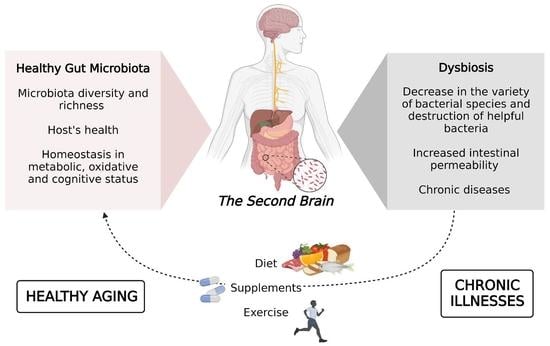
Interventions on Gut Microbiota for Healthy Aging
Abstract
:1. Introduction
2. Gut Microbiome and Aging
3. Gut Microbiome and Cognitive Health
4. Diet and Aging
5. Supplements
5.1. Probiotics
5.2. Prebiotics
5.3. Ω-3 Fatty Acids
5.4. Synbiotics
5.5. Brain Health and Psychobiotics
5.6. Antioxidant
5.6.1. Resveratrol
5.6.2. Flavonoids
5.6.3. Curcumin
6. Exercise
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niccoli, T.; Partridge, L. Ageing as a Risk Factor for Disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemoine, M. The Evolution of the Hallmarks of Aging. Front. Genet. 2021, 12, 1511. [Google Scholar] [CrossRef] [PubMed]
- Ros, M.; Carrascosa, J.M. Current Nutritional and Pharmacological Anti-Aging Interventions. Biochim. Biophys. Acta—Mol. Basis Dis. 2020, 1866, 165612. [Google Scholar] [CrossRef] [PubMed]
- McPhee, J.S.; French, D.P.; Jackson, D.; Nazroo, J.; Pendleton, N.; Degens, H. Physical Activity in Older Age: Perspectives for Healthy Ageing and Frailty. Biogerontology 2016, 17, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a Promising Target in Slowing down the Ageing Process. Biogerontology 2017, 18, 447–476. [Google Scholar] [CrossRef] [Green Version]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut Microbiota: The Neglected Endocrine Organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef] [Green Version]
- Das, B.; Nair, G.B. Homeostasis and Dysbiosis of the Gut Microbiome in Health and Disease. J. Biosci. 2019, 44, 117. [Google Scholar] [CrossRef]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and Anti-Inflammaging: A Systemic Perspective on Aging and Longevity Emerged from Studies in Humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef]
- Boyajian, J.L.; Ghebretatios, M.; Schaly, S.; Islam, P.; Prakash, S. Microbiome and Human Aging: Probiotic and Prebiotic Potentials in Longevity, Skin Health and Cellular Senescence. Nutrients 2021, 13, 4550. [Google Scholar] [CrossRef]
- De la Cuesta-Zuluaga, J.; Kelley, S.T.; Chen, Y.; Escobar, J.S.; Mueller, N.T.; Ley, R.E.; McDonald, D.; Huang, S.; Swafford, A.D.; Knight, R.; et al. Age- and Sex-Dependent Patterns of Gut Microbial Diversity in Human Adults. mSystems 2019, 4, e00261-19. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human Gut Microbiome Viewed across Age and Geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [Green Version]
- Simon, J.-C.; Marchesi, J.R.; Mougel, C.; Selosse, M.-A. Host-Microbiota Interactions: From Holobiont Theory to Analysis. Microbiome 2019, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, H. Biologic Versus Chronologic Age. J. Gerontol. 1947, 2, 217–227. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Jeffery, I.B. Gut Microbiota and Aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef]
- Srivastava, S.; Singh, A.; Sandeep, K.; Yadav, D. Epigenetic Regulation of Gut Microbial Dysbiosis. Indian J. Microbiol. 2021, 61, 125–129. [Google Scholar] [CrossRef]
- Sepp, E.; Smidt, I.; Rööp, T.; Štšepetova, J.; Kõljalg, S.; Mikelsaar, M.; Soidla, I.; Ainsaar, M.; Kolk, H.; Vallas, M.; et al. Comparative Analysis of Gut Microbiota in Centenarians and Young People: Impact of Eating Habits and Childhood Living Environment. Front. Cell. Infect. Microbiol. 2022, 12, 228. [Google Scholar] [CrossRef]
- Kim, B.-S.; Choi, C.W.; Shin, H.; Jin, S.-P.; Bae, J.-S.; Han, M.; Seo, E.Y.; Chun, J.; Chung, J.H. Comparison of the Gut Microbiota of Centenarians in Longevity Villages of South Korea with Those of Other Age Groups. J. Microbiol. Biotechnol. 2019, 29, 429–440. [Google Scholar] [CrossRef]
- Wu, L.; Zeng, T.; Zinellu, A.; Rubino, S.; Kelvin, D.J.; Carru, C. A Cross-Sectional Study of Compositional and Functional Profiles of Gut Microbiota in Sardinian Centenarians. mSystems 2019, 4, e00325-19. [Google Scholar] [CrossRef] [Green Version]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [Green Version]
- Waters, J.L.; Ley, R.E. The Human Gut Bacteria Christensenellaceae Are Widespread, Heritable, and Associated with Health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Bosco, N.; Noti, M. The Aging Gut Microbiome and Its Impact on Host Immunity. Genes Immun. 2021, 22, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhu, H.; Qiu, P. Aging Progression of Human Gut Microbiota. BMC Microbiol. 2019, 19, 236. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, I.B.; Lynch, D.B.; O’Toole, P.W. Composition and Temporal Stability of the Gut Microbiota in Older Persons. ISME J. 2016, 10, 170–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- DeJong, E.N.; Surette, M.G.; Bowdish, D.M.E. The Gut Microbiota and Unhealthy Aging: Disentangling Cause from Consequence. Cell Host Microbe 2020, 28, 180–189. [Google Scholar] [CrossRef]
