The Male-Biased Expression of miR-2954 Is Involved in the Male Pathway of Chicken Sex Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Embryo Sexing
2.3. Solexa Deep Sequencing and Data Analysis of MiRNAs
2.4. In Vivo-Morpholino of miR-2954
2.5. Aromatase Inhibitor (AI) Treatments in Ovo
2.6. Quantitative Reverse Transcription-PCR (qRT-PCR)
2.7. Hematoxylin-Eosin (HE) Staining
2.8. Immunofluorescence Analysis
2.9. Fluorescence In Situ Hybridization
3. Results
3.1. Identification of Sexual Differentially Expressed miRNAs in Chicken Embryos and Gonads
3.2. The Spatiotemporal Expression Pattern of miR-2954 in Chicken Embryonic Gonads
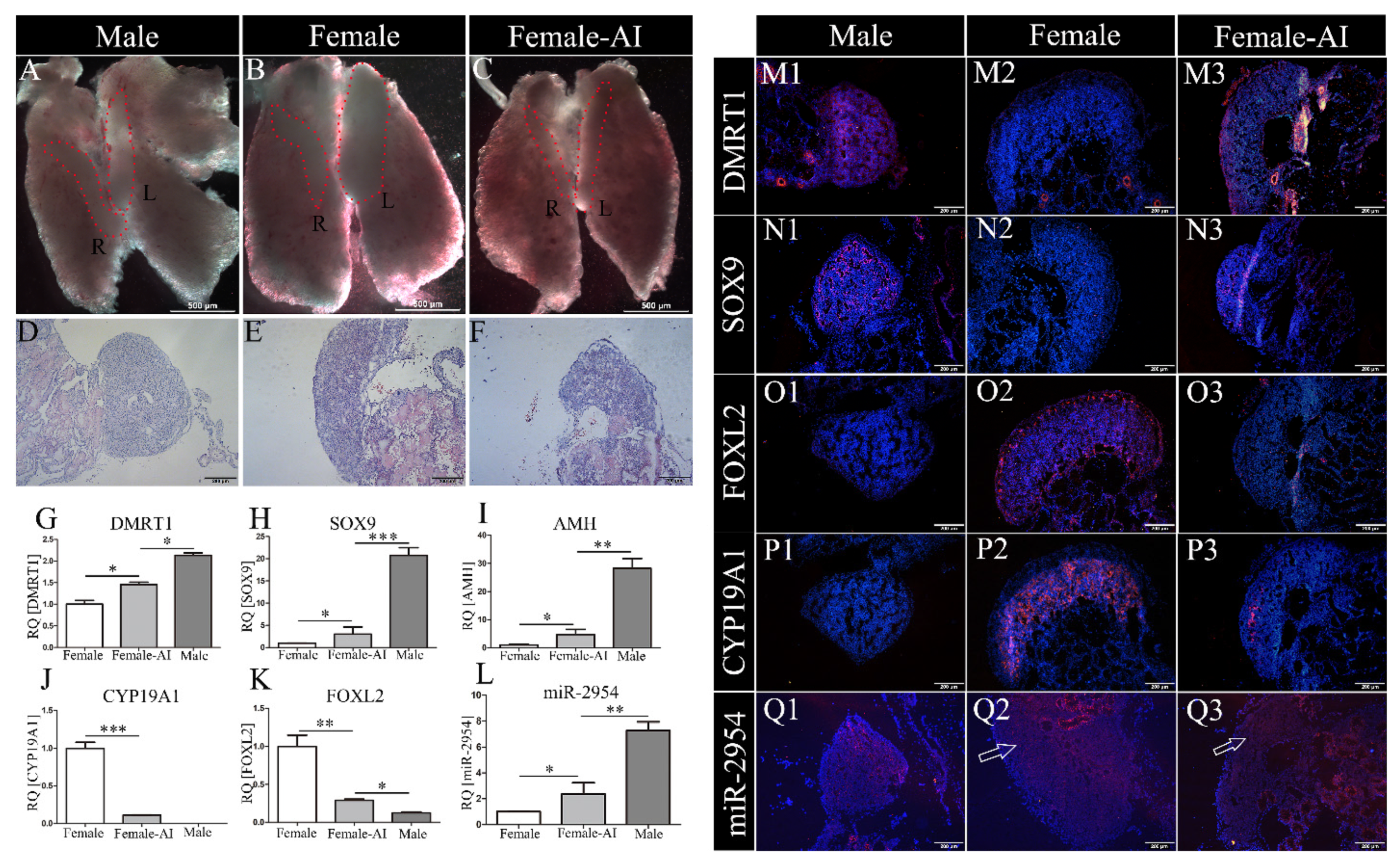
3.3. MiR-2954 Expression in AI-Treated ZW Gonads
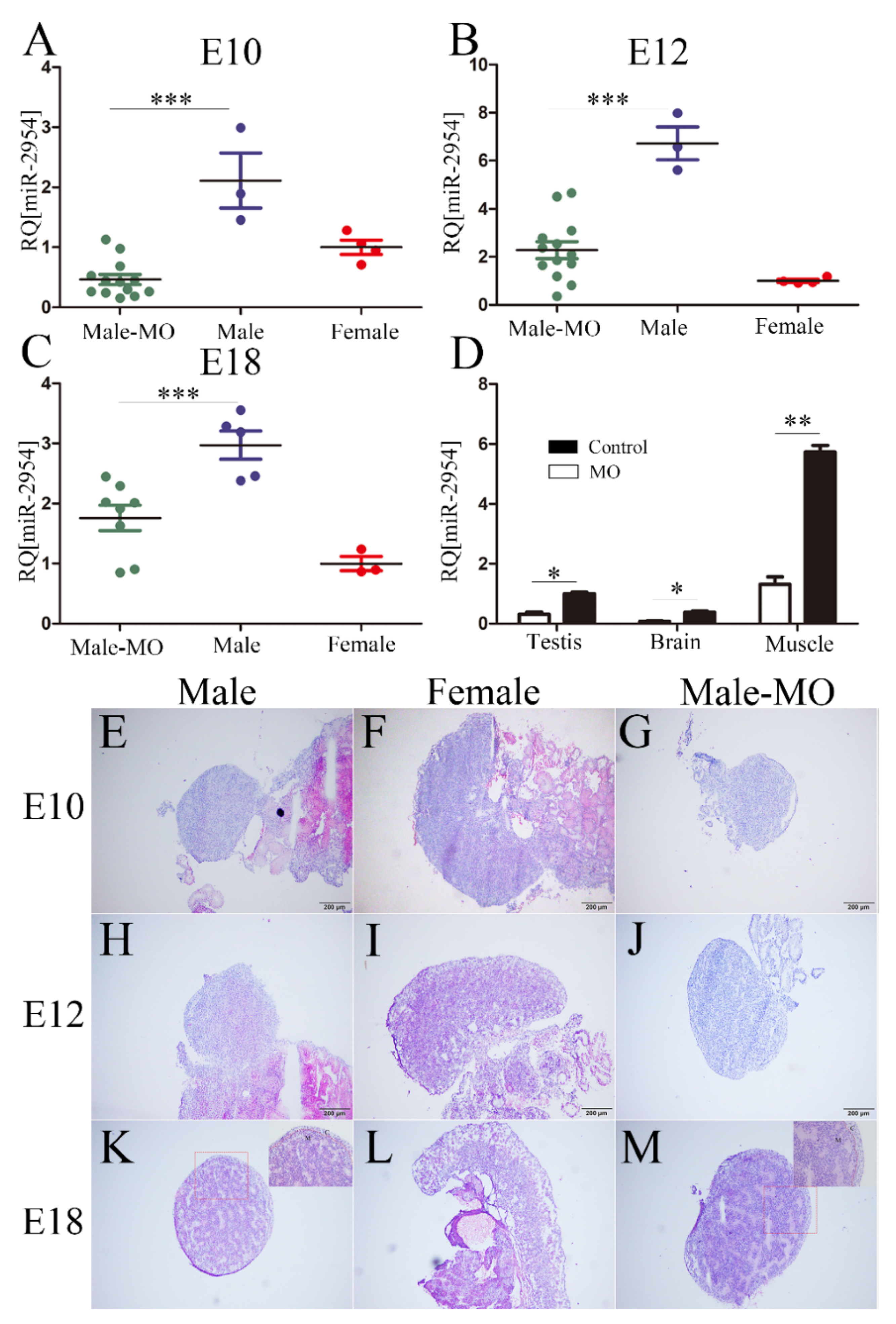
3.4. The Inhibitory Effect and Histomorphological Changes of Embryonic Gonads after VMO-miR-2954 Injection
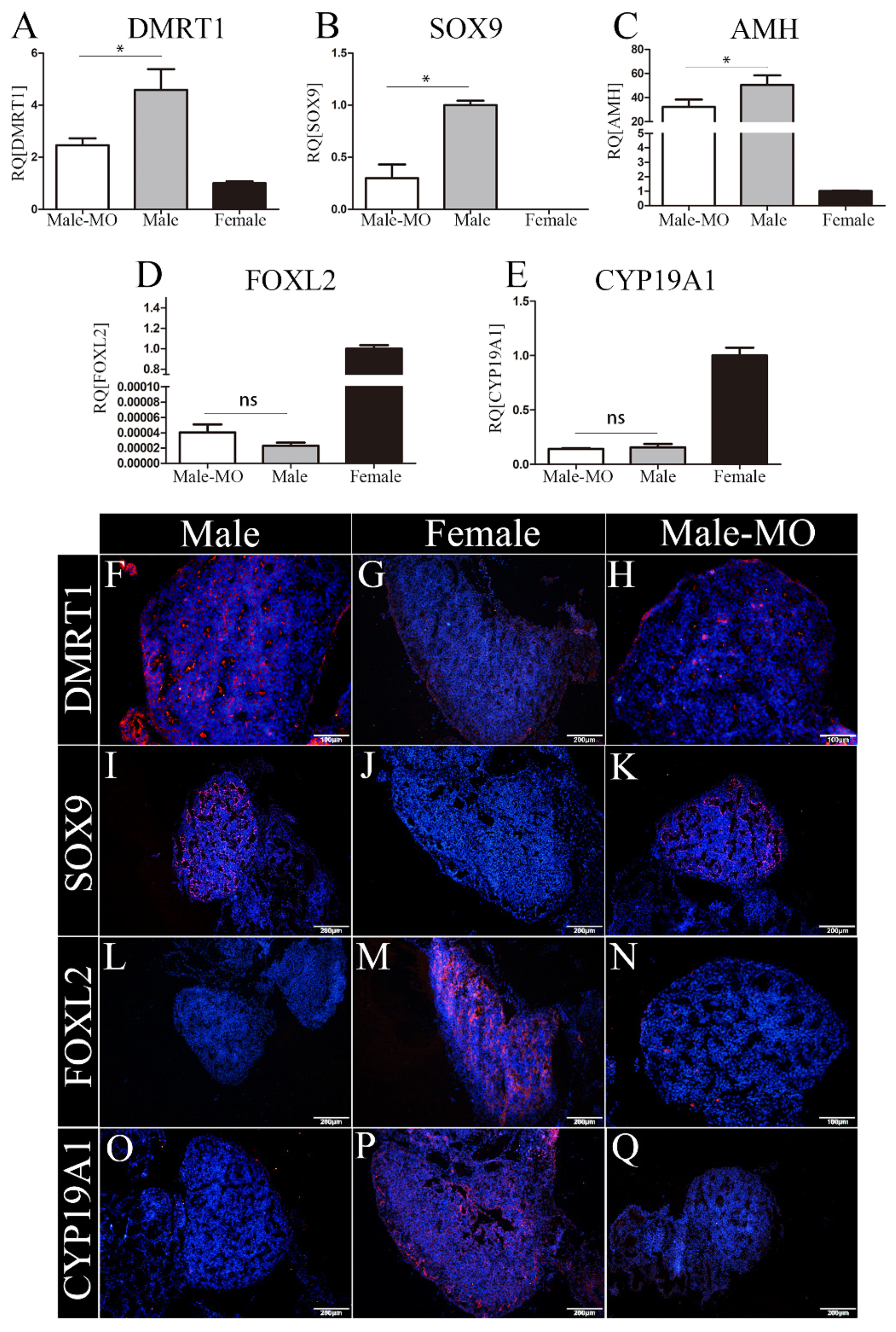
3.5. The Expression of Testicular and Ovarian Marker Genes after miR-2954 Inhibition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kashimada, K.; Koopman, P. Sry: The master switch in mammalian sex determination. Development 2010, 137, 3921–3930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, A.P.; Chen, X.; Itoh, Y. What a difference an X or Y makes: Sex chromosomes, gene dose, and epigenetics in sexual differentiation. In Sex and Gender Differences in Pharmacology; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 67–88. [Google Scholar] [CrossRef] [Green Version]
- Arnold, A.P.; Chen, X.; Link, J.C.; Itoh, Y.; Reue, K. Cell-autonomous sex determination outside of the gonad. Dev. Dyn. 2013, 242, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; McBride, D.; Nandi, S.; McQueen, H.A.; McGrew, M.J.; Hocking, P.M.; Lewis, P.D.; Sang, H.M.; Clinton, M. Somatic sex identity is cell autonomous in the chicken. Nature 2010, 464, 237–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, K.R.; Hirst, C.E.; Major, A.T.; Ezaz, T.; Ford, M.; Bibby, S.; Doran, T.J.; Smith, C.A. Gonadal and Endocrine Analysis of a Gynandromorphic Chicken. Endocrinology 2018, 159, 3492–3502. