APN Expression in Serum and Corpus Luteum: Regulation of Luteal Steroidogenesis Is Possibly Dependent on the AdipoR2/AMPK Pathway in Goats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Luteal Cell Isolation
2.3. Luteal Cell Culture
2.4. Western Blotting
2.5. Multiplex Immunohistochemistry/Immunocytochemistry
2.6. RNA Silencing
2.7. ELISA
2.8. RIA
2.9. Statistical Analyses
3. Results
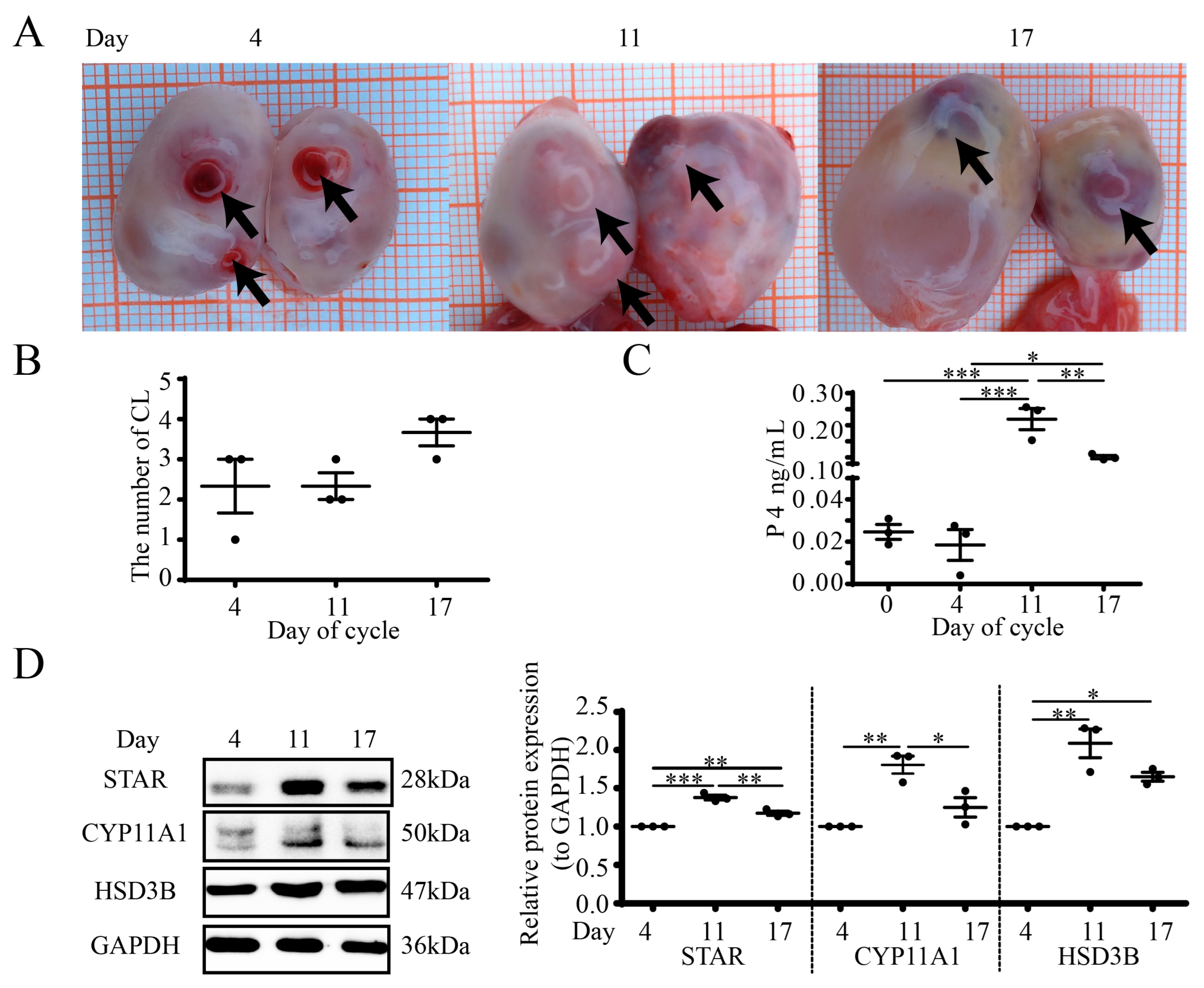
3.1. Luteal Morphology and Steroidogenesis during the Estrous Cycle in Goat Ovaries
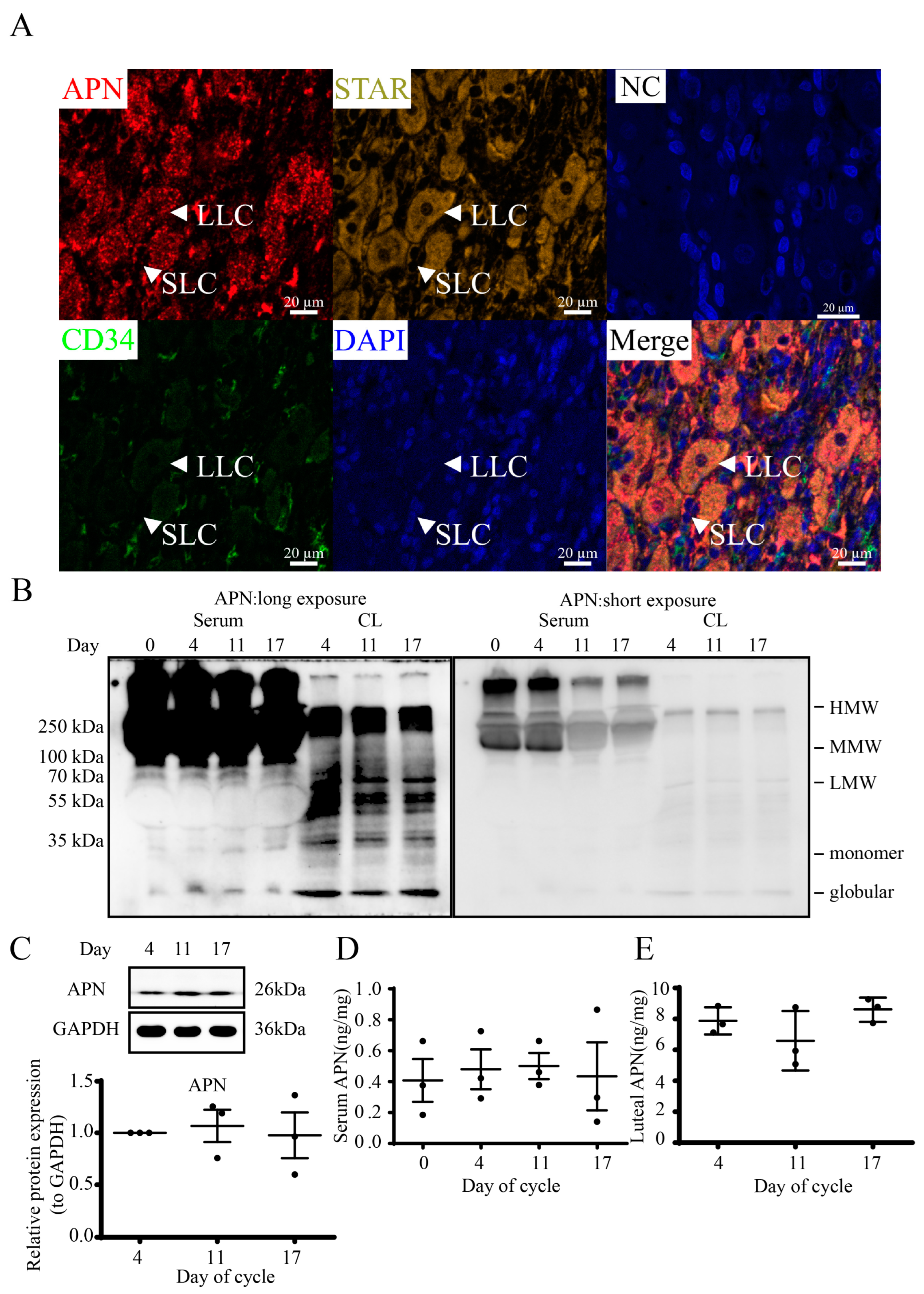
3.2. Localization and Structures of Luteal APN during the Estrous Cycle in Goats
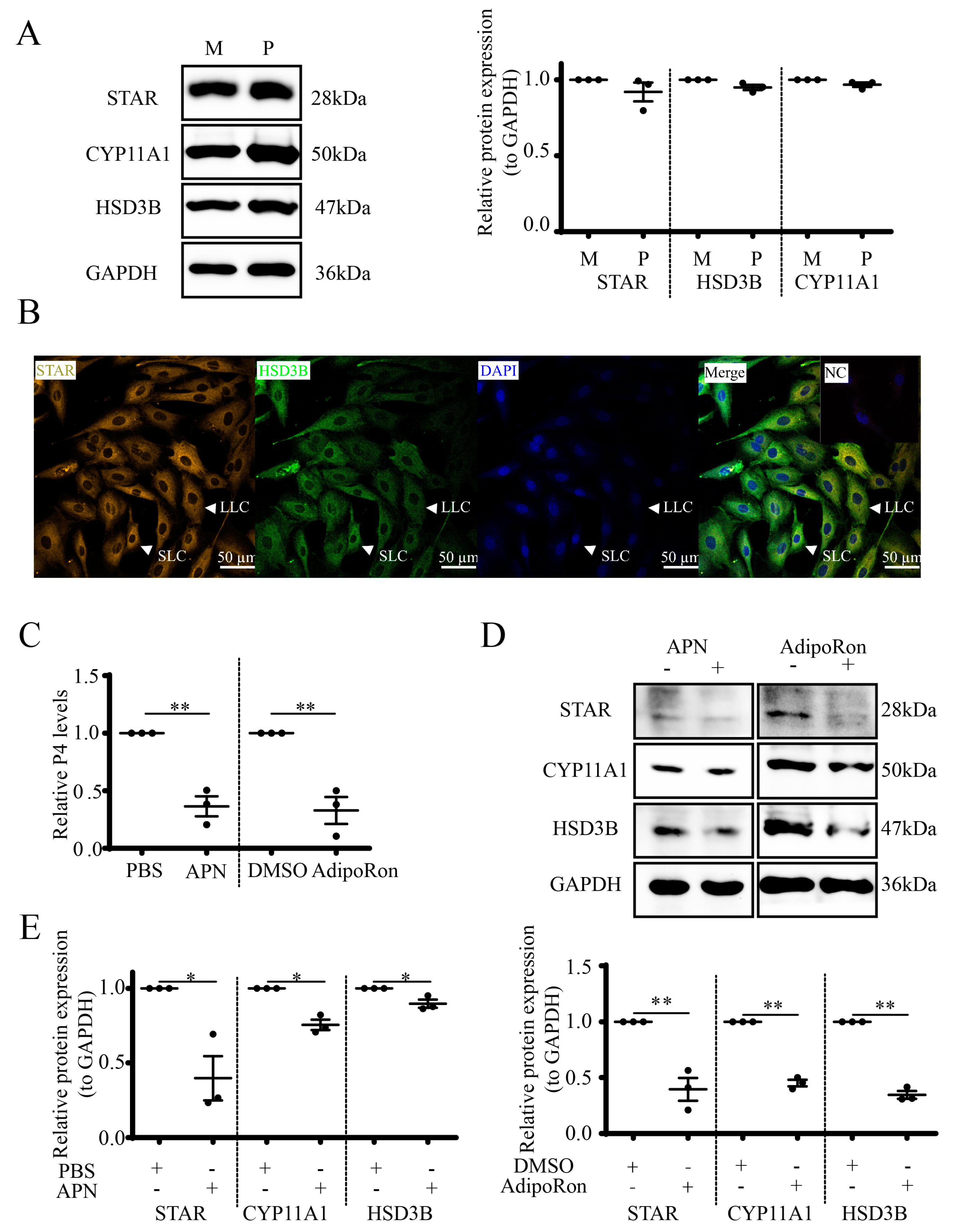
3.3. APN Inhibits P4 Synthesis and Steroidogenic Protein Expression in Goat Luteal Cells
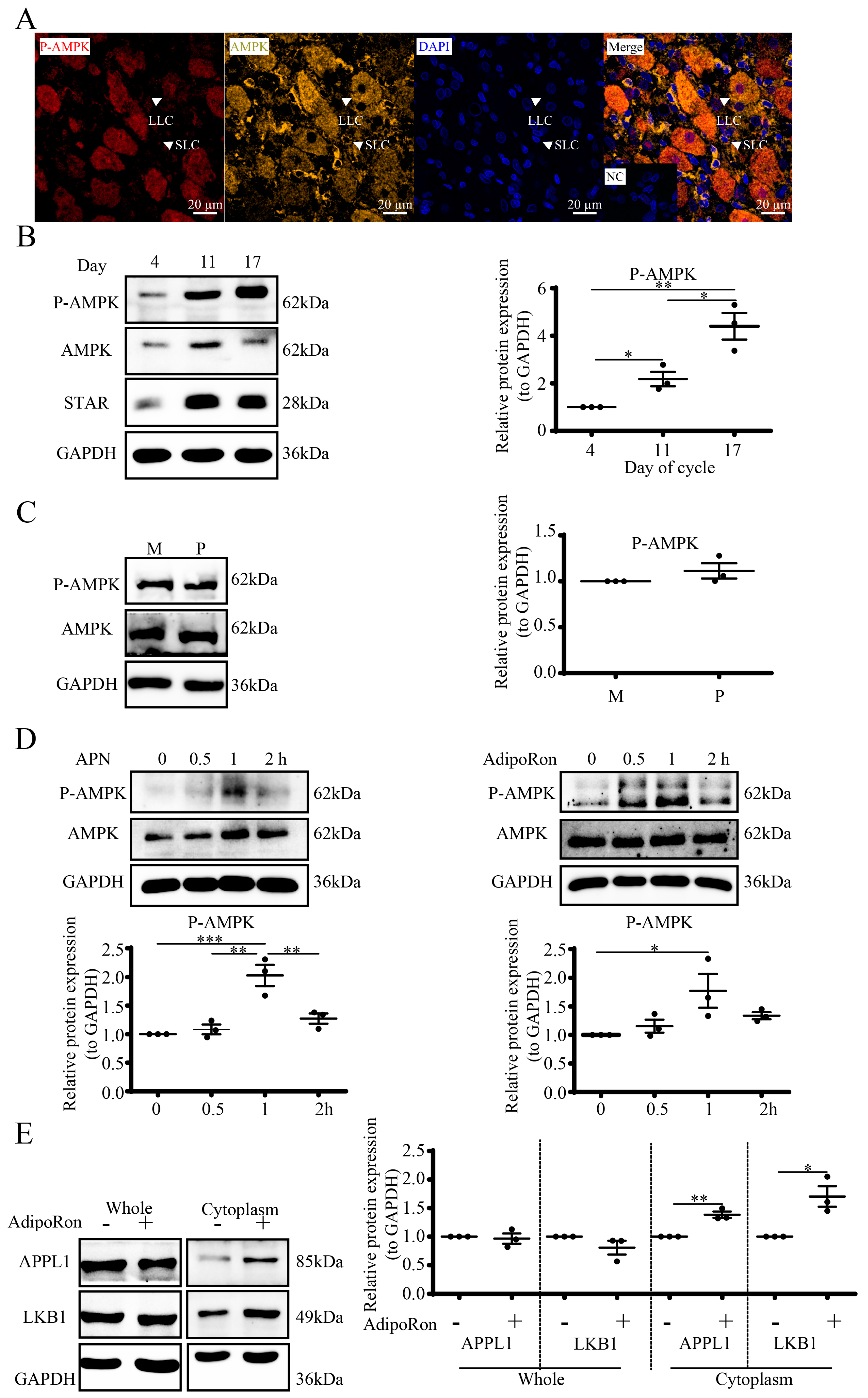
3.4. APN Activates the APPL1-LKB1/AMPK Pathway in Goat Luteal Cells
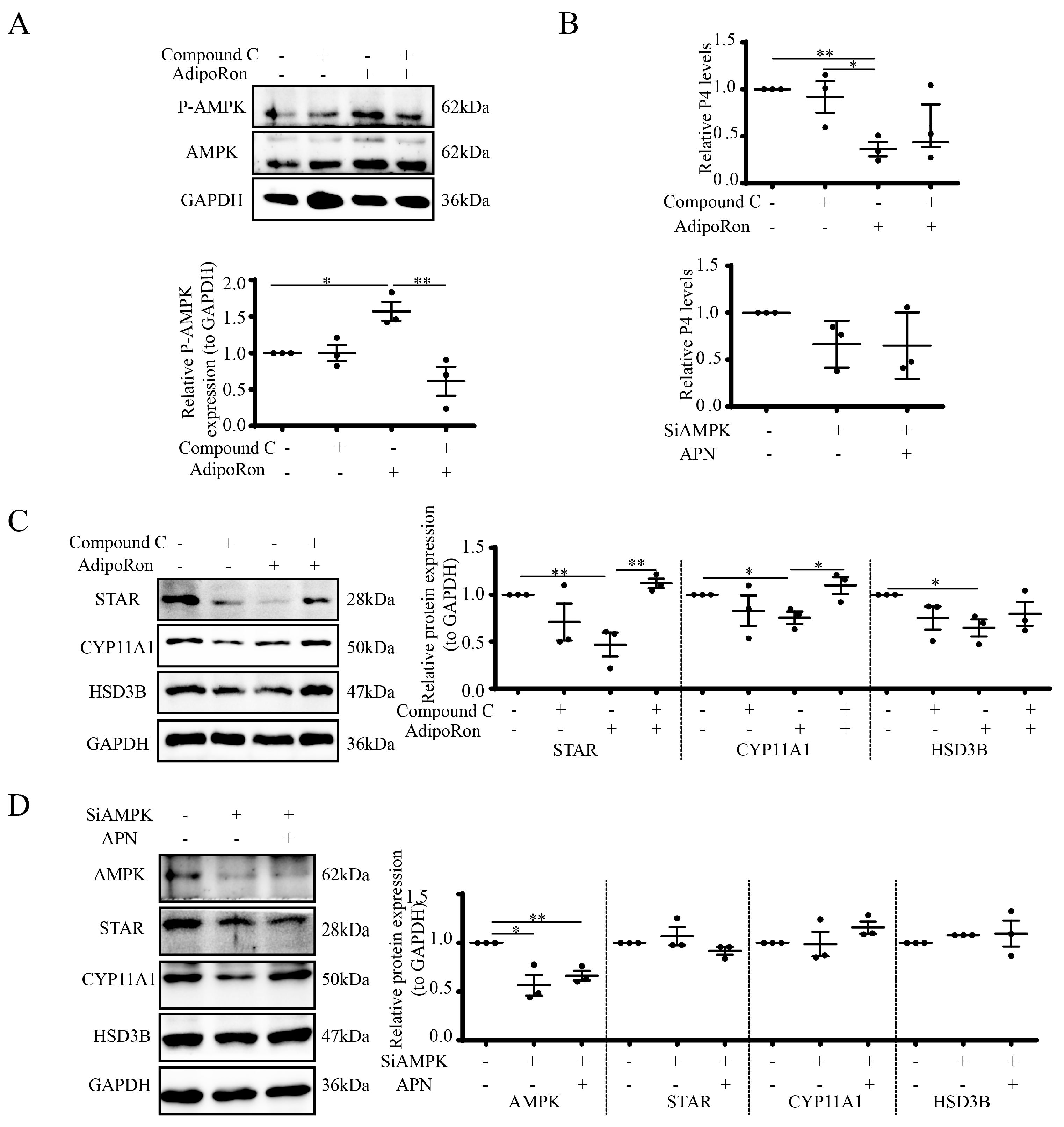
3.5. AdipoRon Regulates P4 by AMPK in Goat Luteal Cells
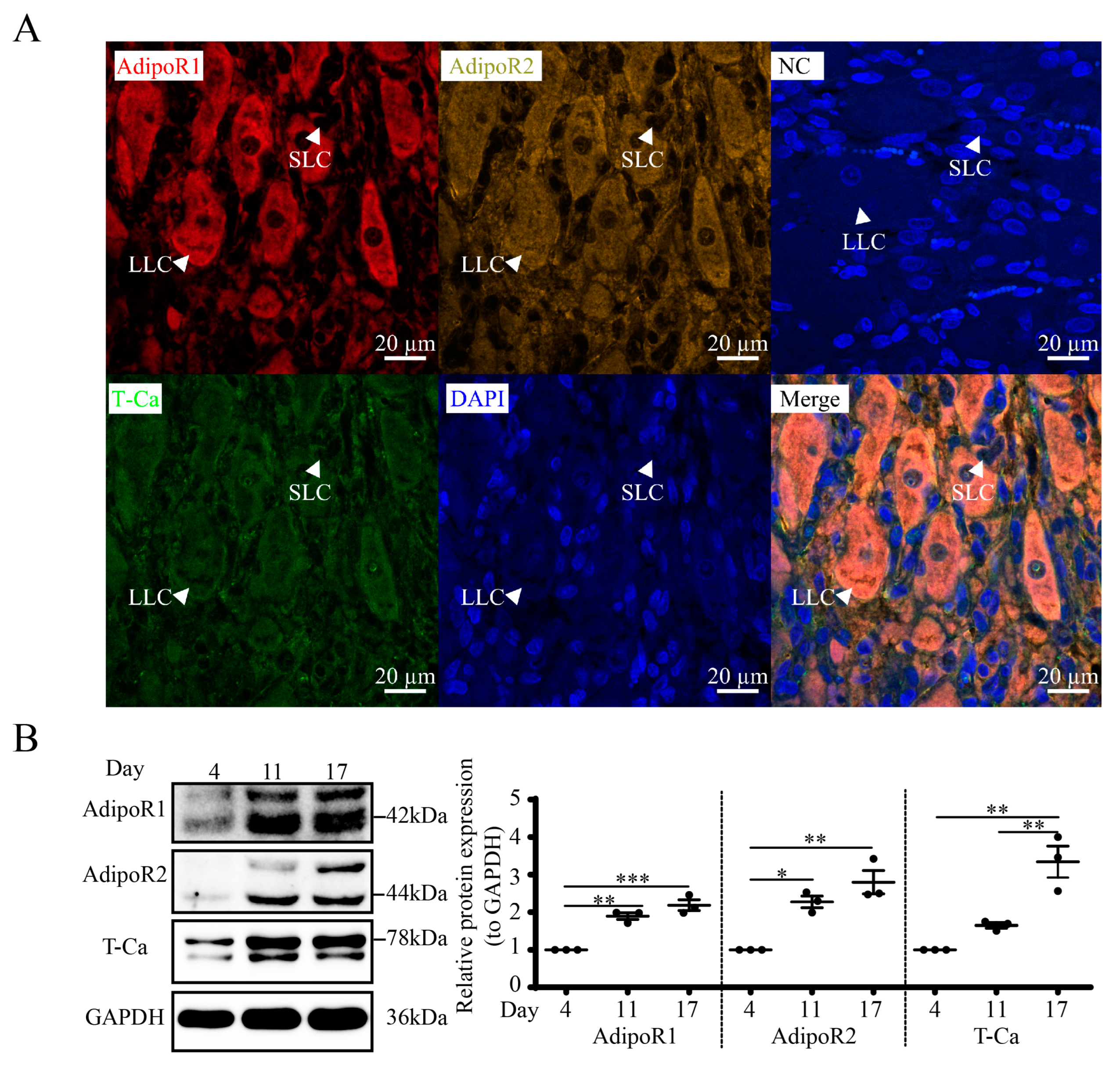
3.6. Expression and Localization of APN Receptors in Goat CLs
3.7. APN Increases P-AMPK through AdipoR2 in Goat Luteal Cells
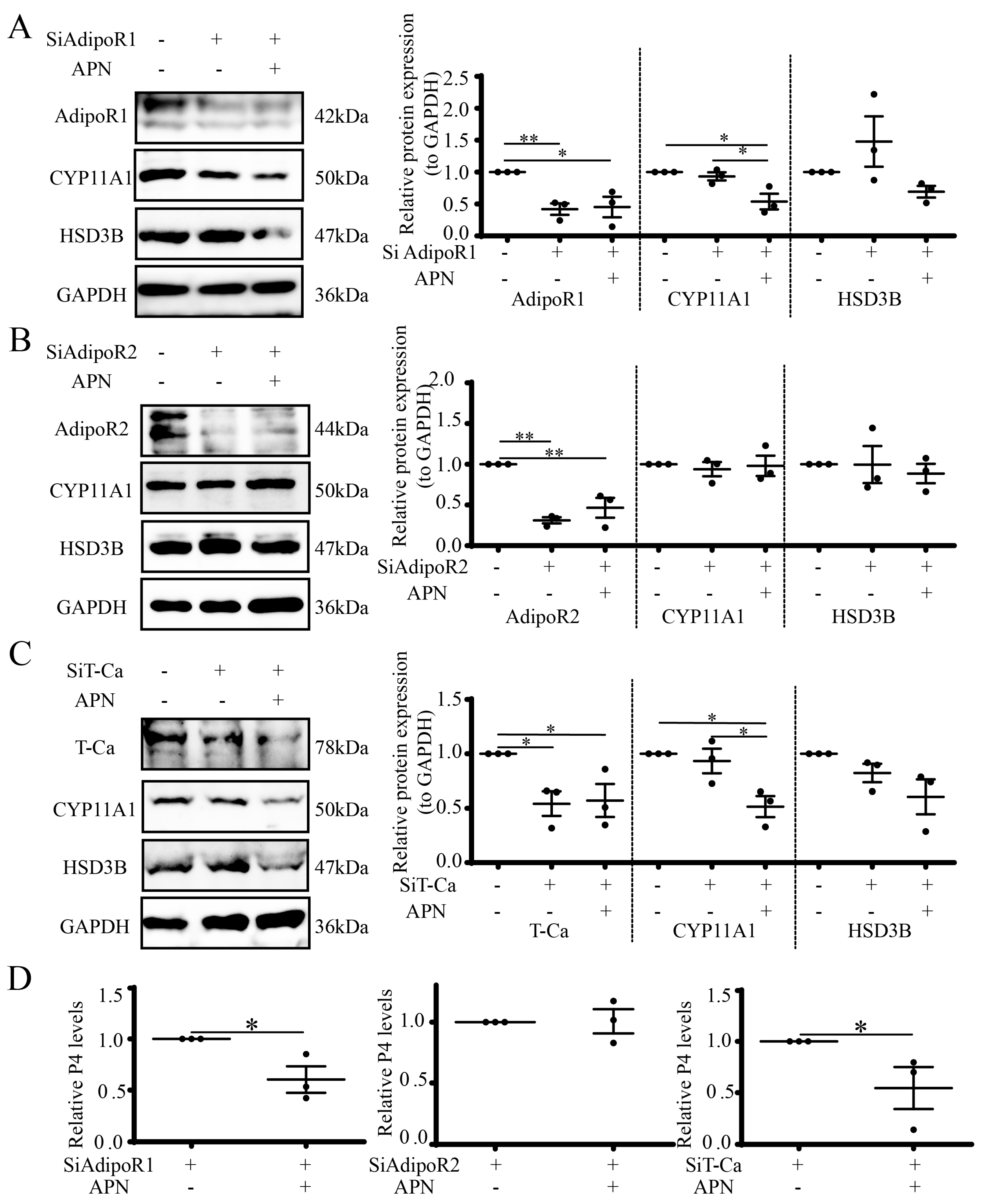
3.8. APN Inhibits Steroidogenesis through AdipoR2 in Goat Luteal Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Baddela, V.S.; Koczan, D.; Viergutz, T.; Vernunft, A.; Vanselow, J. Global gene expression analysis indicates that small luteal cells are involved in extracellular matrix modulation and immune cell recruitment in the bovine corpus luteum. Mol. Cell. Endocrinol. 2018, 474, 201–213. [Google Scholar] [CrossRef]
- Brylla, E.; Aust, G.; Geyer, M.; Uckermann, O.; Löffler, S.; Spanel-Borowski, K. Coexpression of preprotachykinin A and B transcripts in the bovine corpus luteum and evidence for functional neurokinin receptor activity in luteal endothelial cells and ovarian macrophages. Regul. Pept. 2005, 125, 125–133. [Google Scholar] [CrossRef]
- Basini, G.; Falasconi, I.; Bussolati, S.; Grolli, S.; Ramoni, R.; Grasselli, F. Isolation of endothelial cells and pericytes from swine corpus luteum. Domest. Anim. Endocrinol. 2014, 48, 100–109. [Google Scholar] [CrossRef]
- Yu, D.; Jiang, X.; Ge, W.; Qiao, B.; Zhang, D.; Liu, H.; Kuang, H. Gestational exposure to acrylamide suppresses luteal endocrine function through dysregulation of ovarian angiogenesis, oxidative stress and apoptosis in mice. Food Chem. Toxicol. 2022, 159, 112766. [Google Scholar] [CrossRef] [PubMed]
- Hünigen, H.; Bisplinghoff, P.; Plendl, J.; Bahramsoltani, M. Vascular dynamics in relation to immunolocalisation of VEGF-A, VEGFR-2 and Ang-2 in the bovine corpus luteum. Acta Histochem. 2008, 110, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Atli, M.O.; Akbalik, M.E.; Kose, M.; Alak, I.; Atli, Z.; Hitit, M. Expression pattern and cellular localization of two critical non-nuclear progesterone receptors in the ovine corpus luteum during the estrous cycle and early pregnancy. Anim. Reprod. Sci. 2022, 243, 107026. [Google Scholar] [CrossRef] [PubMed]
- Hitit, M.; Kose, M.; Kocak, N.; Atli, M.O. Expression patterns of genes in steroidogenic, cholesterol uptake, and liver x receptor-mediated cholesterol efflux pathway regulating cholesterol homeostasis in natural and PGF2α induced luteolysis as well as early pregnancy in ovine corpus luteum. Anim. Reprod. Sci. 2022, 240, 106988. [Google Scholar] [CrossRef]
- Silva, R.S.; Mattoso Miskulin Cardoso, A.P.; Giometti, I.C.; D’Aprile, L.; Garcia Santos, F.A.; Maruyama, A.S.; Medeiros de Carvalho Sousa, L.M.; Unniappan, S.; Kowalewski, M.P.; de Carvalho Papa, P. Insulin induces steroidogenesis in canine luteal cells via PI3K-MEK-MAPK. Mol. Cell. Endocrinol. 2022, 540, 111518. [Google Scholar] [CrossRef]
