Investigating the Association between Diabetic Neuropathy and Vitamin D in Emirati Patients with Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methods
Study Population and Design
2.2. Statistical Analysis
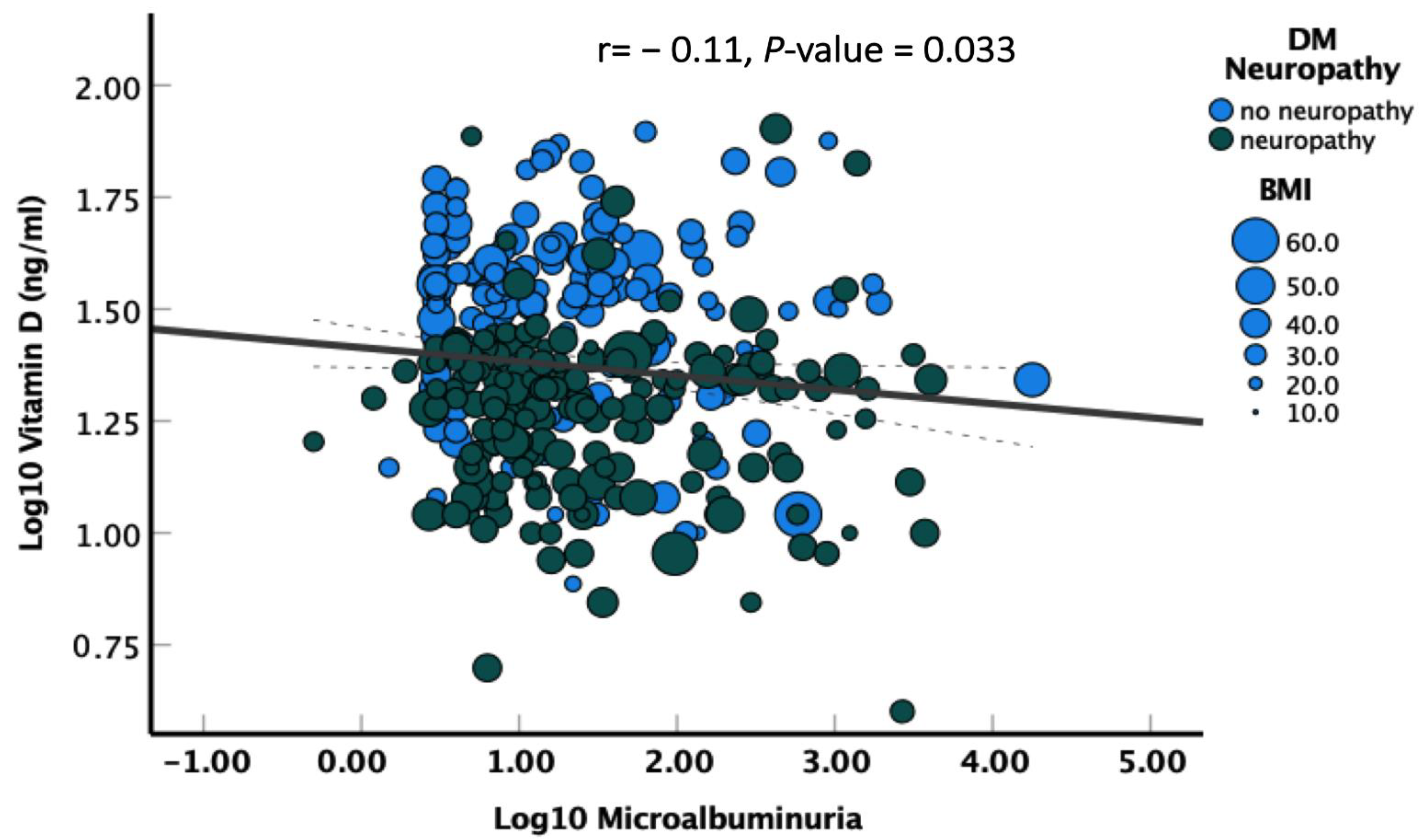
3. Results
3.1. Anthropometric, Clinical Characteristics and Vitamin D Supplement Consumption of the Study Population
3.2. Comparison of Anthropometric, Clinical Characteristics and Vitamin D Supplement Consumption of Patients between Group 1 (Neuropathy) and Group 2 (No Neuropathy)
3.3. Correlation of Diabetic Neuropathy with Different Variables
3.4. Strength of Association between Different Factors and Diabetic Neuropathy
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Sabbah, H.; Alketbi, M.; Dghaim, R. Prevalence of Complications in Type 2 Diabetics in Dubai, UAE: A Cross-sectional Study. 2019. Available online: https://knepublishing.com/index.php/AJNE/article/view/5161 (accessed on 12 September 2022).
