Role of Decorin in the Lens and Ocular Diseases
Abstract
:1. Introduction
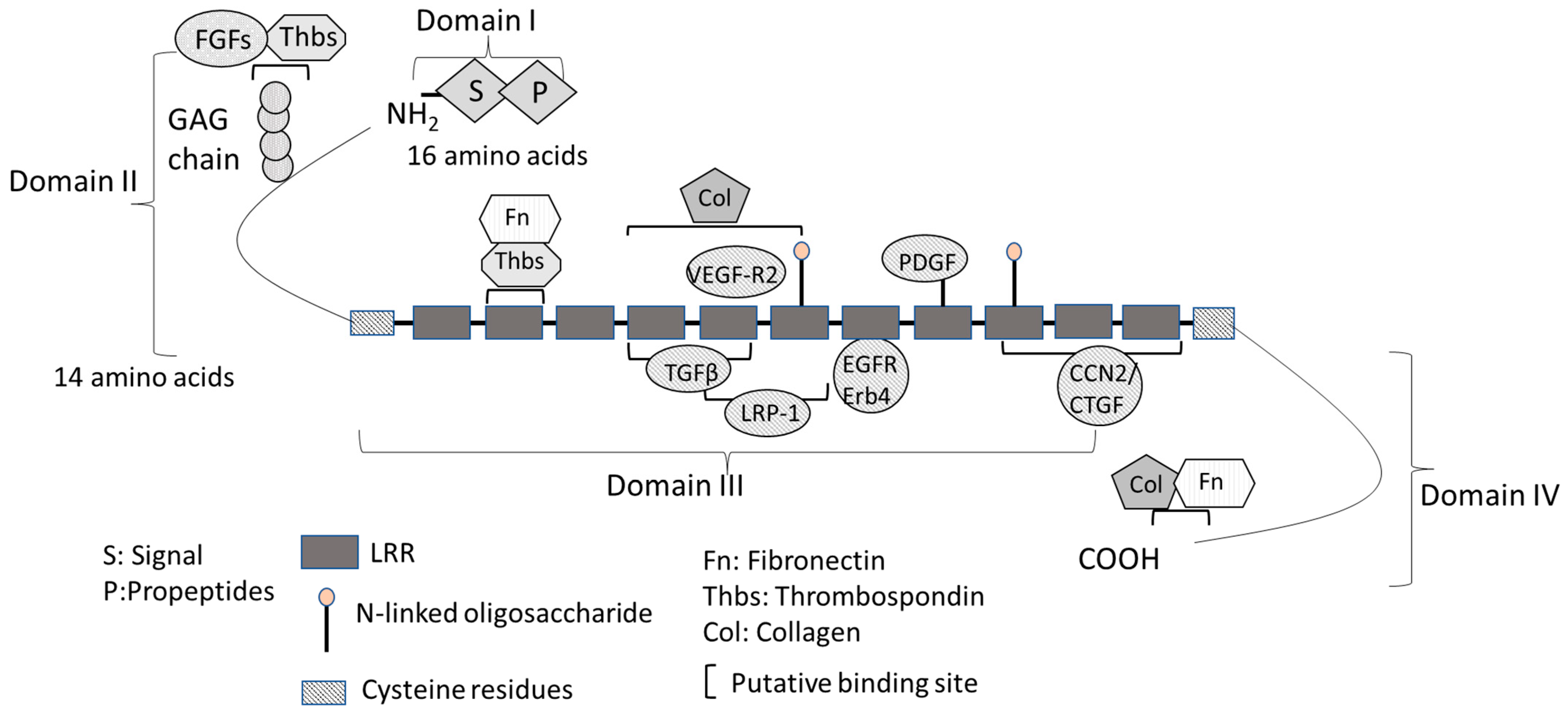
2. Structure and Function of Decorin
3. Interaction between Decorin and TGF-β
4. Distribution of Decorin in the Eye
5. The Role of Decorin in the Lens
6. The Role of Decorin in Other Eye Diseases
6.1. Cornea
6.2. Glaucoma
6.3. Retinal Diseases
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohan, R.R.; Tovey, J.C.; Gupta, R.; Sharma, A.; Tandon, A. Decorin biology, expression, function and therapy in the cornea. Curr. Mol. Med. 2011, 11, 110–128. [Google Scholar] [CrossRef] [PubMed]
- Azuma, N.; Hara, T.; Hara, T. Extracellular matrix of opacified anterior capsule after endocapsular cataract surgery. Graefes Arch. Clin. Exp. Ophthalmol. 1998, 236, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Villodre, E.S.; Larson, R.; Rahal, O.M.; Wang, X.; Gong, Y.; Song, J.; Krishnamurthy, S.; Ueno, N.T.; Tripathy, D.; et al. Decorin-mediated suppression of tumorigenesis, invasion, and metastasis in inflammatory breast cancer. Commun. Biol. 2021, 4, 72. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V. Matrix proteoglycans: From molecular design to cellular function. Annu. Rev. Biochem. 1998, 67, 609–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iozzo, R.V.; Schaefer, L. Proteoglycans in health and disease: Novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 2010, 277, 3864–3875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvelainen, H.; Puolakkainen, P.; Pakkanen, S.; Brown, E.L.; Hook, M.; Iozzo, R.V.; Sage, E.H.; Wight, T.N. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen. 2006, 14, 443–452. [Google Scholar] [CrossRef]
- Shibata, S.; Shibata, N.; Ohtsuka, S.; Yoshitomi, Y.; Kiyokawa, E.; Yonekura, H.; Singh, D.P.; Sasaki, H.; Kubo, E. Role of decorin in posterior capsule opacification and eye lens development. Cells 2021, 10, 863. [Google Scholar] [CrossRef]
- Hill, L.J.; Mead, B.; Blanch, R.J.; Ahmed, Z.; De Cogan, F.; Morgan-Warren, P.J.; Mohamed, S.; Leadbeater, W.; Scott, R.A.; Berry, M.; et al. Decorin reduces intraocular pressure and retinal ganglion cell loss in rodents through fibrolysis of the scarred trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3743–3757. [Google Scholar] [CrossRef]
- Low, S.W.Y.; Vaidya, T.; Gadde, S.G.K.; Mochi, T.B.; Kumar, D.; Kassem, I.S.; Costakos, D.M.; Ahmad, B.; Sethu, S.; Ghosh, A.; et al. Decorin concentrations in aqueous humor of patients with diabetic retinopathy. Life 2021, 11, 1421. [Google Scholar] [CrossRef]
- Oruc, Y.; Keser, S.; Yusufoglu, E.; Celik, F.; Sahin, I.; Yardim, M.; Aydin, S. Decorin, tenascin C, total antioxidant, and total oxidant level changes in patients with pseudoexfoliation syndrome. J. Ophthalmol. 2018, 2018, 7459496. [Google Scholar] [CrossRef]
- Xie, X.; Li, D.; Cui, Y.; Xie, T.; Cai, J.; Yao, Y. Decorin protects retinal pigment epithelium cells from oxidative stress and apoptosis via AMPK-mTOR-regulated autophagy. Oxid. Med. Cell. Longev. 2022, 2022, 3955748. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.D.; Clark, S.J.; Unwin, R.D.; Ridge, L.A.; Day, A.J.; Bishop, P.N. Mapping the differential distribution of proteoglycan core proteins in the adult human retina, choroid, and sclera. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7528–7538. [Google Scholar] [CrossRef] [PubMed]
- Low, S.W.Y.; Connor, T.B.; Kassem, I.S.; Costakos, D.M.; Chaurasia, S.S. Small leucine-rich proteoglycans (SLRPs) in the retina. Int. J. Mol. Sci. 2021, 22, 7293. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Pawlak, R.; Weber, G.R.; Dillinger, A.E.; Kuespert, S.; Iozzo, R.V.; Quigley, H.A.; Ohlmann, A.; Tamm, E.R.; Fuchshofer, R. A novel ocular function for decorin in the aqueous humor outflow. Matrix Biol. 2021, 97, 1–19. [Google Scholar] [CrossRef]
- Vogel, K.G.; Paulsson, M.; Heinegard, D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem. J. 1984, 223, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Nareyeck, G.; Seidler, D.G.; Troyer, D.; Rauterberg, J.; Kresse, H.; Schonherr, E. Differential interactions of decorin and decorin mutants with type I and type VI collagens. Eur. J. Biochem. 2004, 271, 3389–3398. [Google Scholar] [CrossRef]
- Ruhland, C.; Schonherr, E.; Robenek, H.; Hansen, U.; Iozzo, R.V.; Bruckner, P.; Seidler, D.G. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. FEBS J. 2007, 274, 4246–4255. [Google Scholar] [CrossRef]
- Neill, T.; Schaefer, L.; Iozzo, R.V. Decorin: A guardian from the Matrix. Am. J. Pathol. 2012, 181, 380–387. [Google Scholar] [CrossRef] [Green Version]
- Merline, R.; Moreth, K.; Beckmann, J.; Nastase, M.V.; Zeng-Brouwers, J.; Tralhao, J.G.; Lemarchand, P.; Pfeilschifter, J.; Schaefer, R.M.; Iozzo, R.V.; et al. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci. Signal. 2011, 4, ra75. [Google Scholar] [CrossRef] [Green Version]
- Baghy, K.; Iozzo, R.V.; Kovalszky, I. Decorin-TGFbeta axis in hepatic fibrosis and cirrhosis. J. Histochem. Cytochem. 2012, 60, 262–268. [Google Scholar] [CrossRef]