- Kiezun, M.; Dobrzyn, K.; Zaobidna, E.; Rytelewska, E.; Kisielewska, K.; Gudelska, M.; Orzechowska, K.; Kopij, G.; Szymanska, K.; Kaminska, B.; et al. Adiponectin affects uterine steroidogenesis during early pregnancy and the oestrous cycle: An in vitro study. Anim. Reprod. Sci. 2022, 245, 107067. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Hua, L.; Feng, B.; Jiang, X.; Li, J.; Jiang, D.; Huang, X.; Zhu, Y.; Li, Z.; Yan, L.; et al. Fibroblast growth factor 21 coordinates adiponectin to mediate the beneficial effects of low-protein diet on primordial follicle reserve. EBioMedicine 2019, 41, 623–635. [Google Scholar] [CrossRef]
- Oliveira, B.S.P.; Costa, J.A.S.; Gomes, E.T.; Silva, D.M.F.; Torres, S.M.; Monteiro, P.L.J., Jr.; Santos Filho, A.S.; Guerra, M.M.P.; Carneiro, G.F.; Wischral, A.; et al. Expression of adiponectin and its receptors (AdipoR1 and AdipoR2) in goat ovary and its effect on oocyte nuclear maturation in vitro. Theriogenology 2017, 104, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Thakre, A.; Bahiram, K.B.; Sardar, V.M.; Dudhe, S.D.; Korde, J.P.; Bonde, S.W.; Kurkure, N.V. Abundance of adiponectin mRNA transcript in the buffalo corpus luteum during the estrous cycle and effects on progesterone secretion in vitro. Anim. Reprod. Sci. 2019, 208, 106110. [Google Scholar] [CrossRef]
- Nowak, M.; Rehrauer, H.; Ay, S.S.; Findik, M.; Boos, A.; Kautz, E.; Kowalewski, M.P. Gene expression profiling of the canine placenta during normal and antigestagen-induced luteolysis. Gen. Comp. Endocrinol. 2019, 282, 113194. [Google Scholar] [CrossRef] [PubMed]
- Kafi, M.; Tamadon, A.; Saeb, M. The relationship between serum adiponectin and postpartum luteal activity in high-producing dairy cows. Theriogenology 2015, 83, 1264–1271. [Google Scholar] [CrossRef]
- Mohammed, M.H.; Al-Thuwaini, T.M.; Al-Shuhaib, M.J.J.o.t.S.S.o.A.S. High association of a novel variant in the adiponectin gene with the litter size in Awassi ewes. J. Saudi Soc. Agric. Sci. 2022, 21, 296–301. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, S.; Zhuang, H.; Fan, S.; Yang, J.; Zhao, L.; Bao, W.; Gao, F.; Zhang, H.; Yuan, Z.; et al. The role of the adiponectin system in acute fasting-impaired mouse ovaries. Reproduction 2019, 158, 429–440. [Google Scholar] [CrossRef]
- Wang, R.; Kuang, M.; Nie, H.; Bai, W.; Sun, L.; Wang, F.; Mao, D.; Wang, Z. Impact of Food Restriction on the Expression of the Adiponectin System and Genes in the Hypothalamic-Pituitary-Ovarian Axis of Pre-Pubertal Ewes. Reprod. Domest. Anim. 2016, 51, 657–664. [Google Scholar] [CrossRef]
- Waki, H.; Yamauchi, T.; Kamon, J.; Ito, Y.; Uchida, S.; Kita, S.; Hara, K.; Hada, Y.; Vasseur, F.; Froguel, P.; et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J. Biol. Chem. 2003, 278, 40352–40363. [Google Scholar] [CrossRef]
- Min, X.; Lemon, B.; Tang, J.; Liu, Q.; Zhang, R.; Walker, N.; Li, Y.; Wang, Z. Crystal structure of a single-chain trimer of human adiponectin globular domain. FEBS Lett. 2012, 586, 912–917. [Google Scholar] [CrossRef]
- Kubota, N.; Yano, W.; Kubota, T.; Yamauchi, T.; Itoh, S.; Kumagai, H.; Kozono, H.; Takamoto, I.; Okamoto, S.; Shiuchi, T.; et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007, 6, 55–68. [Google Scholar] [CrossRef]
- Tanabe, H.; Fujii, Y.; Okada-Iwabu, M.; Iwabu, M.; Nakamura, Y.; Hosaka, T.; Motoyama, K.; Ikeda, M.; Wakiyama, M.; Terada, T.; et al. Crystal structures of the human adiponectin receptors. Nature 2015, 520, 312–316. [Google Scholar] [CrossRef]
- Vasiliauskaité-Brooks, I.; Healey, R.D.; Rochaix, P.; Saint-Paul, J.; Sounier, R.; Grison, C.; Waltrich-Augusto, T.; Fortier, M.; Hoh, F.; Saied, E.M.; et al. Structure of a human intramembrane ceramidase explains enzymatic dysfunction found in leukodystrophy. Nat. Commun. 2018, 9, 5437. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, Y.; Maeda, N.; Matsuda, K.; Masuda, S.; Mori, T.; Fukuda, S.; Sekimoto, R.; Yamaoka, M.; Obata, Y.; Kita, S.; et al. Adiponectin association with T-cadherin protects against neointima proliferation and atherosclerosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Kita, S.; Tanaka, Y.; Fukuda, S.; Obata, Y.; Okita, T.; Nishida, H.; Takahashi, Y.; Kawachi, Y.; Tsugawa-Shimizu, Y.; et al. Adiponectin Stimulates Exosome Release to Enhance Mesenchymal Stem-Cell-Driven Therapy of Heart Failure in Mice. Mol. Ther. J. Am. Soc. Gene Ther. 2020, 28, 2203–2219. [Google Scholar] [CrossRef]