- Al Maskari, F. Prevalence and Determinants of Peripheral Neuropathy in Patients with Type 2 Diabetes Attending a Tertiary Care Center in the United Arab Emirates. J. Diabetes Metab. 2014, 5, 2. Available online: https://www.omicsonline.org/open-access/prevalence-and-determinants-of-peripheral-neuropathy-in-patients-with-type-diabetes-2155-6156.1000346.php?aid=24491 (accessed on 11 September 2022). [CrossRef]
- Shah, S.M.; Ali, R.; Loney, T.; Aziz, F.; ElBarazi, I.; Al Dhaheri, S.; Farooqi, M.H.; Blair, I. Prevalence of Diabetes among Migrant Women and Duration of Residence in the United Arab Emirates: A Cross Sectional Study. Renzaho AMN, editor. PLoS ONE 2017, 12, e0169949. [Google Scholar] [CrossRef] [Green Version]
- Scragg, R.; Sowers, M.; Bell, C. Serum 25-Hydroxyvitamin D, Diabetes, and Ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004, 27, 2813–2818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haidari, F.; Zakerkish, M.; Karandish, M.; Saki, A.; Pooraziz, S. Association between Serum Vitamin D Level and Glycemic and Inflammatory Markers in Non-obese Patients with Type 2 Diabetes. Iran. J. Med. Sci. 2016, 41, 367–373. [Google Scholar]
- Sadiya, A.; Ahmed, S.M.; Carlsson, M.; Tesfa, Y.; George, M.; Ali, S.H.; Siddieg, H.H.; Abusnana, S. Vitamin D supplementation in obese type 2 diabetes subjects in Ajman, UAE: A randomized controlled double-blinded clinical trial. Eur. J. Clin. Nutr. 2015, 69, 707–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymczak-Pajor, I.; Drzewoski, J.; Śliwińska, A. The Molecular Mechanisms by Which Vitamin D Prevents Insulin Resistance and Associated Disorders. Int. J. Mol. Sci. 2020, 21, 6644. [Google Scholar] [CrossRef]
- Palomer, X.; González-Clemente, J.M.; Blanco-Vaca, F.; Mauricio, D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes. Metab. 2008, 10, 185–197. [Google Scholar] [CrossRef]
- Lee, P. Vitamin D as an Analgesic for Patients With Type 2 Diabetes and Neuropathic Pain. Arch. Intern. Med. 2008, 168, 771. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Vitamin D Status: Measurement, Interpretation, and Clinical Application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszek-Matuszek, B.; Lenart-Lipińska, M.; Woźniakowska, E. Featured paper Clinical implications of vitamin D deficiency. Menopausal Rev. 2015, 2, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Naderpoor, N.; Teede, H.; Scragg, R.; de Courten, B. Vitamin D supplementation for improvement of chronic low-grade inflammation in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2018, 76, 380–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The Role of Vitamin D and Calcium in Type 2 Diabetes. A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Abdelsadek, S.E.; El Saghier, E.O.; Abdel Raheem, S.I. Serum 25(OH) vitamin D level and its relation to diabetic peripheral neuropathy in Egyptian patients with type 2 diabetes mellitus. Egypt. J. Neurol. Psychiatry Neurosurg. 2018, 54, 36. [Google Scholar] [CrossRef]
