12-O-tetradecanoylphorbol-13-acetate Reduces Activation of Hepatic Stellate Cells by Inhibiting the Hippo Pathway Transcriptional Coactivator YAP
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. MTT Assay
2.4. qPCR
2.5. Immunoblotting Analysis
2.6. Immunocytochemical Analysis
2.7. Cell Cycle Analysis
2.8. Statistical Analyses
3. Results
3.1. TPA-Induced Inhibition of Activation of Hepatic Stellate Cells
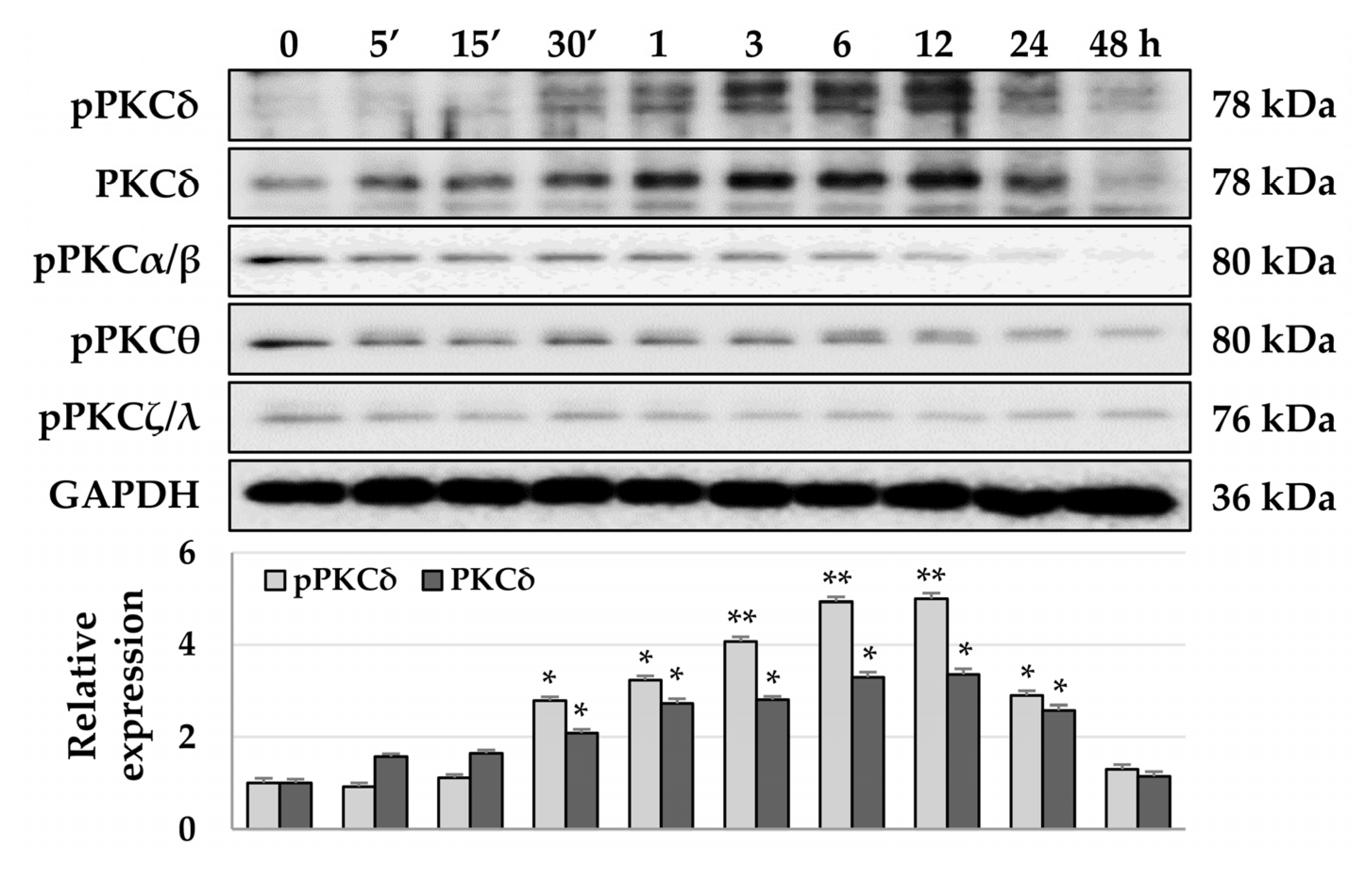
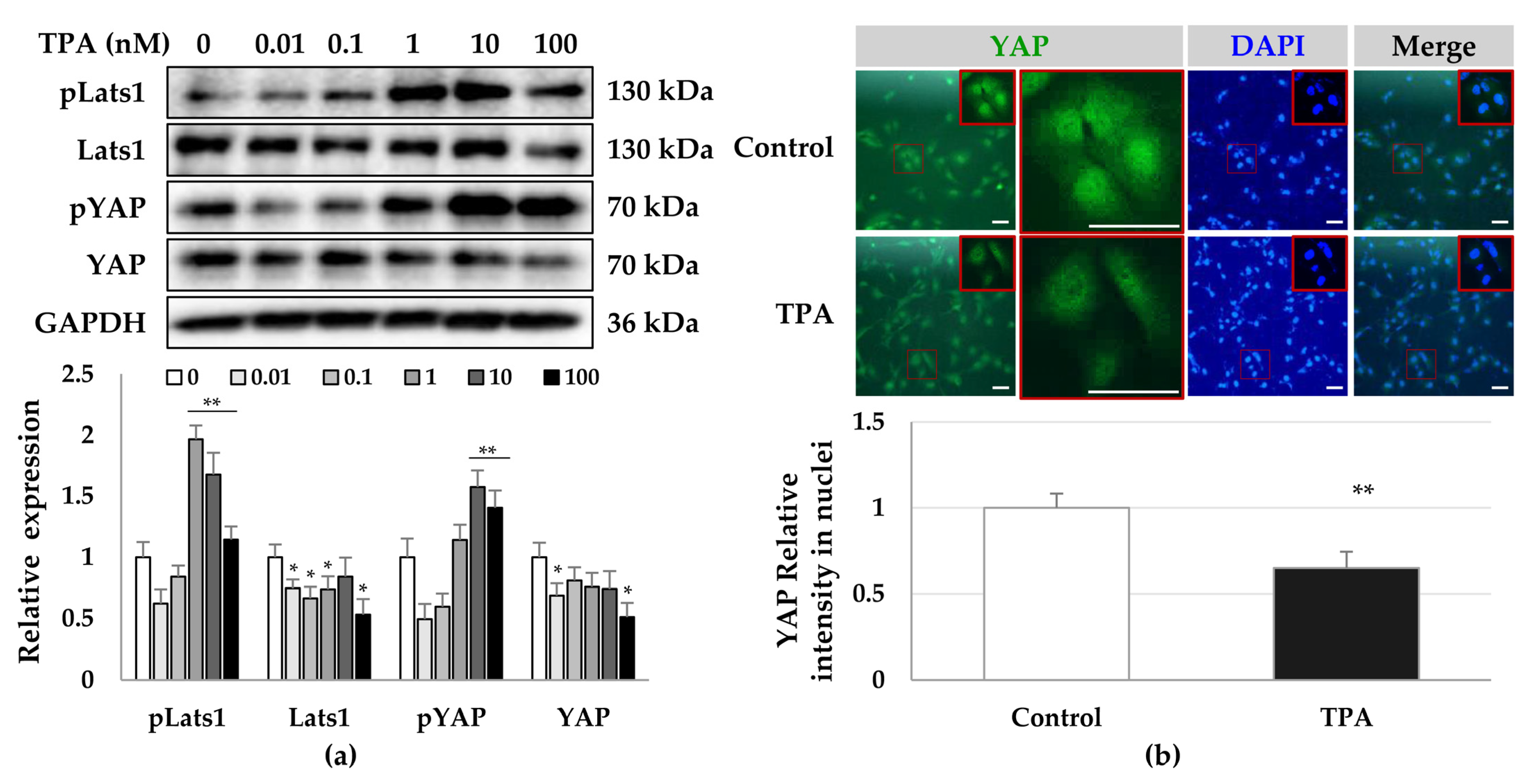
3.2. TPA-Induced Phosphorylation of PKCδ and YAP in HSCs
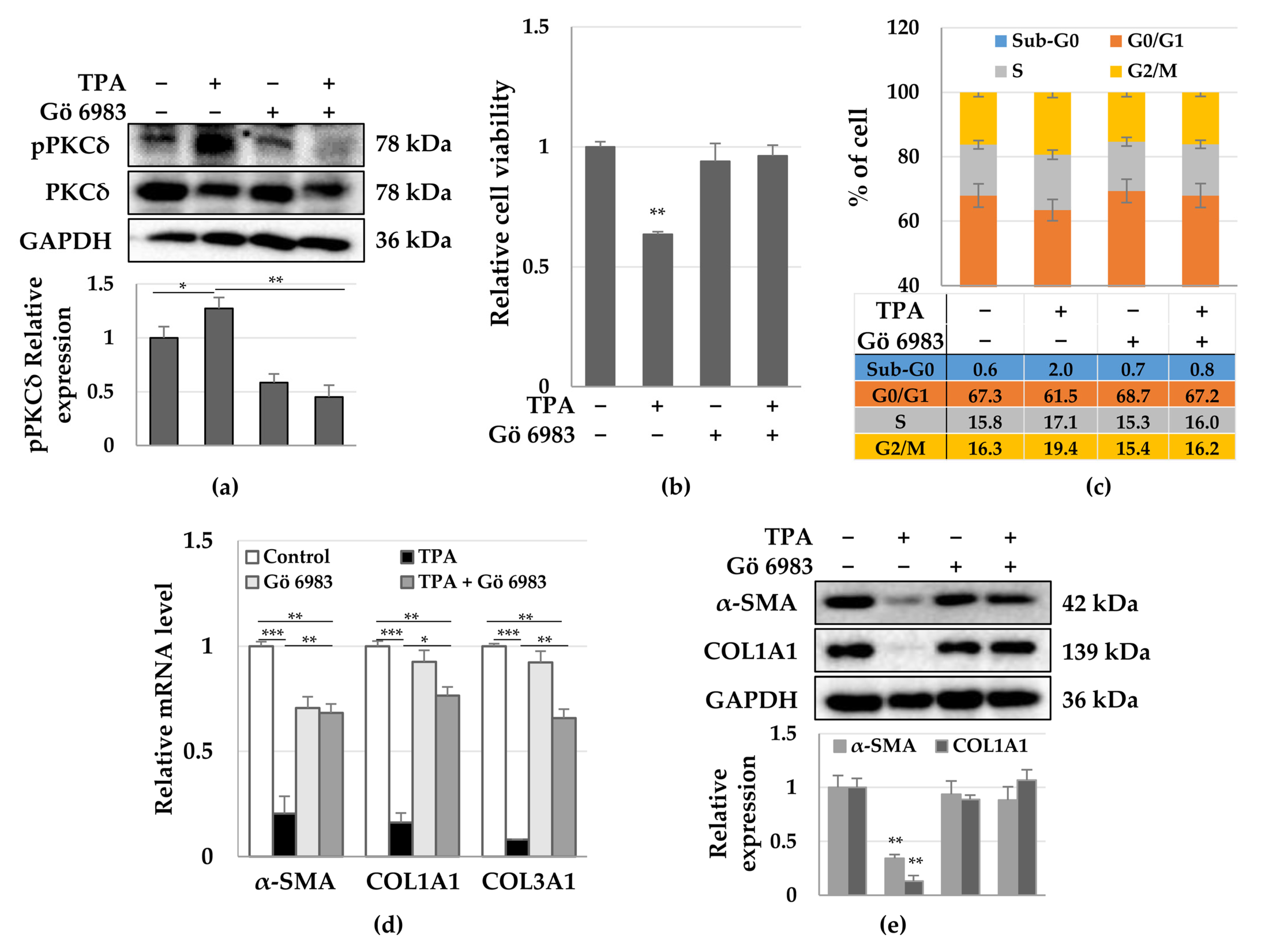
3.3. Roles of PKCδ and YAP in HSC Activation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reynaert, H.; Thompson, M.G.; Thomas, T.; Geerts, A. Hepatic stellate cells: Role in microcirculation and pathophysiology of portal hypertension. Gut 2002, 50, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Geerts, A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin. Liver Dis. 2001, 21, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef]
- Trivedi, P.; Wang, S.; Friedman, S.L. The Power of Plasticity-Metabolic Regulation of Hepatic Stellate Cells. Cell Metab. 2021, 33, 242–257. [Google Scholar] [CrossRef]
- Baghaei, K.; Mazhari, S.; Tokhanbigli, S.; Parsamanesh, G.; Alavifard, H.; Schaafsma, D.; Ghavami, S. Therapeutic potential of targeting regulatory mechanisms of hepatic stellate cell activation in liver fibrosis. Drug Discov. Today 2022, 27, 1044–1061. [Google Scholar] [CrossRef]
- Fabregat, I.; Moreno-Caceres, J.; Sanchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; Ten Dijke, P.; Consortium, I.-L. TGF-beta signalling and liver disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
- Bartneck, M.; Koppe, C.; Fech, V.; Warzecha, K.T.; Kohlhepp, M.; Huss, S.; Weiskirchen, R.; Trautwein, C.; Luedde, T.; Tacke, F. Roles of CCR2 and CCR5 for Hepatic Macrophage Polarization in Mice With Liver Parenchymal Cell-Specific NEMO Deletion. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 327–347. [Google Scholar] [CrossRef]
- Hellerbrand, C.; Jobin, C.; Licato, L.L.; Sartor, R.B.; Brenner, D.A. Cytokines induce NF-kappaB in activated but not in quiescent rat hepatic stellate cells. Am. J. Physiol. 1998, 275, G269–G278. [Google Scholar] [CrossRef]