- Comim, F.V.; Hardy, K.; Franks, S. Adiponectin and its receptors in the ovary: Further evidence for a link between obesity and hyperandrogenism in polycystic ovary syndrome. PLoS ONE 2013, 8, e80416. [Google Scholar] [CrossRef] [PubMed]
- Rubina, K.A.; Semina, E.V.; Kalinina, N.I.; Sysoeva, V.Y.; Balatskiy, A.V.; Tkachuk, V.A. Revisiting the multiple roles of T-cadherin in health and disease. Eur. J. Cell Biol. 2021, 100, 151183. [Google Scholar] [CrossRef]
- Okita, T.; Kita, S.; Fukuda, S.; Fukuoka, K.; Kawada-Horitani, E.; Iioka, M.; Nakamura, Y.; Fujishima, Y.; Nishizawa, H.; Kawamori, D.; et al. Soluble T-cadherin promotes pancreatic β-cell proliferation by upregulating Notch signaling. iScience 2022, 25, 105404. [Google Scholar] [CrossRef]
- Denzel, M.S.; Scimia, M.C.; Zumstein, P.M.; Walsh, K.; Ruiz-Lozano, P.; Ranscht, B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J. Clin. Investig. 2010, 120, 4342–4352. [Google Scholar] [CrossRef]
- Rabinovitch, R.C.; Samborska, B.; Faubert, B.; Ma, E.H.; Gravel, S.P.; Andrzejewski, S.; Raissi, T.C.; Pause, A.; St-Pierre, J.; Jones, R.G. AMPK Maintains Cellular Metabolic Homeostasis through Regulation of Mitochondrial Reactive Oxygen Species. Cell Rep. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Che, J.; Ma, C.; Lu, J.; Chen, B.; Shi, Q.; Jin, X.; Song, R.; Xu, F.; Gan, L.; Li, J.; et al. Discovery of seneciobipyrrolidine derivatives for the amelioration of glucose homeostasis disorders through 4E-BP1/Akt/AMPK signaling activation. Eur. J. Med. Chem. 2022, 228, 113954. [Google Scholar] [CrossRef]
- Marzano, M.; Herzmann, S.; Elsbroek, L.; Sanal, N.; Tarbashevich, K.; Raz, E.; Krahn, M.P.; Rumpf, S. AMPK adapts metabolism to developmental energy requirement during dendrite pruning in Drosophila. Cell Rep. 2021, 37, 110024. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhang, L.; Hou, Y.; Zhong, J.; Hettinga, K.; Zhou, P. Phosphoproteomics reveals that camel and goat milk improve glucose homeostasis in HDF/STZ-induced diabetic rats through activation of hepatic AMPK and GSK3-GYS axis. Food Res. Int. 2022, 157, 111254. [Google Scholar] [CrossRef]
- Zhou, L.; Deepa, S.S.; Etzler, J.C.; Ryu, J.; Mao, X.; Fang, Q.; Liu, D.D.; Torres, J.M.; Jia, W.; Lechleiter, J.D.; et al. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J. Biol. Chem. 2009, 284, 22426–22435. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, J.; Chen, P.; Hao, Q.; Cheong, L.K.; Tang, M.; Hong, L.L.; Hu, X.Y.; Celestial, T.Y.; Bay, B.H.; et al. Oxidative stress-mediated AMPK inactivation determines the high susceptibility of LKB1-mutant NSCLC cells to glucose starvation. Free Radic. Biol. Med. 2021, 166, 128–139. [Google Scholar] [CrossRef]
- Sheng, Z.; Xu, J.; Li, F.; Yuan, Y.; Peng, X.; Chen, S.; Zhou, R.; Huang, W. The RING-domain E3 ubiquitin ligase RNF146 promotes cardiac hypertrophy by suppressing the LKB1/AMPK signaling pathway. Exp. Cell Res. 2022, 410, 112954. [Google Scholar] [CrossRef]
- Li, G.H.; Lin, X.L.; Zhang, H.; Li, S.; He, X.L.; Zhang, K.; Peng, J.; Tang, Y.L.; Zeng, J.F.; Zhao, Y.; et al. Ox-Lp(a) transiently induces HUVEC autophagy via an ROS-dependent PAPR-1-LKB1-AMPK-mTOR pathway. Atherosclerosis 2015, 243, 223–235. [Google Scholar] [CrossRef]