- Elderman, M.; Sovran, B.; Hugenholtz, F.; Graversen, K.; Huijskes, M.; Houtsma, E.; Belzer, C.; Boekschoten, M.; de Vos, P.; Dekker, J.; et al. The Effect of Age on the Intestinal Mucus Thickness, Microbiota Composition and Immunity in Relation to Sex in Mice. PLoS ONE 2017, 12, e0184274. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus Barrier, Mucins and Gut Microbiota: The Expected Slimy Partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Hansson, G.C. Role of Mucus Layers in Gut Infection and Inflammation. Curr. Opin. Microbiol. 2012, 15, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut Microbiota and Extreme Longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef] [Green Version]
- Bárcena, C.; Valdés-Mas, R.; Mayoral, P.; Garabaya, C.; Durand, S.; Rodríguez, F.; Fernández-García, M.T.; Salazar, N.; Nogacka, A.M.; Garatachea, N.; et al. Healthspan and Lifespan Extension by Fecal Microbiota Transplantation into Progeroid Mice. Nat. Med. 2019, 25, 1234–1242. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia Muciniphila and Its Role in Regulating Host Functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Van den Abbeele, P.; Belzer, C.; Goossens, M.; Kleerebezem, M.; de Vos, W.M.; Thas, O.; de Weirdt, R.; Kerckhof, F.-M.; van de Wiele, T. Butyrate-Producing Clostridium Cluster XIVa Species Specifically Colonize Mucins in an In Vitro Gut Model. ISME J. 2013, 7, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Mabbott, N.A. A Breakdown in Communication? Understanding the Effects of Aging on the Human Small Intestine Epithelium. Clin. Sci. 2015, 129, 529–531. [Google Scholar] [CrossRef] [Green Version]
- Parker, A.; Romano, S.; Ansorge, R.; Aboelnour, A.; le Gall, G.; Savva, G.M.; Pontifex, M.G.; Telatin, A.; Baker, D.; Jones, E.; et al. Fecal Microbiota Transfer between Young and Aged Mice Reverses Hallmarks of the Aging Gut, Eye, and Brain. Microbiome 2022, 10, 68. [Google Scholar] [CrossRef]
- Ticinesi, A.; Tana, C.; Nouvenne, A.; Prati, B.; Lauretani, F.; Meschi, T. Gut Microbiota, Cognitive Frailty and Dementia in Older Individuals: A Systematic Review. Clin. Interv. Aging 2018, 13, 1497–1511. [Google Scholar] [CrossRef] [Green Version]
- Zhong, F.; Wen, X.; Yang, M.; Lai, H.-Y.; Momma, H.; Cheng, L.; Sun, X.; Nagatomi, R.; Huang, C. Effect of an 8-Week Exercise Training on Gut Microbiota in Physically Inactive Older Women. Int. J. Sports Med. 2021, 42, 610–623. [Google Scholar] [CrossRef]
- Johnson, D.; Letchumanan, V.; Thurairajasingam, S.; Lee, L.-H. A Revolutionizing Approach to Autism Spectrum Disorder Using the Microbiome. Nutrients 2020, 12, 1983. [Google Scholar] [CrossRef]
- Johnson, K.V.-A. Gut Microbiome Composition and Diversity Are Related to Human Personality Traits. Hum. Microb. J. 2020, 15, 100069. [Google Scholar] [CrossRef]
- Jackson, M.A.; Jeffery, I.B.; Beaumont, M.; Bell, J.T.; Clark, A.G.; Ley, R.E.; O’Toole, P.W.; Spector, T.D.; Steves, C.J. Signatures of Early Frailty in the Gut Microbiota. Genome Med. 2016, 8, 8. [Google Scholar] [CrossRef]
- Maffei, V.J.; Kim, S.; Blanchard, E.; Luo, M.; Jazwinski, S.M.; Taylor, C.M.; Welsh, D.A. Biological Aging and the Human Gut Microbiota. J. Gerontol. Ser. A 2017, 72, 1474–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut Microbiota Composition Correlates with Diet and Health in the Elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut Microbiome and Aging: Physiological and Mechanistic Insights. Nutr. Healthy Aging 2018, 4, 267–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Kong, F.; Hua, Y.; Zeng, B.; Ning, R.; Li, Y.; Zhao, J. Gut Microbiota Signatures of Longevity. Curr. Biol. 2016, 26, R832–R833. [Google Scholar] [CrossRef] [Green Version]
- Tuikhar, N.; Keisam, S.; Labala, R.K.; Imrat; Ramakrishnan, P.; Arunkumar, M.C.; Ahmed, G.; Biagi, E.; Jeyaram, K. Comparative Analysis of the Gut Microbiota in Centenarians and Young Adults Shows a Common Signature across Genotypically Non-Related Populations. Mech. Ageing Dev. 2019, 179, 23–35. [Google Scholar] [CrossRef]
- Santoro, A.; Ostan, R.; Candela, M.; Biagi, E.; Brigidi, P.; Capri, M.; Franceschi, C. Gut Microbiota Changes in the Extreme Decades of Human Life: A Focus on Centenarians. Cell. Mol. Life Sci. 2018, 75, 129–148. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Ke, Y.; Zhan, R.; Liu, C.; Zhao, M.; Zeng, A.; Shi, X.; Ji, L.; Cheng, S.; Pan, B.; et al. Trimethylamine- N -Oxide Promotes Brain Aging and Cognitive Impairment in Mice. Aging Cell 2018, 17, e12768. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Young SN How to Increase Serotonin in the Human Brain without Drugs. J. Psychiatry Neurosci. JPN 2007, 32, 394.