- Lee, M.; Lee, Y.; Song, J.; Lee, J.; Chang, S.Y. Tissue-specific Role of CX3CR1 Expressing Immune Cells and Their Relationships with Human Disease. Immune Netw. 2018, 18, e5. [Google Scholar] [CrossRef]
- Sahin, H.; Borkham-Kamphorst, E.; Kuppe, C.; Zaldivar, M.M.; Grouls, C.; Al-samman, M.; Nellen, A.; Schmitz, P.; Heinrichs, D.; Berres, M.L.; et al. Chemokine Cxcl9 attenuates liver fibrosis-associated angiogenesis in mice. Hepatology 2012, 55, 1610–1619. [Google Scholar] [CrossRef]
- Ballardini, G.; Degli Esposti, S.; Bianchi, F.B.; de Giorgi, L.B.; Faccani, A.; Biolchini, L.; Busachi, C.A.; Pisi, E. Correlation between Ito cells and fibrogenesis in an experimental model of hepatic fibrosis. A sequential stereological study. Liver 1983, 3, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Reeves, H.L.; Burt, A.D.; Wood, S.; Day, C.P. Hepatic stellate cell activation occurs in the absence of hepatitis in alcoholic liver disease and correlates with the severity of steatosis. J. Hepatol. 1996, 25, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Mochly-Rosen, D.; Das, K.; Grimes, K.V. Protein kinase C, an elusive therapeutic target? Nat. Rev. Drug Discov. 2012, 11, 937–957. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Sellin, J.H.; Morris, A.P. Increased nuclear translocation of catalytically active PKC-zeta during mouse colonocyte hyperproliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G223–G237. [Google Scholar] [CrossRef]
- Musashi, M.; Ota, S.; Shiroshita, N. The role of protein kinase C isoforms in cell proliferation and apoptosis. Int. J. Hematol. 2000, 72, 12–19. [Google Scholar]
- Murphy, T.L.; Sakamoto, T.; Hinton, D.R.; Spee, C.; Gundimeda, U.; Soriano, D.; Gopalakrishna, R.; Ryan, S.J. Migration of retinal pigment epithelium cells in vitro is regulated by protein kinase C. Exp. Eye Res. 1995, 60, 683–695. [Google Scholar] [CrossRef]
- Steinberg, S.F. Structural basis of protein kinase C isoform function. Physiol. Rev. 2008, 88, 1341–1378. [Google Scholar] [CrossRef] [PubMed]
- Palaniyandi, S.S.; Sun, L.; Ferreira, J.C.; Mochly-Rosen, D. Protein kinase C in heart failure: A therapeutic target? Cardiovasc. Res. 2009, 82, 229–239. [Google Scholar] [CrossRef]
- O'Brian, C.A.; Liskamp, R.M.; Solomon, D.H.; Weinstein, I.B. Inhibition of protein kinase C by tamoxifen. Cancer Res. 1985, 45, 2462–2465. [Google Scholar]