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, J.; Taylor, G.; Zhao, D.; Liu, L.; Idoko-Akoh, A.; Gong, D.; Lovell-Badge, R.; Guioli, S.; McGrew, M.J.; Clinton, M. Primary sex determination in birds depends on DMRT1 dosage, but gonadal sex does not determine adult secondary sex characteristics. Proc. Natl. Acad. Sci. USA 2021, 118, e2020909118. [Google Scholar] [CrossRef] [PubMed]
- Rakoczy, J.; Fernandez-Valverde, S.L.; Glazov, E.A.; Wainwright, E.N.; Sato, T.; Takada, S.; Combes, A.N.; Korbie, D.J.; Miller, D.; Grimmond, S.M.; et al. MicroRNAs-140-5p/140-3p modulate Leydig cell numbers in the developing mouse testis. Biol. Reprod. 2013, 88, 143. [Google Scholar] [CrossRef] [PubMed]
- Tripurani, S.K.; Xiao, C.; Salem, M.; Yao, J. Cloning and analysis of fetal ovary microRNAs in cattle. Anim. Reprod. Sci. 2010, 120, 16–22. [Google Scholar] [CrossRef]
- Torley, K.J.; da Silveira, J.C.; Smith, P.; Anthony, R.V.; Veeramachaneni, D.N.; Winger, Q.A.; Bouma, G.J. Expression of miRNAs in ovine fetal gonads: Potential role in gonadal differentiation. Reprod. Biol. Endocrinol. 2011, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Jing, J.; Wu, J.; Liu, W.; Xiong, S.; Ma, W.; Zhang, J.; Wang, W.; Gui, J.F.; Mei, J. Sex-biased miRNAs in gonad and their potential roles for testis development in yellow catfish. PLoS ONE 2014, 9, e107946. [Google Scholar] [CrossRef]
- Presslauer, C.; Tilahun Bizuayehu, T.; Kopp, M.; Fernandes, J.M.; Babiak, I. Dynamics of miRNA transcriptome during gonadal development of zebrafish. Sci. Rep. 2017, 7, 43850. [Google Scholar] [CrossRef] [Green Version]
- Bannister, S.C.; Tizard, M.L.; Doran, T.J.; Sinclair, A.H.; Smith, C.A. Sexually dimorphic microRNA expression during chicken embryonic gonadal development. Biol. Reprod. 