Radiotherapy of the Hepatocellular Carcinoma in Mice Has a Time-Of-Day-Dependent Impact on the Mouse Hippocampus
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals
2.2. Irradiation
2.3. Real Time qPCR
2.4. Immunohistochemistry
2.5. Image Analysis
2.6. Western Blot
2.7. Statistical Analysis
3. Results
3.1. Effect of HCC and Irradiation on the Expression Levels of Genes Encoding for Pro-Inflammatory Cytokines
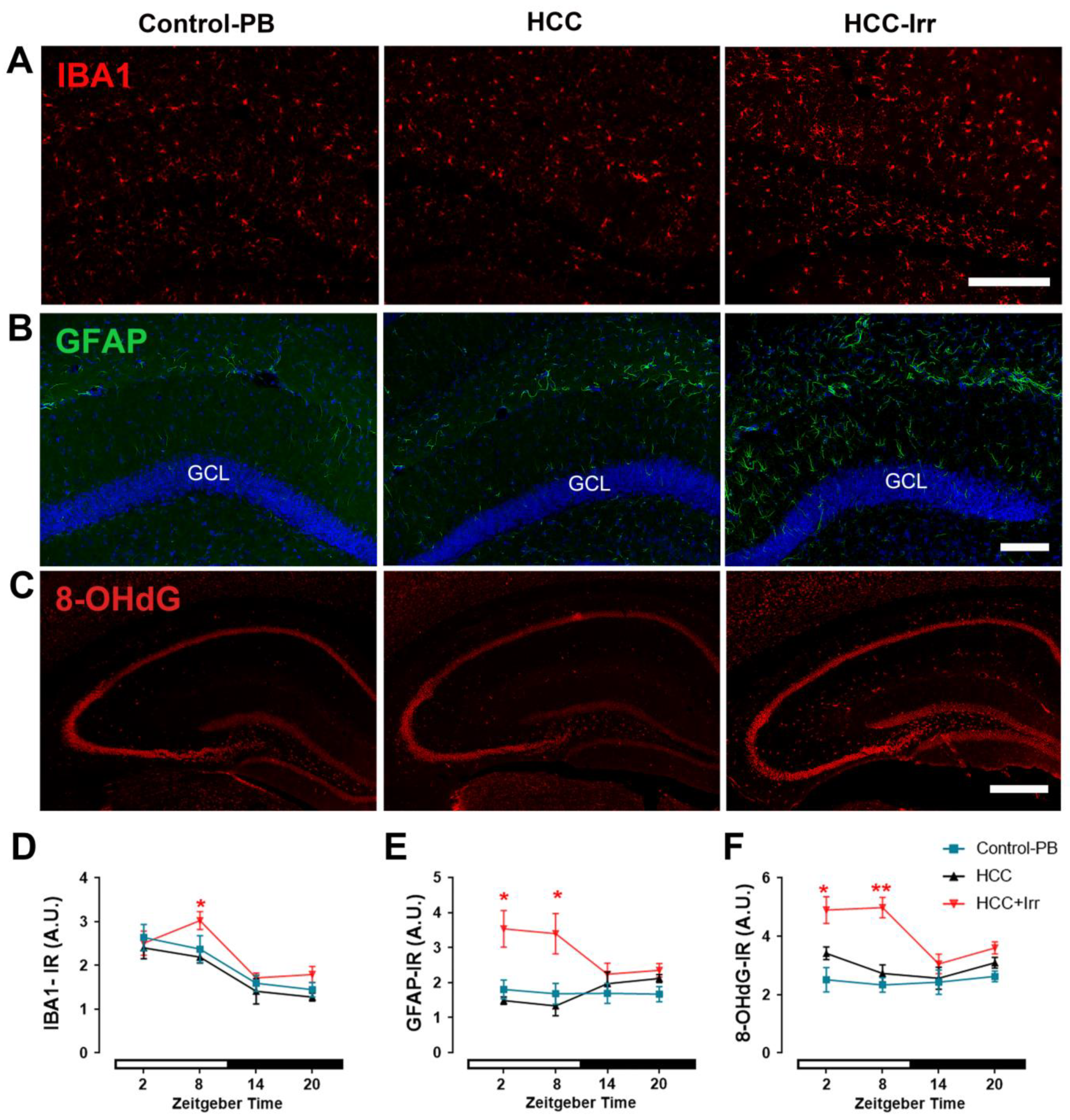
3.2. Effect of HCC and Irradiation on Glial Cell Activation and Oxidative Stress Levels
3.3. Effect of HCC and Irradiation on Neuronal Activity and Neural Progenitor Cell (NPC) Proliferation
3.4. Effect of HCC and Irradiation on Expression Levels of Clock Genes in the Hippocampus
3.5. Effect of HCC and Irradiation on the Expression Levels of p-ERK in the Hippocampus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Globocan. Worldwide Liver Cancer Fact Sheet. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf (accessed on 16 December 2022).
