Les. Mov. Disord. 2020, 35, 495–499. [Google Scholar] [CrossRef]
- Nalls, M.A.; Duran, R.; Lopez, G.; Kurzawa-Akanbi, M.; McKeith, I.G.; Chinnery, P.F.; Morris, C.M.; Theuns, J.; Crosiers, D.; Cras, P.; et al. A Multicenter Study of Glucocerebrosidase Mutations in Dementia with Lewy Bodies. JAMA Neurol. 2013, 70, 727. [Google Scholar] [CrossRef] [PubMed]
- Greuel, A.; Trezzi, J.P.; Glaab, E.; Ruppert, M.C.; Maier, F.; Jäger, C.; Hodak, Z.; Lohmann, K.; Ma, Y.; Eidelberg, D.; et al. GBA Variants in Parkinson’s Disease: Clinical, Metabolomic, and Multimodal Neuroimaging Phenotypes. Mov. Disord. 2020, 35, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Krohn, L.; Ruskey, J.A.; Rudakou, U.; Leveille, E.; Asayesh, F.; Hu, M.T.M.; Arnulf, I.; Dauvilliers, Y.; Högl, B.; Stefani, A.; et al. GBA Variants in REM Sleep Behavior Disorder: A Multicenter Study. Neurology 2020, 95, e1008–e1016. [Google Scholar] [CrossRef] [PubMed]
- Outeiro, T.F.; Koss, D.J.; Erskine, D.; Walker, L.; Kurzawa-Akanbi, M.; Burn, D.; Donaghy, P.; Morris, C.; Taylor, J.-P.; Thomas, A.; et al. Dementia with Lewy Bodies: An Update and Outlook. Mol. Neurodegener. 2019, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Sklerov, M.; Kang, U.J.; Liong, C.; Clark, L.; Marder, K.; Pauciulo, M.; Nichols, W.C.; Chung, W.K.; Honig, L.S.; Cortes, E.; et al. Frequency of GBA Variants in Autopsy-Proven Multiple System Atrophy. Mov. Disord. Clin. Pract. 2017, 4, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, K.; Hagita, H.; Horiguchi, T.; Tanimura, A.; Noma, T. Redefining GBA Gene Structure Unveils the Ability of Cap-Independent, IRES-Dependent Gene Regulation. Commun. Biol. 2022, 5, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Wilder, S.; Horowitz, Z.; Reiner, O.; Gelbart, T.; Beutler, E. The Human Glucocerebrosidase Gene and Pseudogene: Structure and Evolution. Genomics 1989, 4, 87–96. [Google Scholar] [CrossRef]
- Straniero, L.; Rimoldi, V.; Samarani, M.; Goldwurm, S.; Di Fonzo, A.; Krüger, R.; Deleidi, M.; Aureli, M.; Soldà, G.; Duga, S.; et al. The GBAP1 Pseudogene Acts as a CeRNA for the Glucocerebrosidase Gene GBA by Sponging MiR-22-3p. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Brady, R.O.; Kanfer, J.; Shapiro, D. The Metabolism of Glucocerebrosides. I. Purification and Properties of a Glucocerebroside-cleaving Enzyme From Spleen Tissue. J. Biol. Chem. 1965, 240, 39–43. [Google Scholar] [CrossRef]
- Dvir, H.; Harel, M.; McCarthy, A.A.; Toker, L.; Silman, I.; Futerman, A.H.; Sussman, J.L. X-ray Structure of Human Acid-β-glucosidase, the Defective Enzyme in Gaucher Disease. EMBO Rep. 2003, 4, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Mullin, S.; Schapira, A.H.V. Insights into the Structural Biology of Gaucher Disease. Exp. Neurol. 2017, 298, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Reczek, D.; Schwake, M.; Schröder, J.; Hughes, H.; Blanz, J.; Jin, X.; Brondyk, W.; Van Patten, S.; Edmunds, T.; Saftig, P. LIMP-2 Is a Receptor for Lysosomal Mannose-6-Phosphate-Independent Targeting of β-Glucocerebrosidase. Cell 2007, 131, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Malini, E.; Zampieri, S.; Deganuto, M.; Romanello, M.; Sechi, A.; Bembi, B.; Dardis, A. Role of LIMP-2 in the Intracellular Trafficking of β-Glucosidase in Different Human Cellular Models. FASEB J. 2015, 29, 3839–3852. [Google Scholar] [CrossRef]
- Blanz, J.; Zunke, F.; Markmann, S.; Damme, M.; Braulke, T.; Saftig, P.; Schwake, M. Mannose 6-Phosphate-Independent Lysosomal Sorting of LIMP-2. Traffic 2015, 16, 1127–1136. [Google Scholar] [CrossRef]
- Zachos, C.; Blanz, J.; Saftig, P.; Schwake, M. A Critical Histidine Residue Within LIMP-2 Mediates PH Sensitive Binding to Its Ligand β-Glucocerebrosidase. Traffic 2012, 13, 1113–1123. [Google Scholar] [CrossRef]
- Liou, B.; Haffey, W.D.; Greis, K.D.; Grabowski, G.A. The LIMP-2/SCARB2 Binding Motif on Acid β-Glucosidase. J. Biol. Chem. 2014, 289, 30063–30074. [Google Scholar] [CrossRef]
- Zunke, F.; Andresen, L.; Wesseler, S.; Groth, J.; Arnold, P.; Rothaug, M.; Mazzulli, J.R.; Krainc, D.; Blanz, J.; Saftig, P.; et al. Characterization of the Complex Formed by β-Glucocerebrosidase and the Lysosomal Integral Membrane Protein Type-2. Proc. Natl. Acad. Sci. USA 2016, 113, 3791–3796. [Google Scholar] [CrossRef]
- Abdul-Hammed, M.; Breiden, B.; Schwarzmann, G.; Sandhoff, K. Lipids Regulate the Hydrolysis of Membrane Bound Glucosylceramide by Lysosomal β-Glucocerebrosidase. J. Lipid Res. 2017, 58, 563–577. [Google Scholar] [CrossRef]
- Atrian, S.; López-Viñas, E.; Gómez-Puertas, P.; Chabás, A.; Vilageliu, L.; Grinberg, D. An Evolutionary and Structure-based Docking Model for Glucocerebrosidase–saposin C and Glucocerebrosidase–substrate Interactions—Relevance for Gaucher Disease. Proteins Struct. Funct. Bioinforma. 2008, 70, 882–891. [Google Scholar] [CrossRef]
- Aerts, J.M.F.G.; Sa Miranda, M.C.; Brouwer-Kelder, E.M.; Van Weely, S.; Barranger, J.A.; Tager, J.M. Conditions Affecting the Activity of Glucocerebrosidase Purified from Spleens of Control Subjects and Patients with Type 1 Gaucher Disease. Biochim. Biophys. Acta 1990, 1041, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wilkening, G.; Linke, T.; Sandhoff, K. Lysosomal Degradation on Vesicular Membrane Surfaces. J. Biol. Chem. 1998, 273, 30271–30278. [Google Scholar] [CrossRef] [PubMed]