- Bi, X.; Pohl, N.M.; Qian, Z.; Yang, G.R.; Gou, Y.; Guzman, G.; Kajdacsy-Balla, A.; Iozzo, R.V.; Yang, W. Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice. Carcinogenesis 2012, 33, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Sanderson, R.D. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J. Cell. Mol. Med. 2011, 15, 1013–1031. [Google Scholar] [CrossRef] [PubMed]
- Gubbiotti, M.A.; Neill, T.; Frey, H.; Schaefer, L.; Iozzo, R.V. Decorin is an autophagy-inducible proteoglycan and is required for proper in vivo autophagy. Matrix Biol. 2015, 48, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Young, M.F.; Chakravarti, S.; Birk, D.E. Interclass small leucine-rich repeat proteoglycan interactions regulate collagen fibrillogenesis and corneal stromal assembly. Matrix Biol. 2014, 35, 103–111. [Google Scholar] [CrossRef]
- Santra, M.; Eichstetter, I.; Iozzo, R.V. An anti-oncogenic role for decorin. Down-regulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J. Biol. Chem. 2000, 275, 35153–35161. [Google Scholar] [CrossRef] [Green Version]
- Csordas, G.; Santra, M.; Reed, C.C.; Eichstetter, I.; McQuillan, D.J.; Gross, D.; Nugent, M.A.; Hajnoczky, G.; Iozzo, R.V. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J. Biol. Chem. 2000, 275, 32879–32887. [Google Scholar] [CrossRef] [Green Version]
- Jarvinen, T.A.; Prince, S. Decorin: A growth factor antagonist for tumor growth inhibition. BioMed Res. Int. 2015, 2015, 654765. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, L.; Schaefer, R.M. Proteoglycans: From structural compounds to signaling molecules. Cell Tissue Res. 2010, 339, 237–246. [Google Scholar] [CrossRef]
- Gubbiotti, M.A.; Vallet, S.D.; Ricard-Blum, S.; Iozzo, R.V. Decorin interacting network: A comprehensive analysis of decorin-binding partners and their versatile functions. Matrix Biol. 2016, 55, 7–21. [Google Scholar] [CrossRef]
- Stander, M.; Naumann, U.; Wick, W.; Weller, M. Transforming growth factor-beta and p-21: Multiple molecular targets of decorin-mediated suppression of neoplastic growth. Cell Tissue Res. 1999, 296, 221–227. [Google Scholar] [CrossRef]
- Chen, S.; Birk, D.E. Focus on molecules: Decorin. Exp. Eye Res. 2011, 92, 444–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, M.W.; Chamberlain, C.G.; McAvoy, J.W. Binding of FGF-1 and FGF-2 to heparan sulphate proteoglycans of the mammalian lens capsule. Growth Factors 1997, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Seidler, D.G.; Dreier, R. Decorin and its galactosaminoglycan chain: Extracellular regulator of cellular function? IUBMB Life 2008, 60, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Hocking, A.M.; Shinomura, T.; McQuillan, D.J. Leucine-rich repeat glycoproteins of the extracellular Matrix. Matrix Biol. 1998, 17, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Danielson, K.G.; Baribault, H.; Holmes, D.F.; Graham, H.; Kadler, K.E.; Iozzo, R.V. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef] [Green Version]
- Dunkman, A.A.; Buckley, M.R.; Mienaltowski, M.J.; Adams, S.M.; Thomas, S.J.; Satchell, L.; Kumar, A.; Pathmanathan, L.; Beason, D.P.; Iozzo, R.V.; et al. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013, 32, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, Y.; Kodama, Y.; Matsumoto, T. Bone matrix decorin binds transforming growth factor-beta and enhances its bioactivity. J. Biol. Chem. 1994, 269, 32634–32638. [Google Scholar] [CrossRef]
- Brandan, E.; Retamal, C.; Cabello-Verrugio, C.; Marzolo, M.P. The low density lipoprotein receptor-related protein functions as an endocytic receptor for decorin. J. Biol. Chem. 2006, 281, 31562–31571. [Google Scholar] [CrossRef]
- Cabello-Verrugio, C.; Brandan, E. A novel modulatory mechanism of transforming growth factor-beta signaling through decorin and LRP-1. J. Biol. Chem. 2007, 282, 18842–18850. [Google Scholar] [CrossRef] [Green Version]
- Cabello-Verrugio, C.; Santander, C.; Cofre, C.; Acuna, M.J.; Melo, F.; Brandan, E. The internal region leucine-rich repeat 6 of decorin interacts with low density lipoprotein receptor-related protein-1, modulates transforming growth factor (TGF)-beta-dependent signaling, and inhibits TGF-beta-dependent fibrotic response in skeletal muscles. J. Biol. Chem. 2012, 287, 6773–6787. [Google Scholar] [CrossRef]
- Ungvari, Z.; Valcarcel-Ares, M.N.; Tarantini, S.; Yabluchanskiy, A.; Fulop, G.A.; Kiss, T.; Csiszar, A. Connective tissue growth factor (CTGF) in age-related vascular pathologies. Geroscience 2017, 39, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Lipson, K.E.; Wong, C.; Teng, Y.; Spong, S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 2012, 5, S24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vial, C.; Gutierrez, J.; Santander, C.; Cabrera, D.; Brandan, E. Decorin interacts with connective tissue growth factor (CTGF)/CCN2 by LRR12 inhibiting its biological activity. J. Biol. Chem. 2011, 286, 24242–24252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, L.; Iozzo, R.V. Biological functions of the small leucine-rich proteoglycans: From genetics to signal transduction. J. Biol. Chem. 2008, 283, 21305–21309. [Google Scholar] [CrossRef] [Green Version]
