Tissue Derivation and Biological Sex Uniquely Mediate Endothelial Cell Protein Expression, Redox Status, and Nitric Oxide Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Western Blot
2.4. mRNA Analysis
2.5. Measurement of ROS
2.6. Immunocytochemistry
2.7. Intracellular NO
2.8. Statistical Analysis
3. Results
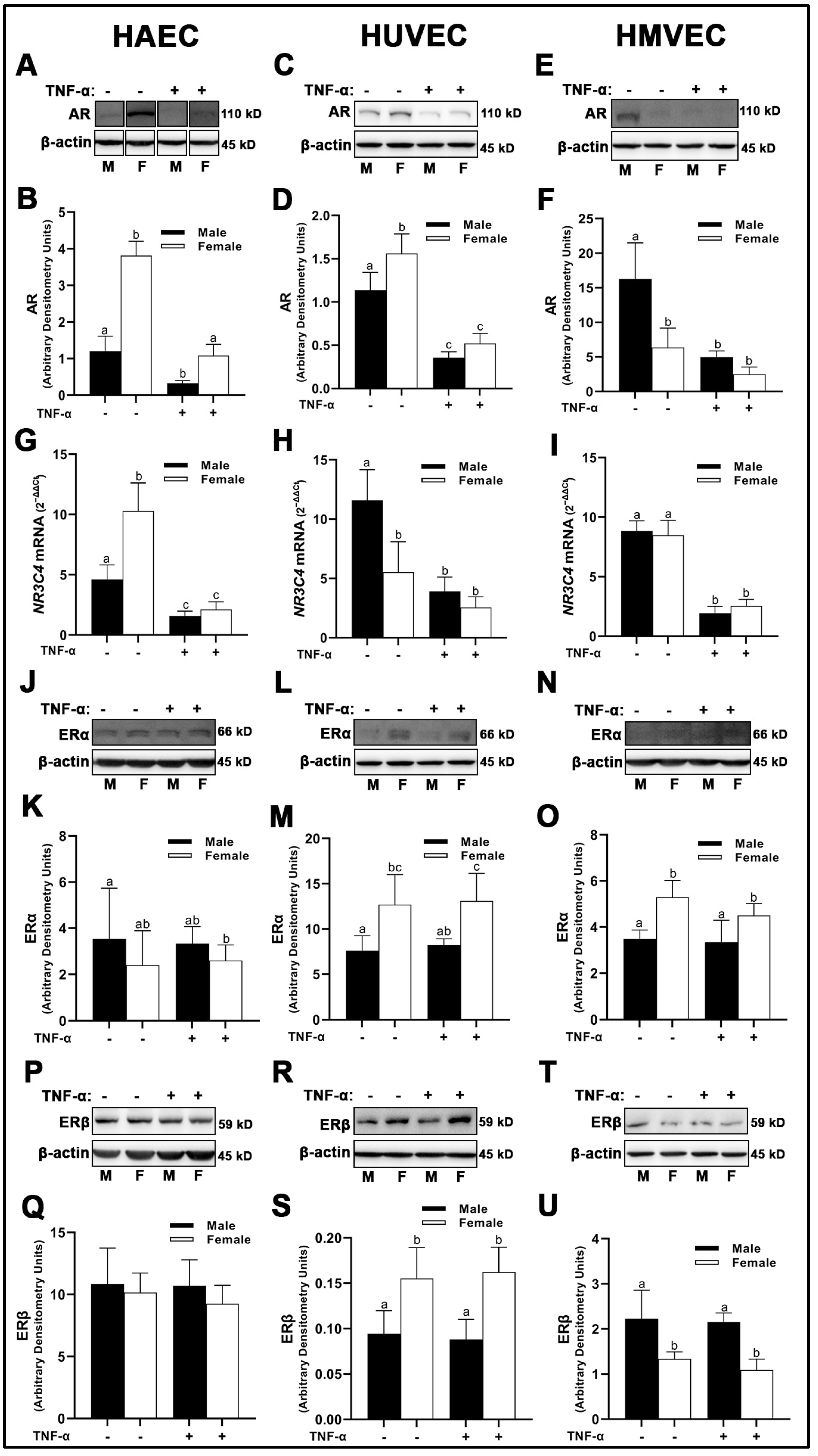
3.1. Sex Receptor Expression
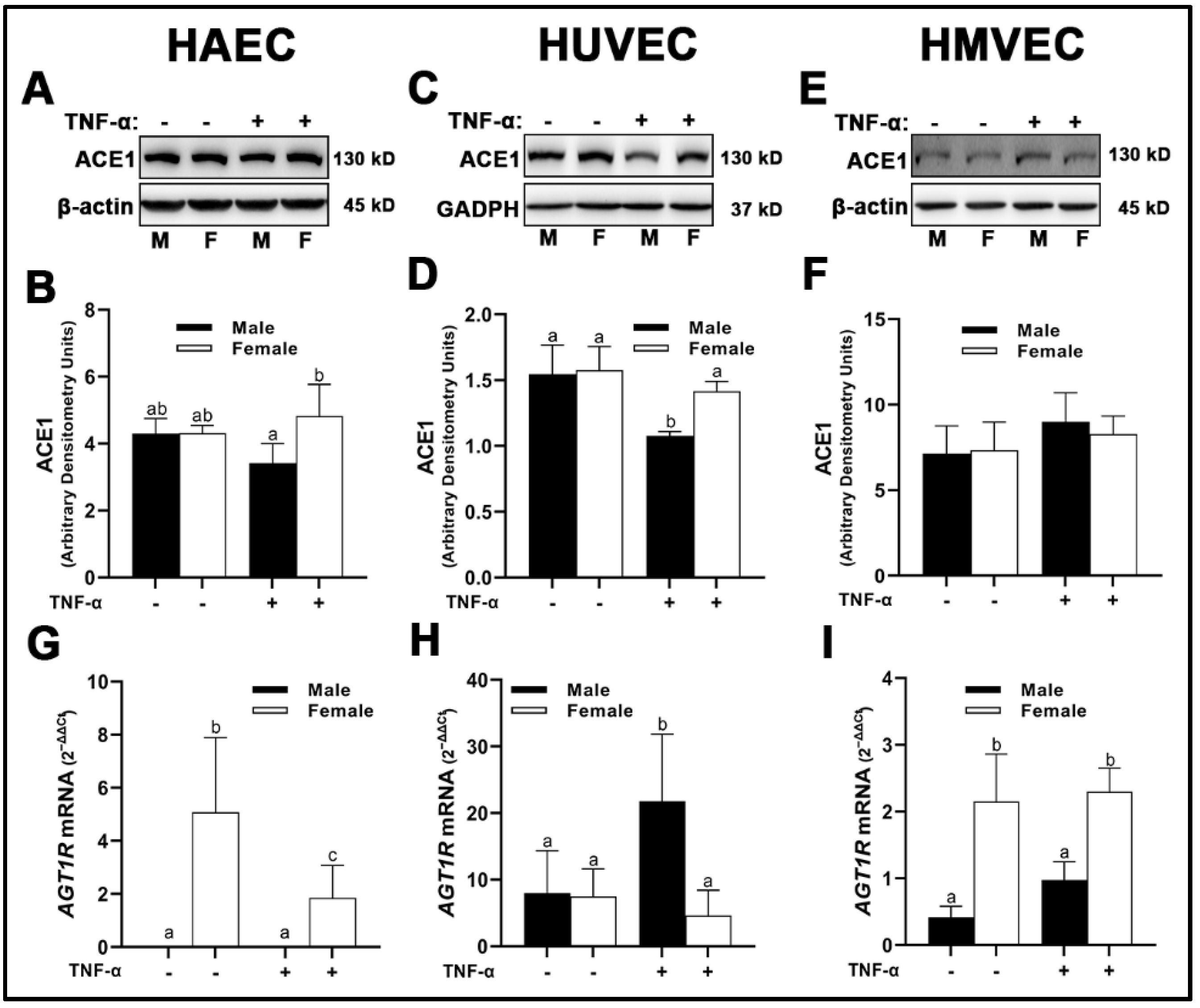
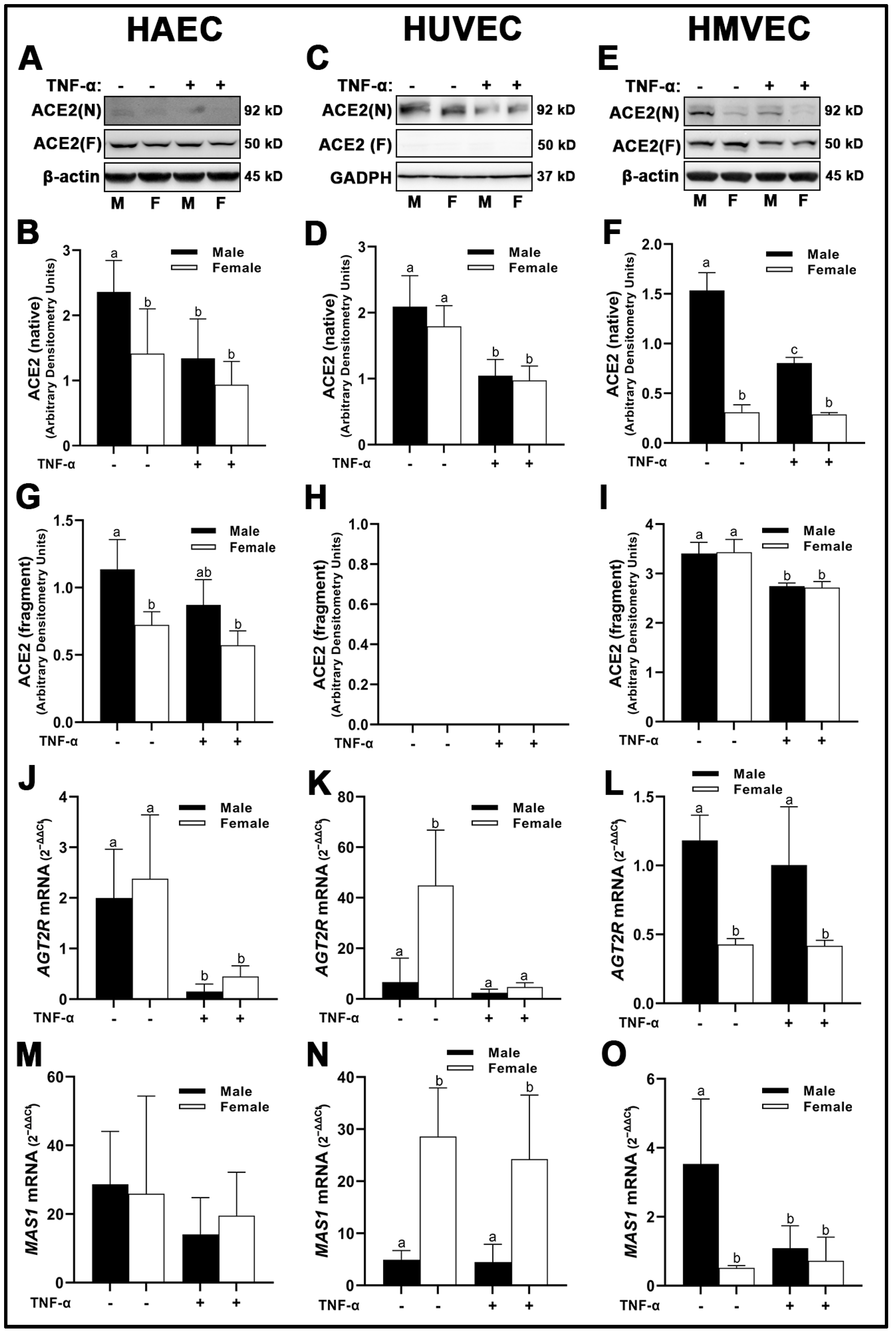
3.2. Renin-Angiotensin System (RAS) Components
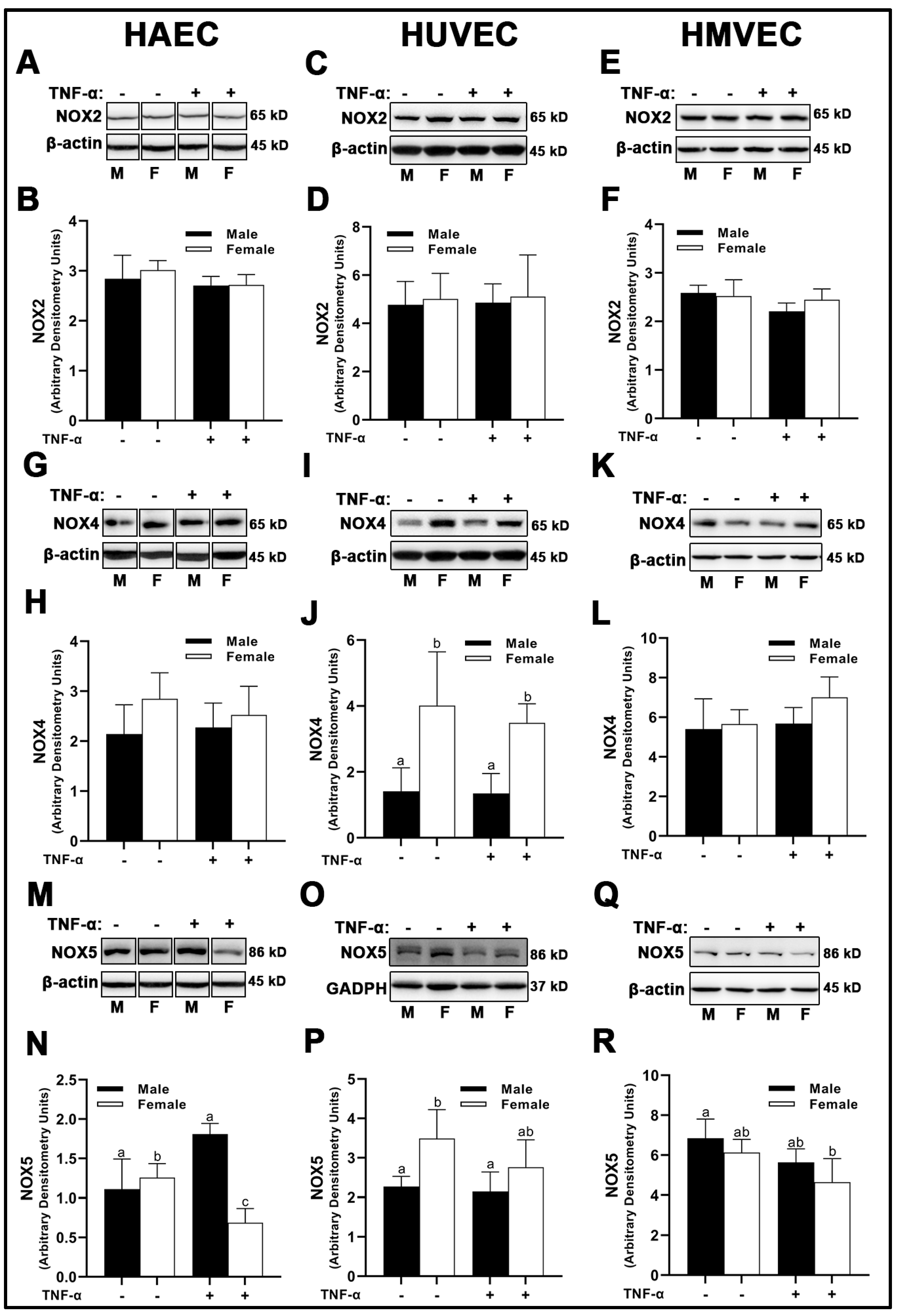
3.3. NADPH-Oxidase (NOX) Expression
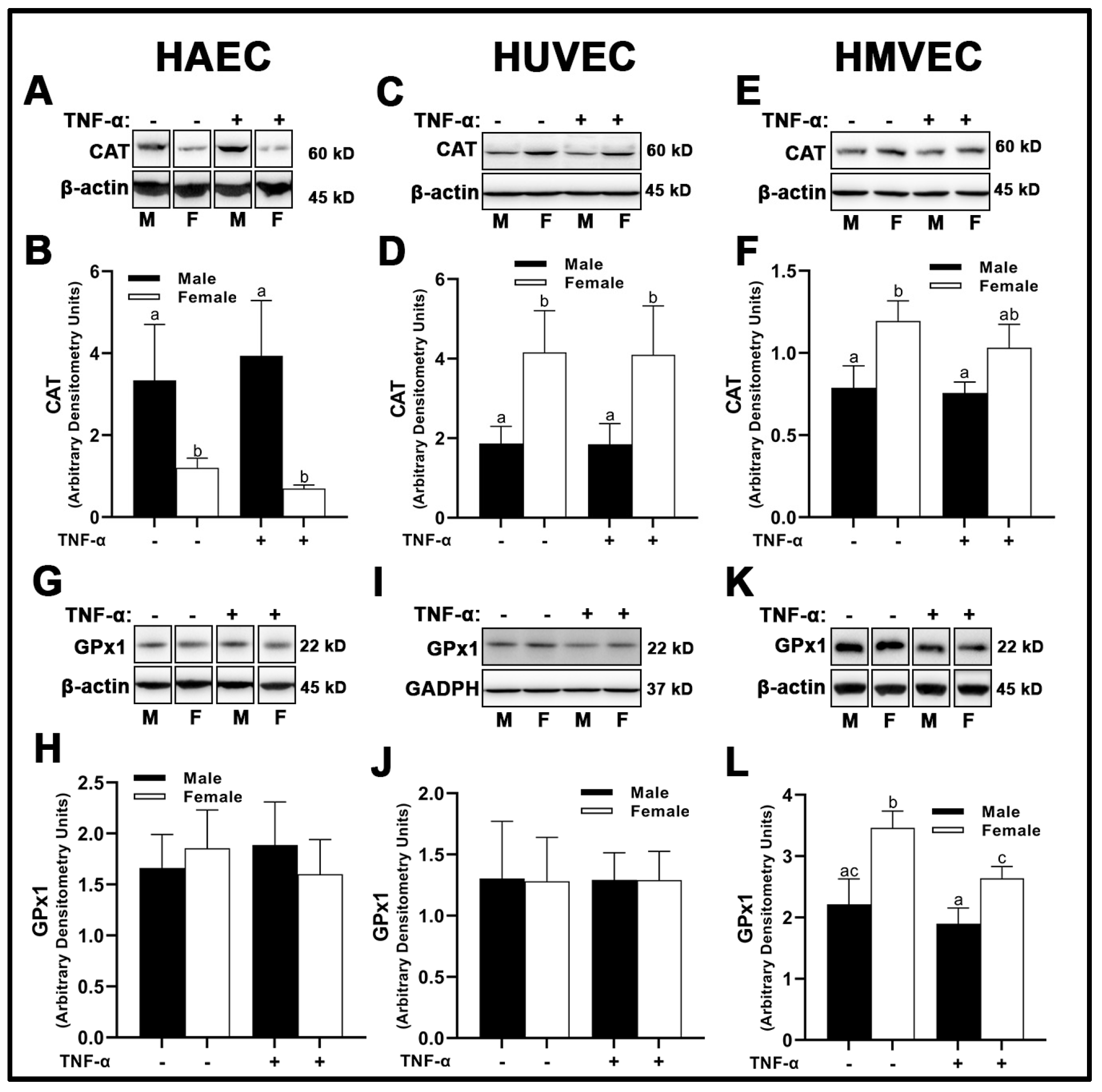
3.4. Endogenous Antioxidant Expression
3.5. Cellular Reactive Oxygen Species
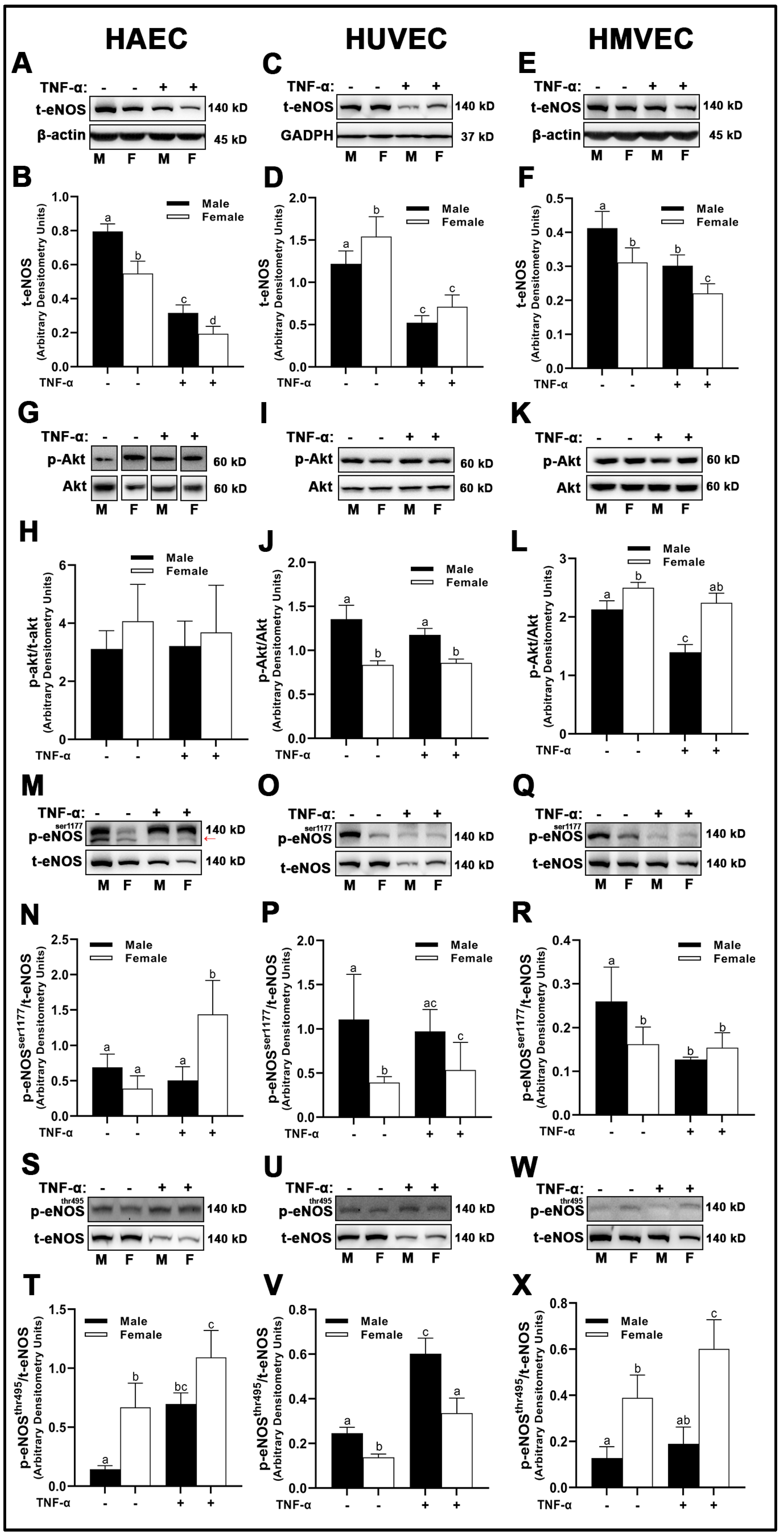
3.6. Endothelial Nitric Oxide Synthase (eNOS) and Nitric Oxide (NO) Bioavailability
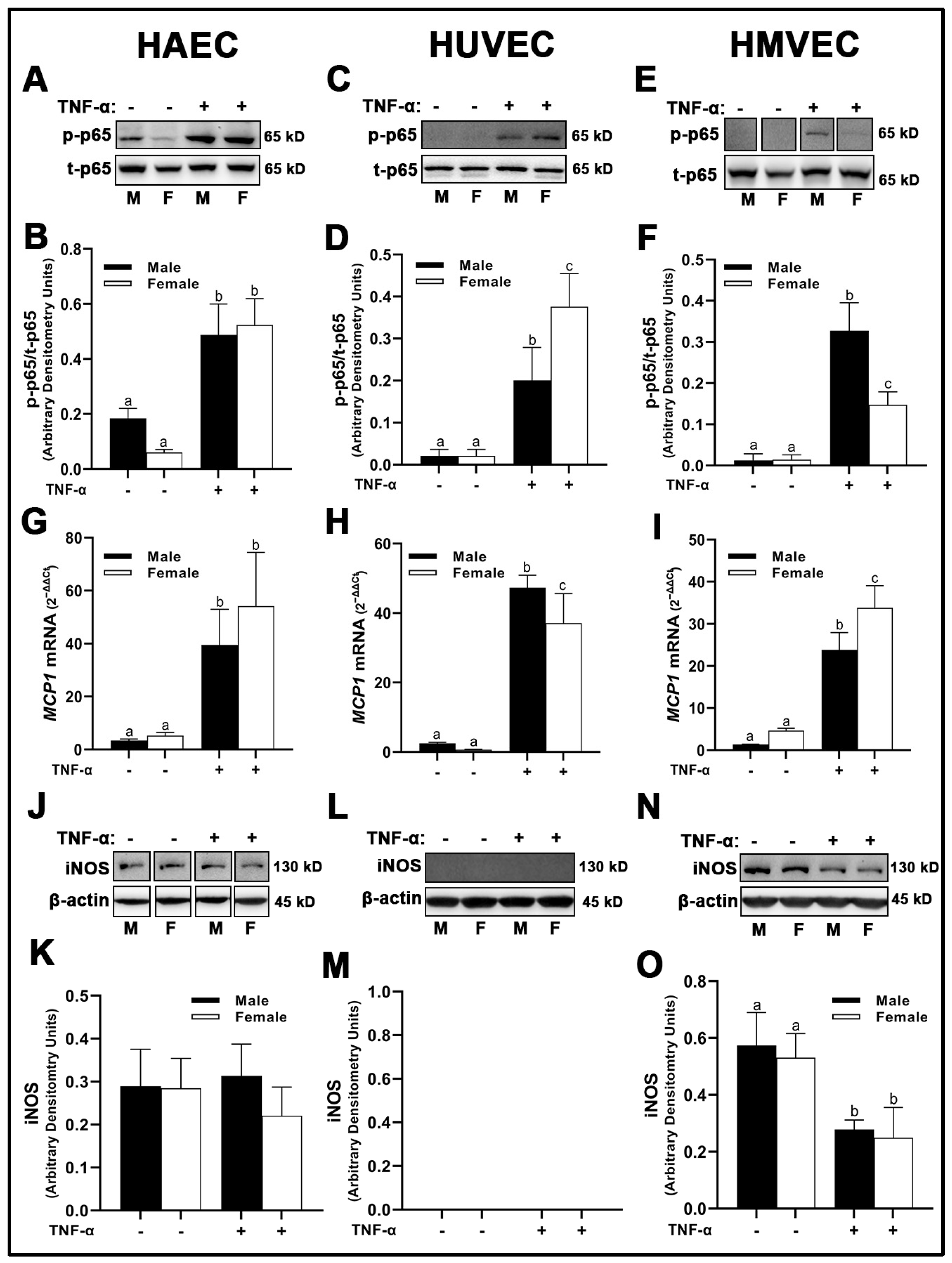
3.7. Inflammation
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, Y.; Liu, X.; Chen, Q.; Chen, T.; Jiang, N.; Guo, Z. Myricetin ameliorates ox-LDL-induced HUVECs apoptosis and inflammation via lncRNA GAS5 upregulating the expression of miR-29a-3p. Sci. Rep. 2021, 11, 19637. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Ostchega, Y.; Fryar, C.D.; Nwankwo, T.; Nguyen, D.T. Hypertension Prevalence among Adults Aged 18 and over: United States, 2017–2018. NCHS Data Brief 2020, 364, 1–8. [Google Scholar]
