Optimizing THP-1 Macrophage Culture for an Immune-Responsive Human Intestinal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. THP-1 Monocyte Cultures
2.2. THP-1m Induction
2.3. THP-1m Culture
2.4. THP-1m Morphology and Confluency
2.5. THP-1m Detachment Rate
2.6. Crystal Violet Staining
2.7. LysoTracker and Nuclear Staining
2.8. Morphological Analysis
2.9. Culture of Intestinal Epithelial Cells
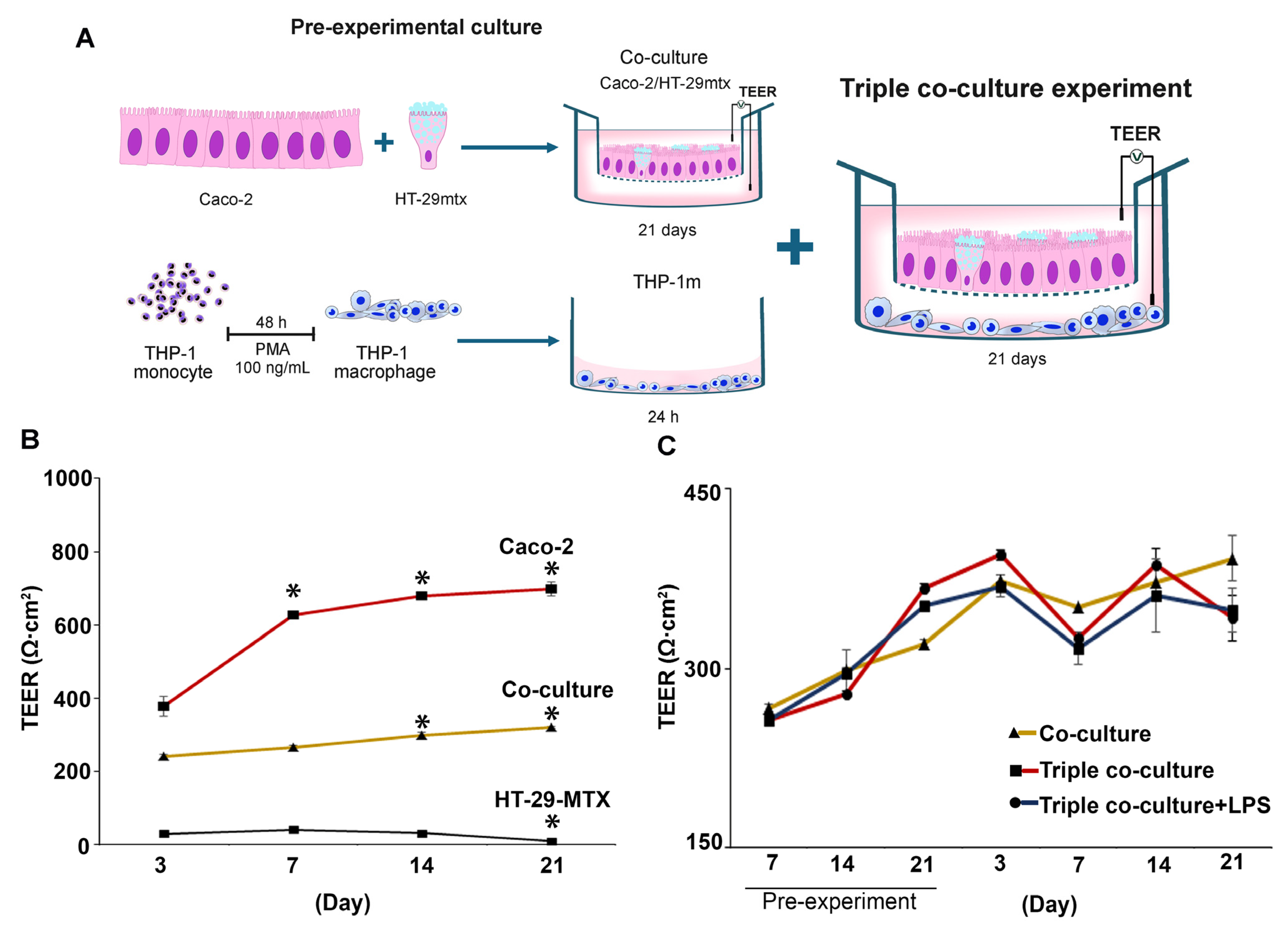
2.10. Triple Co-Culture of Caco-2, HT-29-MTX, and THP-1m Cells
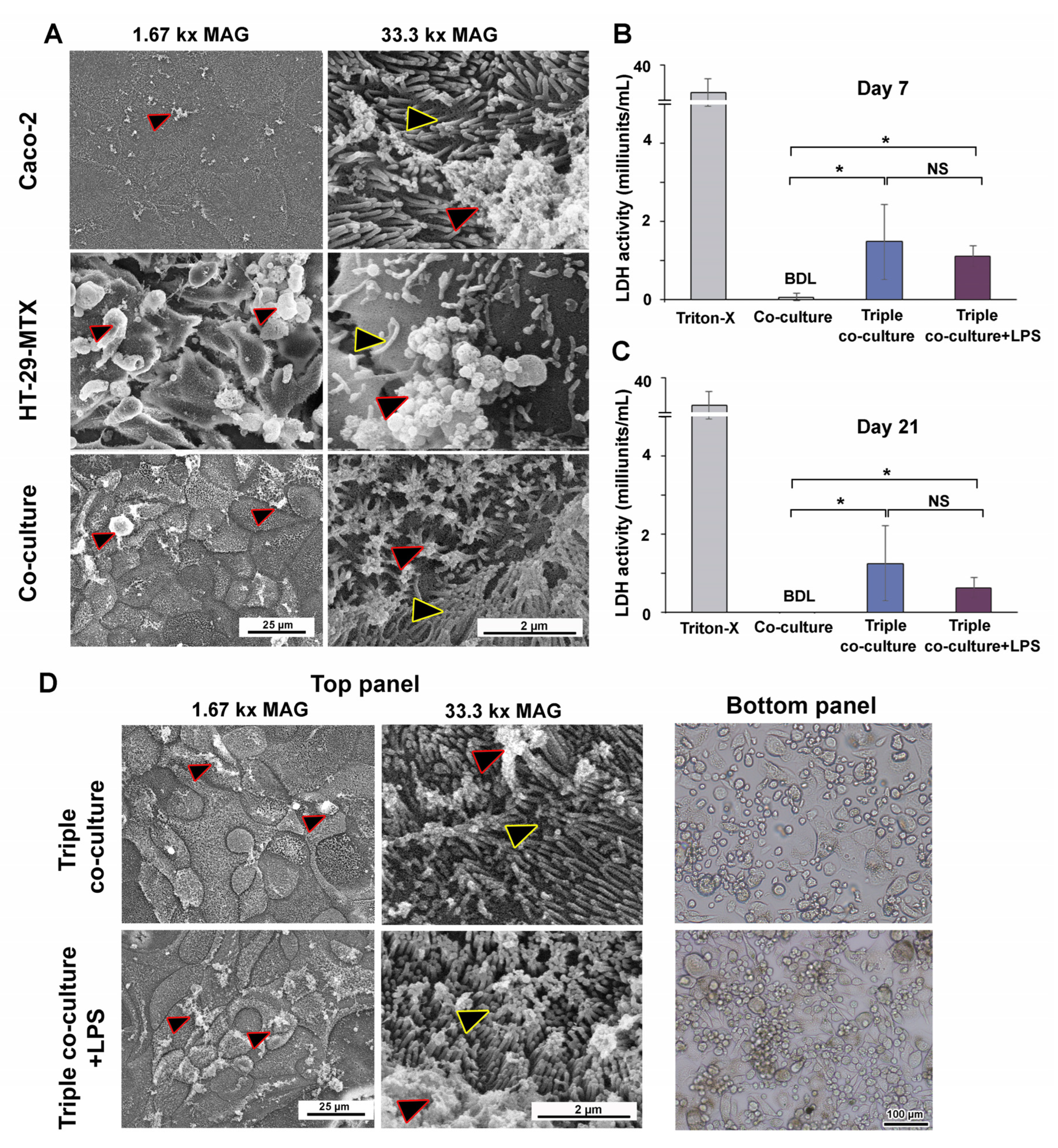
2.11. Scanning Electron Microscopy (SEM)
2.12. Transepithelial Electrical Resistance (TEER) Assay
2.13. Cytokine Secretion Measurement
2.14. Lactate Dehydrogenase (LDH) Activity
2.15. Statistical Analysis
3. Results
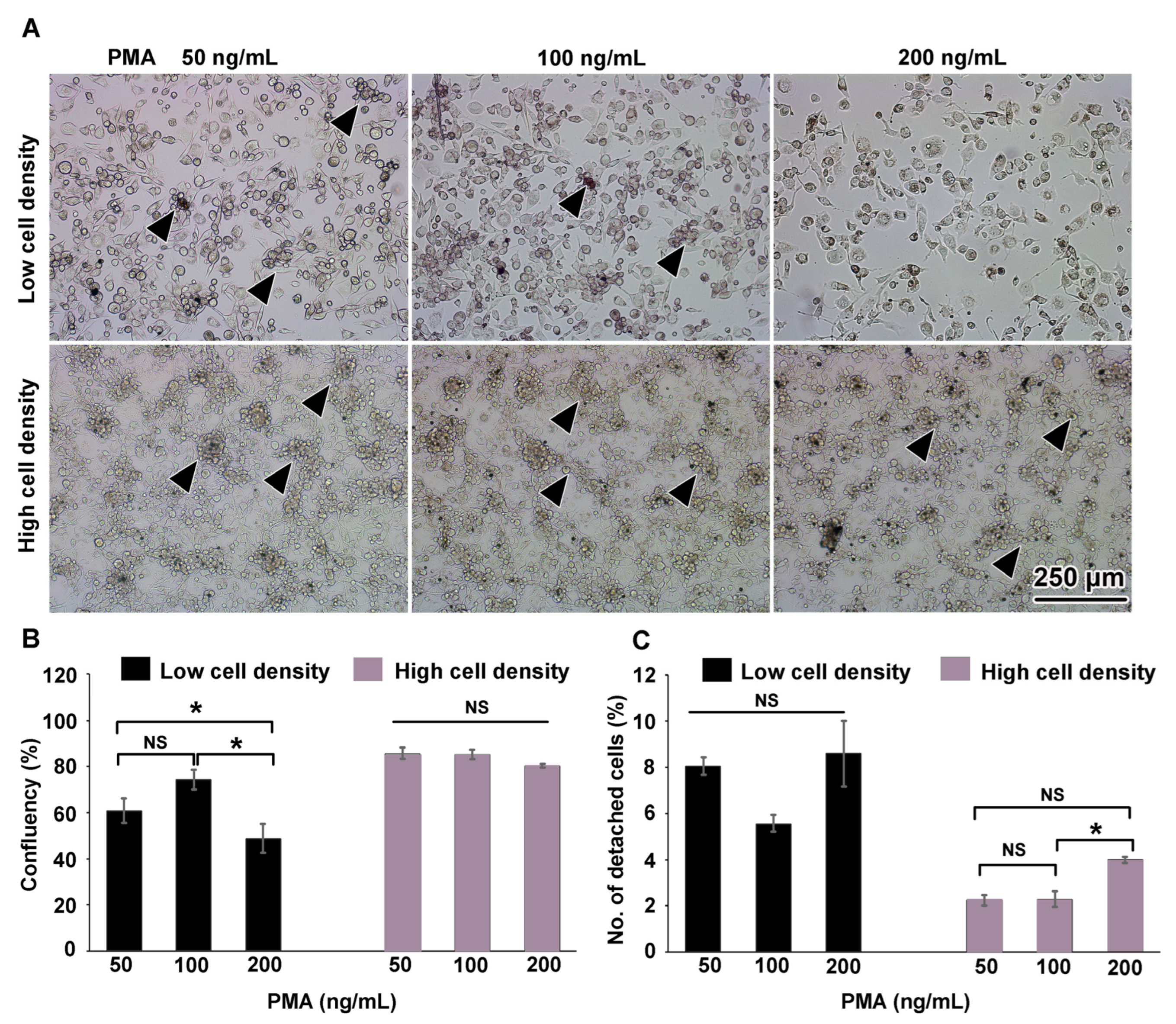
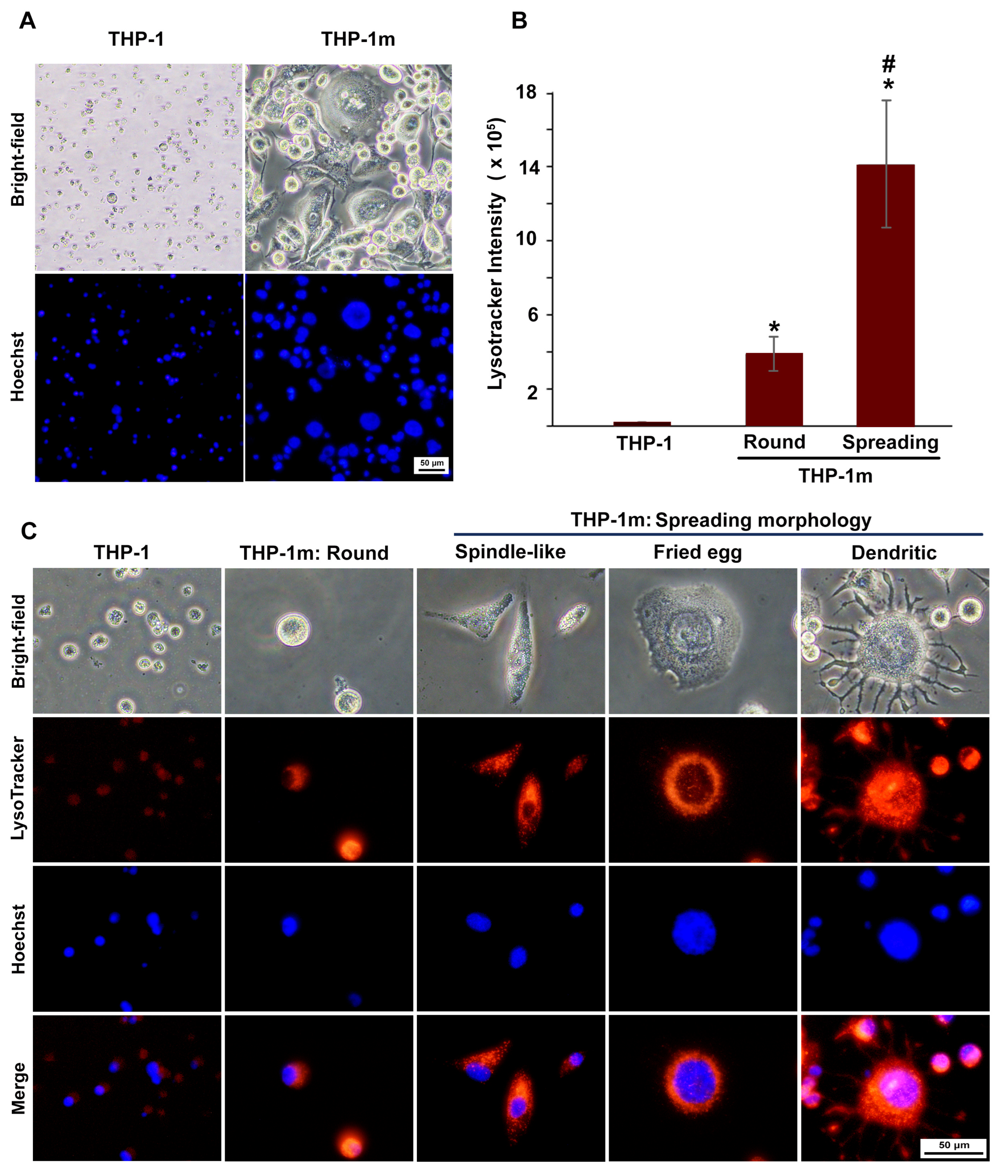
3.1. THP-1m Generation
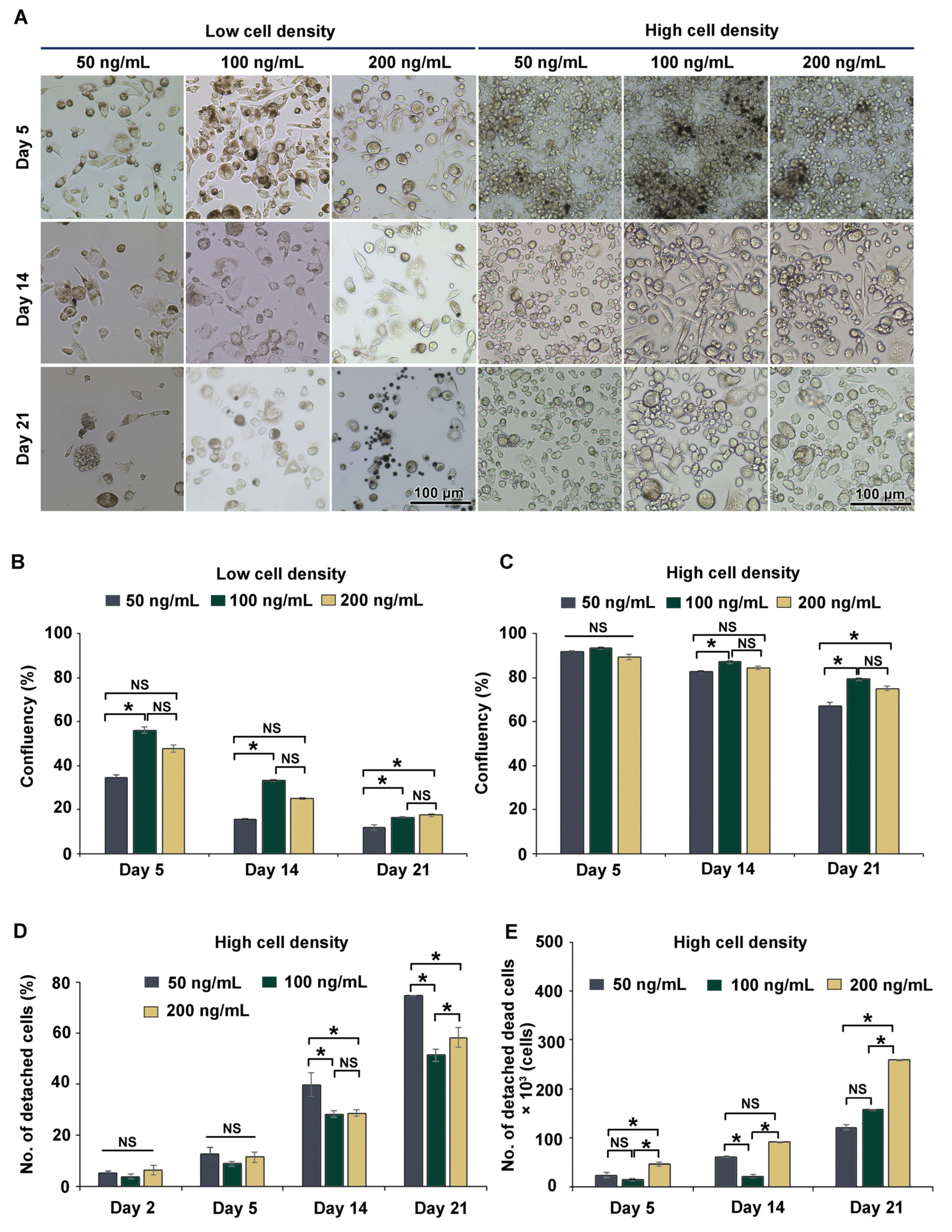
3.2. Long-Term (21-Day) Culture of THP-1m
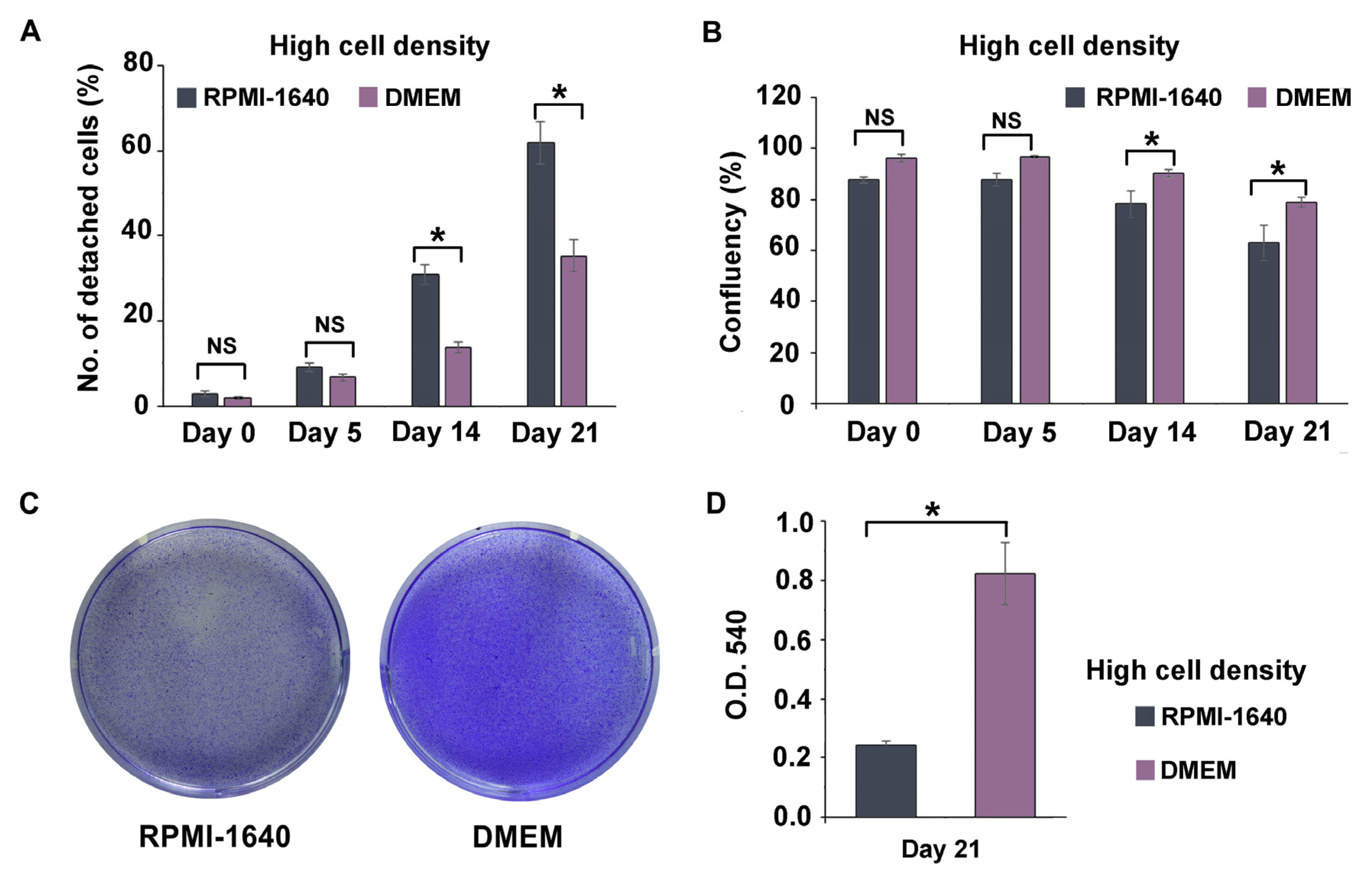
3.3. DMEM Promoted the Long-Term Maintenance of THP-1m Cultures
3.4. Inducible Cytokine Secretions While Maintaining Membrane Integrity in Long-Term (21-Day) Triple Co-Culture of THP-1m with Intestinal Epithelium Caco-2/HT-29-MTX
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blom, R.A.; Erni, S.T.; Krempaská, K.; Schaerer, O.; Van Dijk, R.M.; Amacker, M.; Moser, C.; Hall, S.R.; Von Garnier, C.; Blank, F. A triple co-culture model of the human respiratory tract to study immune-modulatory effects of liposomes and virosomes. PLoS ONE 2016, 11, e0163539. [Google Scholar] [CrossRef]
- Kämpfer, A.A.; Urbán, P.; Gioria, S.; Kanase, N.; Stone, V.; Kinsner-Ovaskainen, A. Development of an in vitro co-culture model to mimic the human intestine in healthy and diseased state. Toxicol. In Vitro 2017, 45, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Fedi, A.; Vitale, C.; Ponschin, G.; Ayehunie, S.; Fato, M.; Scaglione, S. In vitro models replicating the human intestinal epithelium for absorption and metabolism studies: A systematic review. J. Control. Release 2021, 335, 247–268. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Kaissi, S.; Homaidan, F.R.; Naim, H.Y.; El-Sabban, M.E. Cross-talk between intestinal epithelial cells and immune cells in inflammatory bowel disease. Sci. Rep. 2016, 6, 29783. [Google Scholar] [CrossRef]