- Yan, Y.; Du, C.; Li, G.; Chen, L.; Yan, Y.; Chen, G.; Hu, W.; Chang, L. CO suppresses prostate cancer cell growth by directly targeting LKB1/AMPK/mTOR pathway in vitro and in vivo. Urol. Oncol. 2018, 36, 312.e1–312.e8. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.K.; Sharma, A.; Singh, N.; Kakkar, P. Activation of autophagic flux via LKB1/AMPK/mTOR axis against xenoestrogen Bisphenol-A exposure in primary rat hepatocytes. Food Chem. Toxicol. 2020, 141, 111314. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Arvisais, E.W.; Davis, J.S. Luteinizing hormone stimulates mammalian target of rapamycin signaling in bovine luteal cells via pathways independent of AKT and mitogen-activated protein kinase: Modulation of glycogen synthase kinase 3 and AMP-activated protein kinase. Endocrinology 2010, 151, 2846–2857. [Google Scholar] [CrossRef]
- Tosca, L.; Chabrolle, C.; Uzbekova, S.; Dupont, J. Effects of metformin on bovine granulosa cells steroidogenesis: Possible involvement of adenosine 5’ monophosphate-activated protein kinase (AMPK). Biol. Reprod. 2007, 76, 368–378. [Google Scholar] [CrossRef]
- Bowdridge, E.C.; Goravanahally, M.P.; Inskeep, E.K.; Flores, J.A. Activation of Adenosine Monophosphate-Activated Protein Kinase Is an Additional Mechanism That Participates in Mediating Inhibitory Actions of Prostaglandin F2Alpha in Mature, but Not Developing, Bovine Corpora Lutea. Biol. Reprod. 2015, 93, 7. [Google Scholar] [CrossRef] [PubMed]
- Mihanfar, A.; Nouri, M.; Roshangar, L.; Khadem-Ansari, M.H. Therapeutic potential of quercetin in an animal model of PCOS: Possible involvement of AMPK/SIRT-1 axis. Eur. J. Pharmacol. 2021, 900, 174062. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huang, X.J.; Yang, C.H.; Deng, S.S.; Qian, M.; Zang, Y.; Li, J. 5′-AMP-activated protein kinase (AMPK) regulates progesterone receptor transcriptional activity in breast cancer cells. Biochem. Biophys. Res. Commun. 2011, 416, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Tosca, L.; Chabrolle, C.; Crochet, S.; Tesseraud, S.; Dupont, J. IGF-1 receptor signaling pathways and effects of AMPK activation on IGF-1-induced progesterone secretion in hen granulosa cells. Domest. Anim. Endocrinol. 2008, 34, 204–216. [Google Scholar] [CrossRef]
- Arosh, J.A.; Parent, J.; Chapdelaine, P.; Sirois, J.; Fortier, M.A. Expression of cyclooxygenases 1 and 2 and prostaglandin E synthase in bovine endometrial tissue during the estrous cycle. Biol. Reprod. 2002, 67, 161–169. [Google Scholar] [CrossRef]
- Jin, H.Z.; Wang, Y.; Liu, Y.; Li, X.P.; Jin, Y. Changes in cFLIP expression in the corpus luteum throughout the estrous cycle of Shibagoats. Genet. Mol. Res. GMR 2015, 14, 12955–12966. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.M.; Chiu, H.Y.; Chang, C.S.; Chien, Y.Y.; Jong, D.S.; Wu, L.S.; Chiu, C.H. Role of kisspeptin on cell proliferation and steroidogenesis in luteal cells in vitro and in vivo. J. Chin. Med. Assoc. 2021, 84, 389–399. [Google Scholar] [CrossRef]
- Sivachelvan, M.N.; Ali, M.G.; Chibuzo, G.A.J.S.R.R. Foetal age estimation in sheep and goats. Small Rumin. Res. 1996, 19, 69–76. [Google Scholar] [CrossRef]
- Wen, X.; Liu, L.; Li, S.; Lin, P.; Chen, H.; Zhou, D.; Tang, K.; Wang, A.; Jin, Y. Prostaglandin F2alpha Induces Goat Corpus Luteum Regression via Endoplasmic Reticulum Stress and Autophagy. Front. Physiol. 2020, 11, 868. [Google Scholar] [CrossRef]
- Mao, D.; Hou, X.; Talbott, H.; Cushman, R.; Cupp, A.; Davis, J.S. ATF3 expression in the corpus luteum: Possible role in luteal regression. Mol. Endocrinol. 2013, 27, 2066–2079. [Google Scholar] [CrossRef]
- Grandhaye, J.; Hmadeh, S.; Plotton, I.; Levasseur, F.; Estienne, A.; LeGuevel, R.; Levern, Y.; Rame, C.; Jeanpierre, E.; Guerif, F.; et al. The adiponectin agonist, AdipoRon, inhibits steroidogenesis and cell proliferation in human luteinized granulosa cells. Mol. Cell. Endocrinol. 2021, 520, 111080. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Wang, J.G.; Liu, C.L.; Yan, H.J. AdipoRon improves cognitive dysfunction of Alzheimer’s disease and rescues impaired neural stem cell proliferation through AdipoR1/AMPK pathway. Exp. Neurol. 2020, 327, 113249. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ng, T.S.C.; Wang, S.J.; Prytyskach, M.; Rodell, C.B.; Mikula, H.; Kohler, R.H.; Garlin, M.A.; Lauffenburger, D.A.; Parangi, S.; et al. Therapeutically reprogrammed nutrient signalling enhances nanoparticulate albumin bound drug uptake and efficacy in KRAS-mutant cancer. Nat. Nanotechnol. 2021, 16, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Sambrock, J.; Russel, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory: Laurel Hollow, NY, USA, 2001. [Google Scholar]