- Schwartz, G.K.; Jiang, J.; Kelsen, D.; Albino, A.P. Protein kinase C: A novel target for inhibiting gastric cancer cell invasion. J. Natl. Cancer Inst. 1993, 85, 402–407. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, X.; Macdonnell, S.M.; Kranias, E.G.; Lorenz, J.N.; Leitges, M.; Houser, S.R.; Molkentin, J.D. Protein kinase C{alpha}, but not PKC{beta} or PKC{gamma}, regulates contractility and heart failure susceptibility: Implications for ruboxistaurin as a novel therapeutic approach. Circ. Res. 2009, 105, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.; Koyanagi, T.; Palaniyandi, S.S.; Fajardo, G.; Churchill, E.N.; Budas, G.; Disatnik, M.H.; Bernstein, D.; Brum, P.C.; Mochly-Rosen, D. Pharmacological inhibition of betaIIPKC is cardioprotective in late-stage hypertrophy. J. Mol. Cell. Cardiol. 2011, 51, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, K.; Koyanagi, T.; Berry, N.C.; Sun, L.; Mochly-Rosen, D. Pharmacological inhibition of epsilon-protein kinase C attenuates cardiac fibrosis and dysfunction in hypertension-induced heart failure. Hypertension 2008, 51, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Piersma, B.; Bank, R.A.; Boersema, M. Signaling in Fibrosis: TGF-beta, WNT, and YAP/TAZ Converge. Front. Med. 2015, 2, 59. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Uchinami, H.; Feirt, N.; Quintana-Bustamante, O.; Segovia, J.C.; Schwabe, R.F.; Brenner, D.A. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J. Hepatol. 2006, 45, 429–438. [Google Scholar] [CrossRef]
- Shi, Y.; Massague, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Bujak, M.; Ren, G.; Kweon, H.J.; Dobaczewski, M.; Reddy, A.; Taffet, G.; Wang, X.F.; Frangogiannis, N.G. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation 2007, 116, 2127–2138. [Google Scholar] [CrossRef]
- Wang, B.; Omar, A.; Angelovska, T.; Drobic, V.; Rattan, S.G.; Jones, S.C.; Dixon, I.M. Regulation of collagen synthesis by inhibitory Smad7 in cardiac myofibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1282–H1290. [Google Scholar] [CrossRef]
- Springer, J.; Scholz, F.R.; Peiser, C.; Groneberg, D.A.; Fischer, A. SMAD-signaling in chronic obstructive pulmonary disease: Transcriptional down-regulation of inhibitory SMAD 6 and 7 by cigarette smoke. Biol. Chem. 2004, 385, 649–653. [Google Scholar] [CrossRef]