- Satsu, H.; Ishimoto, Y.; Nakano, T.; Mochizuki, T.; Iwanaga, T.; Shimizu, M. Induction by activated macrophage-like THP-1 cells of apoptotic and necrotic cell death in intestinal epithelial Caco-2 monolayers via tumor necrosis factor-alpha. Exp. Cell Res. 2006, 312, 3909–3919. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.; von der Weid, P.-Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microb. 2020, 11, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Duweb, A.; Gaiser, A.K.; Stiltz, I.; El Gaafary, M.; Simmet, T.; Syrovets, T. The SC cell line as an in vitro model of human monocytes. J. Leukoc. Biol. 2022, 112, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Kämpfer, A.A.; Urbán, P.; La Spina, R.; Jiménez, I.O.; Kanase, N.; Stone, V.; Kinsner-Ovaskainen, A. Ongoing inflammation enhances the toxicity of engineered nanomaterials: Application of an in vitro co-culture model of the healthy and inflamed intestine. Toxicol. In Vitro 2020, 63, 104738. [Google Scholar] [CrossRef]
- Marescotti, D.; Lo Sasso, G.; Guerrera, D.; Renggli, K.; Ruiz Castro, P.A.; Piault, R.; Jaquet, V.; Moine, F.; Luettich, K.; Frentzel, S. Development of an Advanced Multicellular Intestinal Model for Assessing Immunomodulatory Properties of Anti-Inflammatory Compounds. Front. Pharmacol. 2021, 12, 639716. [Google Scholar] [CrossRef] [PubMed]
- Moyes, S.M.; Morris, J.F.; Carr, K.E. Macrophages increase microparticle uptake by enterocyte-like Caco-2 cell monolayers. J. Anat. 2010, 217, 740–754. [Google Scholar] [CrossRef]
- Pinto, S.M.; Kim, H.; Subbannayya, Y.; Giambelluca, M.; Bösl, K.; Kandasamy, R.K. Dose-dependent phorbol 12-myristate-13-acetate-mediated monocyte-to-macrophage differentiation induces unique proteomic signatures in THP-1 cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lund, M.E.; To, J.; O’Brien, B.A.; Donnelly, S. The choice of phorbol 12-myristate 13-acetate differentiation protocol influences the response of THP-1 macrophages to a pro-inflammatory stimulus. J. Immunol. Methods 2016, 430, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ota, T.; Jiang, Y.S.; Fujiwara, M.; Tatsuka, M. Apoptosis-independent cleavage of RhoGDIβ at Asp19 during PMA-stimulated differentiation of THP-1 cells to macrophages. Mol. Med. Rep. 2017, 15, 1722–1726. [Google Scholar] [CrossRef]