- Pajvani, U.B.; Du, X.; Combs, T.P.; Berg, A.H.; Rajala, M.W.; Schulthess, T.; Engel, J.; Brownlee, M.; Scherer, P.E. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J. Biol. Chem. 2003, 278, 9073–9085. [Google Scholar] [CrossRef]
- Wright, J.H.; Huang, L.Y.; Weaver, S.; Archila, L.D.; McAfee, M.S.; Hirayama, A.V.; Chapuis, A.G.; Bleakley, M.; Rongvaux, A.; Turtle, C.J.; et al. Detection of engineered T cells in FFPE tissue by multiplex in situ hybridization and immunohistochemistry. J. Immunol. Methods 2021, 492, 112955. [Google Scholar] [CrossRef]
- Fang, Y.; Braley-Mullen, H. Cultured murine thyroid epithelial cells expressing transgenic Fas-associated death domain-like interleukin-1beta converting enzyme inhibitory protein are protected from fas-mediated apoptosis. Endocrinology 2008, 149, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Choi, S.M.; Kim, B.C. Adiponectin Regulates the Polarization and Function of Microglia via PPAR-γ Signaling Under Amyloid β Toxicity. Front. Cell. Neurosci. 2017, 11, 64. [Google Scholar] [CrossRef]
- Cawthorn, W.P.; Scheller, E.L.; Learman, B.S.; Parlee, S.D.; Simon, B.R.; Mori, H.; Ning, X.; Bree, A.J.; Schell, B.; Broome, D.T.; et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014, 20, 368–375. [Google Scholar] [CrossRef]
- Chedid, P.; Hurtado-Nedelec, M.; Marion-Gaber, B.; Bournier, O.; Hayem, G.; Gougerot-Pocidalo, M.A.; Frystyk, J.; Flyvbjerg, A.; El Benna, J.; Marie, J.C. Adiponectin and its globular fragment differentially modulate the oxidative burst of primary human phagocytes. Am. J. Pathol. 2012, 180, 682–692. [Google Scholar] [CrossRef]
- Obata, Y.; Kita, S.; Koyama, Y.; Fukuda, S.; Takeda, H.; Takahashi, M.; Fujishima, Y.; Nagao, H.; Masuda, S.; Tanaka, Y.; et al. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight 2018, 3, e99680. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef]
- Maleszka, A.; Smolinska, N.; Nitkiewicz, A.; Kiezun, M.; Dobrzyn, K.; Czerwinska, J.; Szeszko, K.; Kaminski, T. Expression of adiponectin receptors 1 and 2 in the ovary and concentration of plasma adiponectin during the oestrous cycle of the pig. Acta Vet. Hung. 2014, 62, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Takahashi, N.; Hileman, S.M.; Patel, H.R.; Berg, A.H.; Pajvani, U.B.; Scherer, P.E.; Ahima, R.S. Adiponectin acts in the brain to decrease body weight. Nat. Med. 2004, 10, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, C.; Sun, Q.; van Dam, R.M.; Meigs, J.B.; Zhang, C.; Tworoger, S.S.; Mantzoros, C.S.; Hu, F.B. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann. Intern. Med. 2008, 149, 307–316. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ouchi, N.; Kihara, S.; Walsh, K.; Kumada, M.; Abe, Y.; Funahashi, T.; Matsuzawa, Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ. Res. 2004, 94, e27–e31. [Google Scholar] [CrossRef]
- China, S.P.; Pal, S.; Chattopadhyay, S.; Porwal, K.; Kushwaha, S.; Bhattacharyya, S.; Mittal, M.; Gurjar, A.A.; Barbhuyan, T.; Singh, A.K.; et al. Globular adiponectin reverses osteo-sarcopenia and altered body composition in ovariectomized rats. Bone 2017, 105, 75–86. [Google Scholar] [CrossRef]
- Chatzidimitriou, K.; Gougoura, S.G.; Bargiota, A.; Koukoulis, G.N. Normal menstrual cycle steroid hormones variation does not affect the blood levels of total adiponectin and its multimer forms. J. Clin. Transl. Endocrinol. 2015, 2, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Hall, N.; White, C.; O’Sullivan, A.J. The relationship between adiponectin, progesterone, and temperature across the menstrual cycle. J. Endocrinol. Investig. 2009, 32, 279–283. [Google Scholar] [CrossRef]
