Genome-Wide Analysis of MYB Transcription Factors in the Wheat Genome and Their Roles in Salt Stress Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of MYB Sequences
2.2. Transcription Factor Binding Site Prediction
2.3. Phylogenetic Analysis
2.4. Plant Growth Conditions and Treatments
2.5. Quantitative Real-Time PCR (qPCR)
3. Results
3.1. Identification of MYB Transcription Factors in Wheat
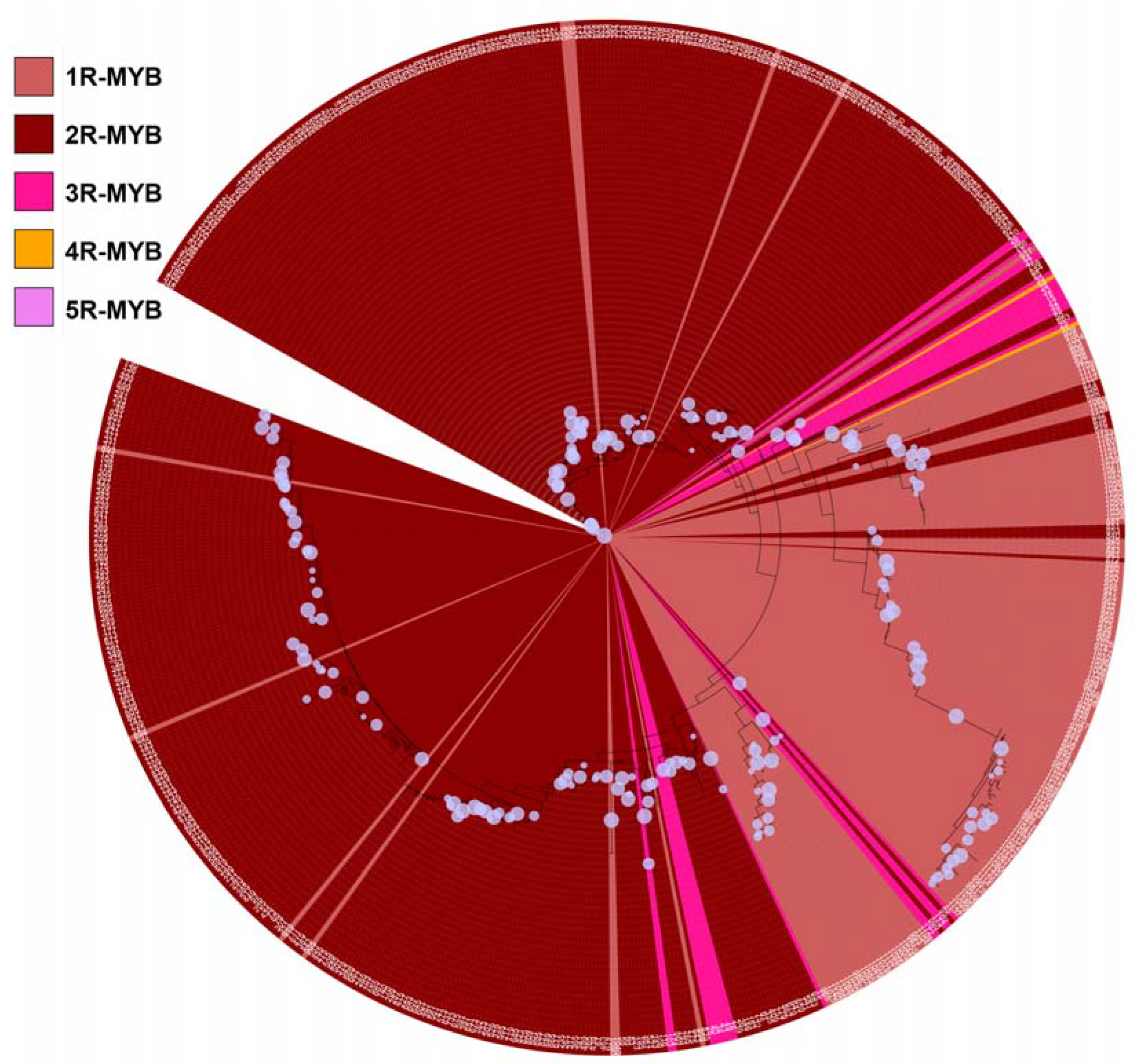
3.2. Phylogenetic Tree
3.3. Structure-Based Analysis of MYB Transcription Factors
3.4. MYB Transcription Factor Expression under Salt Stress
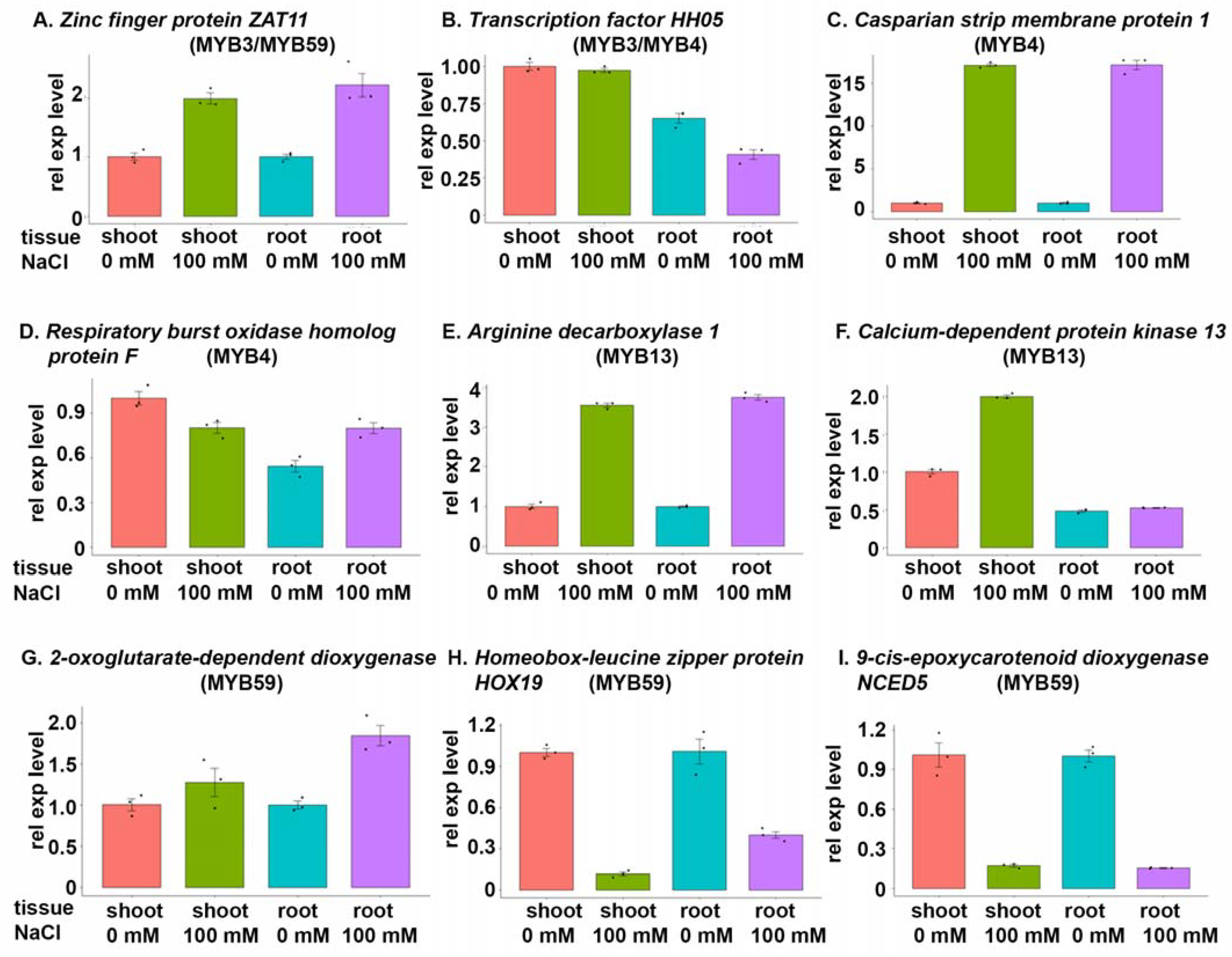
3.5. Target Genes Involved in Salt Stress That Are Regulated by MYB Transcription Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dolferus, R. To grow or not to grow: A stressful decision for plants. Plant Sci. 2014, 229, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Shahid, S.A.; Heng, L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- FAO. Global Map of Salt-Affected Soils; FAO: Rome, Italy, 2021; pp. 1–20. [Google Scholar]
- Hossain, M.S. Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci. 2019, 1, 1–3. [Google Scholar]
- Sutton, R.B.; Davletov, B.A.; Berghuis, A.M.; Sudhof, T.C.; Sprang, S.R. Structure of the first c2 domain of synaptotagmin i: A novel ca2+/phospholipid-binding fold. Cell 1995, 80, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The development of halophyte-based agriculture: Past and present. Ann. Bot. 2015, 115, 529–540. [Google Scholar] [CrossRef]
- Sheldon, A.R.; Dalal, R.C.; Kirchhof, G.; Kopittke, P.M.; Menzies, N.W. The effect of salinity on plant-available water. Plant Soil 2017, 418, 477–491. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Blumwald, E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Shabala, S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef]
- Zhu, J.-K. Genetic analysis of plant salt tolerance using arabidopsis. Plant Physiol. 2000, 124, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.-A.; Li, M.-Z.; Wang, S.-M.; Yin, H.-J. Revisiting the role of plant transcription factors in the battle against abiotic stress. Int. J. Mol. Sci. 2018, 19, 1634. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, X.; Dong, N.; Liu, X.; Zhang, H.; Zhang, Z. Expression of a wheat myb gene in transgenic tobacco enhances resistance to ralstonia solanacearum, and to drought and salt stresses. Funct. Integr. Genom. 2011, 11, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Mujeeb-Kazi, A.; Ogbonnaya, F.C.; He, Z.; Rajaram, S. Wheat genetic resources in the post-genomics era: Promise and challenges. Ann. Bot. 2018, 121, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Luo, Q.; Wang, R.; Zhang, F.; He, Y.; Zhang, Y.; Qiu, D.; Li, K.; Chang, J.; Yang, G. A wheat r2r3-type myb transcription factor taodorant1 positively regulates drought and salt stress responses in transgenic tobacco plants. Front. Plant Sci. 2017, 8, 1374. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, S.; Chen, P.; Cai, J.; Tang, S.; Yang, W.; Cao, F.; Zheng, P.; Sun, B. Systematic analysis of the r2r3-myb family in camellia sinensis: Evidence for galloylated catechins biosynthesis regulation. Front. Plant Sci. 2021, 12, 782220. [Google Scholar] [CrossRef]
- Yin, Y.; Guo, C.; Shi, H.; Zhao, J.; Ma, F.; An, W.; He, X.; Luo, Q.; Cao, Y.; Zhan, X. Genome-wide comparative analysis of the r2r3-myb gene family in five solanaceae species and identification of members regulating carotenoid biosynthesis in wolfberry. Int. J. Mol. Sci. 2022, 23, 2259. [Google Scholar] [CrossRef]
- Katiyar, A.; Smita, S.; Lenka, S.K.; Rajwanshi, R.; Chinnusamy, V.; Bansal, K.C. Genome-wide classification and expression analysis of myb transcription factor families in rice and arabidopsis. BMC Genom. 2012, 13, 544. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. Myb transcription factors in arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Lippold, F.; Sanchez, D.H.; Musialak, M.; Schlereth, A.; Scheible, W.-R.; Hincha, D.K.; Udvardi, M.K. Atmyb41 regulates transcriptional and metabolic responses to osmotic stress in arabidopsis. Plant Physiol. 2009, 149, 1761–1772. [Google Scholar] [CrossRef]
- Segarra, G.; Van der Ent, S.; Trillas, I.; Pieterse, C. Myb72, a node of convergence in induced systemic resistance triggered by a fungal and a bacterial beneficial microbe. Plant Biol. 2009, 11, 90–96. [Google Scholar] [CrossRef]