- Khan, G.A.; Girish, G.V.; Lala, N.; Di Guglielmo, G.M.; Lala, P.K. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast. Mol. Endocrinol. 2011, 25, 1431–1443. [Google Scholar] [CrossRef]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef]
- Itoh, Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015, 44–46, 207–223. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015, 44–46, 94–112. [Google Scholar] [CrossRef]
- Imai, K.; Hiramatsu, A.; Fukushima, D.; Pierschbacher, M.D.; Okada, Y. Degradation of decorin by matrix metalloproteinases: Identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release. Biochem. J. 1997, 322 Pt 3, 809–814. [Google Scholar] [CrossRef]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar] [CrossRef]
- Dallas, S.L.; Rosser, J.L.; Mundy, G.R.; Bonewald, L.F. Proteolysis of latent transforming growth factor-beta (TGF-beta)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone Matrix. J. Biol. Chem 2002, 277, 21352–21360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandon, A.; Tovey, J.C.; Sharma, A.; Gupta, R.; Mohan, R.R. Role of transforming growth factor Beta in corneal function, biology and pathology. Curr. Mol. Med. 2010, 10, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.K.; Flavell, R.A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef] [PubMed]
- Hales, A.M.; Schulz, M.W.; Chamberlain, C.G.; McAvoy, J.W. TGF-beta 1 induces lens cells to accumulate alpha-smooth muscle actin, a marker for subcapsular cataracts. Curr. Eye Res. 1994, 13, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Kita, T.; Hata, Y.; Kano, K.; Miura, M.; Nakao, S.; Noda, Y.; Shimokawa, H.; Ishibashi, T. Transforming growth factor-beta2 and connective tissue growth factor in proliferative vitreoretinal diseases: Possible involvement of hyalocytes and therapeutic potential of Rho kinase inhibitor. Diabetes 2007, 56, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.M.; Desai, L.P. Reciprocal regulation of TGF-beta and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Lovicu, F.J.; Schulz, M.W.; Hales, A.M.; Vincent, L.N.; Overbeek, P.A.; Chamberlain, C.G.; McAvoy, J.W. TGFbeta induces morphological and molecular changes similar to human anterior subcapsular cataract. Br. J. Ophthalmol. 2002, 86, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Broekelmann, T.J.; Limper, A.H.; Colby, T.V.; McDonald, J.A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 1991, 88, 6642–6646. [Google Scholar] [CrossRef] [Green Version]
- Massague, J. The transforming growth factor-beta family. Annu. Rev. Cell Biol. 1990, 6, 597–641. [Google Scholar] [CrossRef]
- Ask, K.; Bonniaud, P.; Maass, K.; Eickelberg, O.; Margetts, P.J.; Warburton, D.; Groffen, J.; Gauldie, J.; Kolb, M. Progressive pulmonary fibrosis is mediated by TGF-beta isoform 1 but not TGF-beta3. Int. J. Biochem. Cell Biol. 2008, 40, 484–495. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, X.; Tan, J.; Wang, J.; Xing, Y. Effect of recombinant adeno-associated virus mediated transforming growth factor-beta1 on corneal allograft survival after high-risk penetrating keratoplasty. Transpl. Immunol. 2013, 28, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massague, J.; Seoane, J.; Wotton, D. Smad transcription factors. Genes Dev. 2005, 19, 2783–2810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmierer, B.; Hill, C.S. TGFbeta-SMAD signal transduction: Molecular specificity and functional flexibility. Nat. Rev. Mol. Cell Biol. 2007, 8, 970–982. [Google Scholar] [CrossRef]
- Yu, L.; Hebert, M.C.; Zhang, Y.E. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002, 21, 3749–3759. [Google Scholar] [CrossRef] [Green Version]
- Stratton, R.; Rajkumar, V.; Ponticos, M.; Nichols, B.; Shiwen, X.; Black, C.M.; Abraham, D.J.; Leask, A. Prostacyclin derivatives prevent the fibrotic response to TGF-beta by inhibiting the Ras/MEK/ERK pathway. FASEB J. 2002, 16, 1949–1951. [Google Scholar] [CrossRef]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-beta signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Kubo, E.; Shibata, S.; Shibata, T.; Kiyokawa, E.; Sasaki, H.; Singh, D.P. FGF2 antagonizes aberrant TGFbeta regulation of tropomyosin: Role for posterior capsule opacity. J. Cell. Mol. Med. 2017, 21, 916–928. [Google Scholar] [CrossRef]
- Kubo, E.; Shibata, T.; Singh, D.P.; Sasaki, H. Roles of TGF beta and FGF signals in the lens: Tropomyosin regulation for posterior capsule cpacity. Int. J. Mol. Sci. 2018, 19, 3093. [Google Scholar] [CrossRef] [Green Version]
- Kurosaka, D.; Kato, K.; Nagamoto, T.; Negishi, K. Growth factors influence contractility and alpha-smooth muscle actin expression in bovine lens epithelial cells. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1701–1708. [Google Scholar]
- Yamaguchi, Y.; Mann, D.M.; Ruoslahti, E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 1990, 346, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E.; Yamaguchi, Y. Proteoglycans as modulators of growth factor activities. Cell 1991, 64, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Kerr, L.D.; Miller, D.B.; Matrisian, L.M. TGF-beta 1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell 1990, 61, 267–278. [Google Scholar] [CrossRef] [PubMed]