- Pulichino, A.M.; Wang, I.M.; Caron, A.; Mortimer, J.; Auger, A.; Boie, Y.; Elias, J.A.; Kartono, A.; Xu, L.; Menetski, J.; et al. Identification of transforming growth factor beta1-driven genetic programs of acute lung fibrosis. Am. J. Respir. Cell. Mol. Biol. 2008, 39, 324–336. [Google Scholar] [CrossRef]
- Liu, C.; Gaca, M.D.; Swenson, E.S.; Vellucci, V.F.; Reiss, M.; Wells, R.G. Smads 2 and 3 are differentially activated by transforming growth factor-beta (TGF-beta ) in quiescent and activated hepatic stellate cells. Constitutive nuclear localization of Smads in activated cells is TGF-beta-independent. J. Biol. Chem. 2003, 278, 11721–11728. [Google Scholar] [CrossRef] [PubMed]
- Kaimori, A.; Potter, J.; Kaimori, J.Y.; Wang, C.; Mezey, E.; Koteish, A. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J. Biol. Chem. 2007, 282, 22089–22101. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, A.C.; Schnaper, H.W.; Tan, R.; Liu, Y.; Runyan, C.E. Cell phenotype-specific down-regulation of Smad3 involves decreased gene activation as well as protein degradation. J. Biol. Chem. 2007, 282, 15534–15540. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Huang, X.R.; Chung, A.C.; Qin, W.; Shao, X.; Igarashi, P.; Ju, W.; Bottinger, E.P.; Lan, H.Y. Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J. Am. Soc. Nephrol. 2010, 21, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, G.S.; Yang, X.; Glick, A.B.; Weinstein, M.; Letterio, J.L.; Mizel, D.E.; Anzano, M.; Greenwell-Wild, T.; Wahl, S.M.; Deng, C.; et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell. Biol. 1999, 1, 260–266. [Google Scholar] [CrossRef]
- Wei, J.; Fang, F.; Lam, A.P.; Sargent, J.L.; Hamburg, E.; Hinchcliff, M.E.; Gottardi, C.J.; Atit, R.; Whitfield, M.L.; Varga, J. Wnt/beta-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum. 2012, 64, 2734–2745. [Google Scholar] [CrossRef]
- Kapoor, M.; Liu, S.; Xu, S.; Huh, K.; McCann, M.; Denton, C.P.; Woodgett, J.R.; Abraham, D.J.; Leask, A. GSK-3beta in mouse fibroblasts controls wound healing and fibrosis through an endothelin-1-dependent mechanism. J. Clin. Investig. 2008, 118, 3279–3290. [Google Scholar] [CrossRef]
- Hamburg, E.J.; Atit, R.P. Sustained beta-catenin activity in dermal fibroblasts is sufficient for skin fibrosis. J. Investig. Dermatol 2012, 132, 2469–2472. [Google Scholar] [CrossRef]