- Smith, A.G.; Singleton, J.R. Obesity and hyperlipidemia are risk factors for early diabetic neuropathy. J. Diabetes Its Complicat. 2013, 27, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Alamdari, A.; Mozafari, R.; Tafakhori, A.; Faghihi-Kashani, S.; Hafezi-Nejad, N.; Sheikhbahaei, S.; Naderi, N.; Ebadi, M.; Esteghamati, A. An inverse association between serum vitamin D levels with the presence and severity of impaired nerve conduction velocity and large fiber peripheral neuropathy in diabetic subjects. Neurol. Sci. 2015, 36, 1121–1126. [Google Scholar] [CrossRef]
- Putz, Z.; Martos, T.; Nemeth, N.; Körei, A.E.; Szabó, M.; Vági, O.E.; Kempler, M.S.; Kempler, P. Vitamin D and neuropathy. Orv. Hetil. 2013, 154, 2012–2015. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.; Kotake, M.; Ono, Y.; Kato, T.; Oda, N.; Hayakawa, N.; Hashimoto, S.; Itoh, M. Hypovitaminosis D in Type 2 Diabetes Mellitus: Association with Microvascular Complications and Type of Treatment. Endocr. J. 2006, 53, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, S.; Singh, R.P.; Dwivedi, N.C.; Singh, K.; Gupta, A.; Mathur, M. Vitamin D levels and microvascular complications in type 2 diabetes. Indian J. Endocrinol. Metab. 2014, 18, 537–541. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Alkharfy, K.M.; Al-Othman, A.; El-Kholie, E.; Moharram, O.; Alokail, M.S.; Al-Saleh, Y.; Sabico, S.; Kumar, S.; Chrousos, G.P. Vitamin D supplementation as an adjuvant therapy for patients with T2DM: An 18-month prospective interventional study. Cardiovasc. Diabetol. 2012, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.B.; Wang, L.L.; Tang, X.; Wu, W.; Sun, Y.H. The association between vitamin D level and diabetic peripheral neuropathy in patients with type 2 diabetes mellitus: An update systematic review and meta-analysis. J. Clin. Transl. Endocrinol. 2017, 9, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, P.; Schernthaner, G.; Woloszczuk, W. Serum osteocalcin levels in diabetes mellitus: Analysis of the type of diabetes and microvascular complications. Diabetologia 1988, 31, 892–895. Available online: http://link.springer.com/10.1007/BF00265373 (accessed on 11 September 2022). [CrossRef] [Green Version]
- Isaia, G.; Giorgino, R.; Adami, S. High Prevalence of Hypovitaminosis D in Female Type 2 Diabetic Population. Diabetes Care 2001, 24, 1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehab, D.; Al-Jarallah, K.; Mojiminiyi, O.A.; Al Mohamedy, H.; Abdella, N.A. Does Vitamin D deficiency play a role in peripheral neuropathy in Type 2 diabetes?: Vitamin D deficiency and peripheral neuropathy in type 2 diabetes. Diabet. Med. 2012, 29, 43–49. [Google Scholar] [CrossRef]