2009, 81, 165–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bizuayehu, T.T.; Babiak, J.; Norberg, B.; Fernandes, J.M.; Johansen, S.D.; Babiak, I. Sex-biased miRNA expression in Atlantic halibut (Hippoglossus hippoglossus) brain and gonads. Sex. Dev. 2012, 6, 257–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.; Gong, Y.; Peng, X.; Li, S.; Yang, Y.; Feng, Y. Cloning, identification, and expression analysis at the stage of gonadal sex differentiation of chicken miR-363 and 363*. Acta Biochim. Biophys. Sin. 2010, 42, 522–529. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.Z.; Hafner, M.; Shi, Z.; Brown, M.; Feng, G.H.; Tuschl, T.; Wang, X.J.; Li, X. Genome-wide annotation and analysis of zebra finch microRNA repertoire reveal sex-biased expression. BMC Genom. 2012, 13, 727. [Google Scholar] [CrossRef] [Green Version]
- Gunaratne, P.H.; Lin, Y.C.; Benham, A.L.; Drnevich, J.; Coarfa, C.; Tennakoon, J.B.; Creighton, C.J.; Kim, J.H.; Milosavljevic, A.; Watson, M.; et al. Song exposure regulates known and novel microRNAs in the zebra finch auditory forebrain. BMC Genom. 2011, 12, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.C.; Balakrishnan, C.N.; Clayton, D.F. Functional genomic analysis and neuroanatomical localization of miR-2954, a song-responsive sex-linked microRNA in the zebra finch. Front. Neurosci. 2014, 8, 409. [Google Scholar] [CrossRef] [Green Version]
- Warnefors, M.; Mössinger, K.; Halbert, J.; Studer, T.; VandeBerg, J.L.; Lindgren, I.; Fallahshahroudi, A.; Jensen, P.; Kaessmann, H. Sex-biased microRNA expression in mammals and birds reveals underlying regulatory mechanisms and a role in dosage compensation. Genome Res. 2017, 27, 1961–1973. [Google Scholar] [CrossRef]
- Wen, M.; Shen, Y.; Shi, S.; Tang, T. miREvo: An integrative microRNA evolutionary analysis platform for next-generation sequencing experiments. BMC Bioinform. 2012, 13, 140. [Google Scholar] [CrossRef] [Green Version]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Lambeth, L.S.; Raymond, C.S.; Roeszler, K.N.; Kuroiwa, A.; Nakata, T.; Zarkower, D.; Smith, C.A. Over-expression of DMRT1 induces the male pathway in embryonic chicken gonads. Dev. Biol. 2014, 389, 160–172. [Google Scholar] [CrossRef] [Green Version]