- Bairey Merz, C.N.; Pepine, C.J.; Walsh, M.N.; Fleg, J.L. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation 2017, 135, 1075–1092. [Google Scholar] [CrossRef]
- Forstermann, U.; Munzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef]
- Cai, H.; Li, Z.; Dikalov, S.; Holland, S.M.; Hwang, J.; Jo, H.; Dudley, S.C., Jr.; Harrison, D.G. NAD(P)H oxidase-derived hydrogen peroxide mediates endothelial nitric oxide production in response to angiotensin II. J. Biol. Chem. 2002, 277, 48311–48317. [Google Scholar] [CrossRef]
- Doughan, A.K.; Harrison, D.G.; Dikalov, S.I. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008, 102, 488–496. [Google Scholar] [CrossRef]
- Najjar, R.S.; Mu, S.; Feresin, R.G. Blueberry Polyphenols Increase Nitric Oxide and Attenuate Angiotensin II-Induced Oxidative Stress and Inflammatory Signaling in Human Aortic Endothelial Cells. Antioxidants 2022, 11, 616. [Google Scholar] [CrossRef]
- Watanabe, T.; Barker, T.A.; Berk, B.C. Angiotensin II and the endothelium: Diverse signals and effects. Hypertension 2005, 45, 163–169. [Google Scholar] [CrossRef]