- Celikbilek, A.; Gocmen, A.Y.; Tanik, N.; Börekçi, E.; Adam, M.; Celikbilek, M.; Suher, M.; Delibas, N. Decreased serum vitamin D levels are associated with diabetic peripheral neuropathy in a rural area of Turkey. Acta Neurol. Belg. 2015, 115, 47–52. [Google Scholar] [CrossRef]
- Saadi, H.F.; Dawodu, A.; Afandi, B.O.; Zayed, R.; Benedict, S.; Nagelkerke, N. Efficacy of daily and monthly high-dose calciferol in vitamin D–deficient nulliparous and lactating women. Am. J. Clin. Nutr. 2007, 85, 1565–1571. [Google Scholar] [CrossRef] [Green Version]
- Xiaohua, G.; Dongdong, L.; Xiaoting, N.; Shuoping, C.; Feixia, S.; Huajun, Y.; Qi, Z.; Zimiao, C. Severe Vitamin D Deficiency Is Associated With Increased Expression of Inflammatory Cytokines in Painful Diabetic Peripheral Neuropathy. Front. Nutr. 2021, 8, 612068. [Google Scholar] [CrossRef]
- Herder, C.; Kannenberg, J.M.; Huth, C.; Carstensen-Kirberg, M.; Rathmann, W.; Koenig, W.; Heier, M.; Püttgen, S.; Thorand, B.; Peters, A.; et al. Proinflammatory Cytokines Predict the Incidence and Progression of Distal Sensorimotor Polyneuropathy: KORA F4/FF4 Study. Diabetes Care 2017, 40, 569–576. [Google Scholar] [CrossRef]
| Variables | n | % | Mean ± SD | Variables | n | % | Mean ± SD | |
|---|---|---|---|---|---|---|---|---|
| Gender | Vitamin D levels | |||||||
| Females | 353 | 59% | Low | 300 | 50% | 27.3 ± 15.2 | ||
| Males | 247 | 41% | Normal | 148 | 25% | |||
| Age (years) | CRP | |||||||
| 20–40 | 36 | 6% | 62.85 ± 11.35 | High | 206 | 34% | 18.1 ± 33.1 | |
| 41–60 | 139 | 23% | Normal | 315 | 53% | |||
| 61–80 | 425 | 71% | Total Cholesterol | |||||
| Duration of diabetes | High | 107 | 18% | 44.34 ± 1.15 | ||||
| 1–10 years | 125 | 21% | Normal | 416 | 69% | |||
| 11–20 years | 120 | 20% | Triglycerides | |||||
| 21–30 years | 78 | 13% | High | 133 | 22% | 1.56 ± 0.82 | ||
| >30 years | 34 | 6% | Normal | 374 | 62% | |||
| Height (cm) | 159.3 ± 11.37 | HDL | ||||||
| High | 66 | 11% | 1.48 ± 6.2 | |||||
| weight (cm) | 79.3 ± 16.8 | Normal | 270 | 45% | ||||
| BMI | Low | 158 | 26% | |||||
| Normal weight | 79 | 13% | 31.1 ± 6.0 | LDL | ||||
| Overweight | 192 | 32% | High | 62 | 10% | 2.85 ± 3.26 | ||
| Obese | 310 | 52% | Normal | 462 | 77% | |||
| Underweight | 1 | 0% | Creatinine | |||||
| Vitamin D deficiency | High | 69 | 12% | 85.6 ± 52.8 | ||||
| Yes | 363 | 61% | Normal | 522 | 87% | |||
| No | 237 | 40% | Microalbumin Urine | |||||
| Consumption of Vitamin D supplements | High | 159 | 27% | 176.8 ± 919.2 | ||||
| Yes | 490 | 82% | Normal | 326 | 54% | |||
| No | 110 | 18% | Creatinine Urine | |||||
| Neuropathy | High | 8 | 1% | 7832.9 ± 5536 | ||||
| Yes | 300 | 50% | Normal | 446 | 74% | |||
| No | 300 | 50% | ||||||
| HbA1c | ||||||||