- Kletting, S.; Barthold, S.; Repnik, U.; Griffiths, G.; Loretz, B.; Schneider-Daum, N.; de Souza Carvalho-Wodarz, C.; Lehr, C.-M. Co-culture of human alveolar epithelial (hAELVi) and macrophage (THP-1) cell lines. ALTEX Altern. Anim. Exp. 2018, 35, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Aldo, P.B.; Craveiro, V.; Guller, S.; Mor, G. Effect of Culture Conditions on the Phenotype of THP-1 Monocyte Cell Line; Wiley Online Library: Hoboken, NJ, USA, 2013; pp. 1046–7408. [Google Scholar]
- Sureshbabu, A.; Okajima, H.; Yamanaka, D.; Tonner, E.; Shastri, S.; Maycock, J.; Szymanowska, M.; Shand, J.; Takahashi, S.-I.; Beattie, J. IGFBP5 induces cell adhesion, increases cell survival and inhibits cell migration in MCF-7 human breast cancer cells. J. Cell Sci. 2012, 125, 1693–1705. [Google Scholar] [PubMed]
- Jakic, B.; Buszko, M.; Cappellano, G.; Wick, G. Elevated sodium leads to the increased expression of HSP60 and induces apoptosis in HUVECs. PLoS ONE 2017, 12, e0179383. [Google Scholar] [CrossRef]
- Grytting, V.S.; Olderbø, B.P.; Holme, J.A.; Samuelsen, J.T.; Solhaug, A.; Becher, R.; Bølling, A.K. Di-n-butyl phthalate modifies PMA-induced macrophage differentiation of THP-1 monocytes via PPARγ. Toxicol. In Vitro 2019, 54, 168–177. [Google Scholar] [CrossRef]
- Lesuffleur, T.; Barbat, A.; Dussaulx, E.; Zweibaum, A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990, 50, 6334–6343. [Google Scholar]
- Schimpel, C.; Teubl, B.; Absenger, M.; Meindl, C.; Fröhlich, E.; Leitinger, G.; Zimmer, A.; Roblegg, E. Development of an advanced intestinal in vitro triple culture permeability model to study transport of nanoparticles. Mol. Pharm. 2014, 11, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. SLAS Technol. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Ann. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Austermann, J.; Roth, J.; Barczyk-Kahlert, K. The good and the bad: Monocytes’ and macrophages’ diverse functions in inflammation. Cells 2022, 11, 1979. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez-Pomares, L. Physiological roles of macrophages. Pflügers Arch. Eur. J. Physiol. 2017, 469, 365–374. [Google Scholar] [CrossRef]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.; Dockrell, D.H. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef]