- Chabrolle, C.; Tosca, L.; Dupont, J. Regulation of adiponectin and its receptors in rat ovary by human chorionic gonadotrophin treatment and potential involvement of adiponectin in granulosa cell steroidogenesis. Reproduction 2007, 133, 719–731. [Google Scholar] [CrossRef]
- Maillard, V.; Uzbekova, S.; Guignot, F.; Perreau, C.; Ramé, C.; Coyral-Castel, S.; Dupont, J. Effect of adiponectin on bovine granulosa cell steroidogenesis, oocyte maturation and embryo development. Reprod. Biol. Endocrinol. RBE 2010, 8, 23. [Google Scholar] [CrossRef]
- Gupta, M.; Dangi, S.S.; Chouhan, V.S.; Hyder, I.; Babitha, V.; Yadav, V.P.; Khan, F.A.; Sonwane, A.; Singh, G.; Das, G.K.; et al. Expression and localization of ghrelin and its functional receptor in corpus luteum during different stages of estrous cycle and the modulatory role of ghrelin on progesterone production in cultured luteal cells in buffalo. Domest. Anim. Endocrinol. 2014, 48, 21–32. [Google Scholar] [CrossRef]
- Carballo, M.; Pinto, L.; Brito, M.J.E. The role of adiponectin in ischemia-reperfusion syndrome: A literature review. Einstein 2020, 18, 51–60. [Google Scholar] [CrossRef]
- Singh, A.; Bora, P.; Krishna, A. Direct action of adiponectin ameliorates increased androgen synthesis and reduces insulin receptor expression in the polycystic ovary. Biochem. Biophys. Res. Commun. 2017, 488, 509–515. [Google Scholar] [CrossRef]
- Tabandeh, M.R.; Hosseini, A.; Saeb, M.; Kafi, M.; Saeb, S. Changes in the gene expression of adiponectin and adiponectin receptors (AdipoR1 and AdipoR2) in ovarian follicular cells of dairy cow at different stages of development. Theriogenology 2010, 73, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Joharapurkar, A.; Das, N.; Khatoon, S.; Kushwaha, S.; Gurjar, A.A.; Singh, A.K.; Shree, S.; Ahmed, M.Z.; China, S.P.; et al. Estradiol overcomes adiponectin-resistance in diabetic mice by regulating skeletal muscle adiponectin receptor 1 expression. Mol. Cell. Endocrinol. 2022, 540, 111525. [Google Scholar] [CrossRef]
- Sattar, A.A.; Sattar, R. Globular adiponectin activates Akt in cultured myocytes. Biochem. Biophys. Res. Commun. 2012, 424, 753–757. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, D.; Wang, B.; Zhang, Y.; Wang, L.; Chen, X.; Li, M.; Tang, Z.; Wang, C. APPL1-mediated activation of STAT3 contributes to inhibitory effect of adiponectin on hepatic gluconeogenesis. Mol. Cell. Endocrinol. 2016, 433, 12–19. [Google Scholar] [CrossRef]
- Abd El-Fatah, I.M.; Abdelrazek, H.M.A.; Ibrahim, S.M.; Abdallah, D.M.; El-Abhar, H.S. Dimethyl fumarate abridged tauo-/amyloidopathy in a D-Galactose/ovariectomy-induced Alzheimer’s-like disease: Modulation of AMPK/SIRT-1, AKT/CREB/BDNF, AKT/GSK-3β, adiponectin/Adipo1R, and NF-κB/IL-1β/ROS trajectories. Neurochem. Int. 2021, 148, 105082. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.T.; Zhao, B.; Yang, J. Enhanced muscle by myostatin propeptide increases adipose tissue adiponectin, PPAR-alpha, and PPAR-gamma expressions. Biochem. Biophys. Res. Commun. 2008, 369, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Lam, K.S.; Wang, Y.; Huang, Y.; Carling, D.; Wu, D.; Wong, C.; Xu, A. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes 2007, 56, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xin, X.; Xiang, R.; Ramos, F.J.; Liu, M.; Lee, H.J.; Chen, H.; Mao, X.; Kikani, C.K.; Liu, F.; et al. Yin-Yang regulation of adiponectin signaling by APPL isoforms in muscle cells. J. Biol. Chem. 2009, 284, 31608–31615. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Gao, Y.; Deng, C.; Wan, Y.; Zhuang, Y.; Hu, X.; Wang, Y. Therapeutic effects of AdipoRon on liver inflammation and fibrosis induced by CCl(4) in mice. Int. Immunopharmacol. 2020, 79, 106157. [Google Scholar] [CrossRef]