- Nakata, T.; Ishiguro, M.; Aduma, N.; Izumi, H.; Kuroiwa, A. Chicken hemogen homolog is involved in the chicken-specific sex-determining mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 3417–3422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattsson, A.; Olsson, J.A.; Brunström, B. Activation of estrogen receptor alpha disrupts differentiation of the reproductive organs in chicken embryos. Gen. Comp. Endocrinol. 2011, 172, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Guioli, S.; Zhao, D.; Nandi, S.; Clinton, M.; Lovell-Badge, R. Oestrogen in the chick embryo can induce chromosomally male ZZ left gonad epithelial cells to form an ovarian cortex that can support oogenesis. Development 2020, 147, dev181693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbrecht, A.; Smith, R.G. Aromatase enzyme activity and sex determination in chickens. Science 1992, 255, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Katz, M.; Sinclair, A.H. DMRT1 is upregulated in the gonads during female-to-male sex reversal in ZW chicken embryos. Biol. Reprod. 2003, 68, 560–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, K.; Zuo, Q.; Song, J.; Zhang, Y.; Chen, G.; Li, B. CYP19A1 (aromatase) dominates female gonadal differentiation in chicken (Gallus gallus) embryos sexual differentiation. Biosci. Rep. 2020, 40, BSR20201576. [Google Scholar] [CrossRef]
- Moulton, J.D. Using morpholinos to control gene expression. Curr. Protoc. Nucleic Acid Chem. 2006, 27, 4.30.1–4.30.24. [Google Scholar] [CrossRef]
- Morcos, P.A.; Li, Y.; Jiang, S. Vivo-Morpholinos: A non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. Biotechniques 2008, 45, 613–614, 616, 618 passim. [Google Scholar] [CrossRef]
- Reissner, K.J.; Sartor, G.C.; Vazey, E.M.; Dunn, T.E.; Aston-Jones, G.; Kalivas, P.W. Use of vivo-morpholinos for control of protein expression in the adult rat brain. J. Neurosci. Methods 2012, 203, 354–360. [Google Scholar] [CrossRef]