- Salvioli, R.; Tatti, M.; Scarpa, S.; Moavero, S.M.; Ciaffoni, F.; Felicetti, F.; Kaneski, C.R.; Brady, R.O.; Vaccaro, A.M. The N370S (Asn370→Ser) Mutation Affects the Capacity of Glucosylceramidase to Interact with Anionic Phospholipid-Containing Membranes and Saposin C. Biochem. J. 2005, 390, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Futerman, A.H.; Platt, F.M. The Metabolism of Glucocerebrosides—From 1965 to the Present. Mol. Genet. Metab. 2017, 120, 22–26. [Google Scholar] [CrossRef]
- Hein, L.K.; Rozaklis, T.; Adams, M.K.; Hopwood, J.J.; Karageorgos, L. Lipid Composition of Microdomains Is Altered in Neuronopathic Gaucher Disease Sheep Brain and Spleen. Mol. Genet. Metab. 2017, 121, 259–270. [Google Scholar] [CrossRef]
- Karageorgos, L.; Hein, L.; Rozaklis, T.; Adams, M.; Duplock, S.; Snel, M.; Hemsley, K.; Kuchel, T.; Smith, N.; Hopwood, J.J. Glycosphingolipid Analysis in a Naturally Occurring Ovine Model of Acute Neuronopathic Gaucher Disease. Neurobiol. Dis. 2016, 91, 143–154. [Google Scholar] [CrossRef]
- Ghauharali-van der Vlugt, K.; Langeveld, M.; Poppema, A.; Kuiper, S.; Hollak, C.E.M.; Aerts, J.M.; Groener, J.E.M. Prominent Increase in Plasma Ganglioside GM3 Is Associated with Clinical Manifestations of Type I Gaucher Disease. Clin. Chim. Acta 2008, 389, 109–113. [Google Scholar] [CrossRef]
- Rolfs, A.; Giese, A.K.; Grittner, U.; Mascher, D.; Elstein, D.; Zimran, A.; Böttcher, T.; Lukas, J.; Hübner, R.; Gölnitz, U.; et al. Glucosylsphingosine Is a Highly Sensitive and Specific Biomarker for Primary Diagnostic and Follow-up Monitoring in Gaucher Disease in a Non-Jewish, Caucasian Cohort of Gaucher Disease Patients. PLoS ONE 2013, 11, e79732. [Google Scholar] [CrossRef]
- Murugesan, V.; Chuang, W.L.; Liu, J.; Lischuk, A.; Kacena, K.; Lin, H.; Pastores, G.M.; Yang, R.; Keutzer, J.; Zhang, K.; et al. Glucosylsphingosine Is a Key Biomarker of Gaucher Disease. Am. J. Hematol. 2016, 91, 1082–1089. [Google Scholar] [CrossRef]
- Dekker, N.; Van Dussen, L.; Hollak, C.E.M.; Overkleeft, H.; Scheij, S.; Ghauharali, K.; Van Breemen, M.J.; Ferraz, M.J.; Groener, J.E.M.; Maas, M.; et al. Elevated Plasma Glucosylsphingosine in Gaucher Disease: Relation to Phenotype, Storage Cell Markers, and Therapeutic Response. Blood 2011, 118, e118–e127. [Google Scholar] [CrossRef]
- Muñoz, S.S.; Petersen, D.; Marlet, F.R.; Kücükköse, E.; Galvagnion, C. The Interplay between Glucocerebrosidase, α-Synuclein and Lipids in Human Models of Parkinson’s Disease. Biophys. Chem. 2021, 273, 106534. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.M.; Smith, G.A.; Park, E.; Cao, H.; Brown, E.; Hallett, P.; Isacson, O. Progressive Decline of Glucocerebrosidase in Aging and Parkinson’s Disease. Ann. Clin. Transl. Neurol. 2015, 2, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Romero, A.; Fernandez-Gonzalez, I.; Riera, J.; Montpeyo, M.; Albert-Bayo, M.; Lopez-Royo, T.; Castillo-Sanchez, P.; Carnicer-Caceres, C.; Arranz-Amo, J.A.; Castillo-Ribelles, L.; et al. Lysosomal Lipid Alterations Caused by Glucocerebrosidase Deficiency Promote Lysosomal Dysfunction, Chaperone-Mediated-Autophagy Deficiency, and Alpha-Synuclein Pathology. npj Park. Dis. 2022, 8, 126. [Google Scholar] [CrossRef]
- Srikanth, M.P.; Jones, J.W.; Kane, M.; Awad, O.; Park, T.S.; Zambidis, E.T.; Feldman, R.A. Elevated Glucosylsphingosine in Gaucher Disease Induced Pluripotent Stem Cell Neurons Deregulates Lysosomal Compartment through Mammalian Target of Rapamycin Complex 1. Stem Cells Transl. Med. 2021, 10, 1081–1094. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Sasagasako, N.; Goto, I.; Kobayashi, T. The Synthetic Pathway for Glucosylsphingosine in Cultured Fibroblasts. J. Biochem. 1994, 116, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Schueler, U.H.; Kolter, T.; Kaneski, C.R.; Blusztajn, J.K.; Herkenham, M.; Sandhoff, K.; Brady, R.O. Toxicity of Glucosylsphingosine (Glucopsychosine) to Cultured Neuronal Cells: A Model System for Assessing Neuronal Damage in Gaucher Disease Type 2 and 3. Neurobiol. Dis. 2003, 14, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, M.; Ferra, M.J.; Boot, R.G.; Aerts, J.M.F.G. Lyso-Glycosphingolipids: Presence and Consequences. Essays Biochem. 2020, 64, 565–578. [Google Scholar]
- van der Poel, S.; Wolthoorn, J.; van den Heuvel, D.; Egmond, M.; Groux-Degroote, S.; Neumann, S.; Gerritsen, H.; van Meer, G.; Sprong, H. Hyperacidification of Trans-Golgi Network and Endo/Lysosomes in Melanocytes by Glucosylceramide-Dependent V-ATPase Activity. Traffic 2011, 12, 1634–1647. [Google Scholar] [CrossRef]
- Sillence, D.J. Glucosylceramide Modulates Endolysosomal PH in Gaucher Disease. Mol. Genet. Metab. 2013, 109, 194–200. [Google Scholar] [CrossRef]