- Ide, T.; Tsutsui, H.; Ohashi, N.; Hayashidani, S.; Suematsu, N.; Tsuchihashi, M.; Tamai, H.; Takeshita, A. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 438–442. [Google Scholar] [CrossRef]
- Xue, B.; Pamidimukkala, J.; Lubahn, D.B.; Hay, M. Estrogen receptor-alpha mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1770–H1776. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bian, Z.; Lu, P.; Karas, R.H.; Bao, L.; Cox, D.; Hodgin, J.; Shaul, P.W.; Thoren, P.; Smithies, O.; et al. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 2002, 295, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Jazbutyte, V.; Arias-Loza, P.A.; Hu, K.; Widder, J.; Govindaraj, V.; von Poser-Klein, C.; Bauersachs, J.; Fritzemeier, K.H.; Hegele-Hartung, C.; Neyses, L.; et al. Ligand-dependent activation of ER{beta} lowers blood pressure and attenuates cardiac hypertrophy in ovariectomized spontaneously hypertensive rats. Cardiovasc. Res. 2008, 77, 774–781. [Google Scholar] [CrossRef]
- Widder, J.; Pelzer, T.; von Poser-Klein, C.; Hu, K.; Jazbutyte, V.; Fritzemeier, K.H.; Hegele-Hartung, C.; Neyses, L.; Bauersachs, J. Improvement of endothelial dysfunction by selective estrogen receptor-alpha stimulation in ovariectomized SHR. Hypertension 2003, 42, 991–996. [Google Scholar] [CrossRef]