- Madhvi, A.; Mishra, H.; Leisching, G.; Mahlobo, P.; Baker, B. Comparison of human monocyte derived macrophages and THP1-like macrophages as in vitro models for M. tuberculosis infection. Comp. Immunol. Microbiol. Infect. Dis. 2019, 67, 101355. [Google Scholar] [CrossRef]
- Jimenez-Duran, G.; Luque-Martin, R.; Patel, M.; Koppe, E.; Bernard, S.; Sharp, C.; Buchan, N.; Rea, C.; de Winther, M.P.; Turan, N. Pharmacological validation of targets regulating CD14 during macrophage differentiation. EBioMedicine 2020, 61, 103039. [Google Scholar] [CrossRef]
- Li, Z.H.; Si, Y.; Xu, G.; Chen, X.M.; Xiong, H.; Lai, L.; Zheng, Y.Q.; Zhang, Z.G. High-dose PMA with RANKL and MCSF induces THP-1 cell differentiation into human functional osteoclasts in vitro. Mol. Med. Rep. 2017, 16, 8380–8384. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Jung, H.S.; Yang, H.I.; Yoo, M.C.; Kim, C.; Kim, K.S. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflammat. Res. 2007, 56, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Starr, T.; Bauler, T.J.; Malik-Kale, P.; Steele-Mortimer, O. The phorbol 12-myristate-13-acetate differentiation protocol is critical to the interaction of THP-1 macrophages with Salmonella Typhimurium. PLoS ONE 2018, 13, e0193601. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, L.; Duan, C.; Li, L.; Chen, G. Total saponin of Dioscorea collettii attenuates MSU crystal-induced inflammation via inhibiting the activation of the NALP3 inflammasome and caspase-1 in THP-1 macrophages. Mol. Med. Rep. 2020, 21, 2466–2474. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, C.; Feng, L.; Ma, L.; Wei, Y.; Sheng, G. Sorting, identification and enrichment of side population cells in THP-1 acute monocytic leukemia cells. Oncol. Rep. 2013, 29, 1923–1931. [Google Scholar] [CrossRef]