- Fairaq, A.; Shawky, N.M.; Osman, I.; Pichavaram, P.; Segar, L. AdipoRon, an adiponectin receptor agonist, attenuates PDGF-induced VSMC proliferation through inhibition of mTOR signaling independent of AMPK: Implications toward suppression of neointimal hyperplasia. Pharmacol. Res. 2017, 119, 289–302. [Google Scholar] [CrossRef]
- Widjaja, A.A.; Viswanathan, S.; Wei Ting, J.G.; Tan, J.; Shekeran, S.G.; Carling, D.; Lim, W.W.; Cook, S.A. IL11 stimulates ERK/P90RSK to inhibit LKB1/AMPK and activate mTOR initiating a mesenchymal program in stromal, epithelial, and cancer cells. iScience 2022, 25, 104806. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hudson, E.; Chi, M.M.; Chang, A.S.; Moley, K.H.; Hardie, D.G.; Downs, S.M. AMPK regulation of mouse oocyte meiotic resumption in vitro. Dev. Biol. 2006, 291, 227–238. [Google Scholar] [CrossRef]
- Ji, R.; Jia, F.Y.; Chen, X.; Wang, Z.H.; Jin, W.Y.; Yang, J. Salidroside alleviates oxidative stress and apoptosis via AMPK/Nrf2 pathway in DHT-induced human granulosa cell line KGN. Arch. Biochem. Biophys. 2022, 715, 109094. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Li, C.; Tao, J.; Liu, Z.; Li, X.; Zang, Z.; Fu, C.; Wei, J.; Yang, Y.; Zhu, Q.; et al. FSH mediates estradiol synthesis in hypoxic granulosa cells by activating glycolytic metabolism through the HIF-1alpha-AMPK-GLUT1 signaling pathway. J. Biol. Chem. 2022, 298, 101830. [Google Scholar] [CrossRef]
- De Silva, M.S.I.; Dayton, A.W.; Rhoten, L.R.; Mallett, J.W.; Reese, J.C.; Squires, M.D.; Dalley, A.P.; Porter, J.P.; Judd, A.M. Involvement of adenosine monophosphate activated kinase in interleukin-6 regulation of steroidogenic acute regulatory protein and cholesterol side chain cleavage enzyme in the bovine zona fasciculata and zona reticularis. Steroids 2018, 134, 53–66. [Google Scholar] [CrossRef]
- Fang, L.; Jia, Q.; Liu, B.; Yan, Y.; Han, X.; Guo, Y.; Cheng, J.C.; Sun, Y.P. BMP-9 downregulates StAR expression and progesterone production by activating both SMAD1/5/8 and SMAD2/3 signaling pathways in human granulosa-lutein cells obtained from gonadotropins induced ovarian cycles. Cell. Signal. 2021, 86, 110089. [Google Scholar] [CrossRef]
- Przygrodzka, E.; Hou, X.; Zhang, P.; Plewes, M.R.; Franco, R.; Davis, J.S. PKA and AMPK Signaling Pathways Differentially Regulate Luteal Steroidogenesis. Endocrinology 2021, 162, bqab015. [Google Scholar] [CrossRef]
- Huang, Y.; Xia, Q.; Cui, Y.; Qu, Q.; Jiang, Q.J.J.o.F.F. Resveratrol increase the proportion of oxidative muscle fiber through the AdipoR1-AMPK-PGC-1α pathway in pigs. J. Funct. Foods 2020, 73, 104090. [Google Scholar] [CrossRef]
- Nguyen, T.M.D. Adiponectin: Role in Physiology and Pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef]
- Sharma, A.; Mah, M.; Ritchie, R.H.; De Blasio, M.J. The adiponectin signalling pathway—A therapeutic target for the cardiac complications of type 2 diabetes? Pharmacol. Ther. 2022, 232, 108008. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhang, Y.; Doycheva, D.M.; Ding, Y.; Zhang, Y.; Tang, J.; Guo, H.; Zhang, J.H. Adiponectin attenuates neuronal apoptosis induced by hypoxia-ischemia via the activation of AdipoR1/APPL1/LKB1/AMPK pathway in neonatal rats. Neuropharmacology 2018, 133, 415–428. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, Z.; Liang, F.; Xu, W.; Lu, J.; Shi, L.; Shao, A.; Yu, J.; Zhang, J. AdipoRon Attenuates Neuroinflammation After Intracerebral Hemorrhage Through AdipoR1-AMPK Pathway. Neuroscience 2019, 412, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.O.; Lee, H.I.; Ham, J.R.; Seo, K.I.; Lee, M.K. Long-term supplementation of esculetin ameliorates hepatosteatosis and insulin resistance partly by activating AdipoR2–AMPK pathway in diet-induced obese mice. J. Funct. Foods 2015, 15, 160–171. [Google Scholar] [CrossRef]
- Ning, Z.; Gu, P.; Zhang, J.; Cheung, C.W.; Lao, L.; Chen, H.; Zhang, Z.J. Adiponectin regulates electroacupuncture-produced analgesic effects in association with a crosstalk between the peripheral circulation and the spinal cord. Brain Behav. Immun. 2022, 99, 43–52. [Google Scholar] [CrossRef]