- White, L.A.; Mitchell, T.I.; Brinckerhoff, C.E. Transforming growth factor beta inhibitory element in the rabbit matrix metalloproteinase-1 (collagenase-1) gene functions as a repressor of constitutive transcription. Biochim. Biophys. Acta 2000, 1490, 259–268. [Google Scholar] [CrossRef]
- Sharma, P.; Fatma, N.; Kubo, E.; Shinohara, T.; Chylack, L.T., Jr.; Singh, D.P. Lens epithelium-derived growth factor relieves transforming growth factor-beta1-induced transcription repression of heat shock proteins in human lens epithelial cells. J. Biol. Chem. 2003, 278, 20037–20046. [Google Scholar] [CrossRef] [Green Version]
- Danielson, K.G.; Siracusa, L.D.; Donovan, P.J.; Iozzo, R.V. Decorin, epiphycan, and lumican genes are closely linked on murine Chromosome 10 and are deleted in lethal steel mutants. Mamm. Genome 1999, 10, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Demoor-Fossard, M.; Galera, P.; Santra, M.; Iozzo, R.V.; Pujol, J.P.; Redini, F. A composite element binding the vitamin D receptor and the retinoic X receptor alpha mediates the transforming growth factor-beta inhibition of decorin gene expression in articular chondrocytes. J. Biol. Chem. 2001, 276, 36983–36992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iozzo, R.V.; Cohen, I. Altered proteoglycan gene expression and the tumor stroma. Experientia 1993, 49, 447–455. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Moscatello, D.K.; McQuillan, D.J.; Eichstetter, I. Decorin is a biological ligand for the epidermal growth factor receptor. J. Biol. Chem. 1999, 274, 4489–4492. [Google Scholar] [CrossRef] [Green Version]
- Mauviel, A.; Santra, M.; Chen, Y.Q.; Uitto, J.; Iozzo, R.V. Transcriptional regulation of decorin gene expression. Induction by quiescence and repression by tumor necrosis factor-alpha. J. Biol. Chem. 1995, 270, 11692–11700. [Google Scholar] [CrossRef] [Green Version]
- Mauviel, A.; Korang, K.; Santra, M.; Tewari, D.; Uitto, J.; Iozzo, R.V. Identification of a bimodal regulatory element encompassing a canonical AP-1 binding site in the proximal promoter region of the human decorin gene. J. Biol. Chem. 1996, 271, 24824–24829. [Google Scholar] [CrossRef] [PubMed]
- Demoor-Fossard, M.; Redini, F.; Boittin, M.; Pujol, J.P. Expression of decorin and biglycan by rabbit articular chondrocytes. Effects of cytokines and phenotypic modulation. Biochim. Biophys. Acta 1998, 1398, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Border, W.A.; Okuda, S.; Languino, L.R.; Ruoslahti, E. Transforming growth factor-beta regulates production of proteoglycans by mesangial cells. Kidney. Int. 1990, 37, 689–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, D.Y.; Lovicu, F.J. Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis. Prog. Retin. Eye Res. 2017, 60, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Lois, N.; Taylor, J.; McKinnon, A.D.; Smith, G.C.; van’t Hof, R.; Forrester, J.V. Effect of TGF-beta2 and anti-TGF-beta2 antibody in a new in vivo rodent model of posterior capsule opacification. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4260–4266. [Google Scholar] [CrossRef] [Green Version]
- McDonnell, P.J.; Rowen, S.L.; Glaser, B.M.; Sato, M. Posterior capsule opacification. An in vitro model. Arch. Ophthalmol. 1985, 103, 1378–1381. [Google Scholar] [CrossRef]
- Nikhalashree, S.; George, R.; Shantha, B.; Lingam, V.; Vidya, W.; Panday, M.; Sulochana, K.N.; Coral, K. Detection of proteins associated with extracellular matrix regulation in the aqueous humour of patients with primary glaucoma. Curr. Eye Res. 2019, 44, 1018–1025. [Google Scholar] [CrossRef]
- NikhalaShree, S.; Karthikkeyan, G.; George, R.; Shantha, B.; Vijaya, L.; Ratra, V.; Sulochana, K.N.; Coral, K. Lowered decorin with aberrant extracellular matrix remodeling in aqueous humor and tenon’s tissue from primary glaucoma patients. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4661–4669. [Google Scholar] [CrossRef] [Green Version]
- Guerin, C.J.; Hu, L.; Scicli, G.; Scicli, A.G. Transforming growth factor beta in experimentally detached retina and periretinal membranes. Exp. Eye Res. 2001, 73, 753–764. [Google Scholar] [CrossRef]
- Jampel, H.D.; Roche, N.; Stark, W.J.; Roberts, A.B. Transforming growth factor-beta in human aqueous humor. Curr. Eye Res. 1990, 9, 963–969. [Google Scholar] [CrossRef]
- Wallentin, N.; Wickstrom, K.; Lundberg, C. Effect of cataract surgery on aqueous TGF-beta and lens epithelial cell proliferation. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1410–1418. [Google Scholar]
- Hu, D.N.; McCormick, S.A.; Lin, A.Y.; Lin, J.Y. TGF-beta2 inhibits growth of uveal melanocytes at physiological concentrations. Exp. Eye Res. 1998, 67, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.; Nazari, H.; Sreekumar, P.G.; Kannan, R.; Dustin, L.; Zhu, D.; Barron, E.; Hinton, D.R. TGF-beta2 secretion from RPE decreases with polarization and becomes apically oriented. Cytokine 2015, 71, 394–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokawa, N.; Sotozono, C.; Nishida, K.; Kinoshita, S. High total TGF-beta 2 levels in normal human tears. Curr. Eye. Res. 1996, 15, 341–343. [Google Scholar] [CrossRef]