- Liu, X.; Ma, D.; Zhang, Z.; Wang, S.; Du, S.; Deng, X.; Yin, L. Plant lipid remodeling in response to abiotic stresses. Environ. Exp. Bot. 2019, 165, 174–184. [Google Scholar] [CrossRef]
- Wei, Q.; Chen, R.; Wei, X.; Liu, Y.; Zhao, S.; Yin, X.; Xie, T. Genome-wide identification of r2r3-myb family in wheat and functional characteristics of the abiotic stress responsive gene tamyb344. BMC Genom. 2020, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Lethin, J.; Blomberg, R.; Mousavi, H.; Aronsson, H. In silico based screening of wrky genes for identifying functional genes regulated by wrky under salt stress. Comput. Biol. Chem. 2019, 83, 107131. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhang, Y.; Cui, H.; Liu, J.; Wu, Y.; Cheng, Y.; Xu, H.; Huang, X.; Li, S.; Zhou, A. Wego 2.0: A web tool for analyzing and plotting go annotations, 2018 update. Nucleic Acids Res. 2018, 46, W71–W75. [Google Scholar] [CrossRef]
- Hübner, L.; Kozlov, A.M.; Hespe, D.; Sanders, P.; Stamatakis, A. Exploring parallel mpi fault tolerance mechanisms for phylogenetic inference with raxml-ng. Bioinformatics 2021, 37, 4056–4063. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Liu, H.; Chu, X.; Niu, Y.; Wang, C.; Markov, G.V.; Teng, L. Independent evolution of the myb family in brown algae. Front. Genet. 2021, 12, 2866. [Google Scholar] [CrossRef]
- Ponting, C.P.; Blake, D.J.; Davies, K.E.; Kendrick-Jones, J.; Winder, S.J. Zz and taz: New putative zinc fingers in dystrophin and other proteins. Trends Biochem. Sci. 1996, 21, 11–13. [Google Scholar] [CrossRef]
- Chen, Y.; Song, W.; Xie, X.; Wang, Z.; Guan, P.; Peng, H.; Jiao, Y.; Ni, Z.; Sun, Q.; Guo, W. A collinearity-incorporating homology inference strategy for connecting emerging assemblies in the triticeae tribe as a pilot practice in the plant pangenomic era. Mol. Plant 2020, 13, 1694–1708. [Google Scholar] [CrossRef]
- Arce-Rodríguez, M.L.; Martínez, O.; Ochoa-Alejo, N. Genome-wide identification and analysis of the myb transcription factor gene family in chili pepper (Capsicum spp.). Int. J. Mol. Sci. 2021, 22, 2229. [Google Scholar] [CrossRef]