- Park, J.-S.; Shin, E.; Hong, H.; Shin, H.-J.; Cho, Y.-H.; Ahn, K.-H.; Paek, K.; Lee, Y. Characterization of Lactobacillus Fermentum PL9988 Isolated from Healthy Elderly Korean in a Longevity Village. J. Microbiol. Biotechnol. 2015, 25, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, T.B.L. Evolution of Ageing. Nature 1977, 270, 301–304. [Google Scholar] [CrossRef] [PubMed]
- The Evolution of Ageing and Longevity. Proc. R. Soc. Lond. B Biol. Sci. 1979, 205, 531–546. [CrossRef] [PubMed]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef]
- Noureldein, M.H.; Eid, A.A. Gut Microbiota and MTOR Signaling: Insight on a New Pathophysiological Interaction. Microb Pathog 2018, 118, 98–104. [Google Scholar] [CrossRef]
- Deleyto-Seldas, N.; Efeyan, A. The MTOR–Autophagy Axis and the Control of Metabolism. Front. Cell Dev. Biol. 2021, 9, 1519. [Google Scholar] [CrossRef]
- Donati Zeppa, S.; Agostini, D.; Gervasi, M.; Annibalini, G.; Amatori, S.; Ferrini, F.; Sisti, D.; Piccoli, G.; Barbieri, E.; Sestili, P.; et al. Mutual Interactions among Exercise, Sport Supplements and Microbiota. Nutrients 2019, 12, 17. [Google Scholar] [CrossRef] [Green Version]
- Kapahi, P.; Chen, D.; Rogers, A.N.; Katewa, S.D.; Li, P.W.-L.; Thomas, E.L.; Kockel, L. With TOR, Less Is More: A Key Role for the Conserved Nutrient-Sensing TOR Pathway in Aging. Cell Metab. 2010, 11, 453–465. [Google Scholar] [CrossRef] [Green Version]
- De Lucia, C.; Murphy, T.; Steves, C.J.; Dobson, R.J.B.; Proitsi, P.; Thuret, S. Lifestyle Mediates the Role of Nutrient-Sensing Pathways in Cognitive Aging: Cellular and Epidemiological Evidence. Commun. Biol. 2020, 3, 157. [Google Scholar] [CrossRef] [Green Version]
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R. A C. Elegans Mutant That Lives Twice as Long as Wild Type. Nature 1993, 366, 461–464. [Google Scholar] [CrossRef]
- Puig, O.; Tjian, R. Transcriptional Feedback Control of Insulin Receptor by DFOXO/FOXO1. Genes Dev. 2005, 19, 2435–2446. [Google Scholar] [CrossRef] [PubMed]
- Van der Horst, A.; Burgering, B.M.T. Stressing the Role of FoxO Proteins in Lifespan and Disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Daitoku, H.; Fukamizu, A. FOXO Transcription Factors in the Regulatory Networks of Longevity. J. Biochem. 2007, 141, 769–774. [Google Scholar] [CrossRef] [PubMed]
- García-Prat, L.; Perdiguero, E.; Alonso-Martín, S.; Dell’Orso, S.; Ravichandran, S.; Brooks, S.R.; Juan, A.H.; Campanario, S.; Jiang, K.; Hong, X.; et al. FoxO Maintains a Genuine Muscle Stem-Cell Quiescent State until Geriatric Age. Nat. Cell Biol. 2020, 22, 1307–1318. [Google Scholar] [CrossRef]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. MTOR Is a Key Modulator of Ageing and Age-Related Disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric Restriction Delays Disease Onset and Mortality in Rhesus Monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef] [Green Version]
- Hwangbo, D.-S.; Lee, H.-Y.; Abozaid, L.S.; Min, K.-J. Mechanisms of Lifespan Regulation by Calorie Restriction and Intermittent Fasting in Model Organisms. Nutrients 2020, 12, 1194. [Google Scholar] [CrossRef]
- Rahmani, J.; Montesanto, A.; Giovannucci, E.; Zand, H.; Barati, M.; Kopchick, J.J.; Mirisola, M.G.; Lagani, V.; Bawadi, H.; Vardavas, R.; et al. Association between IGF-1 Levels Ranges and All-cause Mortality: A Meta-analysis. Aging Cell 2022, 21, e13540. [Google Scholar] [CrossRef]
- Vasto, S.; Buscemi, S.; Barera, A.; di Carlo, M.; Accardi, G.; Caruso, C. Mediterranean Diet and Healthy Ageing: A Sicilian Perspective. Gerontology 2014, 60, 508–518. [Google Scholar] [CrossRef]
- Fontana, L.; Adelaiye, R.M.; Rastelli, A.L.; Miles, K.M.; Ciamporcero, E.; Longo, V.D.; Nguyen, H.; Vessella, R.; Pili, R. Dietary Protein Restriction Inhibits Tumor Growth in Human Xenograft Models of Prostate and Breast Cancer. Oncotarget 2013, 4, 2451–2461. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, A.; Speakman, J.R.; Soltani, S.; Djafarian, K. Effect of Calorie Restriction or Protein Intake on Circulating Levels of Insulin like Growth Factor I in Humans: A Systematic Review and Meta-Analysis. Clin. Nutr. 2020, 39, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Alì, S.; Davinelli, S.; Accardi, G.; Aiello, A.; Caruso, C.; Duro, G.; Ligotti, M.E.; Pojero, F.; Scapagnini, G.; Candore, G. Healthy Ageing and Mediterranean Diet: A Focus on Hormetic Phytochemicals. Mech. Ageing Dev. 2021, 200, 111592. [Google Scholar] [CrossRef] [PubMed]
- Bona, D.; Accardi, G.; Virruso, C.; Candore, G.; Caruso, C. Association between Genetic Variations in the Insulin/Insulin-like Growth Factor (Igf-1) Signaling Pathway and Longevity: A Systematic Review and Meta-Analysis. Curr. Vasc. Pharmacol. 2013, 12, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Joven, J.; Aragonès, G.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Borrás-Linares, I.; Camps, J.; Corominas-Faja, B.; Cufí, S.; Fernández-Arroyo, S.; et al. Xenohormetic and Anti-Aging Activity of Secoiridoid Polyphenols Present in Extra Virgin Olive Oil. Cell Cycle 2013, 12, 555–578. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.H.; Ro, S.-H.; Cao, J.; Otto, N.M.; Kim, D.-H. MTOR Regulation of Autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.H.; Cao, L.; Mostoslavsky, R.; Lombard, D.B.; Liu, J.; Bruns, N.E.; Tsokos, M.; Alt, F.W.; Finkel, T. A Role for the NAD-Dependent Deacetylase Sirt1 in the Regulation of Autophagy. Proc. Natl. Acad. Sci. USA 2008, 105, 3374–3379. [Google Scholar] [CrossRef] [Green Version]