- Pascolutti, R.; Erlandson, S.C.; Burri, D.J.; Zheng, S.; Kruse, A.C. Mapping and engineering the interaction between adiponectin and T-cadherin. J. Biol. Chem. 2020, 295, 2749–2759. [Google Scholar] [CrossRef] [PubMed]
- Kyriakakis, E.; Frismantiene, A.; Dasen, B.; Pfaff, D.; Rivero, O.; Lesch, K.P.; Erne, P.; Resink, T.J.; Philippova, M. T-cadherin promotes autophagy and survival in vascular smooth muscle cells through MEK1/2/Erk1/2 axis activation. Cell. Signal. 2017, 35, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Kyriakakis, E.; Philippova, M.; Joshi, M.B.; Pfaff, D.; Bochkov, V.; Afonyushkin, T.; Erne, P.; Resink, T.J. T-cadherin attenuates the PERK branch of the unfolded protein response and protects vascular endothelial cells from endoplasmic reticulum stress-induced apoptosis. Cell. Signal. 2010, 22, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kita, S.; Nishizawa, H.; Fukuda, S.; Fujishima, Y.; Obata, Y.; Nagao, H.; Masuda, S.; Nakamura, Y.; Shimizu, Y.; et al. Adiponectin promotes muscle regeneration through binding to T-cadherin. Sci. Rep. 2019, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulos, C.G.; Spiroglou, S.G.; Varakis, J.N.; Apostolakis, E.; Papadaki, H.H. Adiponectin/T-cadherin and apelin/APJ expression in human arteries and periadventitial fat: Implication of local adipokine signaling in atherosclerosis? Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2014, 23, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Pierre, P.; Froment, P.; Nègre, D.; Ramé, C.; Barateau, V.; Chabrolle, C.; Lecomte, P.; Dupont, J.J.H.R. Role of adiponectin receptors, AdipoR1 and AdipoR2, in the steroidogenesis of the human granulosa tumor cell line, KGN. Hum. Reprod. 2009, 24, 2890–2901. [Google Scholar] [CrossRef] [PubMed]

| Antibodies | Cat No. | MFRS | Antigen Source | Host | Dilution |
|---|---|---|---|---|---|
| STAR | A1035 | ABclonal | Human | Rabbit | WB: 1:1000; mIHC: 1:100 |
| HSD3B | A1823 | ABclonal | Human | Rabbit | WB: 1:1000; mIHC: 1:100 |
| CYP11A1 | A1713 | ABclonal | Human | Rabbit | WB: 1:1000 |
| APN | A2543 | ABclonal | Human | Rabbit | WB: 1:1000; mIHC: 1:200 |
| T-Ca | A1761 | ABclonal | Human | Rabbit | WB: 1:1000; mIHC: 1:200 |
| AdipoR1 | A1509 | ABclonal | Human | Rabbit | WB: 1:2000; mIHC: 1:100 |
| AdipoR2 | A12777 | ABclonal | Human | Rabbit | WB: 1:2000; mIHC: 1:100 |
| APPL1 | A4606 | ABclonal | Human | Rabbit | WB: 1:2000 |
| AMPK | A7339 | ABclonal | Human | Rabbit | WB:1:2000; mIHC: 1:100 |
| P-AMPK | AP0116 | ABclonal | Human | Rabbit | WB:1:2000; mIHC: 1:100 |
| GAPDH | T0004 | Affinity | Human | Mouse | WB: 1:5000 |
| LKB1 | A2122 | ABclonal | Human | Rabbit | WB:1:2000 |
| HRP Anti-Mouse IgG | AS003 | Abclonal | Mouse | Goat | WB: 1:20,000 |
| HRP Anti-Rabbit IgG | AS014 | Abclonal | Rabbit | Goat | WB: 1:20,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, X.; Li, H.; Yu, H.; Wang, W.; Mao, D. APN Expression in Serum and Corpus Luteum: Regulation of Luteal Steroidogenesis Is Possibly Dependent on the AdipoR2/AMPK Pathway in Goats. Cells 2023, 12, 1393. https://doi.org/10.3390/cells12101393
Pei X, Li H, Yu H, Wang W, Mao D. APN Expression in Serum and Corpus Luteum: Regulation of Luteal Steroidogenesis Is Possibly Dependent on the AdipoR2/AMPK Pathway in Goats. Cells. 2023; 12(10):1393. https://doi.org/10.3390/cells12101393
Chicago/Turabian StylePei, Xiaomeng, Haolin Li, Hao Yu, Wei Wang, and Dagan Mao. 2023. "APN Expression in Serum and Corpus Luteum: Regulation of Luteal Steroidogenesis Is Possibly Dependent on the AdipoR2/AMPK Pathway in Goats" Cells 12, no. 10: 1393. https://doi.org/10.3390/cells12101393
APA StylePei, X., Li, H., Yu, H., Wang, W., & Mao, D. (2023). APN Expression in Serum and Corpus Luteum: Regulation of Luteal Steroidogenesis Is Possibly Dependent on the AdipoR2/AMPK Pathway in Goats. Cells, 12(10), 1393. https://doi.org/10.3390/cells12101393