- Ren, Z.; Ma, X.; Duan, Z.; Chen, X. Diagnosis, Therapy, and Prognosis for Hepatocellular Carcinoma. Anal. Cell. Pathol. 2020, 2020, 8157406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- I Mishra, S.; Scherer, R.W.; Snyder, C.; Geigle, P.M.; Berlanstein, D.; Topaloglu, O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst. Rev. 2012, 2012, CD008465. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, Y.; Kiyohara, H.; Teratani, T.; Mikami, Y.; Kanai, T. Organ and brain crosstalk: The liver-brain axis in gastrointestinal, liver, and pancreatic diseases. Neuropharmacology 2021, 205, 108915. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- D'Mello, C.; Ronaghan, N.; Zaheer, R.; Dicay, M.; Le, T.; Macnaughton, W.K.; Surrette, M.G.; Swain, M.G. Probiotics Improve Inflammation-Associated Sickness Behavior by Altering Communication between the Peripheral Immune System and the Brain. J. Neurosci. 2015, 35, 10821–10830. [Google Scholar] [CrossRef] [Green Version]
- Chesnokova, V.; Pechnick, R.N.; Wawrowsky, K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav. Immun. 2016, 58, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Swain, M.G.; Jones, D.E.J. Fatigue in chronic liver disease: New insights and therapeutic approaches. Liver Int. 2019, 39, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Silver, R.; Kriegsfeld, L.J. Circadian rhythms have broad implications for understanding brain and behavior. Eur. J. Neurosci. 2014, 39, 1866–1880. [Google Scholar] [CrossRef] [Green Version]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef]
- Korf, H.-W.; von Gall, C. Circadian Physiology. In Neuroscience in the 21st Century, 3rd ed.; Pfaff, D.W., Volkow, N.D., Eds.; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Damato, A.R.; Herzog, E.D. Circadian clock synchrony and chronotherapy opportunities in cancer treatment. Semin. Cell Dev. Biol. 2022, 126, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, T.; Neves, A.R.; Quintela, T.; Costa, D. Exploring the link between chronobiology and drug delivery: Effects on cancer therapy. J. Mol. Med. 2021, 99, 1349–1371. [Google Scholar] [CrossRef] [PubMed]
- Damato, A.R.; Luo, J.; Katumba, R.G.N.; Talcott, G.R.; Rubin, J.B.; Herzog, E.D.; Campian, J.L. Temozolomide chronotherapy in patients with glioblastoma: A retrospective single-institute study. Neuro-Oncol. Adv. 2021, 3, vdab041. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.A.; Ali, A.A.H.; Sohn, D.; Flögel, U.; Jänicke, R.U.; Korf, H.; Gall, C. Does timing matter in radiotherapy of hepatocellular carcinoma? An experimental study in mice. Cancer Med. 2021, 10, 7712–7725. [Google Scholar] [CrossRef]
- Hassan, S.A.; Ali, A.A.; Yassine, M.; Sohn, D.; Pfeffer, M.; Jänicke, R.U.; Korf, H.; Gall, C. Relationship between locomotor activity rhythm and corticosterone levels during HCC development, progression, and treatment in a mouse model. J. Pineal Res. 2021, 70, e12724. [Google Scholar] [CrossRef]