- Sillence, D.J.; Puri, V.; Marks, D.L.; Butters, T.D.; Dwek, R.A.; Pagano, R.E.; Platt, F.M. Glucosylceramide Modulates Membrane Traffic along the Endocytic Pathway. J. Lipid Res. 2002, 43, 1837–1845. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeon, S.; Burbulla, L.F.; Krainc, D. Acid Ceramidase Inhibition Ameliorates a Synuclein Accumulation upon Loss of GBA1 Function. Hum. Mol. Genet. 2018, 27, 1972–1988. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liou, B.; Lin, Y.; Fannin, V.; Zhang, W.; Feldman, R.A.; Setchell, K.D.R.R.; Grabowski, G.A.; Sun, Y. Substrate Reduction Therapy Reverses Mitochondrial, MTOR, and Autophagy Alterations in a Cell Model of Gaucher Disease. Cells 2021, 10, 2286. [Google Scholar] [CrossRef] [PubMed]
- Ballabio, A.; Bonifacino, J.S. Lysosomes as Dynamic Regulators of Cell and Organismal Homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.M.; Zoncu, R. The Lysosome as a Regulatory Hub. Annu. Rev. Cell Dev. Biol. 2016, 32, 223–253. [Google Scholar] [CrossRef]
- Rabanal-Ruiz, Y.; Korolchuk, V. MTORC1 and Nutrient Homeostasis: The Central Role of the Lysosome. Int. J. Mol. Sci. 2018, 19, 818. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. MTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Rabanal-Ruiz, Y.; Otten, E.G.; Korolchuk, V.I. MTORC1 as the Main Gateway to Autophagy. Essays Biochem. 2017, 61, 565–584. [Google Scholar]
- Meng, Y.; Heybrock, S.; Neculai, D.; Saftig, P. Cholesterol Handling in Lysosomes and Beyond. Trends Cell Biol. 2020, 30, 452–466. [Google Scholar] [CrossRef]
- García-Sanz, P.; Orgaz, L.; Bueno-Gil, G.; Espadas, I.; Rodríguez-Traver, E.; Kulisevsky, J.; Gutierrez, A.; Dávila, J.C.; González-Polo, R.A.; Fuentes, J.M.; et al. N370S -GBA1 Mutation Causes Lysosomal Cholesterol Accumulation in Parkinson’s Disease. Mov. Disord. 2017, 32, 1409–1422. [Google Scholar] [CrossRef]
- Akiyama, H.; Kobayashi, S.; Hirabayashi, Y.; Murakami-Murofushi, K. Cholesterol Glucosylation Is Catalyzed by Transglucosylation Reaction of β-Glucosidase 1. Biochem. Biophys. Res. Commun. 2013, 441, 838–843. [Google Scholar] [CrossRef]
- Marques, A.A.R.A.; Mirzaian, M.; Akiyama, H.; Wisse, P.; Ferraz, M.J.; Gaspar, P.; Ghauharali-van der Vlugt, K.; Meijer, R.; Giraldo, P.; Alfonso, P.; et al. Glucosylated Cholesterol in Mammalian Cells and Tissues: Formation and Degradation by Multiple Cellular β-Glucosidases. J. Lipid Res. 2016, 57, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Sánchez-Arias, J.A.; Navarro, G.; Lanciego, J.L. Glucocerebrosidase Mutations and Synucleinopathies. Potential Role of Sterylglucosides and Relevance of Studying both GBA1 and GBA2 Genes. Front. Neuroanat. 2018, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.S.; Cheng, T.-W.; Ysselstein, D.; Heybrock, S.; Hoth, L.R.; Chrunyk, B.A.; am Ende, C.W.; Krainc, D.; Schwake, M.; Saftig, P.; et al. Lysosomal Integral Membrane Protein-2 as a Phospholipid Receptor Revealed by Biophysical and Cellular Studies. Nat. Commun. 2017, 8, 1908. [Google Scholar] [CrossRef] [PubMed]
- Neculai, D.; Schwake, M.; Ravichandran, M.; Zunke, F.; Collins, R.F.; Peters, J.; Neculai, M.; Plumb, J.; Loppnau, P.; Pizarro, J.C.; et al. Structure of LIMP-2 Provides Functional Insights with Implications for SR-BI and CD36. Nature 2013, 504, 172–176. [Google Scholar] [CrossRef]
- Heybrock, S.; Kanerva, K.; Meng, Y.; Ing, C.; Liang, A.; Xiong, Z.-J.J.; Weng, X.; Ah Kim, Y.; Collins, R.; Trimble, W.; et al. Lysosomal Integral Membrane Protein-2 (LIMP-2/SCARB2) Is Involved in Lysosomal Cholesterol Export. Nat. Commun. 2019, 10, 3521. [Google Scholar] [CrossRef]
- van der Lienden, M.J.C.; Aten, J.; Marques, A.R.A.; Waas, I.S.E.; Larsen, P.W.B.; Claessen, N.; van der Wel, N.N.; Ottenhoff, R.; van Eijk, M.; Aerts, J.M.F.G. GCase and LIMP2 Abnormalities in the Liver of Niemann Pick Type C Mice. Int. J. Mol. Sci. 2021, 22, 2532. [Google Scholar] [CrossRef]
- Castellano, B.M.; Thelen, A.M.; Moldavski, O.; Feltes, M.; van der Welle, R.E.N.; Mydock-McGrane, L.; Jiang, X.; van Eijkeren, R.J.; Davis, O.B.; Louie, S.M.; et al. Lysosomal Cholesterol Activates MTORC1 via an SLC38A9–Niemann-Pick C1 Signaling Complex. Science 2017, 355, 1306–1311. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, J.A.; Cuervo, A.M. Dietary Lipids and Aging Compromise Chaperone-Mediated Autophagy by Similar Mechanisms. Autophagy 2012, 8, 1152–1154. [Google Scholar] [CrossRef][Green Version]
- Kaushik, S.; Massey, A.C.; Cuervo, A.M. Lysosome Membrane Lipid Microdomains: Novel Regulators of Chaperone-Mediated Autophagy. EMBO J. 2006, 25, 3921–3933. [Google Scholar] [CrossRef]
- Villar-Piqué, A.; Lopes da Fonseca, T.; Outeiro, T.F. Structure, Function and Toxicity of Alpha-Synuclein: The Bermuda Triangle in Synucleinopathies. J. Neurochem. 2016, 139, 240–255. [Google Scholar] [CrossRef]
- Ulmer, T.S.; Bax, A.; Cole, N.B.; Nussbaum, R.L. Structure and Dynamics of Micelle-Bound Human Alpha-Synuclein. J. Biol. Chem. 2005, 280, 9595–9603. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.R.; Rhoades, E. Effects of Curvature and Composition on α-Synuclein Binding to Lipid Vesicles. Biophys. J. 2010, 99, 2279–2288. [Google Scholar] [CrossRef] [PubMed]