- Fukushima, K.; Takahashi, K.; Fukushima, N.; Honoki, K.; Tsujiuchi, T. Different effects of GPR120 and GPR40 on cellular functions stimulated by 12-O-tetradecanoylphorbol-13-acetate in melanoma cells. Biochem. Biophys. Res. Commun. 2016, 475, 25–30. [Google Scholar] [CrossRef]
- Lii, C.K.; Chang, J.W.; Chen, J.J.; Chen, H.W.; Liu, K.L.; Yeh, S.L.; Wang, T.S.; Liu, S.H.; Tsai, C.H.; Li, C.C. Docosahexaenoic acid inhibits 12-O-tetradecanoylphorbol-13- acetate-induced fascin-1-dependent breast cancer cell migration by suppressing the PKCdelta- and Wnt-1/beta-catenin-mediated pathways. Oncotarget 2016, 7, 25162–25179. [Google Scholar] [CrossRef]
- Slaga, T.J.; Scribner, J.D.; Viaje, A. Epidermal cell proliferation and promoting ability of phorbol esters. J. Natl. Cancer Inst. 1976, 57, 1145–1149. [Google Scholar] [CrossRef]
- Alfredsson, C.F.; Rendel, F.; Liang, Q.L.; Sundstrom, B.E.; Nanberg, E. Altered sensitivity to ellagic acid in neuroblastoma cells undergoing differentiation with 12-O-tetradecanoylphorbol-13-acetate and all-trans retinoic acid. Biomed. Pharmacother. 2015, 76, 39–45. [Google Scholar] [CrossRef]
- Strair, R.K.; Schaar, D.; Goodell, L.; Aisner, J.; Chin, K.V.; Eid, J.; Senzon, R.; Cui, X.X.; Han, Z.T.; Knox, B.; et al. Administration of a phorbol ester to patients with hematological malignancies: Preliminary results from a phase I clinical trial of 12-O-tetradecanoylphorbol-13-acetate. Clin. Cancer Res. 2002, 8, 2512–2518. [Google Scholar] [PubMed]
- Zhu, G.; Chen, Y.; Zhang, X.; Wu, Q.; Zhao, Y.; Chen, Y.; Sun, F.; Qiao, Y.; Wang, J. 12-O-Tetradecanoylphorbol-13-acetate (TPA) is anti-tumorigenic in liver cancer cells via inhibiting YAP through AMOT. Sci. Rep. 2017, 7, 44940. [Google Scholar] [CrossRef] [PubMed]
- Mulsow, J.J.; Watson, R.W.; Fitzpatrick, J.M.; O’Connell, P.R. Transforming growth factor-beta promotes pro-fibrotic behavior by serosal fibroblasts via PKC and ERK1/2 mitogen activated protein kinase cell signaling. Ann. Surg. 2005, 242, 880–887; discussion 887–889. [Google Scholar] [CrossRef]
- Slattery, C.; Ryan, M.P.; McMorrow, T. Protein kinase C beta overexpression induces fibrotic effects in human proximal tubular epithelial cells. Int. J. Biochem. Cell. Biol. 2008, 40, 2218–2229. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Qian, X.; Shen, M.; Jiang, R.; Wagner, M.B.; Ding, G.; Chen, G.; Shen, B. Protein kinase C promotes cardiac fibrosis and heart failure by modulating galectin-3 expression. Biochim. Biophys. Acta 2015, 1853, 513–521. [Google Scholar] [CrossRef]