- Shahar, M.; Szalat, A.; Rosen, H. Pathogenic Stress Induces Human Monocyte to Express an Extracellular Web of Tunneling Nanotubes. Front. Immunol. 2021, 12, 620734. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Leshchyns’ka, I.; Sytnyk, V. Cell adhesion and intracellular calcium signaling in neurons. Cell Commun. Signal. 2013, 11, 1–13. [Google Scholar] [CrossRef]
- Smith, B.M.; Sturm, R.J.; Carchman, R.A. Calcium modulation of phorbol ester-induced alterations in murine macrophage morphology. Cancer Res. 1983, 43, 3385–3391. [Google Scholar] [PubMed]
- Chauhan, A.; Sun, Y.; Sukumaran, P.; Zangbede, F.O.Q.; Jondle, C.N.; Sharma, A.; Evans, D.L.; Chauhan, P.; Szlabick, R.E.; Aaland, M.O. M1 macrophage polarization is dependent on TRPC1-mediated calcium entry. iScience 2018, 8, 85–102. [Google Scholar] [CrossRef]
- Długosz, E.; Basałaj, K.; Zawistowska-Deniziak, A. Cytokine production and signalling in human THP-1 macrophages is dependent on Toxocara canis glycans. Parasitol. Res. 2019, 118, 2925–2933. [Google Scholar] [CrossRef]
- Serrano, C.; Galán, S.; Rubio, J.F.; Candelario-Martínez, A.; Montes-Gómez, A.E.; Chánez-Paredes, S.; Cedillo-Barrón, L.; Schnoor, M.; Meraz-Ríos, M.A.; Villegas-Sepúlveda, N. Compartmentalized response of IL-6/STAT3 signaling in the colonic mucosa mediates colitis development. J. Immunol. 2019, 202, 1239–1249. [Google Scholar] [CrossRef]
- Skronska-Wasek, W.; Durlanik, S.; Garnett, J.; Pflanz, S. Polarized cytokine release from airway epithelium differentially influences macrophage phenotype. Mol. Immunol. 2021, 132, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Armstead, A.L.; Li, B. In vitro inflammatory effects of hard metal (WC–Co) nanoparticle exposure. Int. J. Nanomed. 2016, 11, 6195. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.; Vadeboncoeur, N.; Gottschalk, M. CD14-dependent and-independent cytokine and chemokine production by human THP-1 monocytes stimulated by Streptococcus suis capsular type 2. Clin. Exp. Immunol. 2002, 127, 243–254. [Google Scholar] [CrossRef]
- Groeneweg, M.; Kanters, E.; Vergouwe, M.N.; Duerink, H.; Kraal, G.; Hofker, M.H.; de Winther, M.P. Lipopolysaccharide-induced gene expression in murine macrophages is enhanced by prior exposure to oxLDL. J. Lipid Res. 2006, 47, 2259–2267. [Google Scholar] [CrossRef]