- Kamp, F.; Beyer, K. Binding of α-Synuclein Affects the Lipid Packing in Bilayers of Small Vesicles. J. Biol. Chem. 2006, 281, 9251–9259. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Wang, S.; Slone, S.R.; Yacoubian, T.A.; Witt, S.N. Defects in Very Long Chain Fatty Acid Synthesis Enhance Alpha-Synuclein Toxicity in a Yeast Model of Parkinson’s Disease. PLoS ONE 2011, 6, e15946. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leftin, A.; Job, C.; Beyer, K.; Brown, M.F. Solid-State 13C NMR Reveals Annealing of Raft-like Membranes Containing Cholesterol by the Intrinsically Disordered Protein α-Synuclein. J. Mol. Biol. 2013, 425, 2973–2987. [Google Scholar] [CrossRef]
- Varkey, J.; Isas, J.M.; Mizuno, N.; Jensen, M.B.; Bhatia, V.K.; Jao, C.C.; Petrlova, J.; Voss, J.C.; Stamou, D.G.; Steven, A.C.; et al. Membrane Curvature Induction and Tubulation Are Common Features of Synucleins and Apolipoproteins. J. Biol. Chem. 2010, 285, 32486–32493. [Google Scholar] [CrossRef]
- Rocha, S.; Kumar, R.; Nordén, B.; Wittung-Stafshede, P. Orientation of α-Synuclein at Negatively Charged Lipid Vesicles: Linear Dichroism Reveals Time-Dependent Changes in Helix Binding Mode. J. Am. Chem. Soc. 2021, 143, 18899–18906. [Google Scholar] [CrossRef]
- Fantini, J.; Carlus, D.; Yahi, N. The Fusogenic Tilted Peptide (67–78) of α-Synuclein Is a Cholesterol Binding Domain. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2343–2351. [Google Scholar] [CrossRef]
- Varkey, J.; Mizuno, N.; Hegde, B.G.; Cheng, N.; Steven, A.C.; Langen, R. α-Synuclein Oligomers with Broken Helical Conformation Form Lipoprotein Nanoparticles. J. Biol. Chem. 2013, 288, 17620–17630. [Google Scholar] [CrossRef]
- Koob, A.O.; Paulino, A.D.; Masliah, E. GFAP Reactivity, Apolipoprotein E Redistribution and Cholesterol Reduction in Human Astrocytes Treated with α-Synuclein. Neurosci. Lett. 2010, 469, 11–14. [Google Scholar] [CrossRef]
- Ronzitti, G.; Bucci, G.; Emanuele, M.; Leo, D.; Sotnikova, T.D.; Mus, L.V.; Soubrane, C.H.; Dallas, M.L.; Thalhammer, A.; Cingolani, L.A.; et al. Exogenous -Synuclein Decreases Raft Partitioning of Cav2.2 Channels Inducing Dopamine Release. J. Neurosci. 2014, 34, 10603–10615. [Google Scholar] [CrossRef] [PubMed]
- Perissinotto, F.; Rondelli, V.; Parisse, P.; Tormena, N.; Zunino, A.; Almásy, L.; Merkel, D.G.; Bottyán, L.; Sajti, S.; Casalis, L. GM1 Ganglioside Role in the Interaction of Alpha-Synuclein with Lipid Membranes: Morphology and Structure. Biophys. Chem. 2019, 255, 106272. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.; Pallbo, J.; Weininger, U.; Linse, S.; Sparr, E. Ganglioside Lipids Accelerate α-Synuclein Amyloid Formation. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Martinez, Z.; Zhu, M.; Han, S.; Fink, A.L. GM1 Specifically Interacts with α-Synuclein and Inhibits Fibrillation. Biochemistry 2007, 46, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, E.I.; Jiang, Z.; Strub, M.P.; Lee, J.C. Effects of Phosphatidylcholine Membrane Fluidity on the Conformation and Aggregation of N-Terminally Acetylated α-Synuclein. J. Biol. Chem. 2018, 293, 11195–11205. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Jacoby, G.; Laor Bar-Yosef, D.; Beck, R.; Gazit, E.; Segal, D. Glucosylceramide Associated with Gaucher Disease Forms Amyloid-like Twisted Ribbon Fibrils That Induce α-Synuclein Aggregation. ACS Nano 2021, 15, 11854–11868. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, Y.V.; Liu, J.; Ruan, J.; Pacheco, J.; Zhang, X.; Abbasi, J.; Keutzer, J.; Mistry, P.K.; Chandra, S.S. Glucosylsphingosine Promotes α-Synuclein Pathology in Mutant GBA-Associated Parkinson’s Disease. J. Neurosci. 2017, 37, 9617–9631. [Google Scholar] [CrossRef]
- Yap, T.L.; Gruschus, J.M.; Velayati, A.; Westbroek, W.; Goldin, E.; Moaven, N.; Sidransky, E.; Lee, J.C. Alpha-Synuclein Interacts with Glucocerebrosidase Providing a Molecular Link between Parkinson and Gaucher Diseases. J. Biol. Chem. 2011, 286, 28080–28088. [Google Scholar] [CrossRef]
- Kaur, U.; Lee, J.C. Membrane Interactions of α-Synuclein Probed by Neutrons and Photons. Acc. Chem. Res. 2021, 54, 302–310. [Google Scholar] [CrossRef]
- Yap, T.L.; Jiang, Z.; Heinrich, F.; Gruschus, J.M.; Pfefferkorn, C.M.; Barros, M.; Curtis, J.E.; Sidransky, E.; Lee, J.C. Structural Features of Membrane-Bound Glucocerebrosidase and α-Synuclein Probed by Neutron Reflectometry and Fluorescence Spectroscopy. J. Biol. Chem. 2015, 290, 744–754. [Google Scholar] [CrossRef]
- Yap, T.L.; Velayati, A.; Sidransky, E.; Lee, J.C. Membrane-Bound α-Synuclein Interacts with Glucocerebrosidase and Inhibits Enzyme Activity. Mol. Genet. Metab. 2013, 108, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.L.; Gruschus, J.M.; Velayati, A.; Sidransky, E.; Lee, J.C. Saposin C Protects Glucocerebrosidase against α-Synuclein Inhibition. Biochemistry 2013, 52, 7161–7163. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B. Autophagy in Human Diseases. N. Engl. J. Med. 2020, 383, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Lamark, T.; Johansen, T. Mechanisms of Selective Autophagy. Annu. Rev. Cell Dev. Biol. 2021, 37, 143–169. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [PubMed]