- Si, Z.; Wang, L.; Ji, Z.; Zhao, M.; Zhang, K.; Qiao, Y. Comparative analysis of the myb gene family in seven ipomoea species. Front. Plant Sci. 2023, 14, 1155018. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.-H.; Zhang, S.-Z.; Wang, R.-K.; Zhang, R.-F.; Hao, Y.-J. Genome wide analysis of the apple myb transcription factor family allows the identification of mdomyb121 gene confering abiotic stress tolerance in plants. PLoS ONE 2013, 8, e69955. [Google Scholar] [CrossRef] [PubMed]
- Kanei-Ishii, C.; Sarai, A.; Sawazaki, T.; Nakagoshi, H.; He, D.-N.; Ogata, K.; Nishimura, Y.; Ishii, S. The tryptophan cluster: A hypothetical structure of the DNA-binding domain of the myb protooncogene product. J. Biol. Chem. 1990, 265, 19990–19995. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhou, J.; Deng, R.-Y.; Zhao, H.-X.; Li, C.-L.; Chen, H.; Suzuki, T.; Park, S.-U.; Wu, Q. Overexpression of a tartary buckwheat r2r3-myb transcription factor gene, ftmyb9, enhances tolerance to drought and salt stresses in transgenic arabidopsis. J. Plant Physiol. 2017, 214, 81–90. [Google Scholar] [CrossRef]
- Cui, J.; Jiang, N.; Zhou, X.; Hou, X.; Yang, G.; Meng, J.; Luan, Y. Tomato myb49 enhances resistance to phytophthora infestans and tolerance to water deficit and salt stress. Planta 2018, 248, 1487–1503. [Google Scholar] [CrossRef]
- Yang, A.; Dai, X.; Zhang, W.-H. A r2r3-type myb gene, osmyb2, is involved in salt, cold, and dehydration tolerance in rice. J. Exp. Bot. 2012, 63, 2541–2556. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Yan, F.; Li, J.; Zhao, Y.; Zhao, X.; Zhai, Y.; Wang, Q. Overexpression of soybean r2r3-myb transcription factor, gmmyb12b2, and tolerance to uv radiation and salt stress in transgenic arabidopsis. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Liu, H.-C.; Zhang, X.-S.; Guo, Q.-X.; Bian, S.-M.; Wang, J.-Y.; Zhai, L.-L. Vcmyb4a, an r2r3-myb transcription factor from vaccinium corymbosum, negatively regulates salt, drought, and temperature stress. Gene 2020, 757, 144935. [Google Scholar] [CrossRef]
- Colmer, T.D.; Flowers, T.J.; Munns, R. Use of wild relatives to improve salt tolerance in wheat. J. Exp. Bot. 2006, 57, 1059–1078. [Google Scholar] [CrossRef] [PubMed]
- Debat, V.; David, P. Mapping phenotypes: Canalization, plasticity and developmental stability. Trends Ecol. Evol. 2001, 16, 555–561. [Google Scholar] [CrossRef]