- Calatayud, M.; Dezutter, O.; Hernandez-Sanabria, E.; Hidalgo-Martinez, S.; Meysman, F.J.; Van de Wiele, T. Development of a host-microbiome model of the small intestine. FASEB J. 2019, 33, 3985–3996. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Wohlleben, W.; Septiadi, D.; Landsiedel, R.; Petri-Fink, A.; Rothen-Rutishauser, B. A novel 3D intestine barrier model to study the immune response upon exposure to microplastics. Arch. Toxicol. 2020, 94, 2463–2479. [Google Scholar] [CrossRef]
- Gibb, M.; Pradhan, S.H.; Mulenos, M.R.; Lujan, H.; Liu, J.; Ede, J.D.; Shatkin, J.A.; Sayes, C.M. Characterization of a human in vitro intestinal model for the hazard assessment of nanomaterials used in cancer immunotherapy. Appl. Sci. 2021, 11, 2113. [Google Scholar] [CrossRef]
- Weber, L.; Kuck, K.; Jürgenliemk, G.; Heilmann, J.; Lipowicz, B.; Vissiennon, C. Anti-inflammatory and barrier-stabilising effects of myrrh, coffee charcoal and chamomile flower extract in a co-culture cell model of the intestinal mucosa. Biomolecules 2020, 10, 1033. [Google Scholar] [CrossRef]
- Ling, X.; Linglong, P.; Weixia, D.; Hong, W. Protective effects of bifidobacterium on intestinal barrier function in LPS-induced enterocyte barrier injury of Caco-2 monolayers and in a rat NEC model. PLoS ONE 2016, 11, e0161635. [Google Scholar] [CrossRef]
- Tu, J.; Xu, Y.; Xu, J.; Ling, Y.; Cai, Y. Chitosan nanoparticles reduce LPS-induced inflammatory reaction via inhibition of NF-κB pathway in Caco-2 cells. Int. J. Biol. Macromol. 2016, 86, 848–856. [Google Scholar] [CrossRef]
- Felix, K.; Tobias, S.; Jan, H.; Nicolas, S.; Michael, M. Measurements of transepithelial electrical resistance (TEER) are affected by junctional length in immature epithelial monolayers. Histochem. Cell Biol. 2021, 156, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Burmester, M.; Langeheine, M.; Brehm, R.; Empl, M.T.; Seeger, B.; Breves, G. Caco-2/HT29-MTX co-cultured cells as a model for studying physiological properties and toxin-induced effects on intestinal cells. PLoS ONE 2021, 16, e0257824. [Google Scholar] [CrossRef]