| <5.7% | 24 | 4% | 7.68 ± 1.65 | |||||
| 5.7–6.5% | 101 | 17% | ||||||
| >6.5% | 461 | 77% | ||||||
| Variables | Neuropathy | No Neuropathy | Neuropathy | No Neuropathy | Variables | Neuropathy | No Neuropathy | Neuropathy | No Neuropathy |
|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | Mean ± SD | Mean ± SD | n (%) | n (%) | Mean ± SD | Mean ± SD | ||
| Gender | Vitamin D levels | ||||||||
| Females (n = 353) | 176 (49.8%) | 177 (50.1%) | Low (n = 300) | 209 (69.6%) | 91 (30.3%) | 21.37 ± 11.9 | 33.41 ± 15.7 | ||
| Males (n = 247) | 124 (50.2%) | 123 (49.7%) | Normal (n = 148) | 19 (12.8%) | 129 (87.2%) | ||||
| Age (years) | CRP | ||||||||
| 20-40 (n = 36) | 7 (19.4%) | 29 (80.5%) | 63.45 ± 9.3 | 62.25 ± 13.0 | High (n = 206) | 123 (59.7%) | 83 (40.2%) | 19.5 ± 31.4 | 16.7 ± 34.9 |
| 41-60 (n = 139) | 81 (58.2%) | 58 (41.7%) | Normal (n = 315) | 145 (46%) | 170 (53.9%) | ||||
| 61-80 (n = 425) | 212 (49.8%) | 213 (50.1%) | Total Cholesterol | ||||||
| Height (cm) | 159.9 ± 9.0 | 158.7 ± 13.3 | High (n = 107) | 60 (56%) | 47 (43.9%) | 4.37 ± 1.19 | 4.3 ± 1.1 | ||
| Weight (kg) | 80.2 ± 16.5 | 78.3 ± 17.1 | Normal (n = 416) | 220 (52.8%) | 196 (47.1%) | ||||
| Duration of diabetes | Triglycerides | ||||||||
| 1–10 years (n = 153) | 72 (47%) | 53 (34.6%) | High (n = 133) | 82 (61.6%) | 51 (38.3%) | 1.62 ± 0.94 | 1.47 ± 0.65 | ||
| 11–20 years (n = 120) | 87 (72.5%) | 33 (27.5%) | Normal (n = 374) | 198 (52.9%) | 176 (47%) | ||||
| 21–30 years (n = 78) | 49 (62.8%) | 29 (37.2%) | |||||||
| >30 years (n = 34) | 24 (70.5%) | 10 (29.4%) | HDL | Median (IQR) | |||||
| BMI | High (n = 66) | 44 (66.6%) | 22 (33.3%) | 1.19 (1.43-0.98) | 1.13 (1.35–0.95) | ||||
| Normal weight (n = 79) | 31 (39.2%) | 48 (60.7%) | 31.3 ± 5.9 | 30.8 ± 6.1 | Normal (n = 270) | 148 (54.8%) | 122 (45.1%) | ||
| Overweight (n = 192) | 110 (57.2%) | 82 (42.7%) | Low (n = 158) | 81 (51.2%) | 77 (48.7%) | ||||
| Obese (n = 310) | 155 (50%) | 155 (50%) | LDL | ||||||
| Underweight (n = 1) | 0 (0%) | 1 (0.2%) | High (n = 62) | 32 (51.6%) | 30 (48%) | 2.67 ± 1.1 | 3.05 ± 4.6 | ||
| Vitamin D deficiency | Normal (n = 462) | 249 (53.8%) | 213 (46.1%) | ||||||
| Yes (n = 363) | 261 (71.9%) | 102 (28%) | Creatinine | ||||||
| No (n = 237) | 39 (16.4%) | 198 (83.5%) | High (n = 69) | 39 (56.5%) | 30 (43.4%) | 89.4 ± 63.1 | 81.8 ± 39.3 | ||
| Consumption of Vitamin D supplements | Normal (n = 522) | 259 (49.6%) | 263 (50.3%) | ||||||
| Yes (n = 490) | 272 (55.5%) | 218 (44.4%) | |||||||
| No (n = 110) | 28 (25.4%) | 82 (74.5%) | Microalbumin Urine | ||||||
| HbA1C | High (n = 159) | 90 (56.6%) | 69 (43.3%) | 172 ± 530 | 182 ± 1238.6 | ||||
| <5.7% (n = 101) | 43 (42.5%) | 58 (57.4%) | 7.7 ± 1.6 | 7.6 ± 1.7 | Normal (n = 326) | 176 (53.9%) | 150 (46%) | ||
| 5.7–6.5% (n = 24) | 16 (66.7%) | 8 (33.3%) | |||||||