- Tolba, R.; Kraus, T.; Liedtke, C.; Schwarz, M.; Weiskirchen, R. Diethylnitrosamine (DEN)-induced carcinogenic liver injury in mice. Lab. Anim. 2015, 49 (Suppl. S1), 59–69. [Google Scholar] [CrossRef] [Green Version]
- Bakiri, L.; Wagner, E.F. Mouse models for liver cancer. Mol. Oncol. 2013, 7, 206–223. [Google Scholar] [CrossRef]
- Loeppen, S.; Schneider, D.; Gaunitz, F.; Gebhardt, R.; Kurek, R.; Buchmann, A.; Schwarz, M. Overexpression of glutamine synthetase is associated with beta-catenin-mutations in mouse liver tumors during promotion of hepatocarcinogenesis by phenobarbital. Cancer Res. 2002, 62, 5685–5688. [Google Scholar]
- Rignall, B.; Braeuning, A.; Buchmann, A.; Schwarz, M. Tumor formation in liver of conditional -catenin-deficient mice exposed to a diethylnitrosamine/phenobarbital tumor promotion regimen. Carcinogenesis 2011, 32, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Vucic, V.; Isenovic, E.; Adzic, M.; Ruzdijic, S.; Radojcic, M. Effects of gamma-radiation on cell growth, cycle arrest, death, and superoxide dismutase expression by DU 145 human prostate cancer cells. Braz. J. Med Biol. Res. 2006, 39, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M. Quantification strategies in real-time PCR, p 87–112. In AZ of Quantitative PCR; International University Line: La Jolla, CA, USA, 2004. [Google Scholar]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Environ. Sci. Heal. Part C 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.H.; Schwarz-Herzke, B.; Rollenhagen, A.; Anstötz, M.; Holub, M.; Lübke, J.; Rose, C.R.; Schnittler, H.; von Gall, C. Bmal1-deficiency affects glial synaptic coverage of the hippocampal mossy fiber synapse and the actin cytoskeleton in astrocytes. Glia 2020, 68, 947–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.A.H.; Abdel-Hafiz, L.; Tundo-Lavalle, F.; Hassan, S.A.; von Gall, C. P2Y(2) deficiency impacts adult neurogenesis and related forebrain functions. Faseb j. 2021, 35, e21546. [Google Scholar] [CrossRef] [PubMed]
- Smarr, B.L.; Jennings, K.J.; Driscoll, J.R.; Kriegsfeld, L.J. A time to remember: The role of circadian clocks in learning and memory. Behav. Neurosci. 2014, 128, 283–303. [Google Scholar] [CrossRef] [Green Version]
- Jilg, A.; Lesny, S.; Peruzki, N.; Schwegler, H.; Selbach, O.; Dehghani, F.; Stehle, J.H. Temporal dynamics of mouse hippocampal clock gene expression support memory processing. Hippocampus 2010, 20, 377–388. [Google Scholar] [CrossRef]
- Debski, K.J.; Ceglia, N.; Ghestem, A.; Ivanov, A.I.; Brancati, G.E.; Bröer, S.; Bot, A.M.; Müller, J.A.; Schoch, S.; Becker, A.; et al. The circadian dynamics of the hippocampal transcriptome and proteome is altered in experimental temporal lobe epilepsy. Sci. Adv. 2020, 6, eaat5979. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Gibbons, A.J. Neuroinflammation, Sleep, and Circadian Rhythms. Front. Cell. Infect. Microbiol. 2022, 12, 853096. [Google Scholar] [CrossRef]