- Hasbullah, N.I.; Syed Mohamad, S.A.; Hasan, N.A.; Ahmad, N.; Johari, N.A.; Abd Manap, M.N.; Mohd Amin, M.C.I. The role of mucus in adhesion and invasion of foodborne pathogens: Challenges in current human intestinal model. Food Res. 2022, 6, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Feng, P.; Zhang, M.; Tian, T.; Wang, S.; Zhao, B.; Li, Y.; Wang, S.; Wu, C. Endotoxins Induced ECM-Receptor Interaction Pathway Signal Effect on the Function of MUC2 in Caco2/HT29 Co-Culture Cells. Front. Immunol. 2022, 13, 916933. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef]
- Busch, M.; Bredeck, G.; Kämpfer, A.A.; Schins, R.P. Investigations of acute effects of polystyrene and polyvinyl chloride micro-and nanoplastics in an advanced in vitro triple culture model of the healthy and inflamed intestine. Environ. Res. 2021, 193, 110536. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ye, Q.; Zeng, X.; Qiao, S. Functions of macrophages in the maintenance of intestinal homeostasis. J. Immunol. Res. 2019, 2019, 1512969. [Google Scholar] [CrossRef]
- Kaulmann, A.; Legay, S.; Schneider, Y.J.; Hoffmann, L.; Bohn, T. Inflammation related responses of intestinal cells to plum and cabbage digesta with differential carotenoid and polyphenol profiles following simulated gastrointestinal digestion. Mol. Nutrit. Food Res. 2016, 60, 992–1005. [Google Scholar] [CrossRef]
- Ponce de León-Rodríguez, M.d.C.; Guyot, J.-P.; Laurent-Babot, C. Intestinal in vitro cell culture models and their potential to study the effect of food components on intestinal inflammation. Crit. Rev. Food Sci. Nutrit. 2019, 59, 3648–3666. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Ahluwalia, A. Advances and current challenges in intestinal in vitro model engineering: A digest. Front. Bioeng. Biotechnol. 2019, 7, 144. [Google Scholar] [CrossRef]
- Kakni, P.; Truckenmüller, R.; Habibović, P.; van Griensven, M.; Giselbrecht, S. A Microwell-Based Intestinal Organoid-Macrophage Co-Culture System to Study Intestinal Inflammation. Int. J. Mol. Sci. 2022, 23, 15364. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Shirure, V.S.; Liu, R.; Cunningham, C.; Ding, L.; Meacham, J.M.; Goedegebuure, S.P.; George, S.C.; Fields, R.C. Tumor-on-a-chip platform to interrogate the role of macrophages in tumor progression. Integrat. Biol. 2020, 12, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Prestigiacomo, V.; Weston, A.; Messner, S.; Lampart, F.; Suter-Dick, L. Pro-fibrotic compounds induce stellate cell activation, ECM-remodelling and Nrf2 activation in a human 3D-multicellular model of liver fibrosis. PLoS ONE 2017, 12, e0179995. [Google Scholar] [CrossRef] [PubMed]