- Villablanca, A.; Lubahn, D.; Shelby, L.; Lloyd, K.; Barthold, S. Susceptibility to early atherosclerosis in male mice is mediated by estrogen receptor alpha. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.Y.; Song, K.S.; Lee, Y.H.; Seo, J.S.; Jelinek, J.; Goldschmidt-Clermont, P.J.; Issa, J.P. Epigenetic changes in estrogen receptor beta gene in atherosclerotic cardiovascular tissues and in-vitro vascular senescence. Biochim. Biophys. Acta 2007, 1772, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.K.; Lee, S.O.; Chang, E.; Pang, H.; Chang, C. Androgen receptor (AR) in cardiovascular diseases. J. Endocrinol. 2016, 229, R1–R16. [Google Scholar] [CrossRef]
- Yu, J.; Akishita, M.; Eto, M.; Ogawa, S.; Son, B.K.; Kato, S.; Ouchi, Y.; Okabe, T. Androgen receptor-dependent activation of endothelial nitric oxide synthase in vascular endothelial cells: Role of phosphatidylinositol 3-kinase/akt pathway. Endocrinology 2010, 151, 1822–1828. [Google Scholar] [CrossRef]
- Death, A.K.; McGrath, K.C.; Sader, M.A.; Nakhla, S.; Jessup, W.; Handelsman, D.J.; Celermajer, D.S. Dihydrotestosterone promotes vascular cell adhesion molecule-1 expression in male human endothelial cells via a nuclear factor-kappaB-dependent pathway. Endocrinology 2004, 145, 1889–1897. [Google Scholar] [CrossRef]