- Russo, M.; Sansone, L.; Polletta, L.; Runci, A.; Rashid, M.; Santis, E.; Vernucci, E.; Carnevale, I.; Tafani, M. Sirtuins and Resveratrol-Derived Compounds: A Model for Understanding the Beneficial Effects of the Mediterranean Diet. Endocr. Metab. Immune Disord.-Drug Targets 2014, 14, 300–308. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; la Storia, A.; Laghi, L.; Serrazanetti, D.I.; di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-Level Adherence to a Mediterranean Diet Beneficially Impacts the Gut Microbiota and Associated Metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Kawano, Y.; Edwards, M.; Huang, Y.; Bilate, A.M.; Araujo, L.P.; Tanoue, T.; Atarashi, K.; Ladinsky, M.S.; Reiner, S.L.; Wang, H.H.; et al. Microbiota Imbalance Induced by Dietary Sugar Disrupts Immune-Mediated Protection from Metabolic Syndrome. Cell 2022, 185, 3501–3519.e20. [Google Scholar] [CrossRef]
- Alcedo, J.; Kenyon, C. Regulation of C. Elegans Longevity by Specific Gustatory and Olfactory Neurons. Neuron 2004, 41, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Libert, S.; Chao, Y.; Chu, X.; Pletcher, S.D. Trade-Offs between Longevity and Pathogen Resistance in Drosophila Melanogaster Are Mediated by NF? B Signaling. Aging Cell 2006, 5, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, M.L.; Romanuk, T.N. A Meta-Analysis of Probiotic Efficacy for Gastrointestinal Diseases. PLoS ONE 2012, 7, e34938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, A.C.; Quigley, E.M.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.R.; Moayyedi, P. Efficacy of Prebiotics, Probiotics, and Synbiotics in Irritable Bowel Syndrome and Chronic Idiopathic Constipation: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2014, 109, 1547–1561. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of Probiotics on Blood Pressure. Hypertension 2014, 64, 897–903. [Google Scholar] [CrossRef] [Green Version]
- Wallace, C.J.K.; Milev, R. The Effects of Probiotics on Depressive Symptoms in Humans: A Systematic Review. Ann. Gen. Psychiatry 2017, 16, 14. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Yue, M.; Wei, J.; Wang, Y.; Hong, D.; Wang, B.; Zhou, X.; Chen, T. Evaluation of the Anti-Aging Effects of a Probiotic Combination Isolated From Centenarians in a SAMP8 Mouse Model. Front. Immunol. 2021, 12, 5163. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Racedo, S.M.; Ripert, G.; Housez, B.; Cazaubiel, M.; Maudet, C.; Jüsten, P.; Marteau, P.; Urdaci, M.C. Probiotic Strain Bacillus Subtilis CU1 Stimulates Immune System of Elderly during Common Infectious Disease Period: A Randomized, Double-Blind Placebo-Controlled Study. Immun. Ageing 2015, 12, 24. [Google Scholar] [CrossRef] [Green Version]
- Salvesi, C.; Silvi, S.; Fiorini, D.; Scortichini, S.; Sagratini, G.; Palermo, F.A.; de Leone, R.; Egidi, N.; Fatone, L.; Cifani, C.; et al. Impact of a Probiotic Diet on Well-being of Healthy Senior: THE PROBIOSENIOR PROJECT. J. Appl. Microbiol. 2022, 133, 2941–2953. [Google Scholar] [CrossRef]
- Eloe-Fadrosh, E.A.; Brady, A.; Crabtree, J.; Drabek, E.F.; Ma, B.; Mahurkar, A.; Ravel, J.; Haverkamp, M.; Fiorino, A.-M.; Botelho, C.; et al. Functional Dynamics of the Gut Microbiome in Elderly People during Probiotic Consumption. mBio 2015, 6, e00231-15. [Google Scholar] [CrossRef] [Green Version]
- Lawson, P.A.; Citron, D.M.; Tyrrell, K.L.; Finegold, S.M. Reclassification of Clostridium Difficile as Clostridioides Difficile (Hall and O’Toole 1935) Prévot 1938. Anaerobe 2016, 40, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.J.; Macfarlane, G.T. Changes in Predominant Bacterial Populations in Human Faeces with Age and with Clostridium Difficile Infection. J. Med. Microbiol. 2002, 51, 448–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagamine, T.; Matsumoto, Y.; Nakamura, M. Combination Probiotics May Prevent Clostridium difficile Infection among Elderly Patients Undergoing an Orthopedic Surgery. Biosci. Microbiota Food Health 2019, 38, 31–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, G.R.; Probert, H.M.; van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Updating the Concept of Prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Blaut, M. Relationship of Prebiotics and Food to Intestinal Microflora. Eur J. Nutr. 2002, 41, i11–i16. [Google Scholar] [CrossRef]
- Salazar, N.; Valdés-Varela, L.; González, S.; Gueimonde, M.; de los Reyes-Gavilán, C.G. Nutrition and the Gut Microbiome in the Elderly. Gut Microbes 2017, 8, 82–97. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.-C.; Hsu, C.-K.; Ko, C.-Y.; Chan, Y.-C. Dietary Intake of Xylooligosaccharides Improves the Intestinal Microbiota, Fecal Moisture, and PH Value in the Elderly. Nutr. Res. 2007, 27, 756–761. [Google Scholar] [CrossRef]