- Connor, T.B., Jr.; Roberts, A.B.; Sporn, M.B.; Danielpour, D.; Dart, L.L.; Michels, R.G.; de Bustros, S.; Enger, C.; Kato, H.; Lansing, M.; et al. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J. Clin. Investig. 1989, 83, 1661–1666. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Tamm, E.R. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012, 347, 279–290. [Google Scholar] [CrossRef]
- Igarashi, N.; Honjo, M.; Asaoka, R.; Kurano, M.; Yatomi, Y.; Igarashi, K.; Miyata, K.; Kaburaki, T.; Aihara, M. Aqueous autotaxin and TGF-betas are promising diagnostic biomarkers for distinguishing open-angle glaucoma subtypes. Sci. Rep. 2021, 11, 1408. [Google Scholar] [CrossRef]
- Saucedo, L.; Pfister, I.B.; Zandi, S.; Gerhardt, C.; Garweg, J.G. Ocular TGF-beta, matrix metalloproteinases, and TIMP-1 increase with the development and progression of diabetic retinopathy in Type 2 diabetes mellitus. Mediat. Inflamm. 2021, 2021, 9811361. [Google Scholar] [CrossRef]
- Yu, X.B.; Sun, X.H.; Dahan, E.; Guo, W.Y.; Qian, S.H.; Meng, F.R.; Song, Y.L.; Simon, G.J. Increased levels of transforming growth factor-betal and -beta2 in the aqueous humor of patients with neovascular glaucoma. Ophthalmic Surg. Lasers Imaging 2007, 38, 6–14. [Google Scholar] [CrossRef]
- Bredrup, C.; Knappskog, P.M.; Majewski, J.; Rodahl, E.; Boman, H. Congenital stromal dystrophy of the cornea caused by a mutation in the decorin gene. Investig. Ophthalmol. Vis. Sci. 2005, 46, 420–426. [Google Scholar] [CrossRef]
- Rodahl, E.; Van Ginderdeuren, R.; Knappskog, P.M.; Bredrup, C.; Boman, H. A second decorin frame shift mutation in a family with congenital stromal corneal dystrophy. Am. J. Ophthalmol. 2006, 142, 520–521. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Buyank, F.; Sinha, N.R.; Grant, D.G.; Sinha, P.R.; Iozzo, R.V.; Chaurasia, S.S.; Mohan, R.R. Decorin regulates collagen fibrillogenesis during corneal wound healing in mouse in vivo. Exp. Eye Res. 2022, 216, 108933. [Google Scholar] [CrossRef] [PubMed]
- Begum, G.; O’Neill, J.; Chaudhary, R.; Blachford, K.; Snead, D.R.J.; Berry, M.; Scott, R.A.H.; Logan, A.; Blanch, R.J. Altered decorin biology in proliferative vitreoretinopathy: A mechanistic and cohort study. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4929–4936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Beshri, A.S.; Edward, D.P.; Haiti, K.A.; Craven, E.R. Overhanging-dissecting blebs: Immunohistochemical characterization. J. Glaucoma 2018, 27, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, M.G.; Bressler, S.L.; Wight, T.N. The regulated synthesis of versican, decorin, and biglycan: Extracellular matrix proteoglycans that influence cellular phenotype. Crit. Rev. Eukaryot. Gene Expr. 2004, 14, 203–234. [Google Scholar] [CrossRef]
- Sahay, P.; Chakraborty, M.; Rao, A. Global and comparative proteome signatures in the lens capsule, trabecular meshwork, and iris of patients with pseudoexfoliation glaucoma. Front. Mol. Biosci. 2022, 9, 877250. [Google Scholar] [CrossRef]
- Abdullatif, A.M.; Macky, T.A.; Abdullatif, M.M.; Nassar, K.; Grisanti, S.; Mortada, H.A.; Soliman, M.M. Intravitreal decorin preventing proliferative vitreoretinopathy in perforating injuries: A pilot study. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 2473–2481. [Google Scholar] [CrossRef]
- Wormstone, I.M. Posterior capsule opacification: A cell biological perspective. Exp. Eye Res. 2002, 74, 337–347. [Google Scholar] [CrossRef]
- Marcantonio, J.M.; Syam, P.P.; Liu, C.S.; Duncan, G. Epithelial transdifferentiation and cataract in the human lens. Exp. Eye Res. 2003, 77, 339–346. [Google Scholar] [CrossRef]
- de Iongh, R.U.; Lovicu, F.J.; Overbeek, P.A.; Schneider, M.D.; Joya, J.; Hardeman, E.D.; McAvoy, J.W. Requirement for TGFbeta receptor signaling during terminal lens fiber differentiation. Development 2001, 128, 3995–4010. [Google Scholar] [CrossRef]
- de Iongh, R.U.; Wederell, E.; Lovicu, F.J.; McAvoy, J.W. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: A model for cataract formation. Cells Tissues Organs 2005, 179, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Seomun, Y.; Hwang, K.H.; Kim, J.E.; Kim, I.S.; Kim, J.H.; Joo, C.K. Overexpression of the transforming growth factor-beta-inducible gene betaig-h3 in anterior polar cataracts. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1840–1845. [Google Scholar]
- Saika, S.; Miyamoto, T.; Ishida, I.; Shirai, K.; Ohnishi, Y.; Ooshima, A.; McAvoy, J.W. TGFbeta-Smad signalling in postoperative human lens epithelial cells. Br. J. Ophthalmol. 2002, 86, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Medvedovic, M.; Tomlinson, C.R.; Call, M.K.; Grogg, M.; Tsonis, P.A. Gene expression and discovery during lens regeneration in mouse: Regulation of epithelial to mesenchymal transition and lens differentiation. Mol. Vis. 2006, 12, 422–440. [Google Scholar]