- Xing, C.; Zhou, Y.; Xu, H.; Ding, M.; Zhang, Y.; Zhang, M.; Hu, M.; Huang, X.; Song, L. Sleep disturbance induces depressive behaviors and neuroinflammation by altering the circadian oscillations of clock genes in rats. Neurosci. Res. 2021, 171, 124–132. [Google Scholar] [CrossRef]
- Musiek, E.; Lim, M.M.; Yang, G.; Bauer, A.Q.; Qi, L.; Lee, Y.; Roh, J.H.; Ortiz-Gonzalez, X.; Dearborn, J.; Culver, J.P.; et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Investig. 2013, 123, 5389–5400. [Google Scholar] [CrossRef] [Green Version]
- Griffin, P.; Dimitry, J.M.; Sheehan, P.W.; Lananna, B.V.; Guo, C.J.; Robinette, M.L.; Hayes, M.E.; Cedeño, M.R.; Nadarajah, C.; Ezerskiy, L.A.; et al. Circadian clock protein Rev-erbα regulates neuroinflammation. Proc. Natl. Acad. Sci. USA 2019, 116, 5102–5107. [Google Scholar] [CrossRef] [Green Version]
- Fonken, L.K.; Kitt, M.M.; Gaudet, A.D.; Barrientos, R.M.; Watkins, L.R.; Maier, S.F. Diminished circadian rhythms in hippocampal microglia may contribute to age-related neuroinflammatory sensitization. Neurobiol. Aging 2016, 47, 102–112. [Google Scholar] [CrossRef]
- Ait-Aissa, K.; Nguyen, Q.M.; Gabani, M.; Kassan, A.; Kumar, S.; Choi, S.-K.; Gonzalez, A.A.; Khataei, T.; Sahyoun, A.M.; Chen, C.; et al. MicroRNAs and obesity-induced endothelial dysfunction: Key paradigms in molecular therapy. Cardiovasc. Diabetol. 2020, 19, 136. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wu, Z.; Meng, J.; Saito, T.; Saido, T.C.; Qing, H.; Nakanishi, H. An impaired intrinsic microglial clock system induces neuroinflammatory alterations in the early stage of amyloid precursor protein knock-in mouse brain. J. Neuroinflammation 2019, 16, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawther, A.J.; Phillips, A.J.; Chung, N.-C.; Chang, A.; Ziegler, A.I.; Debs, S.; Sloan, E.K.; Walker, A.K. Disrupting circadian rhythms promotes cancer-induced inflammation in mice. Brain Behav. Immun. Heath. 2022, 21, 100428. [Google Scholar] [CrossRef] [PubMed]
- Buller, K.M. Chapter 9—Central Pathways of Immunoregulation. In NeuroImmune Biology; Arnason, B.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 9, pp. 101–111. [Google Scholar] [CrossRef]
- Belenguer, G.; Duart-Abadia, P.; Jordán-Pla, A.; Domingo-Muelas, A.; Blasco-Chamarro, L.; Ferrón, S.R.; Morante-Redolat, J.M.; Fariñas, I. Adult Neural Stem Cells Are Alerted by Systemic Inflammation through TNF-α Receptor Signaling. Cell Stem Cell 2021, 28, 285–299.e9. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Gallego, P.; Grande, L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J. Hepatol. 2018, 10, 1. [Google Scholar] [CrossRef]
- Sanghera, C.; Teh, J.J.; Pinato, D.J. The systemic inflammatory response as a source of biomarkers and therapeutic targets in hepatocellular carcinoma. Liver Int. 2019, 39, 2008–2023. [Google Scholar] [CrossRef] [Green Version]
- Pekny, M.; Pekna, M. Astrocyte Reactivity and Reactive Astrogliosis: Costs and Benefits. Physiol. Rev. 2014, 94, 1077–1098. [Google Scholar] [CrossRef]