- Vulevic, J.; Juric, A.; Walton, G.E.; Claus, S.P.; Tzortzis, G.; Toward, R.E.; Gibson, G.R. Influence of Galacto-Oligosaccharide Mixture (B-GOS) on Gut Microbiota, Immune Parameters and Metabonomics in Elderly Persons. Br. J. Nutr. 2015, 114, 586–595. [Google Scholar] [CrossRef] [Green Version]
- Scheid, M.M.A.; Genaro, P.S.; Moreno, Y.M.F.; Pastore, G.M. Freeze-Dried Powdered Yacon: Effects of FOS on Serum Glucose, Lipids and Intestinal Transit in the Elderly. Eur J. Nutr. 2014, 53, 1457–1464. [Google Scholar] [CrossRef]
- Muthyala, S.D.V.; Shankar, S.; Klemashevich, C.; Blazier, J.C.; Hillhouse, A.; Wu, C.-S. Differential Effects of the Soluble Fiber Inulin in Reducing Adiposity and Altering Gut Microbiome in Aging Mice. J. Nutr. Biochem. 2022, 105, 108999. [Google Scholar] [CrossRef]
- Theou, O.; Jayanama, K.; Fernández-Garrido, J.; Buigues, C.; Pruimboom, L.; Hoogland, A.J.; Navarro-Martínez, R.; Rockwood, K.; Cauli, O. Can a prebiotic formulation reduce frailty levels in older people? J. Frailty Aging 2018, 8, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Ni Lochlainn, M.; Nessa, A.; Sheedy, A.; Horsfall, R.; García, M.P.; Hart, D.; Akdag, G.; Yarand, D.; Wadge, S.; Baleanu, A.-F.; et al. The PROMOTe Study: Targeting the Gut Microbiome with Prebiotics to Overcome Age-Related Anabolic Resistance: Protocol for a Double-Blinded, Randomised, Placebo-Controlled Trial. BMC Geriatr. 2021, 21, 407. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, K.W.; Cheatham, C.L. Omega-6/Omega-3 Fatty Acid Intake of Children and Older Adults in the U.S.: Dietary Intake in Comparison to Current Dietary Recommendations and the Healthy Eating Index. Lipids Health Dis. 2018, 17, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menni, C.; Zierer, J.; Pallister, T.; Jackson, M.A.; Long, T.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Omega-3 Fatty Acids Correlate with Gut Microbiome Diversity and Production of N-Carbamylglutamate in Middle Aged and Elderly Women. Sci. Rep. 2017, 7, 11079. [Google Scholar] [CrossRef] [Green Version]
- Rajkumar, H.; Mahmood, N.; Kumar, M.; Varikuti, S.R.; Challa, H.R.; Myakala, S.P. Effect of Probiotic (VSL#3) and Omega-3 on Lipid Profile, Insulin Sensitivity, Inflammatory Markers, and Gut Colonization in Overweight Adults: A Randomized, Controlled Trial. Mediat. Inflamm. 2014, 2014, 348959. [Google Scholar] [CrossRef] [Green Version]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [Green Version]
- Bellenger, J.; Bellenger, S.; Escoula, Q.; Bidu, C.; Narce, M. N-3 Polyunsaturated Fatty Acids: An Innovative Strategy against Obesity and Related Metabolic Disorders, Intestinal Alteration and Gut Microbiota Dysbiosis. Biochimie 2019, 159, 66–71. [Google Scholar] [CrossRef]
- Cândido, F.G.; Valente, F.X.; Grześkowiak, Ł.M.; Moreira, A.P.B.; Rocha, D.M.U.P.; Alfenas, R.d.C.G. Impact of Dietary Fat on Gut Microbiota and Low-Grade Systemic Inflammation: Mechanisms and Clinical Implications on Obesity. Int. J. Food Sci. Nutr. 2018, 69, 125–143. [Google Scholar] [CrossRef]
- Hamazaki, K.; Maekawa, M.; Toyota, T.; Dean, B.; Hamazaki, T.; Yoshikawa, T. Fatty Acid Composition of the Postmortem Prefrontal Cortex of Patients with Schizophrenia, Bipolar Disorder, and Major Depressive Disorder. Psychiatry Res. 2015, 227, 353–359. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Shalim, C.P.; Yoewono, A.; Utomo, Y.; Kuswardhani, R.T. The role of probiotic supplementation on the immune system in elderly. Int J. Med. Biomed. Stud. 2019, 3, 250–255. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Tiihonen, K.; Saarinen, M.; Putaala, H.; Rautonen, N. Influence of a Combination of Lactobacillus Acidophilus NCFM and Lactitol on Healthy Elderly: Intestinal and Immune Parameters. Br. J. Nutr. 2008, 101, 367–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Impact of a Short-Term Synbiotic Supplementation on Metabolic Syndrome and Systemic Inflammation in Elderly Patients: A Randomized Placebo-Controlled Clinical Trial. Eur. J. Nutr. 2021, 60, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Padwad, Y. Probiotic Bacteria as Modulators of Cellular Senescence: Emerging Concepts and Opportunities. Gut Microbes 2020, 11, 335–349. [Google Scholar] [CrossRef]
- Westfall, S.; Lomis, N.; Prakash, S. Longevity Extension in Drosophila through Gut-Brain Communication. Sci. Rep. 2018, 8, 8362. [Google Scholar] [CrossRef]
- Donati Zeppa, S.; Ferrini, F.; Agostini, D.; Amatori, S.; Barbieri, E.; Piccoli, G.; Sestili, P.; Stocchi, V. Nutraceuticals and Physical Activity as Antidepressants: The Central Role of the Gut Microbiota. Antioxidants 2022, 11, 236. [Google Scholar] [CrossRef]
- Zhuang, Z.-Q.; Shen, L.-L.; Li, W.-W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Zhu, C.; Tan, Y.-L.; et al. Gut Microbiota Is Altered in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 63, 1337–1346. [Google Scholar] [CrossRef] [Green Version]
- Friedland, R.P. Mechanisms of Molecular Mimicry Involving the Microbiota in Neurodegeneration. J. Alzheimer’s Dis. 2015, 45, 349–362. [Google Scholar] [CrossRef] [Green Version]