- Hasegawa, P.M. Sodium (na+) homeostasis and salt tolerance of plants. Environ. Exp. Bot. 2013, 92, 19–31. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, A.; Zhu, S.; Zhang, L. Expression of aco1, ers1 and erf1 genes in harvested bananas in relation to heat-induced defense against colletotrichum musae. J. Plant Physiol. 2011, 168, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, X.; Wang, X.; Wang, L.; Zhang, M.; Ren, M. Effects of abiotic stress on anthocyanin accumulation and grain weight in purple wheat. Crop Pasture Sci. 2018, 69, 1208–1214. [Google Scholar] [CrossRef]
- Shoeva, O.Y.; Khlestkina, E. Differently expressed ‘early’ flavonoid synthesis genes in wheat seedlings become to be co-regulated under salinity stress. Cereal Res. Commun. 2015, 43, 537–543. [Google Scholar] [CrossRef]
- Han, G.; Qiao, Z.; Li, Y.; Wang, C.; Wang, B. The roles of ccch zinc-finger proteins in plant abiotic stress tolerance. Int. J. Mol. Sci. 2021, 22, 8327. [Google Scholar] [CrossRef]
- Ramírez-González, R.; Borrill, P.; Lang, D.; Harrington, S.; Brinton, J.; Venturini, L.; Davey, M.; Jacobs, J.; Van Ex, F.; Pasha, A. The transcriptional landscape of polyploid wheat. Science 2018, 361, eaar6089. [Google Scholar] [CrossRef]
- Guo, Y.H.; Yu, Y.P.; Wang, D.; Wu, C.A.; Yang, G.D.; Huang, J.G.; Zheng, C.C. Ghzfp1, a novel ccch-type zinc finger protein from cotton, enhances salt stress tolerance and fungal disease resistance in transgenic tobacco by interacting with gzird21a and gzipr5. New Phytol. 2009, 183, 62–75. [Google Scholar] [CrossRef]
- Kilian, J.; Whitehead, D.; Horak, J.; Wanke, D.; Weinl, S.; Batistic, O.; D’Angelo, C.; Bornberg-Bauer, E.; Kudla, J.; Harter, K. The atgenexpress global stress expression data set: Protocols, evaluation and model data analysis of uv-b light, drought and cold stress responses. Plant J. 2007, 50, 347–363. [Google Scholar] [CrossRef]
- Chen, S.; Wu, F.; Li, Y.; Qian, Y.; Pan, X.; Li, F.; Wang, Y.; Wu, Z.; Fu, C.; Lin, H. Ntmyb4 and ntchs1 are critical factors in the regulation of flavonoid biosynthesis and are involved in salinity responsiveness. Front. Plant Sci. 2019, 10, 178. [Google Scholar] [CrossRef]