- Dermentzaki, G.; Dimitriou, E.; Xilouri, M.; Michelakakis, H.; Stefanis, L. Loss of β-Glucocerebrosidase Activity Does Not Affect Alpha-Synuclein Levels or Lysosomal Function in Neuronal Cells. PLoS ONE 2013, 8, e60674. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, J.; Gegg, M.E.; Migdalska-Richards, A.; Doherty, M.K.; Whitfield, P.D.; Schapira, A.H.V. Autophagic Lysosome Reformation Dysfunction in Glucocerebrosidase Deficient Cells: Relevance to Parkinson Disease. Hum. Mol. Genet. 2016, 25, 3432–3445. [Google Scholar] [CrossRef]
- Rocha, E.M.; Smith, G.A.; Park, E.; Cao, H.; Brown, E.; Hayes, M.A.; Beagan, J.; McLean, J.R.; Izen, S.C.; Perez-Torres, E.; et al. Glucocerebrosidase Gene Therapy Prevents α-Synucleinopathy of Midbrain Dopamine Neurons. Neurobiol. Dis. 2015, 82, 495–503. [Google Scholar] [CrossRef]
- Mazzulli, J.R.; Xu, Y.-H.; Sun, Y.; Knight, A.L.; McLean, P.J.; Caldwell, G.A.; Sidransky, E.; Grabowski, G.A.; Krainc, D. Gaucher Disease Glucocerebrosidase and α-Synuclein Form a Bidirectional Pathogenic Loop in Synucleinopathies. Cell 2011, 146, 37–52. [Google Scholar] [CrossRef]
- Du, T.-T.; Wang, L.; Duan, C.-L.; Lu, L.-L.; Zhang, J.-L.; Gao, G.; Qiu, X.-B.; Wang, X.-M.; Yang, H. GBA Deficiency Promotes SNCA/α-Synuclein Accumulation through Autophagic Inhibition by Inactivated PPP2A. Autophagy 2015, 11, 1–43. [Google Scholar] [CrossRef]
- Osellame, L.D.; Rahim, A.A.; Hargreaves, I.P.; Gegg, M.E.; Richard-Londt, A.; Brandner, S.; Waddington, S.N.; Schapira, A.H.V.; Duchen, M.R. Mitochondria and Quality Control Defects in a Mouse Model of Gaucher Disease--Links to Parkinson’s Disease. Cell Metab. 2013, 17, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Kinghorn, K.J.; Grönke, S.; Castillo-Quan, J.I.; Woodling, N.S.; Li, L.; Sirka, E.; Gegg, M.; Mills, K.; Hardy, J.; Bjedov, I.; et al. A Drosophila Model of Neuronopathic Gaucher Disease Demonstrates Lysosomal-Autophagic Defects and Altered MTOR Signalling and Is Functionally Rescued by Rapamycin. J. Neurosci. 2016, 36, 11654–11670. [Google Scholar] [CrossRef] [PubMed]
- McNeill, A.; Magalhaes, J.; Shen, C.; Chau, K.-Y.Y.; Hughes, D.; Mehta, A.; Foltynie, T.; Cooper, J.M.; Abramov, A.Y.; Gegg, M.; et al. Ambroxol Improves Lysosomal Biochemistry in Glucocerebrosidase Mutation-Linked Parkinson Disease Cells. Brain 2014, 137, 1481–1495. [Google Scholar] [CrossRef]
- de la Mata, M.; Cotán, D.; Oropesa-Ávila, M.; Garrido-Maraver, J.; Cordero, M.D.; Villanueva Paz, M.; Delgado Pavón, A.; Alcocer-Gómez, E.; De Lavera, I.; Ybot-González, P.; et al. Pharmacological Chaperones and Coenzyme Q10 Treatment Improves Mutant β-Glucocerebrosidase Activity and Mitochondrial Function in Neuronopathic Forms of Gaucher Disease. Sci. Rep. 2015, 5, 10903. [Google Scholar] [CrossRef]
- Collins, L.M.; Drouin-Ouellet, J.; Kuan, W.-L.; Cox, T.; Barker, R.A. Dermal Fibroblasts from Patients with Parkinson’s Disease Have Normal GCase Activity and Autophagy Compared to Patients with PD and GBA Mutations. F1000Research 2017, 6, 1751. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wang, X.; Feng, X.; Zhang, A.; Li, J.; Gu, K.; Huang, J.; Pang, S.; Dong, H.; Gao, H.; et al. Altered Expression of Autophagic Genes in the Peripheral Leukocytes of Patients with Sporadic Parkinson’s Disease. Brain Res. 2011, 1394, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ham, A.; Ma, T.C.; Kuo, S.-H.H.; Kanter, E.; Kim, D.; Ko, H.S.; Quan, Y.; Sardi, S.P.; Li, A.; et al. Mitochondrial Dysfunction and Mitophagy Defect Triggered by Heterozygous GBA Mutations. Autophagy 2019, 15, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Gegg, M.; Chau, D.; Schapira, A. Glucocerebrosidase Activity, Cathepsin D and Monomeric α-Synuclein Interactions in a Stem Cell Derived Neuronal Model of a PD Associated GBA1 Mutation. Neurobiol. Dis. 2020, 134, 104620. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.H.; Tasset, I.; Cheng, M.M.; Diaz, A.; Pan, M.K.; Lieberman, O.J.; Hutten, S.J.; Alcalay, R.N.; Kim, S.; Ximénez-Embún, P.; et al. Mutant Glucocerebrosidase Impairs α-Synuclein Degradation by Blockade of Chaperone-Mediated Autophagy. Sci. Adv. 2022, 8, eabm6393. [Google Scholar] [CrossRef]
- Bae, E.-J.; Yang, N.-Y.; Song, M.; Lee, C.S.; Lee, J.S.; Jung, B.C.; Lee, H.-J.; Kim, S.; Masliah, E.; Sardi, S.P.; et al. Glucocerebrosidase Depletion Enhances Cell-to-Cell Transmission of α-Synuclein. Nat. Commun. 2014, 5, 4755. [Google Scholar] [CrossRef]
- Schöndorf, D.C.; Aureli, M.; McAllister, F.E.; Hindley, C.J.; Mayer, F.; Schmid, B.; Sardi, S.P.; Valsecchi, M.; Hoffmann, S.; Schwarz, L.K.; et al. IPSC-Derived Neurons from GBA1-Associated Parkinson’s Disease Patients Show Autophagic Defects and Impaired Calcium Homeostasis. Nat. Commun. 2014, 5, 4028. [Google Scholar] [CrossRef] [PubMed]