- Petrov, V.A.; Saltykova, I.V.; Zhukova, I.A.; Alifirova, V.M.; Zhukova, N.G.; Dorofeeva, Y.B.; Tyakht, A.V.; Kovarsky, B.A.; Alekseev, D.G.; Kostryukova, E.S.; et al. Analysis of Gut Microbiota in Patients with Parkinson’s Disease. Bull. Exp. Biol. Med. 2017, 162, 734–737. [Google Scholar] [CrossRef]
- Bourassa, M.W.; Alim, I.; Bultman, S.J.; Ratan, R.R. Butyrate, Neuroepigenetics and the Gut Microbiome: Can a High Fiber Diet Improve Brain Health? Neurosci. Lett. 2016, 625, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.F.; de Oliveira, H.L.; Yamada, E.S.; Neves, B.C.; Pereira, A. The Gut and Parkinson’s Disease—A Bidirectional Pathway. Front. Neurol. 2019, 10, 574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef] [Green Version]
- Patterson, E.; Ryan, P.M.; Wiley, N.; Carafa, I.; Sherwin, E.; Moloney, G.; Franciosi, E.; Mandal, R.; Wishart, D.S.; Tuohy, K.; et al. Gamma-Aminobutyric Acid-Producing Lactobacilli Positively Affect Metabolism and Depressive-like Behaviour in a Mouse Model of Metabolic Syndrome. Sci. Rep. 2019, 9, 16323. [Google Scholar] [CrossRef] [Green Version]
- Kimura-Todani, T.; Hata, T.; Miyata, N.; Takakura, S.; Yoshihara, K.; Zhang, X.-T.; Asano, Y.; Altaisaikhan, A.; Tsukahara, T.; Sudo, N. Dietary Delivery of Acetate to the Colon Using Acylated Starches as a Carrier Exerts Anxiolytic Effects in Mice. Physiol. Behav. 2020, 223, 113004. [Google Scholar] [CrossRef]
- Savignac, H.M.; Corona, G.; Mills, H.; Chen, L.; Spencer, J.P.E.; Tzortzis, G.; Burnet, P.W.J. Prebiotic Feeding Elevates Central Brain Derived Neurotrophic Factor, N-Methyl-d-Aspartate Receptor Subunits and d-Serine. Neurochem. Int. 2013, 63, 756–764. [Google Scholar] [CrossRef] [Green Version]
- Wellman, A.S.; Metukuri, M.R.; Kazgan, N.; Xu, X.; Xu, Q.; Ren, N.S.X.; Czopik, A.; Shanahan, M.T.; Kang, A.; Chen, W.; et al. Intestinal Epithelial Sirtuin 1 Regulates Intestinal Inflammation During Aging in Mice by Altering the Intestinal Microbiota. Gastroenterology 2017, 153, 772–786. [Google Scholar] [CrossRef]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain–Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of Fermented Milk Product With Probiotic Modulates Brain Activity. Gastroenterology 2013, 144, 1394–1401.e4. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.; Sutherland, D.; Hewlett, P. An Investigation of the Acute Effects of Oligofructose-Enriched Inulin on Subjective Wellbeing, Mood and Cognitive Performance. Nutrients 2015, 7, 8887–8896. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, P.; Marchetti, M.; Cioccoloni, G.; de Lorenzo, A.; Romano, L.; Cammarano, A.; Colica, C.; Condò, R.; di Renzo, L. Psychobiotics Regulate the Anxiety Symptoms in Carriers of Allele A of IL-1 β Gene: A Randomized, Placebo-Controlled Clinical Trial. Mediat. Inflamm. 2020, 2020, 2346126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Luo, Q.; Nie, R.; Yang, X.; Tang, Z.; Chen, H. Potential Implications of Polyphenols on Aging Considering Oxidative Stress, Inflammation, Autophagy, and Gut Microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 2175–2193. [Google Scholar] [CrossRef] [PubMed]
- Del Bo’, C.; Bernardi, S.; Cherubini, A.; Porrini, M.; Gargari, G.; Hidalgo-Liberona, N.; González-Domínguez, R.; Zamora-Ros, R.; Peron, G.; Marino, M.; et al. A Polyphenol-Rich Dietary Pattern Improves Intestinal Permeability, Evaluated as Serum Zonulin Levels, in Older Subjects: The MaPLE Randomised Controlled Trial. Clin. Nutr. 2021, 40, 3006–3018. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health Relevance of the Modification of Low Grade Inflammation in Ageing (Inflammageing) and the Role of Nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef]
- Pannu, N.; Bhatnagar, A. Resveratrol: From Enhanced Biosynthesis and Bioavailability to Multitargeting Chronic Diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Lertpiriyapong, K.; Steelman, L.S.; Abrams, S.L.; Yang, L.V.; Murata, R.M.; Rosalen, P.L.; Scalisi, A.; Neri, L.M.; Cocco, L.; et al. Effects of Resveratrol, Curcumin, Berberine and Other Nutraceuticals on Aging, Cancer Development, Cancer Stem Cells and MicroRNAs. Aging 2017, 9, 1477–1536. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Gao, Y.; Zeng, B.; Fan, X.; Yang, D.; Yang, M. Effects of Anti-Aging Interventions on Intestinal Microbiota. Gut Microbes 2021, 13, 1994835. [Google Scholar] [CrossRef]
- Carrera-Quintanar, L.; López Roa, R.I.; Quintero-Fabián, S.; Sánchez-Sánchez, M.A.; Vizmanos, B.; Ortuño-Sahagún, D. Phytochemicals That Influence Gut Microbiota as Prophylactics and for the Treatment of Obesity and Inflammatory Diseases. Mediat. Inflamm. 2018, 2018, 9734845. [Google Scholar] [CrossRef] [Green Version]
- Rehman, K.; Saeed, K.; Munawar, S.M.; Akash, M.S.H. Resveratrol Regulates Hyperglycemia-Induced Modulations in Experimental Diabetic Animal Model. Biomed. Pharmacother. 2018, 102, 140–146. [Google Scholar] [CrossRef]