- Hayashi, N.; Kato, H.; Kiyosawa, T.; Hayashi, H.; Oshima, K.; Yamaoka, M. [The change in immunohistochemical localization of basic fibroblast growth factor (b-FGF) around the lens capsule after extracapsular extraction]. Nihon Ganka Gakkai Zasshi 1991, 95, 621–624. [Google Scholar]
- Lovicu, F.J.; McAvoy, J.W. Localization of acidic fibroblast growth factor, basic fibroblast growth factor, and heparan sulphate proteoglycan in rat lens: Implications for lens polarity and growth patterns. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3355–3365. [Google Scholar]
- Meacock, W.R.; Spalton, D.J.; Stanford, M.R. Role of cytokines in the pathogenesis of posterior capsule opacification. Br. J. Ophthalmol. 2000, 84, 332–336. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, C.G.; McAvoy, J.W. Induction of lens fibre differentiation by acidic and basic fibroblast growth factor (FGF). Growth Factors 1989, 1, 125–134. [Google Scholar] [CrossRef]
- McAvoy, J.W.; Chamberlain, C.G. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development 1989, 107, 221–228. [Google Scholar] [CrossRef]
- Nishi, O.; Nishi, K.; Fujiwara, T.; Shirasawa, E.; Ohmoto, Y. Effects of the cytokines on the proliferation of and collagen synthesis by human cataract lens epithelial cells. Br. J. Ophthalmol. 1996, 80, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Wormstone, I.M.; Del Rio-Tsonis, K.; McMahon, G.; Tamiya, S.; Davies, P.D.; Marcantonio, J.M.; Duncan, G. FGF: An autocrine regulator of human lens cell growth independent of added stimuli. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1305–1311. [Google Scholar]
- Kahari, V.M.; Larjava, H.; Uitto, J. Differential regulation of extracellular matrix proteoglycan (PG) gene expression. Transforming growth factor-beta 1 up-regulates biglycan (PGI), and versican (large fibroblast PG) but down-regulates decorin (PGII) mRNA levels in human fibroblasts in culture. J. Biol. Chem. 1991, 266, 10608–10615. [Google Scholar] [PubMed]
- Vuorio, T.; Kahari, V.M.; Black, C.; Vuorio, E. Expression of osteonectin, decorin, and transforming growth factor-beta 1 genes in fibroblasts cultured from patients with systemic sclerosis and morphea. J. Rheumatol. 1991, 18, 247–251. [Google Scholar] [PubMed]
- Shu, C.; Smith, S.M.; Little, C.B.; Melrose, J. Use of FGF-2 and FGF-18 to direct bone marrow stromal stem cells to chondrogenic and osteogenic lineages. Future Sci. OA 2016, 2, FSO142. [Google Scholar] [CrossRef] [Green Version]
- Tan, E.M.; Hoffren, J.; Rouda, S.; Greenbaum, S.; Fox, J.W.t.; Moore, J.H., Jr.; Dodge, G.R. Decorin, versican, and biglycan gene expression by keloid and normal dermal fibroblasts: Differential regulation by basic fibroblast growth factor. Exp. Cell Res. 1993, 209, 200–207. [Google Scholar] [CrossRef]
- Wang, S.C.; Lin, C.T.; Nie, D.B.; Ouyang, H.S. The effect of basic fibroblast growth factor on the gene expression of decorin by periodontal ligament fibroblasts in culture. Hua Xi Kou Qiang Yi Xue Za Zhi 2008, 26, 352–354. [Google Scholar]
- Sonal, D. Prevention of IGF-1 and TGFbeta stimulated type II collagen and decorin expression by bFGF and identification of IGF-1 mRNA transcripts in articular chondrocytes. Matrix Biol. 2001, 20, 233–242. [Google Scholar] [CrossRef]
- Trowbridge, J.M.; Rudisill, J.A.; Ron, D.; Gallo, R.L. Dermatan sulfate binds and potentiates activity of keratinocyte growth factor (FGF-7). J. Biol. Chem 2002, 277, 42815–42820. [Google Scholar] [CrossRef]
- Penc, S.F.; Pomahac, B.; Winkler, T.; Dorschner, R.A.; Eriksson, E.; Herndon, M.; Gallo, R.L. Dermatan sulfate released after injury is a potent promoter of fibroblast growth factor-2 function. J. Biol. Chem. 1998, 273, 28116–28121. [Google Scholar] [CrossRef] [Green Version]
- Gunning, P. Emerging issues for tropomyosin structure, regulation, function and pathology. Adv. Exp. Med. Biol. 2008, 644, 293–298. [Google Scholar]
- Gunning, P.W.; Schevzov, G.; Kee, A.J.; Hardeman, E.C. Tropomyosin isoforms: Divining rods for actin cytoskeleton function. Trends Cell Biol. 2005, 15, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Shu, D.Y.; Lovicu, F.J. Enhanced EGF receptor-signaling potentiates TGFbeta-induced lens epithelial-mesenchymal transition. Exp. Eye Res. 2019, 185, 107693. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Shibata, S.; Ishigaki, Y.; Kiyokawa, E.; Ikawa, M.; Singh, D.P.; Sasaki, H.; Kubo, E. Tropomyosin 2 heterozygous knockout in mice using CRISPR-Cas9 system displays the inhibition of injury-induced epithelial-mesenchymal transition, and lens opacity. Mech. Ageing Dev. 2018, 171, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wormstone, I.M.; Tamiya, S.; Anderson, I.; Duncan, G. TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2301–2308. [Google Scholar]
- Dwivedi, D.J.; Pino, G.; Banh, A.; Nathu, Z.; Howchin, D.; Margetts, P.; Sivak, J.G.; West-Mays, J.A. Matrix metalloproteinase inhibitors suppress transforming growth factor-beta-induced subcapsular cataract formation. Am. J. Pathol. 2006, 168, 69–79. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; Rizo, C.M.; Overbeek, P.A.; Robinson, M.L. Insertion of a Pax6 consensus binding site into the alphaA-crystallin promoter acts as a lens epithelial cell enhancer in transgenic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1930–1939. [Google Scholar] [CrossRef] [Green Version]