- Awad, O.; Sarkar, C.; Panicker, L.M.; Miller, D.; Zeng, X.; Sgambato, J.A.; Feldman, R.A. Altered TFEB-Mediated Lysosomal Biogenesis in Gaucher Disease IPSC-Derived Neuronal Cells. Hum. Mol. Genet. 2015, 24, 5775–5788. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.J.R.R.; Hartfield, E.M.; Christian, H.C.; Emmanoulidou, E.; Zheng, Y.; Booth, H.; Bogetofte, H.; Lang, C.; Ryan, B.J.; Sardi, S.P.; et al. ER Stress and Autophagic Perturbations Lead to Elevated Extracellular α-Synuclein in GBA-N370S Parkinson’s IPSC-Derived Dopamine Neurons. Stem Cell Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sardi, S.P.; Clarke, J.; Kinnecom, C.; Tamsett, T.J.; Li, L.; Stanek, L.M.; Passini, M.A.; Grabowski, G.A.; Schlossmacher, M.G.; Sidman, R.L.; et al. CNS Expression of Glucocerebrosidase Corrects Alpha-Synuclein Pathology and Memory in a Mouse Model of Gaucher-Related Synucleinopathy. Proc. Natl. Acad. Sci. USA 2011, 108, 12101–12106. [Google Scholar] [CrossRef]
- Polinski, N.K.; Martinez, T.N.; Gorodinsky, A.; Gareus, R.; Sasner, M.; Herberth, M.; Switzer, R.; Ahmad, S.O.; Cosden, M.; Kandebo, M.; et al. Decreased Glucocerebrosidase Activity and Substrate Accumulation of Glycosphingolipids in a Novel GBA1 D409V Knock-in Mouse Model. PLoS ONE 2021, 16, e0252325. [Google Scholar] [CrossRef]
- Johnson, M.E.; Bergkvist, L.; Stetzik, L.; Steiner, J.A.; Meyerdirk, L.; Schulz, E.; Wolfrum, E.; Luk, K.C.; Wesson, D.W.; Krainc, D.; et al. Heterozygous GBA D490V and ATP13a2 Mutations Do Not Exacerbate Pathological α-Synuclein Spread in the Prodromal Preformed Fibrils Model in Young Mice. Neurobiol. Dis. 2021, 159, 105513. [Google Scholar] [CrossRef]
- Aerts, J.M.F.G.; Hollak, C.E.M.; Boot, R.G.; Groener, J.E.M.; Maas, M. Substrate Reduction Therapy of Glycosphingolipid Storage Disorders. J. Inherit. Metab. Dis. 2006, 29, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Stirnemann, J.; Belmatoug, N.; Camou, F.; Serratrice, C.; Froissart, R.; Caillaud, C.; Levade, T.; Astudillo, L.; Serratrice, J.; Brassier, A.; et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int. J. Mol. Sci. 2017, 18, 441. [Google Scholar] [CrossRef]
- Smith, L.J.; Bolsinger, M.M.; Chau, K.-Y.; Gegg, M.E.; Schapira, A.H. V The GBA Variant E326K Is Associated with Alpha-Synuclein Aggregation and Lipid Droplet Accumulation in Human Cell Lines. Hum. Mol. Genet. 2022. [Google Scholar] [CrossRef]
- Jacobson, M.A.; Lipinski, M.M.; Feldman, R.A. MTOR Hyperactivity Mediates Lysosomal Dysfunction in Gaucher’s Disease IPSC- Neuronal Cells. Dis. Model. Mech. 2019, 12, dmm038596. [Google Scholar] [CrossRef]
- Johri, A.; Chandra, A.; Beal, M.F.; Flint Beal, M. PGC-1α, Mitochondrial Dysfunction, and Huntington’s Disease. Free Radic. Biol. Med. 2013, 62, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (4th Edition). Autophagy 2021. [Google Scholar]
- Engedal, N.; Sønstevold, T.; Beese, C.J.; Selladurai, S.; Melcher, T.; Simensen, J.E.; Frankel, L.B.; Urbanucci, A.; Torgersen, M.L. Measuring Autophagic Cargo Flux with Keima-Based Probes. Methods Mol. Biol. 2022, 2445, 99–115. [Google Scholar] [PubMed]
- Luhr, M.; Sætre, F.; Engedal, N. The Long-Lived Protein Degradation Assay: An Efficient Method for Quantitative Determination of the Autophagic Flux of Endogenous Proteins in Adherent Cell Lines. Bio-Protocol 2018, 8, e2836. [Google Scholar] [CrossRef]
- Ivanova, M.M.; Changsila, E.; Iaonou, C.; Goker-Alpan, O. Impaired Autophagic and Mitochondrial Functions Are Partially Restored by ERT in Gaucher and Fabry Diseases. PLoS ONE 2019, 14, e0210617. [Google Scholar] [CrossRef] [PubMed]
- Cleeter, M.W.J.; Chau, K.-Y.; Gluck, C.; Mehta, A.; Hughes, D.A.; Duchen, M.; Wood, N.W.; Hardy, J.; Mark Cooper, J.; Schapira, A.H. Glucocerebrosidase Inhibition Causes Mitochondrial Dysfunction and Free Radical Damage. Neurochem. Int. 2013, 62, 1–7. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, K.; Sun, Y.; Liou, B.; Quinn, B.; Li, R. Multiple Pathogenic Proteins Implicated in Neuronopathic Gaucher Disease Mice. Hum. Mol. Genet. 2014, 23, 3943–3957. [Google Scholar] [CrossRef]
- Yun, S.P.; Kim, D.; Kim, S.; Kim, S.; Karuppagounder, S.S.; Kwon, S.H.; Lee, S.; Kam, T.I.; Lee, S.; Ham, S.; et al. α-Synuclein Accumulation and GBA Deficiency Due to L444P GBA Mutation Contributes to MPTP-Induced Parkinsonism. Mol. Neurodegener. 2018, 13, 1–19. [Google Scholar] [CrossRef]
- Gegg, M.E.; Verona, G.; Schapira, A.H.V. Glucocerebrosidase Deficiency Promotes Release of α-Synuclein Fibrils from Cultured Neurons. Hum. Mol. Genet. 2020, 29, 1716–1728. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Schapira, A.H.; Gardiner, C.; Sargent, I.L.; Wood, M.J.A.; Cooper, J.M. Lysosomal Dysfunction Increases Exosome-Mediated Alpha-Synuclein Release and Transmission. Neurobiol. Dis. 2011, 42, 360–367. [Google Scholar] [CrossRef]
- Cerri, S.; Ghezzi, C.; Ongari, G.; Croce, S.; Avenali, M.; Zangaglia, R.; Di Monte, D.A.; Valente, E.M.; Blandini, F. GBA Mutations Influence the Release and Pathological Effects of Small Extracellular Vesicles from Fibroblasts of Patients with Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 2215. [Google Scholar] [CrossRef] [PubMed]