- Jayakumar, A.R.; Norenberg, M.D. Endothelial-astrocytic interactions in acute liver failure. Metab. Brain Dis. 2013, 28, 183–186. [Google Scholar] [CrossRef]
- Gobernado, R.G.; Reimers, D.; Herranz, A.S.; Díaz-Gil, J.J.; Osuna, C.; Asensio, M.J.; Baena, S.; Serrano, E.M.R.; Bazán, E. Mobilization of Neural Stem Cells and Generation of New Neurons in 6-OHDA–lesioned Rats by Intracerebroventricular Infusion of Liver Growth Factor. J. Histochem. Cytochem. 2009, 57, 491–502. [Google Scholar] [CrossRef]
- Snyder, J.; Hong, N.; McDonald, R.; Wojtowicz, J. A role for adult neurogenesis in spatial long-term memory. Neuroscience 2005, 130, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.H.; von Gall, C. Adult Neurogenesis under Control of the Circadian System. Cells 2022, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nio, K.; Tang, H.; Yamashita, T.; Okada, H.; Li, Y.; Doan, P.T.B.; Li, R.; Lv, J.; Sakai, Y.; et al. BMP9-ID1 Signaling Activates HIF-1α and VEGFA Expression to Promote Tumor Angiogenesis in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 1475. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Deng, Z.; Zeng, Z.; Fan, J.; Feng, Y.; Wang, X.; Cao, D.; Zhang, B.; Yang, L.; Liu, B.; et al. Highly expressed BMP9/GDF2 in postnatal mouse liver and lungs may account for its pleiotropic effects on stem cell differentiation, angiogenesis, tumor growth and metabolism. Genes Dis. 2020, 7, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Mosher, V.A.L.; Swain, M.G.; Pang, J.X.Q.; Kaplan, G.G.; Sharkey, K.; MacQueen, G.M.; Goodyear, B.G. Magnetic resonance imaging evidence of hippocampal structural changes in patients with primary biliary cholangitis. Clin. Transl. Gastroenterol. 2018, 9, e169. [Google Scholar] [CrossRef] [PubMed]
- Perez-Dominguez, M.; Ávila-Muñoz, E.; Domínguez-Rivas, E.; Zepeda, A. The detrimental effects of lipopolysaccharide-induced neuroinflammation on adult hippocampal neurogenesis depend on the duration of the pro-inflammatory response. Neural Regen. Res. 2019, 14, 817–825. [Google Scholar] [CrossRef]
- Chauhan, G.; Kumar, G.; Roy, K.; Kumari, P.; Thondala, B.; Kishore, K.; Panjwani, U.; Ray, K. Hypobaric Hypoxia Induces Deficits in Adult Neurogenesis and Social Interaction via Cyclooxygenase-1/ EP1 Receptor Pathway Activating NLRP3 Inflammasome. Mol. Neurobiol. 2022, 59, 2497–2519. [Google Scholar] [CrossRef]
- Blomstrand, M.; Kalm, M.; Grandér, R.; Björk-Eriksson, T.; Blomgren, K. Different reactions to irradiation in the juvenile and adult hippocampus. Int. J. Radiat. Biol. 2014, 90, 807–815. [Google Scholar] [CrossRef]
- Kempf, S.J.; Casciati, A.; Buratovic, S.; Janik, D.; von Toerne, C.; Ueffing, M.; Neff, F.; Moertl, S.; Stenerlöw, B.; Saran, A.; et al. The cognitive defects of neonatally irradiated mice are accompanied by changed synaptic plasticity, adult neurogenesis and neuroinflammation. Mol. Neurodegener. 2014, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.-T.; Zou, Y.; Corniola, R. Oxidative stress and adult neurogenesis—Effects of radiation and superoxide dismutase deficiency. Semin. Cell Dev. Biol. 2012, 23, 738–744. [Google Scholar] [CrossRef]