- Stratman, A.N.; Farrelly, O.M.; Mikelis, C.M.; Miller, M.F.; Wang, Z.; Pham, V.N.; Davis, A.E.; Burns, M.C.; Pezoa, S.A.; Castranova, D.; et al. Anti-angiogenic effects of VEGF stimulation on endothelium deficient in phosphoinositide recycling. Nat. Commun. 2020, 11, 1204. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.S.; Lu, Y.F.; Liu, Y.H.; Huang, C.J.; Hwang, S.L. Zebrafish Cdx1b modulates epithalamic asymmetry by regulating ndr2 and lft1 expression. Dev. Biol. 2021, 470, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; McDaniel, K.; Wu, N.; Ramos-Lorenzo, S.; Glaser, T.; Venter, J.; Francis, H.; Kennedy, L.; Sato, K.; Zhou, T.; et al. Regulation of Cellular Senescence by miR-34a in Alcoholic Liver Injury. Am. J. Pathol. 2017, 187, 2788–2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasteuning-Vuhman, S.; Boertje-van der Meulen, J.W.; van Putten, M.; Overzier, M.; Ten Dijke, P.; Kiełbasa, S.M.; Arindrarto, W.; Wolterbeek, R.; Lezhnina, K.V.; Ozerov, I.V.; et al. New function of the myostatin/activin type I receptor (ALK4) as a mediator of muscle atrophy and muscle regeneration. FASEB J. 2017, 31, 238–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia de Sousa, M.; Gjorgjieva, M.; Dolicka, D.; Sobolewski, C.; Foti, M. Deciphering miRNAs’ Action through miRNA Editing. Int. J. Mol. Sci. 2019, 20, 6249. [Google Scholar] [CrossRef] [Green Version]
- Hurtado, A.; Real, F.M.; Palomino, R.; Carmona, F.D.; Burgos, M.; Jiménez, R.; Barrionuevo, F.J. Sertoli cell-specific ablation of miR-17-92 cluster significantly alters whole testis transcriptome without apparent phenotypic effects. PLoS ONE 2018, 13, e0197685. [Google Scholar] [CrossRef]
- Liu, Z.L.; Xu, J.; Ling, L.; Yang, D.H.; Chen, S.Q.; Huang, Y.P. MicroRNA-2738 regulates gene expression in the sex determination pathway in Bombyx mori. Insect Sci. 2020, 27, 646–654. [Google Scholar] [CrossRef]