- De Freitas, P.L.; Miranda, J.P.N.; França, L.M.; Paes, A.M.d.A. Plant-Derived (Poly)Phenols and Their Metabolic Outcomes: The Pursuit of a Role for the Gut Microbiota. Nutrients 2022, 14, 3510. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.K.; Luo, J.; Lau, C.W.; Chen, Z.; Tian, X.Y.; Huang, Y. Pharmacological Basis and New Insights of Resveratrol Action in the Cardiovascular System. Br. J. Pharmacol. 2020, 177, 1258–1277. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, D.; Ke, W.; Liang, D.; Hu, X.; Chen, F. Resveratrol-Induced Gut Microbiota Reduces Obesity in High-Fat Diet-Fed Mice. Int J. Obes. 2020, 44, 213–225. [Google Scholar] [CrossRef]
- Chen, M.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.; Zhang, Q.; Mi, M. Resveratrol Attenuates Trimethylamine- N -Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef] [PubMed]
- Wilmanski, T.; Diener, C.; Rappaport, N.; Patwardhan, S.; Wiedrick, J.; Lapidus, J.; Earls, J.C.; Zimmer, A.; Glusman, G.; Robinson, M.; et al. Gut Microbiome Pattern Reflects Healthy Ageing and Predicts Survival in Humans. Nat. Metab. 2021, 3, 274–286. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Yu, B. The Bidirectional Interactions between Resveratrol and Gut Microbiota: An Insight into Oxidative Stress and Inflammatory Bowel Disease Therapy. Biomed. Res. Int. 2019, 2019, 5403761. [Google Scholar] [CrossRef]
- Sonnemann, J.; Kahl, M.; Siranjeevi, P.M.; Blumrich, A.; Blümel, L.; Becker, S.; Wittig, S.; Winkler, R.; Krämer, O.H.; Beck, J.F. Reverse Chemomodulatory Effects of the SIRT1 Activators Resveratrol and SRT1720 in Ewing’s Sarcoma Cells: Resveratrol Suppresses and SRT1720 Enhances Etoposide- and Vincristine-Induced Anticancer Activity. J. Cancer Res. Clin. Oncol. 2016, 142, 17–26. [Google Scholar] [CrossRef]
- Kala, R.; Shah, H.N.; Martin, S.L.; Tollefsbol, T.O. Epigenetic-Based Combinatorial Resveratrol and Pterostilbene Alters DNA Damage Response by Affecting SIRT1 and DNMT Enzyme Expression, Including SIRT1-Dependent γ-H2AX and Telomerase Regulation in Triple-Negative Breast Cancer. BMC Cancer 2015, 15, 672. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Dong, Z.; Li, Q.; Wan, D.; Zhong, J.; Dongzhi, D.; Huang, M. Flavonoids from Rhododendron Nivale Hook. f Delay Aging via Modulation of Gut Microbiota and Glutathione Metabolism. Phytomedicine 2022, 104, 154270. [Google Scholar] [CrossRef]
- Gutiérrez-Díaz, I.; Salazar, N.; Pérez-Jiménez, J.; de los Reyes-Gavilán, C.G.; Gueimonde, M.; González, S. New Players in the Relationship between Diet and Microbiota: The Role of Macromolecular Antioxidant Polyphenols. Eur. J. Nutr. 2021, 60, 1403–1413. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Soja, J.; Gancarz, M.; Wojtunik-Kulesza, K.; Markut-Miotła, E.; Oniszczuk, A. The Efficacy of Black Chokeberry Fruits against Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 6541. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, W.; Suszek, M.; Wnuk, M.; Lewinska, A.; Wasiak, E.; Sikora, E.; Bielak-Zmijewska, A. Curcumin Elevates Sirtuin Level but Does Not Postpone in Vitro Senescence of Human Cells Building the Vasculature. Oncotarget 2016, 7, 19201–19213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dkhar, P.; Sharma, R. Attenuation of Age-Related Increase of Protein Carbonylation in the Liver of Mice by Melatonin and Curcumin. Mol. Cell. Biochem. 2013, 380, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Zhang, H.; Wang, P.; Zhao, Y.; Li, Q.; Wei, X.; Cui, Y.; Sun, J.; Shang, Q.; Liu, D.; et al. Dietary Curcumin Ameliorates Aging-Related Cerebrovascular Dysfunction through the AMPK/Uncoupling Protein 2 Pathway. Cell. Physiol. Biochem. 2013, 32, 1167–1177. [Google Scholar] [CrossRef]
- Bielak-Zmijewska, A.; Grabowska, W.; Ciolko, A.; Bojko, A.; Mosieniak, G.; Bijoch, Ł.; Sikora, E. The Role of Curcumin in the Modulation of Ageing. Int. J. Mol. Sci. 2019, 20, 1239. [Google Scholar] [CrossRef] [Green Version]
- Biagi, E.; Rampelli, S.; Turroni, S.; Quercia, S.; Candela, M.; Brigidi, P. The Gut Microbiota of Centenarians: Signatures of Longevity in the Gut Microbiota Profile. Mech. Ageing Dev. 2017, 165, 180–184. [Google Scholar] [CrossRef]
- Ohno, M.; Nishida, A.; Sugitani, Y.; Nishino, K.; Inatomi, O.; Sugimoto, M.; Kawahara, M.; Andoh, A. Nanoparticle Curcumin Ameliorates Experimental Colitis via Modulation of Gut Microbiota and Induction of Regulatory T Cells. PLoS ONE 2017, 12, e0185999. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Chen, Y.; Xiang, L.; Wang, Z.; Xiao, G.; Hu, J. Effect of Curcumin on the Diversity of Gut Microbiota in Ovariectomized Rats. Nutrients 2017, 9, 1146. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Liu, L.; Ji, H.-F. Regulative Effects of Curcumin Spice Administration on Gut Microbiota and Its Pharmacological Implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef] [Green Version]
