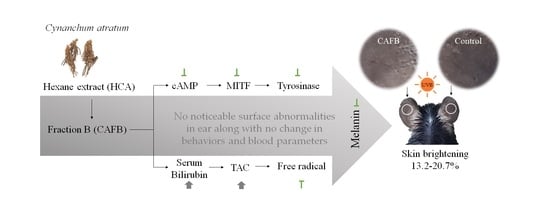
Anti-Melanogenic Effects of Fractioned Cynanchum atratum by Regulation of cAMP/MITF Pathway in a UVB-Stimulated Mice Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cynanchum Atratum Fraction
2.2. HPLC Fingerprinting Analysis
2.3. In Vitro Experiments
2.3.1. Cell Lines and Culture
2.3.2. Cell Viability Assay
2.3.3. Melanin Contents Determination in B16F10 Cell
2.3.4. Tyrosinase Activity Assay in B16F10 Cell
2.3.5. Cyclic Adenosine Monophosphate (cAMP) Assay in B16F10 Cell
2.3.6. Real-Time PCR in B16F10 Cell
2.3.7. Western Blot in B16F10 Cells
2.4. In Vivo Experiments
2.4.1. Experimental Animal Design
2.4.2. Serum Biochemistry Measurement
2.4.3. Ear Skin Histological Observation by Fontana–Masson Staining
2.4.4. The cAMP Assay in Ear Tissue
2.4.5. Western Blot in Ear Tissue
2.4.6. Total Antioxidant Capacity in Ear Tissue
2.5. Statistical Analysis
3. Results
3.1. CAFB Attenuated MSH-Stimulated Melanin in B16F10 Cells
3.2. CAFB Regulates Tyrosinase Activity and Related Signaling in B16F10 Cells
3.3. CAFB Ameliorated UVB-Stimulated Skin Hyperpigmentation in Mouse Ears
3.4. CAFB Inhibited the Melanin Synthesis via Downregulating the cAMP and MITF in Mouse Ears
3.5. Anti-Oxidative Effect of CAFB in Mouse Ears
3.6. CAFB Application Did Not Exhibit Discernible Negative Impacts on Skin and Body in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamaguchi, Y.; Hearing, V.J. Melanocytes and their diseases. Cold Spring Harb. Perspect. Med. 2014, 4, a017046. [Google Scholar] [CrossRef] [PubMed]
- Smit, N.; Vicanova, J.; Pavel, S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef] [PubMed]
- Tran-Ly, A.N.; Reyes, C.; Schwarze, F.W.M.R.; Ribera, J. Microbial production of melanin and its various applications. World J. Microbiol. Biotechnol. 2020, 36, 170. [Google Scholar] [CrossRef]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Kawakami, A.; Fisher, D.E. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab. Investig. 2017, 97, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Fang, Z.; Zhang, P. The melanin inhibitory effect of plants and phytochemicals: A systematic review. Phytomedicine 2022, 107, 154449. [Google Scholar] [CrossRef]
- Liu, J.K. Natural products in cosmetics. Nat. Prod. Bioprospect. 2022, 12, 40. [Google Scholar] [CrossRef]
- Pollock, S.; Taylor, S.; Oyerinde, O.; Nurmohamed, S.; Dlova, N.; Sarkar, R.; Galadari, H.; Manela-Azulay, M.; Chung, H.S.; Handog, E.; et al. The dark side of skin lightening: An international collaboration and review of a public health issue affecting dermatology. Int. J. Womens Dermatol. 2020, 7, 158–164. [Google Scholar] [CrossRef]
- Draelos, Z.D. Skin lightening preparations and the hydroquinone controversy. Dermatol. Ther. 2007, 20, 308–313. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, H.; Huang, J.; Jiang, L.; Chen, J.; Zeng, Q. Traditional Asian Herbs in Skin Whitening: The Current Development and Limitations. Front. Pharmacol. 2020, 11, 982. [Google Scholar] [CrossRef]
- Abdelhameed, R.F.A.; Ibrahim, A.K.; Elfaky, M.A.; Habib, E.S.; Mahamed, M.I.; Mehanna, E.T.; Darwish, K.M.; Khodeer, D.M.; Ahmed, S.A.; Elhady, S.S. Antioxidant and Anti-Inflammatory Activity of Cynanchum acutum L. Isolated Flavonoids Using Experimentally Induced Type 2 Diabetes Mellitus: Biological and In Silico Investigation for NF-κB Pathway/miR-146a Expression Modulation. Antioxidants 2021, 10, 1713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Hwang, S.-J.; Lim, D.-W.; Son, C.-G. Cynanchum atratum Alleviates Non-Alcoholic Fatty Liver by Balancing Lipogenesis and Fatty Acid Oxidation in a High-Fat, High-Fructose Diet Mice Model. Cells 2022, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Day, S.H.; Wang, J.P.; Won, S.J.; Lin, C.N. Bioactive constituents of the roots of Cynanchum atratum. J. Nat. Prod. 2001, 64, 608–611. [Google Scholar] [CrossRef]