- Wysocki, J.; Schulze, A.; Batlle, D. Novel Variants of Angiotensin Converting Enzyme-2 of Shorter Molecular Size to Target the Kidney Renin Angiotensin System. Biomolecules 2019, 9, 886. [Google Scholar] [CrossRef]
- Michalski, R.; Thiebaut, D.; Michalowski, B.; Ayhan, M.M.; Hardy, M.; Ouari, O.; Rostkowski, M.; Smulik-Izydorczyk, R.; Artelska, A.; Marcinek, A.; et al. Oxidation of ethidium-based probes by biological radicals: Mechanism, kinetics and implications for the detection of superoxide. Sci. Rep. 2020, 10, 18626. [Google Scholar] [CrossRef] [PubMed]
- Rathel, T.R.; Leikert, J.J.; Vollmar, A.M.; Dirsch, V.M. Application of 4,5-diaminofluorescein to reliably measure nitric oxide released from endothelial cells in vitro. Biol. Proced. Online 2003, 5, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M.G.; Vanetti, C.; Decimo, I.; Di Chio, M.; Martano, G.; Garrone, G.; Bifari, F.; Vicentini, L.M. Sex-specific eNOS activity and function in human endothelial cells. Sci. Rep. 2017, 7, 9612. [Google Scholar] [CrossRef]
- Su, K.H.; Tsai, J.Y.; Kou, Y.R.; Chiang, A.N.; Hsiao, S.H.; Wu, Y.L.; Hou, H.H.; Pan, C.C.; Shyue, S.K.; Lee, T.S. Valsartan regulates the interaction of angiotensin II type 1 receptor and endothelial nitric oxide synthase via Src/PI3K/Akt signalling. Cardiovasc. Res. 2009, 82, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yuhanna, I.S.; Galcheva-Gargova, Z.; Karas, R.H.; Mendelsohn, M.E.; Shaul, P.W. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J. Clin. Investig. 1999, 103, 401–406. [Google Scholar] [CrossRef]
- Fleming, I.; Fisslthaler, B.; Busse, R. Calcium signaling in endothelial cells involves activation of tyrosine kinases and leads to activation of mitogen-activated protein kinases. Circ. Res. 1995, 76, 522–529. [Google Scholar] [CrossRef]
- Takahashi, S.; Mendelsohn, M.E. Calmodulin-dependent and -independent activation of endothelial nitric-oxide synthase by heat shock protein 90. J. Biol. Chem. 2003, 278, 9339–9344. [Google Scholar] [CrossRef]
- Corda, S.; Laplace, C.; Vicaut, E.; Duranteau, J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am. J. Respir. Cell Mol. Biol. 2001, 24, 762–768. [Google Scholar] [CrossRef]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef]
- Jaiswal, A.K. Regulation of genes encoding NAD(P)H:quinone oxidoreductases. Free Radic. Biol. Med. 2000, 29, 254–262. [Google Scholar] [CrossRef]
- Siegel, D.; Kepa, J.K.; Ross, D. NAD(P)H:quinone oxidoreductase 1 (NQO1) localizes to the mitotic spindle in human cells. PLoS ONE 2012, 7, e44861. [Google Scholar] [CrossRef] [PubMed]