- Yuan, S.; Tang, C.; Zhang, Y.; Wu, J.; Bao, J.; Zheng, H.; Xu, C.; Yan, W. mir-34b/c and mir-449a/b/c are required for spermatogenesis, but not for the first cleavage division in mice. Biol. Open 2015, 4, 212–223. [Google Scholar] [CrossRef] [Green Version]
- Cutting, A.D.; Bannister, S.C.; Doran, T.J.; Sinclair, A.H.; Tizard, M.V.; Smith, C.A. The potential role of microRNAs in regulating gonadal sex differentiation in the chicken embryo. Chromosome Res. 2012, 20, 201–213. [Google Scholar] [CrossRef]


| Gene | Sequence (5′-3′) | Genebank No. |
|---|---|---|
| RT-miR-2954 | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTGCTAGGA | |
| RT-5S rRNA | AACTGGTGTCGTGGAGTCGGC | |
| gga-miR-2954-F | GGTAGGCATCCCCATTCCACTC | MI0013634 |
| gga-miR-2954-R | AACTGGTGTCGTGGAGTCGGC | |
| F-5S rRNA | CCATACCACCCTGGAAACGC | M13919.1 |
| R-5S rRNA | TACTAACCGAGCCCGACCCT | |
| SOX9-F | TTCTCGCTCTCATTCAGCAG | NC_052549 |
| SOX9-R | GTACCCGCATCTGCACAAC | |
| AMH-F | CTCCCTCACCAACTACTCAACC | NC_052559 |
| AMH-R | TGCCAGTCCCCAAAATGCT | |
| FOXL2-F | AGAACAGCATCCGCCACAA | NC_052540 |
| FOXL2-R | GGGTCCAGCGTCCAGTAGT | |
| CYP19A1-F | GCTTGGATTACAGTGCATTG | NC_052541 |
| CYP19A1-R | CCAGGACCAGACAGGGCT | |
| GAPDH-F | GAGGGTAGTGAAGGCTGCTG | NC_052532 |
| GAPDH-R | CACAACACGGTTGCTGTATC | |
| CHD1-R | GTTACTGATTCGTCTACGAGA | NC_052572 |
| CHD1-F | ATTGAAATGATCCAGTGCTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Zhang, Z.; Zhang, G.; Chen, L.; Zeng, C.; Liu, X.; Feng, Y. The Male-Biased Expression of miR-2954 Is Involved in the Male Pathway of Chicken Sex Differentiation. Cells 2023, 12, 4. https://doi.org/10.3390/cells12010004
Cheng Y, Zhang Z, Zhang G, Chen L, Zeng C, Liu X, Feng Y. The Male-Biased Expression of miR-2954 Is Involved in the Male Pathway of Chicken Sex Differentiation. Cells. 2023; 12(1):4. https://doi.org/10.3390/cells12010004
Chicago/Turabian StyleCheng, Yu, Zhen Zhang, Guixin Zhang, Ligen Chen, Cuiping Zeng, Xiaoli Liu, and Yanping Feng. 2023. "The Male-Biased Expression of miR-2954 Is Involved in the Male Pathway of Chicken Sex Differentiation" Cells 12, no. 1: 4. https://doi.org/10.3390/cells12010004