- Son, C.-G.; Jang, E.-S.; Lee, S.-K.; Barng, K.-J. Consumer Perception and Evaluation of Cynanchi Atrati Radix Fermented Oriental Cosmetics for Skin Whitening. J. Fash. Bus. 2019, 23, 137–150. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Yan, C.; Li, J.; Zhang, P.; Liu, R.; He, J.; Chang, Y.-X. A review of the ethnopharmacology, phytochemistry and pharmacology of Cynanchum atratum. J. Ethnopharmacol. 2022, 284, 114748. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Han, X.H.; Yun, C.Y.; Lee, C.; Lee, J.W.; Lee, D.; Lee, M.K.; Jung, S.H.; Hong, J.T.; Kim, Y.; et al. Melanogenesis inhibitory pregnane glycosides from Cynanchum atratum. Bioorg. Med. Chem. Lett. 2018, 28, 1252–1256. [Google Scholar] [CrossRef]
- Chou, S.T.; Jung, F.; Yang, S.H.; Chou, H.L.; Jow, G.M.; Lin, J.C. Antofine suppresses endotoxin-induced inflammation and metabolic disorder via AMP-activated protein kinase. Pharmacol. Res. Perspect. 2017, 5, e00337. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Hwang, S.J.; Shin, K.S.; Lim, D.W.; Son, C.G. Bacillus subtilis-Fermented Amomum xanthioides Ameliorates Metabolic-Syndrome-Like Pathological Conditions in Long-Term HFHFD-Fed Mice. Antioxidants 2022, 11, 2254. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Lee, S.B.; Lee, D.S.; Son, C.G. Total Antioxidant Capacity in HBV Carriers, a Promising Biomarker for Evaluating Hepatic Fibrosis: A Pilot Study. Antioxidants 2021, 10, 77. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Maddodi, N.; Jayanthy, A.; Setaluri, V. Shining light on skin pigmentation: The darker and the brighter side of effects of UV radiation. Photochem. Photobiol. 2012, 88, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Weng, Q.Y.; Fisher, D.E. UV Signaling Pathways within the Skin. J. Investig. Dermatol. 2014, 134, 2080–2085. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.; Heidl, M.; Imfeld, D.; Meeus, L.; Schuetz, R.; Campiche, R. Discovery of a Highly Selective MC1R Agonists Pentapeptide to Be Used as a Skin Pigmentation Enhancer and with Potential Anti-Aging Properties. Int. J. Mol. Sci. 2019, 20, 6143. [Google Scholar] [CrossRef]
- Rzepka, Z.; Buszman, E.; Beberok, A.; Wrześniok, D. From tyrosine to melanin: Signaling pathways and factors regulating melanogenesis. Postep. Higr Med. Dosw. 2016, 70, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, M.; Barek, H. Critical Analysis of the Melanogenic Pathway in Insects and Higher Animals. Int. J. Mol. Sci. 2016, 17, 1753. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Urabe, K.; Winder, A.; Jiménez-Cervantes, C.; Imokawa, G.; Brewington, T.; Solano, F.; García-Borrón, J.C.; Hearing, V.J. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 1994, 13, 5818–5825. [Google Scholar] [CrossRef]
- Solano, F. On the Metal Cofactor in the Tyrosinase Family. Int. J. Mol. Sci. 2018, 19, 633. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment. Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef]
- Bang, J.; Zippin, J.H. Cyclic adenosine monophosphate (cAMP) signaling in melanocyte pigmentation and melanomagenesis. Pigment. Cell Melanoma Res. 2021, 34, 28–43. [Google Scholar] [CrossRef]
- Levy, C.; Khaled, M.; Fisher, D.E. MITF: Master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006, 12, 406–414. [Google Scholar] [CrossRef]