- Karhu, S.T.; Ruskoaho, H.; Talman, V. Distinct Regulation of Cardiac Fibroblast Proliferation and Transdifferentiation by Classical and Novel Protein Kinase C Isoforms: Possible Implications for New Antifibrotic Therapies. Mol. Pharmacol. 2021, 99, 104–113. [Google Scholar] [CrossRef]
- Gong, S.C.; Yoon, Y.; Jung, P.Y.; Kim, M.Y.; Baik, S.K.; Ryu, H.; Eom, Y.W. Antifibrotic TSG-6 Expression Is Synergistically Increased in Both Cells during Coculture of Mesenchymal Stem Cells and Macrophages via the JAK/STAT Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 13122. [Google Scholar] [CrossRef]
- Tahara, E.; Kadara, H.; Lacroix, L.; Lotan, D.; Lotan, R. Activation of protein kinase C by phorbol 12-myristate 13-acetate suppresses the growth of lung cancer cells through KLF6 induction. Cancer Biol. Ther. 2009, 8, 801–807. [Google Scholar] [CrossRef]
- Gomes, G.; Bagri, K.M.; de Andrade Rosa, I.; Jurberg, A.D.; Mermelstein, C.; Costa, M.L. Activation of YAP regulates muscle fiber size in a PKC-dependent mechanism during chick in vitro myogenesis. J. Muscle Res. Cell. Motil. 2022, 43, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Mitani, A.; Nagase, T.; Fukuchi, K.; Aburatani, H.; Makita, R.; Kurihara, H. Transcriptional coactivator with PDZ-binding motif is essential for normal alveolarization in mice. Am. J. Respir. Crit. Care Med. 2009, 180, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Kim, Y.; Sutherland, L.B.; Murakami, M.; Qi, X.; McAnally, J.; Porrello, E.R.; Mahmoud, A.I.; Tan, W.; Shelton, J.M.; et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 13839–13844. [Google Scholar] [CrossRef]
- Gao, P.J.; Li, Y.; Sun, A.J.; Liu, J.J.; Ji, K.D.; Zhang, Y.Z.; Sun, W.L.; Marche, P.; Zhu, D.L. Differentiation of vascular myofibroblasts induced by transforming growth factor-beta1 requires the involvement of protein kinase Calpha. J. Mol. Cell. Cardiol. 2003, 35, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, R.; Maffei, A.; Madaro, L.; Notte, A.; Stanganello, E.; Cifelli, G.; Carullo, P.; Molinaro, M.; Lembo, G.; Bouche, M. Protein kinase Ctheta is required for cardiomyocyte survival and cardiac remodeling. Cell Death Dis. 2010, 1, e45. [Google Scholar] [CrossRef]