- Fussi, N.; Höllerhage, M.; Chakroun, T.; Nykänen, N.-P.P.; Rösler, T.W.; Koeglsperger, T.; Wurst, W.; Behrends, C.; Höglinger, G.U. Exosomal Secretion of α-Synuclein as Protective Mechanism after Upstream Blockage of Macroautophagy. Cell Death Dis. 2018, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Poehler, A.-M.; Xiang, W.; Spitzer, P.; May, V.E.L.; Meixner, H.; Rockenstein, E.; Chutna, O.; Outeiro, T.F.; Winkler, J.; Masliah, E.; et al. Autophagy Modulates SNCA/α-Synuclein Release, Thereby Generating a Hostile Microenvironment. Autophagy 2014, 10, 2171–2192. [Google Scholar] [CrossRef] [PubMed]
- Sagini, K.; Buratta, S.; Delo, F.; Pellegrino, R.M.; Giovagnoli, S.; Urbanelli, L.; Emiliani, C. Drug-Induced Lysosomal Impairment Is Associated with the Release of Extracellular Vesicles Carrying Autophagy Markers. Int. J. Mol. Sci. 2021, 22, 12922. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.E.; Vincow, E.S.; Merrihew, G.E.; MacCoss, M.J.; Davis, M.Y.; Pallanck, L.J. Glucocerebrosidase Deficiency Promotes Protein Aggregation through Dysregulation of Extracellular Vesicles. PLoS Genet. 2018, 14, e1007694. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Dice, J.F. A Receptor for the Selective Uptake and Degradation of Proteins by Lysosomes. Science 1996, 273, 501–503. [Google Scholar] [CrossRef]
- Dice, J.F.; Terlecky, S.R.; Chiang, H.L.; Olson, T.S.; Isenman, L.D.; Short-Russell, S.R.; Freundlieb, S.; Terlecky, L.J. A Selective Pathway for Degradation of Cytosolic Proteins by Lysosomes. Semin. Cell Biol. 1990, 1, 449–455. [Google Scholar]
- Kirchner, P.; BourdenxID, M.; Madrigal-MatuteID, J.; Tiano, S.; Diaz, A.; BartholdyID, B.A.; Will, B.; Maria CuervoID, A.; Bourdenx, M.; Madrigal-Matute, J.; et al. Proteome-Wide Analysis of Chaperone-Mediated Autophagy Targeting Motifs. PLoS Biol. 2019, 17, 1–27. [Google Scholar] [CrossRef]
- Bandyopadhyay, U.; Cuervo, A.M. Entering the Lysosome through a Transient Gate by Chaperone-Mediated Autophagy. Autophagy 2008, 4, 1101–1103. [Google Scholar] [CrossRef]
- Bandyopadhyay, U.; Kaushik, S.; Varticovski, L.; Cuervo, A.M. The Chaperone-Mediated Autophagy Receptor Organizes in Dynamic Protein Complexes at the Lysosomal Membrane. Mol. Cell Biol. 2008, 28, 5747–5763. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Rodriguez-Oroz, M.C.; Cooper, J.M.; Caballero, C.; Ferrer, I.; Obeso, J.A.; Schapira, A.H. V Chaperone-Mediated Autophagy Markers in Parkinson Disease Brains. Arch. Neurol. 2010, 67, 1464–1472. [Google Scholar] [CrossRef]
- Murphy, K.E.; Gysbers, A.M.; Abbott, S.K.; Spiro, A.S.; Furuta, A.; Cooper, A.; Garner, B.; Kabuta, T.; Halliday, G.M. Lysosomal-Associated Membrane Protein 2 Isoforms Are Differentially Affected in Early Parkinson’s Disease. Mov. Disord. 2015, 30, 1639–1647. [Google Scholar] [CrossRef]
- Pang, S.; Chen, D.; Zhang, A.; Qin, X.; Yan, B. Genetic Analysis of the LAMP-2 Gene Promoter in Patients with Sporadic Parkinson’s Disease. Neurosci. Lett. 2012, 526, 63–67. [Google Scholar] [CrossRef]
- Sala, G.; Stefanoni, G.; Arosio, A.; Riva, C.; Melchionda, L.; Saracchi, E.; Fermi, S.; Brighina, L.; Ferrarese, C. Reduced Expression of the Chaperone-Mediated Autophagy Carrier Hsc70 Protein in Lymphomonocytes of Patients with Parkinson’s Disease. Brain Res. 2014, 1546, 46–52. [Google Scholar] [CrossRef]
- Papagiannakis, N.; Xilouri, M.; Koros, C.; Simitsi, A.-M.; Stamelou, M.; Maniati, M.; Stefanis, L. Autophagy Dysfunction in Peripheral Blood Mononuclear Cells of Parkinson’s Disease Patients. Neurosci. Lett. 2019, 704, 112–115. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Stafanis, L.; Fredenburg, R.; Lansbury, P.T.; Sulzer, D. Impaired Degradation of Mutant α-Synuclein by Chaperone-Mediated Autophagy. Science 2004, 305, 1292–1295. [Google Scholar] [CrossRef]
- Martinez-Vicente, M.; Talloczy, Z.; Kaushik, S.; Massey, A.C.; Mazzulli, J.; Mosharov, E.V.; Hodara, R.; Fredenburg, R.; Wu, D.C.; Follenzi, A.; et al. Dopamine-Modified α-Synuclein Blocks Chaperone-Mediated Autophagy. J. Clin. Invest 2008, 118, 777–778. [Google Scholar] [CrossRef]
- Mak, S.K.; McCormack, A.L.; Manning-Bog, A.B.; Cuervo, A.M.; Di Monte, D. a Lysosomal Degradation of Alpha-Synuclein in Vivo. J. Biol. Chem. 2010, 285, 13621–13629. [Google Scholar] [CrossRef]
- Vogiatzi, T.; Xilouri, M.; Vekrellis, K.; Stefanis, L. Wild Type Alpha-Synuclein Is Degraded by Chaperone-Mediated Autophagy and Macroautophagy in Neuronal Cells. J. Biol. Chem. 2008, 283, 23542–23556. [Google Scholar] [CrossRef]
- Xilouri, M.; Vogiatzi, T.; Vekrellis, K.; Park, D.; Stefanis, L. Abberant Alpha-Synuclein Confers Toxicity to Neurons in Part through Inhibition of Chaperone-Mediated Autophagy. PLoS ONE 2009, 4, e5515. [Google Scholar] [CrossRef]
- Xilouri, M.; Vogiatzi, T.; Vekrellis, K.; Stefanis, L. Alpha-Synuclein Degradation by Autophagic Pathways: A Potential Key to Parkinson’s Disease Pathogenesis. Autophagy 2008, 4, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Xilouri, M.; Brekk, O.R.; Kirik, D.; Stefanis, L. LAMP2A as a Therapeutic Target in Parkinson Disease. Autophagy 2013, 9, 2166–2168. [Google Scholar] [CrossRef] [PubMed]