- Carlson, J.A.; Linette, G.P.; Aplin, A.; Ng, B.; Slominski, A. Melanocyte receptors: Clinical implications and therapeutic relevance. Dermatol. Clin. 2007, 25, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Tsuji, Y.; Setaluri, V. Selective down-regulation of tyrosinase family gene TYRP1 by inhibition of the activity of melanocyte transcription factor, MITF. Nucleic Acids Res. 2002, 30, 3096–3106. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Park, S.H.; Oh, S.W.; Yoo, J.A.; Kwon, K.; Park, S.J.; Kim, J.; Lee, H.S.; Cho, J.Y.; Lee, J. Beauvericin inhibits melanogenesis by regulating cAMP/PKA/CREB and LXR-α/p38 MAPK-mediated pathways. Sci. Rep. 2018, 8, 14958. [Google Scholar] [CrossRef] [PubMed]
- Buscà, R.; Abbe, P.; Mantoux, F.; Aberdam, E.; Peyssonnaux, C.; Eychène, A.; Ortonne, J.P.; Ballotti, R. Ras mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J. 2000, 19, 2900–2910. [Google Scholar] [CrossRef]
- Kim, T.; Hyun, C.G. Imperatorin Positively Regulates Melanogenesis through Signaling Pathways Involving PKA/CREB, ERK, AKT, and GSK3β/β-Catenin. Molecules 2022, 27, 6512. [Google Scholar] [CrossRef]
- Papaccio, F.; D Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, H.; Li, W.; Qiang, M.; Dong, T.; Li, H. Role of Vitamin C in Skin Diseases. Front. Physiol. 2018, 9, 819. [Google Scholar] [CrossRef]
- Xing, X.; Dan, Y.; Xu, Z.; Xiang, L. Implications of Oxidative Stress in the Pathogenesis and Treatment of Hyperpigmentation Disorders. Oxidative Med. Cell. Longev. 2022, 2022, 7881717. [Google Scholar] [CrossRef]
- Sedlak, T.W.; Saleh, M.; Higginson, D.S.; Paul, B.D.; Juluri, K.R.; Snyder, S.H. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc. Natl. Acad. Sci. USA 2009, 106, 5171–5176. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Coiffard, L. Overview of Skin Whitening Agents: Drugs and Cosmetic Products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, F.; Peng, Y.; Sun, L.; Chen, C. Nephrotic syndrome of minimal change disease following exposure to mercury-containing skin-lightening cream. Ann. Saudi Med. 2014, 34, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Shin, J.W.; Son, J.Y.; Cho, J.H.; Son, C.G. Antifibrotic effects of CGX, a traditional herbal formula, and its mechanisms in rats. J. Ethnopharmacol. 2010, 127, 534–542. [Google Scholar] [CrossRef]
- Sato, K.; Takei, M.; Iyota, R.; Muraoka, Y.; Nagashima, M.; Yoshimura, Y. Indomethacin inhibits melanogenesis via down-regulation of Mitf mRNA transcription. Biosci. Biotechnol. Biochem. 2017, 81, 2307–2313. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-H.; Hwang, S.-J.; Lee, S.-K.; Choi, Y.; Byun, C.K.; Son, C.-G. Anti-Melanogenic Effects of Fractioned Cynanchum atratum by Regulation of cAMP/MITF Pathway in a UVB-Stimulated Mice Model. Cells 2023, 12, 1390. https://doi.org/10.3390/cells12101390
Wang J-H, Hwang S-J, Lee S-K, Choi Y, Byun CK, Son C-G. Anti-Melanogenic Effects of Fractioned Cynanchum atratum by Regulation of cAMP/MITF Pathway in a UVB-Stimulated Mice Model. Cells. 2023; 12(10):1390. https://doi.org/10.3390/cells12101390
Chicago/Turabian StyleWang, Jing-Hua, Seung-Ju Hwang, Sam-Keun Lee, Yujin Choi, Chang Kyu Byun, and Chang-Gue Son. 2023. "Anti-Melanogenic Effects of Fractioned Cynanchum atratum by Regulation of cAMP/MITF Pathway in a UVB-Stimulated Mice Model" Cells 12, no. 10: 1390. https://doi.org/10.3390/cells12101390
APA StyleWang, J.-H., Hwang, S.-J., Lee, S.-K., Choi, Y., Byun, C. K., & Son, C.-G. (2023). Anti-Melanogenic Effects of Fractioned Cynanchum atratum by Regulation of cAMP/MITF Pathway in a UVB-Stimulated Mice Model. Cells, 12(10), 1390. https://doi.org/10.3390/cells12101390