- Mei, C.; Yang, Y.; Dong, P.; Song, L.; Zhou, Y.; Xu, Y.; Yu, C. Deficiency of PKClambda/iota alleviates the liver pathologic impairment of Schistosoma japonicum infection by thwarting Th2 response. Parasit. Vectors 2022, 15, 154. [Google Scholar] [CrossRef]
- Jackson, D.N.; Foster, D.A. The enigmatic protein kinase Cdelta: Complex roles in cell proliferation and survival. FASEB J. 2004, 18, 627–636. [Google Scholar] [CrossRef]
- Steinberg, S.F. Distinctive activation mechanisms and functions for protein kinase Cdelta. Biochem. J. 2004, 384, 449–459. [Google Scholar] [CrossRef]
- Vallee, S.; Laforest, S.; Fouchier, F.; Montero, M.P.; Penel, C.; Champion, S. Cytokine-induced upregulation of NF-kappaB, IL-8, and ICAM-1 is dependent on colonic cell polarity: Implication for PKCdelta. Exp. Cell Res. 2004, 297, 165–185. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Evers, B.M. Induction of cIAP-2 in human colon cancer cells through PKC delta/NF-kappa B. J. Biol. Chem. 2003, 278, 51091–51099. [Google Scholar] [CrossRef]
- Page, K.; Li, J.; Zhou, L.; Iasvovskaia, S.; Corbit, K.C.; Soh, J.W.; Weinstein, I.B.; Brasier, A.R.; Lin, A.; Hershenson, M.B. Regulation of airway epithelial cell NF-kappa B-dependent gene expression by protein kinase C delta. J. Immunol. 2003, 170, 5681–5689. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, S.A.; Gaidarova, S.; Saitta, B.; Sandorfi, N.; Herrich, D.J.; Rosenbloom, J.C.; Kucich, U.; Abrams, W.R.; Rosenbloom, J. Role of protein kinase C-delta in the regulation of collagen gene expression in scleroderma fibroblasts. J. Clin. Investig. 2001, 108, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Gore-Hyer, E.; Pannu, J.; Smith, E.A.; Grotendorst, G.; Trojanowska, M. Selective stimulation of collagen synthesis in the presence of costimulatory insulin signaling by connective tissue growth factor in scleroderma fibroblasts. Arthritis Rheum. 2003, 48, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Runyan, C.E.; Schnaper, H.W.; Poncelet, A.C. Smad3 and PKCdelta mediate TGF-beta1-induced collagen I expression in human mesangial cells. Am. J. Physiol Renal Physiol 2003, 285, F413–F422. [Google Scholar] [CrossRef]
- Lee, S.J.; Kang, J.H.; Choi, S.Y.; Suk, K.T.; Kim, D.J.; Kwon, O.S. PKCdelta as a regulator for TGFbeta1-induced alpha-SMA production in a murine nonalcoholic steatohepatitis model. PLoS ONE 2013, 8, e55979. [Google Scholar] [CrossRef]