- Aaberg, M.L.; Burch, D.M.; Hud, Z.R.; Zacharias, M.P. Gender differences in the onset of diabetic neuropathy. J. Diabetes Complicat. 2008, 22, 83–87. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Xu, M.; Chen, Z.J. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene 2007, 26, 3214–3226. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kim, S.; Egashira, K.; Takeya, M.; Ikeda, T.; Mimura, O.; Iwao, H. Molecular mechanism and role of endothelial monocyte chemoattractant protein-1 induction by vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Whitmarsh, A.J.; Davis, R.J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 1996, 74, 589–607. [Google Scholar] [CrossRef]
- Herrera, M.; Sparks, M.A.; Alfonso-Pecchio, A.R.; Harrison-Bernard, L.M.; Coffman, T.M. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension 2013, 61, 253–258. [Google Scholar] [CrossRef]
- Hafko, R.; Villapol, S.; Nostramo, R.; Symes, A.; Sabban, E.L.; Inagami, T.; Saavedra, J.M. Commercially available angiotensin II At(2) receptor antibodies are nonspecific. PLoS ONE 2013, 8, e69234. [Google Scholar] [CrossRef]
- Burghi, V.; Fernandez, N.C.; Gandola, Y.B.; Piazza, V.G.; Quiroga, D.T.; Guilhen Mario, E.; Felix Braga, J.; Bader, M.; Santos, R.A.S.; Dominici, F.P.; et al. Validation of commercial Mas receptor antibodies for utilization in Western Blotting, immunofluorescence and immunohistochemistry studies. PLoS ONE 2017, 12, e0183278. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najjar, R.S.; Wong, B.J.; Feresin, R.G. Tissue Derivation and Biological Sex Uniquely Mediate Endothelial Cell Protein Expression, Redox Status, and Nitric Oxide Synthesis. Cells 2023, 12, 93. https://doi.org/10.3390/cells12010093
Najjar RS, Wong BJ, Feresin RG. Tissue Derivation and Biological Sex Uniquely Mediate Endothelial Cell Protein Expression, Redox Status, and Nitric Oxide Synthesis. Cells. 2023; 12(1):93. https://doi.org/10.3390/cells12010093
Chicago/Turabian StyleNajjar, Rami S., Brett J. Wong, and Rafaela G. Feresin. 2023. "Tissue Derivation and Biological Sex Uniquely Mediate Endothelial Cell Protein Expression, Redox Status, and Nitric Oxide Synthesis" Cells 12, no. 1: 93. https://doi.org/10.3390/cells12010093
APA StyleNajjar, R. S., Wong, B. J., & Feresin, R. G. (2023). Tissue Derivation and Biological Sex Uniquely Mediate Endothelial Cell Protein Expression, Redox Status, and Nitric Oxide Synthesis. Cells, 12(1), 93. https://doi.org/10.3390/cells12010093