- Kabuta, T.; Setsuie, R.; Mitsui, T.; Kinugawa, A.; Sakurai, M.; Aoki, S.; Uchida, K.; Wada, K. Aberrant Molecular Properties Shared by Familial Parkinson’s Disease-Associated Mutant UCH-L1 and Carbonyl-Modified UCH-L1. Hum. Mol. Genet. 2008, 17, 1482–1496. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, S.J.; Kuo, S.-H.; Tasset, I.; Arias, E.; Koga, H.; Fernandez-Carasa, I.; Cortes, E.; Honig, L.S.; Dauer, W.; Consiglio, A.; et al. Interplay of LRRK2 with Chaperone-Mediated Autophagy. Nat. Neurosci. 2013, 16, 394–406. [Google Scholar] [CrossRef] [PubMed]

| Model | Sample | MA markers | Interpretation | Reference | |
|---|---|---|---|---|---|
| CBE | In vitro | SH-SY5Y cells and rat cortical neurons | = LC3-II = Syn | No alterations in MA nor CMA | [126] |
| SH-SY5Y cells | ↑ LC3-II, p62, Syn ↓ mTORC1 | defect in the “autolysosome reformation machinery” | [127] | ||
| In vivo | Mouse | ↑ LC3-II, p62 (SNpc) ↑ proteinase-K resistant agg-Syn | Impaired MA flux | [128] | |
| shRNA | In vitro | Human neuroglioma (H4) + shRNA GBA | ↑ LC3-II and LAMPs | GCase depletion causes a decline in lysosomal proteolysis that affects syn homeostasis | [129] |
| SK-N-SH and rat cortical neuron | ↓ LC3-II ↓ autophagic flux ↑ α-Syn | Autophagy pathway is severely compromised inhibiting MA induction via-mTORC1 | [130] | ||
| SH-SY5Y cells | ↑ LC3-II, p62 | Impairment of the lysosomal function | [127] | ||
| KO | In vitro | Mixed cultures of cortical neurons and astrocytes from GBA-/- mice | ↓ LC3-II, Atg5-12 ↑ p62, ↑ α-Syn aggregated | Autophagy pathway is severely compromised | [131] |
| HEK293 GBA-KO | ↑ MA markers (LAMP-2, LC3B, p62, Rab7) | Attributed to the accumulation of toxic GlcSph | [81] | ||
| Immortalized neurons | ↑ LC3-II hyperactivated mTORC1 | Altered lysosomes and autophagy, decrease | [82] | ||
| BE(2)M17 GBA-KO cells | = LC3-II, p62 ↓ Flux | MA is affected very lightly | [73] | ||
| In vivo | Drosophila | ↑ LC3-II, p62 blocked MA flux | Failure in MA and lysosomal dysfunction | [132] | |
| Mutant GBA | In vitro | Fibroblasts PD-GBA patients | = LC3-II | Lysosomal dysfunction but no MA alterations | [133] |
| Fibroblasts from GD and GBA-PD patients | ↑ LC3-II MA flux blockade | Impaired autophagic flux | [134] | ||
| Fibroblasts from PD-GBA patients | ↑ LC3-II, p62, α-Syn ↓ mTORC1 | Defect in the “autolysosome reformation machinery” | [127] | ||
| Fibroblasts from N370/WT patient | ↑ LC3-II, p62 small effect on MA flux | Autophagy impairment cholesterol accumulation | [89] | ||
| Fibroblasts iPD and PD-GBA patients | impaired autophagic flux | Impaired autophagic flux in PD-GBA | [135] | ||
| PBMCs from PD patients | ↑ LC3-II (mRNA and protein) | MA induction probably as a compensatory mechanism of CMA impairment | [136] | ||
| SH-SY5Y + GBA L444P | ↓ Mitophagy MA flux is working but in L444P is lower. | Mitophagy dysfunction and autophagy problesms | [137] | ||
| Neural crest stem cell derived dopaminergic neurons | ↓ Cat D: = Cat B = LAMP-1 | GBA1 mutations lead to a lower level of cathepsin D protein and activity | [138] | ||
| BE(2)M17 GBA-N370S and L444P cells | = LC3-II, p62 ↓ Flux | MA flux is slighted affected, MA induction activated to compensate lysosomal dysfunction. | [73] | ||
| SH-SY5Y + GBA-WT/N370S, L444P, D409H and mouse primary neurons + WT/N370S GBA | ↑ MA flux | MA is OK and over activated to compensate CMA dysfunction. | [139] | ||
| iPSC-DA | ↑ p62 | Lysosomal dysfunction | [140] | ||
| iPSC-DA from GBA-PD patients (N370S/WT and L444P/WT) | ↑ LC3-II ↓ Flux | Autophagic and lysosomal defects. | [141] | ||
| iPSC-DA from neuronopathic GD | ↑ LC3-II ↓ Flux ↓ TFEB expression | Lysosomal dysfunction | [142] | ||
| iPSC-DA N370S | ↑ LC3-II, beclin 1, p62 | Autophagic/lysosomal perturbations. | [143] | ||
| In vivo | Mouse D409V knock-in | = LC3, p62, LAMP-2 ↓ Beclin | No differences in lysosomal and MA markers except for beclin (impairment in initiation of autophagosome). | [144] | |
| GBA L444P knockin mice | ↑ basal LC3-II, p62 Other markers: mTOR, beclin. | impaired basal autophagy and lysosomal degradation MA Flux blocked. mitophagy impairment | [137] | ||
| Mouse D409V knock-in | = LAMP-2 | No changes in autophagy-lysosomal system | [145] | ||
| Mouse D409V (+ ATP13A2) | = LAMP-2 | No changes in autophagy-lysosomal system | [146] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradas, E.; Martinez-Vicente, M. The Consequences of GBA Deficiency in the Autophagy–Lysosome System in Parkinson’s Disease Associated with GBA. Cells 2023, 12, 191. https://doi.org/10.3390/cells12010191
Pradas E, Martinez-Vicente M. The Consequences of GBA Deficiency in the Autophagy–Lysosome System in Parkinson’s Disease Associated with GBA. Cells. 2023; 12(1):191. https://doi.org/10.3390/cells12010191
Chicago/Turabian StylePradas, Eddie, and Marta Martinez-Vicente. 2023. "The Consequences of GBA Deficiency in the Autophagy–Lysosome System in Parkinson’s Disease Associated with GBA" Cells 12, no. 1: 191. https://doi.org/10.3390/cells12010191
APA StylePradas, E., & Martinez-Vicente, M. (2023). The Consequences of GBA Deficiency in the Autophagy–Lysosome System in Parkinson’s Disease Associated with GBA. Cells, 12(1), 191. https://doi.org/10.3390/cells12010191