- Sasserath, T.; Rumsey, J.W.; McAleer, C.W.; Bridges, L.R.; Long, C.J.; Elbrecht, D.; Schuler, F.; Roth, A.; Bertinetti-LaPatki, C.; Shuler, M.L. Differential monocyte actuation in a three-organ functional innate immune system-on-a-chip. Adv. Sci. 2020, 7, 2000323. [Google Scholar] [CrossRef]
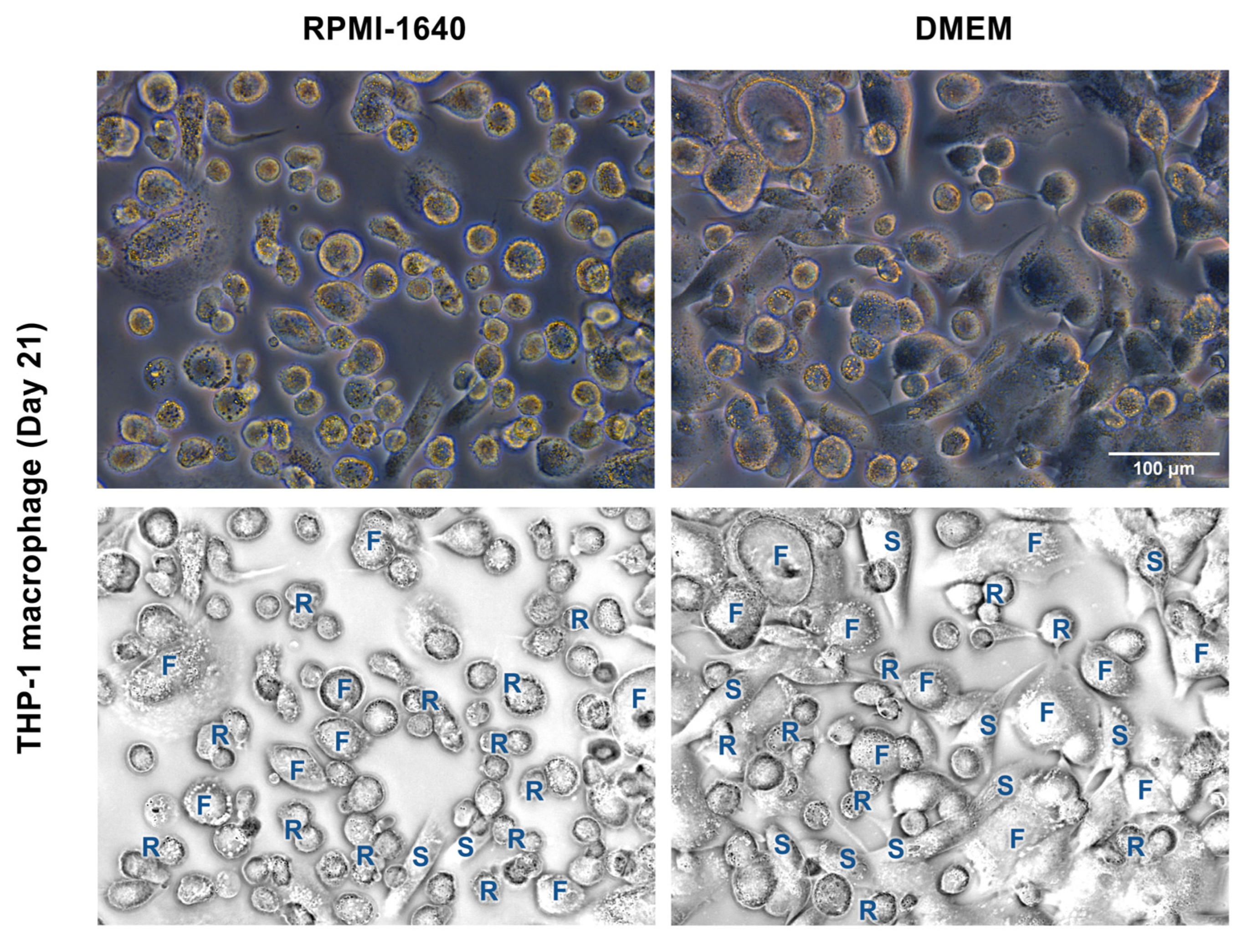

| Class | |||||
|---|---|---|---|---|---|
| Day | Round (%) | Fried Egg (%) | Spindle-like (%) | Total Cell Number/Well | |
| RPMI-1640 | 5 | 84.0 ± 4.1 | 1.8 ± 0.6 | 14.2 ± 2.4 | 4396.7 ± 1516.8 |
| 14 | 65.2 ± 2.1 | 11.0 ± 1.4 | 23.8 ± 1.4 | 2210.0 ± 545.2 | |
| 21 | 82.1 ± 21.7 | 15.7 ± 3.1 | 2.2 ± 0.6 | 1835.0 ± 615.0 | |
| DMEM | 5 | 63.2 ± 1.4 | 0.5 ± 0.1 | 36.3 ± 1.7 | 4338.3 ± 1149.7 |
| 14 | 49.6 ± 2.6 | 8.3 ± 1.5 | 42.2 ± 5.1 | 2481.7 ± 526.9 | |
| 21 | 76.6 ± 4.3 | 7.2 ± 0.8 | 16.1 ± 1.5 | 2140.0 ± 649.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuangbubpha, P.; Thara, S.; Sriboonaied, P.; Saetan, P.; Tumnoi, W.; Charoenpanich, A. Optimizing THP-1 Macrophage Culture for an Immune-Responsive Human Intestinal Model. Cells 2023, 12, 1427. https://doi.org/10.3390/cells12101427
Phuangbubpha P, Thara S, Sriboonaied P, Saetan P, Tumnoi W, Charoenpanich A. Optimizing THP-1 Macrophage Culture for an Immune-Responsive Human Intestinal Model. Cells. 2023; 12(10):1427. https://doi.org/10.3390/cells12101427
Chicago/Turabian StylePhuangbubpha, Pornwipa, Sanya Thara, Patsawee Sriboonaied, Puretat Saetan, Wanwiwa Tumnoi, and Adisri Charoenpanich. 2023. "Optimizing THP-1 Macrophage Culture for an Immune-Responsive Human Intestinal Model" Cells 12, no. 10: 1427. https://doi.org/10.3390/cells12101427
APA StylePhuangbubpha, P., Thara, S., Sriboonaied, P., Saetan, P., Tumnoi, W., & Charoenpanich, A. (2023). Optimizing THP-1 Macrophage Culture for an Immune-Responsive Human Intestinal Model. Cells, 12(10), 1427. https://doi.org/10.3390/cells12101427