- Kim, C.L.; Choi, S.H.; Mo, J.S. Role of the Hippo Pathway in Fibrosis and Cancer. Cells 2019, 8, 468. [Google Scholar] [CrossRef]
- Chu, W.K.; Hung, L.M.; Hou, C.W.; Chen, J.K. PKC Regulates YAP Expression through Alternative Splicing of YAP 3'UTR Pre-mRNA by hnRNP F. Int. J. Mol. Sci. 2021, 22, 694. [Google Scholar] [CrossRef]
- Gong, R.; Hong, A.W.; Plouffe, S.W.; Zhao, B.; Liu, G.; Yu, F.X.; Xu, Y.; Guan, K.L. Opposing roles of conventional and novel PKC isoforms in Hippo-YAP pathway regulation. Cell Res. 2015, 25, 985–988. [Google Scholar] [CrossRef]


| Gene | Forward Primer | Reverse Primer | Size, bp | Accession # |
|---|---|---|---|---|
| COL1A1 | 5′-CAGGAGGCACGCGGAGTGTG-3′ | 5′-GGCAGGGCTCGGGTTTCCAC-3′ | 263 | NM_000088.4 |
| COL3A1 | 5′-TCCCGGTCCTGCTGGTTCCC-3′ | 5′-ATGGCAGCGGCTCCAACACC-3′ | 390 | NM_000090.4 |
| α-SMA | 5′-GACAATGGCTCTGGGCTCTGTAA-3′ | 5′-CTGTGCTTCGTCACCCACGTA-3′ | 149 | NM_001613.4 |
| GAPDH | 5′-CAAGGCTGAGAACGGGAAGC-3′ | 5′-AGGGGGCAGAGATGATGACC-3′ | 194 | NM_001256799.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.W.; Yoon, Y.; Kim, M.Y.; Baik, S.K.; Ryu, H.; Park, I.H.; Eom, Y.W. 12-O-tetradecanoylphorbol-13-acetate Reduces Activation of Hepatic Stellate Cells by Inhibiting the Hippo Pathway Transcriptional Coactivator YAP. Cells 2023, 12, 91. https://doi.org/10.3390/cells12010091
Kim CW, Yoon Y, Kim MY, Baik SK, Ryu H, Park IH, Eom YW. 12-O-tetradecanoylphorbol-13-acetate Reduces Activation of Hepatic Stellate Cells by Inhibiting the Hippo Pathway Transcriptional Coactivator YAP. Cells. 2023; 12(1):91. https://doi.org/10.3390/cells12010091
Chicago/Turabian StyleKim, Chang Wan, Yongdae Yoon, Moon Young Kim, Soon Koo Baik, Hoon Ryu, Il Hwan Park, and Young Woo Eom. 2023. "12-O-tetradecanoylphorbol-13-acetate Reduces Activation of Hepatic Stellate Cells by Inhibiting the Hippo Pathway Transcriptional Coactivator YAP" Cells 12, no. 1: 91. https://doi.org/10.3390/cells12010091
APA StyleKim, C. W., Yoon, Y., Kim, M. Y., Baik, S. K., Ryu, H., Park, I. H., & Eom, Y. W. (2023). 12-O-tetradecanoylphorbol-13-acetate Reduces Activation of Hepatic Stellate Cells by Inhibiting the Hippo Pathway Transcriptional Coactivator YAP. Cells, 12(1), 91. https://doi.org/10.3390/cells12010091