- Du, S.; Shao, J.; Xie, D.; Zhang, F. Decorin inhibits glucose-induced lens epithelial cell apoptosis via suppressing p22phox-p38 MAPK signaling pathway. PLoS ONE 2020, 15, e0224251. [Google Scholar] [CrossRef]
- Ozay, R.; Turkoglu, E.; Gurer, B.; Dolgun, H.; Evirgen, O.; Erguder, B.I.; Hayirli, N.; Gurses, L.; Sekerci, Z.; Yilmaz, E.R. Does Decorin Protect Neuronal Tissue via Its Antioxidant and Antiinflammatory Activity from Traumatic Brain Injury? An Experimental Study. World Neurosurg. 2017, 97, 407–415. [Google Scholar] [CrossRef]
- Fatma, N.; Kubo, E.; Sharma, P.; Beier, D.R.; Singh, D.P. Impaired homeostasis and phenotypic abnormalities in Prdx6-/- mice lens epithelial cells by reactive oxygen species: Increased expression and activation of TGFbeta. Cell Death Differ. 2005, 12, 734–750. [Google Scholar] [CrossRef]
- Bondi, C.D.; Manickam, N.; Lee, D.Y.; Block, K.; Gorin, Y.; Abboud, H.E.; Barnes, J.L. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J. Am. Soc. Nephrol. 2010, 21, 93–102. [Google Scholar] [CrossRef]
- Fatma, N.; Kubo, E.; Toris, C.B.; Stamer, W.D.; Camras, C.B.; Singh, D.P. PRDX6 attenuates oxidative stress- and TGFbeta-induced abnormalities of human trabecular meshwork cells. Free Radic. Res. 2009, 43, 783–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohba, M.; Shibanuma, M.; Kuroki, T.; Nose, K. Production of hydrogen peroxide by transforming growth factor-beta 1 and its involvement in induction of egr-1 in mouse osteoblastic cells. J. Cell Biol. 1994, 126, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.; Murillo, M.M.; Alvarez-Barrientos, A.; Beltran, J.; Fernandez, M.; Fabregat, I. Source of early reactive oxygen species in the apoptosis induced by transforming growth factor-beta in fetal rat hepatocytes. Free Radic. Biol. Med. 2004, 36, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.; Alvarez, A.M.; Sanchez, A.; Fernandez, M.; Roncero, C.; Benito, M.; Fabregat, I. Reactive oxygen species (ROS) mediates the mitochondrial-dependent apoptosis induced by transforming growth factor (beta) in fetal hepatocytes. FASEB J. 2001, 15, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [Green Version]
- Blochberger, T.C.; Cornuet, P.K.; Hassell, J.R. Isolation and partial characterization of lumican and decorin from adult chicken corneas. A keratan sulfate-containing isoform of decorin is developmentally regulated. J. Biol. Chem 1992, 267, 20613–20619. [Google Scholar] [CrossRef]
- Kamma-Lorger, C.S.; Pinali, C.; Martinez, J.C.; Harris, J.; Young, R.D.; Bredrup, C.; Crosas, E.; Malfois, M.; Rodahl, E.; Meek, K.M.; et al. Role of decorin core protein in collagen organisation in congenital stromal corneal dystrophy (CSCD). PLoS ONE 2016, 11, e0147948. [Google Scholar] [CrossRef] [Green Version]
- Bredrup, C.; Stang, E.; Bruland, O.; Palka, B.P.; Young, R.D.; Haavik, J.; Knappskog, P.M.; Rodahl, E. Decorin accumulation contributes to the stromal opacities found in congenital stromal corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5578–5582. [Google Scholar] [CrossRef] [Green Version]
- Mohan, R.R.; Tandon, A.; Sharma, A.; Cowden, J.W.; Tovey, J.C. Significant inhibition of corneal scarring in vivo with tissue-selective, targeted AAV5 decorin gene therapy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4833–4841. [Google Scholar] [CrossRef] [Green Version]
- Mohan, R.R.; Tovey, J.C.; Sharma, A.; Schultz, G.S.; Cowden, J.W.; Tandon, A. Targeted decorin gene therapy delivered with adeno-associated virus effectively retards corneal neovascularization in vivo. PLoS ONE 2011, 6, e26432. [Google Scholar] [CrossRef]
- Mohan, R.R.; Balne, P.K.; Muayad, M.S.; Tripathi, R.; Sinha, N.R.; Gupta, S.; An, J.A.; Sinha, P.R.; Hesemann, N.P. Six-Month In Vivo Safety Profiling of Topical Ocular AAV5-Decorin Gene Transfer. Transl. Vis. Sci. Technol. 2021, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.J.; Moakes, R.J.A.; Vareechon, C.; Butt, G.; Ng, A.; Brock, K.; Chouhan, G.; Vincent, R.C.; Abbondante, S.; Williams, R.L.; et al. Sustained release of decorin to the surface of the eye enables scarless corneal regeneration. NPJ Regen. Med. 2018, 3, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Downie, L.E.; Grover, L.M.; Moakes, R.J.A.; Rauz, S.; Logan, A.; Jiao, H.; Hill, L.J.; Chinnery, H.R. The neuroregenerative effects of topical decorin on the injured mouse cornea. J. Neuroinflamm. 2020, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am. J. Ophthalmol. 1998, 126, 498–505. [Google Scholar] [CrossRef]
- Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am. J. Ophthalmol. 1998, 126, 487–497. [Google Scholar] [CrossRef]
- Moses, R.A. The effect of intraocular pressure on resistance to outflow. Surv. Ophthalmol. 1977, 22, 88–100. [Google Scholar] [CrossRef]
- Wiederholt, M. Direct involvement of trabecular meshwork in the regulation of aqueous humor outflow. Curr. Opin. Ophthalmol. 1998, 9, 46–49. [Google Scholar] [CrossRef]