- Decrock, E.; Hoorelbeke, D.; Ramadan, R.; Delvaeye, T.; De Bock, M.; Wang, N.; Krysko, D.V.; Baatout, S.; Bultynck, G.; Aerts, A.; et al. Calcium, oxidative stress and connexin channels, a harmonious orchestra directing the response to radiotherapy treatment? Biochim. Et Biophys. Acta 2017, 1864, 1099–1120. [Google Scholar] [CrossRef] [PubMed]
- Lensu, S.; Waselius, T.; Mäkinen, E.; Kettunen, H.; Virtanen, A.; Tiirola, M.; Penttonen, M.; Pekkala, S.; Nokia, M.S. Irradiation of the head reduces adult hippocampal neurogenesis and impairs spatial memory, but leaves overall health intact in rats. Eur. J. Neurosci. 2021, 53, 1885–1904. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Q.; Zhang, K.; Teng, H.; Li, M.; Li, D.; Wang, J.; Du, Q.; Zhao, M. The clock-controlled chemokine contributes to neuroinflammation-induced depression. FASEB J. 2020, 34, 8357–8366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korotkov, A.; Broekaart, D.W.M.; Banchaewa, L.; Pustjens, B.; Scheppingen, J.; Anink, J.J.; Baayen, J.C.; Idema, S.; Gorter, J.A.; Vliet, E.A.; et al. microRNA-132 is overexpressed in glia in temporal lobe epilepsy and reduces the expression of pro-epileptogenic factors in human cultured astrocytes. Glia 2020, 68, 60–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Nan, G. The extracellular signal-regulated kinase 1/2 pathway in neurological diseases: A potential therapeutic target (Review). Int. J. Mol. Med. 2017, 39, 1338–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Z.; Sui, G.; Rosa, P.M.; Zhao, W. Radiation-Induced c-Jun Activation Depends on MEK1-ERK1/2 Signaling Pathway in Microglial Cells. PLoS ONE 2012, 7, e36739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betlazar, C.; Middleton, R.J.; Banati, R.B.; Liu, G.-J. The impact of high and low dose ionising radiation on the central nervous system. Redox Biol. 2016, 9, 144–156. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Quintana, R.; Walker, D.J.; Williams, K.J.; Forster, D.M.; Chalmers, A.J. Radiation-induced neuroinflammation: A potential protective role for poly(ADP-ribose) polymerase inhibitors? Neuro-Oncol. Adv. 2022, 4, vdab190. [Google Scholar] [CrossRef]
- Wang, X.-L.; Yuan, K.; Zhang, W.; Li, S.-X.; Gao, G.F.; Lu, L. Regulation of Circadian Genes by the MAPK Pathway: Implications for Rapid Antidepressant Action. Neurosci. Bull. 2020, 36, 66–76. [Google Scholar] [CrossRef]




| Primer | Forward | Reverse | Amplicon Size | GenBank Accession Number |
|---|---|---|---|---|
| IL-1a | CTACAGTTCTGCCATTGACCA′ | ACTCAGCCGTCTCTTCTTCAG | 211 bp | NM_010554.4 |
| IL-1b | AGCCTGTGTTTTCCTCCTTGC | TCAGTGCGGGCTATGACCAA | 171 bp | NM_008361.4 |
| IL-1r1 | ACCAAACCTGTGCAGTCCCT | TGGCCACCAAGTCCTGTTCT | 72 bp | NM_008362.3 |
| IL-6 | AGTCCTTCCTACCCCAATTTCCA | TGGTCTTGGTCCTTAGCCACT | 80 bp | NM_001314054.1 |