- Weyh, C.; Krüger, K.; Strasser, B. Physical Activity and Diet Shape the Immune System during Aging. Nutrients 2020, 12, 622. [Google Scholar] [CrossRef]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The Microbiome of Professional Athletes Differs from That of More Sedentary Subjects in Composition and Particularly at the Functional Metabolic Level. Gut 2017, 67, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory Fitness as a Predictor of Intestinal Microbial Diversity and Distinct Metagenomic Functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Intestinal Microbiota Modulation in Obesity-Related Non-Alcoholic Fatty Liver Disease. Front. Physiol. 2018, 9, 1813. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, L.T. Overtraining Effects on Immunity and Performance in Athletes. Immunol. Cell Biol. 2000, 78, 502–509. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. Exercise-Induced Stress Behavior, Gut-Microbiota-Brain Axis and Diet: A Systematic Review for Athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef] [Green Version]
- Morishima, S.; Aoi, W.; Kawamura, A.; Kawase, T.; Takagi, T.; Naito, Y.; Tsukahara, T.; Inoue, R. Intensive, Prolonged Exercise Seemingly Causes Gut Dysbiosis in Female Endurance Runners. J. Clin. Biochem. Nutr. 2021, 68, 253–258. [Google Scholar] [CrossRef]
- Ticinesi, A.; Lauretani, F.; Tana, C.; Nouvenne, A.; Ridolo, E.; Meschi, T. Exercise and Immune System as Modulators of Intestinal Microbiome: Implications for the Gut-Muscle Axis Hypothesis. Exerc. Immunol. Rev. 2019, 25, 84–95. [Google Scholar]
- Grosicki, G.J.; Durk, R.P.; Bagley, J.R. Rapid Gut Microbiome Changes in a World-class Ultramarathon Runner. Physiol. Rep. 2019, 7, e14313. [Google Scholar] [CrossRef] [Green Version]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, Diet and Stress as Modulators of Gut Microbiota: Implications for Neurodegenerative Diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef]
- Codella, R.; Luzi, L.; Terruzzi, I. Exercise Has the Guts: How Physical Activity May Positively Modulate Gut Microbiota in Chronic and Immune-Based Diseases. Dig. Liver Dis. 2018, 50, 331–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in Gut Microbiota Profile between Women with Active Lifestyle and Sedentary Women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and Disease Markers Correlate with Gut Microbiome Composition across Thousands of People. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Kubo, T.; Hoshino, Y.; Hosokawa, M.; Takeyama, H.; Higuchi, M. Effects of Short-term Endurance Exercise on Gut Microbiota in Elderly Men. Physiol. Rep. 2018, 6, e13935. [Google Scholar] [CrossRef]
- Munukka, E.; Ahtiainen, J.P.; Puigbó, P.; Jalkanen, S.; Pahkala, K.; Keskitalo, A.; Kujala, U.M.; Pietilä, S.; Hollmén, M.; Elo, L.; et al. Six-Week Endurance Exercise Alters Gut Metagenome That Is Not Reflected in Systemic Metabolism in Over-Weight Women. Front. Microbiol. 2018, 9, 2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cronin, O.; Barton, W.; Skuse, P.; Penney, N.C.; Garcia-Perez, I.; Murphy, E.F.; Woods, T.; Nugent, H.; Fanning, A.; Melgar, S.; et al. A Prospective Metagenomic and Metabolomic Analysis of the Impact of Exercise and/or Whey Protein Supplementation on the Gut Microbiome of Sedentary Adults. mSystems 2018, 3, e00044-18. [Google Scholar] [CrossRef] [Green Version]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Bycura, D.; Santos, A.C.; Shiffer, A.; Kyman, S.; Winfree, K.; Sutliffe, J.; Pearson, T.; Sonderegger, D.; Cope, E.; Caporaso, J.G. Impact of Different Exercise Modalities on the Human Gut Microbiome. Sports 2021, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Jiang, S.; Du, G. Effects of Exercise Frequency on the Gut Microbiota in Elderly Individuals. Microbiologyopen 2020, 9, e1053. [Google Scholar] [CrossRef]
- Shi, J.; Yu, D.; Yang, Y.; Cai, H.; Wu, J.; Cai, Q.; Long, J.; Zheng, W.; Xu, W.; Shu, X.-O. Association Between Long-Term Regular Exercise and Gut Microbiota Among Middle-Aged and Older Urban Chinese. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Donati Zeppa, S.; Sisti, D.; Amatori, S.; Gervasi, M.; Agostini, D.; Piccoli, G.; Bertuccioli, A.; Rocchi, M.B.L.; Stocchi, V.; Sestili, P. High-Intensity Interval Training Promotes the Shift to a Health-Supporting Dietary Pattern in Young Adults. Nutrients 2020, 12, 843. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donati Zeppa, S.; Agostini, D.; Ferrini, F.; Gervasi, M.; Barbieri, E.; Bartolacci, A.; Piccoli, G.; Saltarelli, R.; Sestili, P.; Stocchi, V. Interventions on Gut Microbiota for Healthy Aging. Cells 2023, 12, 34. https://doi.org/10.3390/cells12010034
Donati Zeppa S, Agostini D, Ferrini F, Gervasi M, Barbieri E, Bartolacci A, Piccoli G, Saltarelli R, Sestili P, Stocchi V. Interventions on Gut Microbiota for Healthy Aging. Cells. 2023; 12(1):34. https://doi.org/10.3390/cells12010034
Chicago/Turabian StyleDonati Zeppa, Sabrina, Deborah Agostini, Fabio Ferrini, Marco Gervasi, Elena Barbieri, Alessia Bartolacci, Giovanni Piccoli, Roberta Saltarelli, Piero Sestili, and Vilberto Stocchi. 2023. "Interventions on Gut Microbiota for Healthy Aging" Cells 12, no. 1: 34. https://doi.org/10.3390/cells12010034
APA StyleDonati Zeppa, S., Agostini, D., Ferrini, F., Gervasi, M., Barbieri, E., Bartolacci, A., Piccoli, G., Saltarelli, R., Sestili, P., & Stocchi, V. (2023). Interventions on Gut Microbiota for Healthy Aging. Cells, 12(1), 34. https://doi.org/10.3390/cells12010034