- Overby, D.R.; Zhou, E.H.; Vargas-Pinto, R.; Pedrigi, R.M.; Fuchshofer, R.; Braakman, S.T.; Gupta, R.; Perkumas, K.M.; Sherwood, J.M.; Vahabikashi, A.; et al. Altered mechanobiology of Schlemm’s canal endothelial cells in glaucoma. Proc. Natl. Acad. Sci. USA 2014, 111, 13876–13881. [Google Scholar] [CrossRef] [Green Version]
- Kirwan, R.P.; Wordinger, R.J.; Clark, A.F.; O’Brien, C.J. Differential global and extra-cellular matrix focused gene expression patterns between normal and glaucomatous human lamina cribrosa cells. Mol. Vis. 2009, 15, 76–88. [Google Scholar]
- Pena, J.D.; Taylor, A.W.; Ricard, C.S.; Vidal, I.; Hernandez, M.R. Transforming growth factor beta isoforms in human optic nerve heads. Br. J. Ophthalmol. 1999, 83, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.; Dillinger, A.E.; Ohlmann, A.; Iozzo, R.V.; Fuchshofer, R. Decorin-an antagonist of TGF-beta in astrocytes of the optic nerve. Int. J. Mol. Sci. 2021, 22, 7660. [Google Scholar] [CrossRef] [PubMed]
- Addicks, E.M.; Quigley, H.A.; Green, W.R.; Robin, A.L. Histologic characteristics of filtering blebs in glaucomatous eyes. Arch. Ophthalmol. 1983, 101, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Jampel, H.D.; Morrison, J.; Vocci, M.; Quigley, H. Identification of fibrin/fibrinogen in glaucoma filtration surgery wounds. Ophthalmic Surg. Lasers Imaging Retin. 1988, 19, 576–579. [Google Scholar] [CrossRef]
- Jampel, H.D.; McGuigan, L.J.; Dunkelberger, G.R.; L’Hernault, N.L.; Quigley, H.A. Cellular proliferation after experimental glaucoma filtration surgery. Arch. Ophthalmol. 1988, 106, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, S.; Szurman, P.; Warga, M.; Kaczmarek, R.; Ziemssen, F.; Tatar, O.; Bartz-Schmidt, K.U. Decorin modulates wound healing in experimental glaucoma filtration surgery: A pilot study. Investig. Ophthalmol. Vis. Sci. 2005, 46, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Sahay, P.; Reddy, S.; Prusty, B.K.; Modak, R.; Rao, A. TGFbeta1, MMPs and cytokines profiles in ocular surface: Possible tear biomarkers for pseudoexfoliation. PLoS ONE 2021, 16, e0249759. [Google Scholar] [CrossRef]
- Rattner, A.; Nathans, J. Macular degeneration: Recent advances and therapeutic opportunities. Nat. Rev. Neurosci. 2006, 7, 860–872. [Google Scholar] [CrossRef]
- Du, S.; Wang, S.; Wu, Q.; Hu, J.; Li, T. Decorin inhibits angiogenic potential of choroid-retinal endothelial cells by downregulating hypoxia-induced Met, Rac1, HIF-1alpha and VEGF expression in cocultured retinal pigment epithelial cells. Exp. Eye Res. 2013, 116, 151–160. [Google Scholar] [CrossRef]
- Wang, X.; Ma, W.; Han, S.; Meng, Z.; Zhao, L.; Yin, Y.; Wang, Y.; Li, J. TGF-beta participates choroid neovascularization through Smad2/3-VEGF/TNF-alpha signaling in mice with Laser-induced wet age-related macular degeneration. Sci. Rep. 2017, 7, 9672. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.E.; Park, Y.J.; Chung, I.Y.; Seo, S.W.; Park, J.M.; Yoo, J.M.; Song, J.K. Gene expression changes in a rat model of oxygen-induced retinopathy. Korean J. Ophthalmol. 2011, 25, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.J.; Kim, Y.H.; Choi, W.S.; Chung, I.Y.; Yoo, J.M. Treatment with triamcinolone acetonide prevents decreased retinal levels of decorin in a rat model of oxygen-induced retinopathy. Curr. Eye Res. 2010, 35, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Duran, G.L.S.; Balbaba, M.; Colakoglu, N.; Bulmus, O.; Ulas, F.; Eroksuz, Y. Effect of Decorin and Bevacizumab on oxygen-induced retinopathy in rat models: A comparative study. Indian J. Ophthalmol. 2021, 69, 369–373. [Google Scholar] [CrossRef]
- Ryan, S.J. The pathophysiology of proliferative vitreoretinopathy in its management. Am. J. Ophthalmol. 1985, 100, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Hoerauf, H. TGF-ss and RPE-derived cells in taut subretinal strands from patients with proliferative vitreoretinopathy. Eur. J. Ophthalmol. 2011, 21, 422–426. [Google Scholar] [CrossRef]
- Nassar, K.; Luke, J.; Luke, M.; Kamal, M.; Abd El-Nabi, E.; Soliman, M.; Rohrbach, M.; Grisanti, S. The novel use of decorin in prevention of the development of proliferative vitreoretinopathy (PVR). Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1649–1660. [Google Scholar] [CrossRef]
- Jarvinen, T.A. Design of target-seeking antifibrotic compounds. Methods Enzymol. 2012, 509, 243–261. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubo, E.; Shibata, S.; Shibata, T.; Sasaki, H.; Singh, D.P. Role of Decorin in the Lens and Ocular Diseases. Cells 2023, 12, 74. https://doi.org/10.3390/cells12010074
Kubo E, Shibata S, Shibata T, Sasaki H, Singh DP. Role of Decorin in the Lens and Ocular Diseases. Cells. 2023; 12(1):74. https://doi.org/10.3390/cells12010074
Chicago/Turabian StyleKubo, Eri, Shinsuke Shibata, Teppei Shibata, Hiroshi Sasaki, and Dhirendra P. Singh. 2023. "Role of Decorin in the Lens and Ocular Diseases" Cells 12, no. 1: 74. https://doi.org/10.3390/cells12010074
APA StyleKubo, E., Shibata, S., Shibata, T., Sasaki, H., & Singh, D. P. (2023). Role of Decorin in the Lens and Ocular Diseases. Cells, 12(1), 74. https://doi.org/10.3390/cells12010074