| TNF-α | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG | 61 bp | NM_001278601.1 |
| Clock | CAC CGA CAA AGA TCC CTA CTG AT | TGA GAC ATC GCT GGC TGT GT | 151 bp | NM_001305222.1 |
| Bmal1 | GTA GAT CAG AGG GCG ACA GC | CCT GTG ACA TTC TGC GAG GT | 114 bp | NM_001243048.2 |
| Per1 | TGG CTC AAG TGG CAA TGA GTC′ | GGC TCG AGC TGA CTG TTC ACT | 247 bp | NM_001159367.2 |
| Per2 | CCAAACTGCTTGTTCCAGGC | ACCGGCCTGTAGGATCTTCT | 153 bp | NM_011066.3 |
| Cry1 | CTT CTG TCT GAT GAC CAT GAT GA | CCC AGG CCT TTC TTT CCA A | 151 bp | NM_007771.3 |
| Cry2 | AGG GCT GCC AAG TGC ATC AT | AGG AAG GGA CAG ATG CCA ATA G | 151 bp | NM_009963.4 |
| Rev-erba | GGT GCG CTT TGC ATC GTT | GGT TGT GCG GCT CAG GAA | 64 bp | NM_145434.4 |
| Gapdh | CAA CAG CAA CTC CCA CTC TTC | GGT CCA GGG TTT CTT ACT CCT T | 164 bp | NM_001289726.2 |
| ß-Actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT | 154 bp | NM_007393.5 |
| Primary Antibody (Host, Clonality) | Manufacturer | Concentration |
|---|---|---|
| Anti-IBA1 (rabbit, polyclonal, cat # 019-19741) | Fujifilm WAKO (Osaka, Japan) | 1:2000 |
| Anti-GFAP (mouse, monoclonal, cat # 556330) | BD Biosciences (Eysins, Switzerland) | 1:500 |
| Anti-8OHdG (mouse, monoclonal, cat # AM03160PU) | Acris (San Diego, CA, USA) | 1:2000 |
| Anti-c-FOS (rabbit, monoclonal, cat # 2250) | Cell Signaling Technology (Danvers, MA, USA) | 1:5000 |
| Anti-Ki67 (rabbit, polyclonal, cat # ab16667) | DCS Immunoline (Hamburg, Germany) | 1:500 |
| Secondary antibody (host) | Manufacturer | Concentration |
| Anti-rabbit IgG Biotin (goat, cat # BA-1000) | Vector Laboratories (Burlingame, CA, USA) | 1:500 |
| Anti-rabbit IgG Alexa Fluor 568 (goat; cat # A-11036) | Molecular Probes (Eugene, OR, USA) | 1:500 |
| Anti-mouse IgG Alexa Fluor 568 (goat; cat # A-11031) | Molecular Probes (Eugene, OR, USA) | 1:500 |
| Anti-mouse IgG Alexa Fluor 488 (goat, cat # A-21042) | Molecular Probes (Eugene, OR, USA) | 1:500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yassine, M.; Hassan, S.A.; Sommer, S.; Yücel, L.A.; Bellert, H.; Hallenberger, J.; Sohn, D.; Korf, H.-W.; von Gall, C.; Ali, A.A.H. Radiotherapy of the Hepatocellular Carcinoma in Mice Has a Time-Of-Day-Dependent Impact on the Mouse Hippocampus. Cells 2023, 12, 61. https://doi.org/10.3390/cells12010061
Yassine M, Hassan SA, Sommer S, Yücel LA, Bellert H, Hallenberger J, Sohn D, Korf H-W, von Gall C, Ali AAH. Radiotherapy of the Hepatocellular Carcinoma in Mice Has a Time-Of-Day-Dependent Impact on the Mouse Hippocampus. Cells. 2023; 12(1):61. https://doi.org/10.3390/cells12010061
Chicago/Turabian StyleYassine, Mona, Soha A. Hassan, Simon Sommer, Lea Aylin Yücel, Hanna Bellert, Johanna Hallenberger, Dennis Sohn, Horst-Werner Korf, Charlotte von Gall, and Amira A. H. Ali. 2023. "Radiotherapy of the Hepatocellular Carcinoma in Mice Has a Time-Of-Day-Dependent Impact on the Mouse Hippocampus" Cells 12, no. 1: 61. https://doi.org/10.3390/cells12010061