| >6.5% (n = 461) | 238 (51.6%) | 223 (48.3%) | Creatinine Urine | ||||||
| High (n = 8) | 3 (37.5%) | 5 (62.5%) | 7678 ± 5148 | 8036 ± 6017 | |||||
| Normal (n = 440) | 252 (57.2%) | 188 (42.7%) | |||||||
| Variables | Neuropathy | No Neuropathy | p-Value * |
|---|---|---|---|
| n = 300 | n = 300 | ||
| Age (years), mean ± SD | 63 ± 9 | 62 ± 13 | 0.198 |
| Age > 65, n (%) | 151 (50) | 160 (53) | 0.462 |
| Male sex, n (%) | 124 (41) | 123 (41) | 0.934 |
| BMI, n (%) | 0.006 | ||
| BMI 25–29.9 | 110 (37) | 82 (27) | |
| BMI > 30 | 155 (52) | 155 (52) | |
| Laboratory Data | |||
| Vitamin D level (ng/mL), median (IQR) | 20 (14–25) | 33 (20–42) | <0.001 |
| Vitamin D 20–30 (ng/mL), n (%) | 100 (44) | 41 (19) | <0.001 |
| Vitamin D < 20 (ng/mL), n (%) | 117 (39) | 54 (18) | <0.001 |
| HbA1C (109/L), median (IQR) | 7.4 (7–9) | 7.1 (7–8) | 0.279 |
| Total cholesterol (mmol/L), median (IQR) | 4.2 (3–5) | 4.1 (4–5) | 0.534 |
| Triglycerides (mmol/L), median (IQR) | 1.3 (1–2) | 1.4 (1–2) | 0.534 |
| HDL (mmol/L), median (IQR) | 1.2 (1–1) | 1.2 (1–1) | 0.534 |
| LDL (mmol/L), median (IQR) | 2.4 (2–3) | 2.5 (2–3) | 0.534 |
| Serum creatinine (umol/L), median (IQR) | 75 (61–96) | 76 (62–92) | 0.102 |
| Urine creatinine (umol/L), median (IQR) | 6361 (4402–10243) | 6009 (3522–10761) | 0.574 |
| Microalbuminuria (mg/L), median (IQR) | 11.4 (4–61) | 11.1 (4–35) | 0.020 |
| Variables | Adjusted OR (95%CI) | p-Value * |
|---|---|---|
| Age, years | 0.99 (0.97–1.01) | 0.256 |
| Male | 0.87 (0.59–1.29) | 0.491 |
| Vitamin D < 20 (ng/mL) | 2.63 (1.70–4.06) | <0.001 |
| Log10 Microalbuminuria | 1.40 (1.08–1.79) | 0.010 |
| BMI > 25 | 1.93 (1.09–3.41) | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Ali, T.; Ashfaq, A.; Saheb Sharif-Askari, N.; Abusnana, S.; Mussa, B.M. Investigating the Association between Diabetic Neuropathy and Vitamin D in Emirati Patients with Type 2 Diabetes Mellitus. Cells 2023, 12, 198. https://doi.org/10.3390/cells12010198
Al Ali T, Ashfaq A, Saheb Sharif-Askari N, Abusnana S, Mussa BM. Investigating the Association between Diabetic Neuropathy and Vitamin D in Emirati Patients with Type 2 Diabetes Mellitus. Cells. 2023; 12(1):198. https://doi.org/10.3390/cells12010198
Chicago/Turabian StyleAl Ali, Tahra, Alizeh Ashfaq, Narjes Saheb Sharif-Askari, Salah Abusnana, and Bashair M. Mussa. 2023. "Investigating the Association between Diabetic Neuropathy and Vitamin D in Emirati Patients with Type 2 Diabetes Mellitus" Cells 12, no. 1: 198. https://doi.org/10.3390/cells12010198
APA StyleAl Ali, T., Ashfaq, A., Saheb Sharif-Askari, N., Abusnana, S., & Mussa, B. M. (2023). Investigating the Association between Diabetic Neuropathy and Vitamin D in Emirati Patients with Type 2 Diabetes Mellitus. Cells, 12(1), 198. https://doi.org/10.3390/cells12010198