- Oono, Y.; Kobayashi, F.; Kawahara, Y.; Yazawa, T.; Handa, H.; Itoh, T.; Matsumoto, T. Characterisation of the wheat (Triticum aestivum L.) transcriptome by de novo assembly for the discovery of phosphate starvation-responsive genes: Gene expression in pi-stressed wheat. BMC Genom. 2013, 14, 77. [Google Scholar] [CrossRef]
- Pfeifer, M.; Kugler, K.G.; Sandve, S.R.; Zhan, B.; Rudi, H.; Hvidsten, T.R.; Consortium, I.W.G.S.; Mayer, K.F.; Olsen, O.-A. Genome interplay in the grain transcriptome of hexaploid bread wheat. Science 2014, 345, 1250091. [Google Scholar] [CrossRef]
- Aquino, R.S.; Landeira-Fernandez, A.M.; Valente, A.P.; Andrade, L.R.; Mourao, P.A. Occurrence of sulfated galactans in marine angiosperms: Evolutionary implications. Glycobiology 2005, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.L.; Rouzé, P.; Verhelst, B.; Lin, Y.-C.; Bayer, T.; Collen, J.; Dattolo, E.; De Paoli, E.; Dittami, S.; Maumus, F. The genome of the seagrass zostera marina reveals angiosperm adaptation to the sea. Nature 2016, 530, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, M.; Zhigang, A.; Viereck, R.; Löw, R.; Rausch, T. Salt stress induces an increased expression of v-type h+-atpase in mature sugar beet leaves. Plant Mol. Biol. 1996, 32, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, D.; Rost, T. Salinity accelerates endodermal development and induces an exodermis in cotton seedling roots. Environ. Exp. Bot. 1995, 35, 563–574. [Google Scholar] [CrossRef]
- Chen, T.; Cai, X.; Wu, X.; Karahara, I.; Schreiber, L.; Lin, J. Casparian strip development and its potential function in salt tolerance. Plant Signal. Behav. 2011, 6, 1499–1502. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Liang, X.; Zhuang, J.; Wang, X.; Qin, F.; Jiang, C. A dirigent family protein confers variation of casparian strip thickness and salt tolerance in maize. Nat. Commun. 2022, 13, 2222. [Google Scholar] [CrossRef]
- Zongshuai, W.; Xiangnan, L.; Xiancan, Z.; Shengqun, L.; Fengbin, S.; Fulai, L.; Yang, W.; Xiaoning, Q.; Fahong, W.; Zhiyu, Z. Salt acclimation induced salt tolerance is enhanced by abscisic acid priming in wheat. Plant Soil Environ. 2017, 63, 307–314. [Google Scholar]
- Zhang, J.; Yu, H.; Zhang, Y.; Wang, Y.; Li, M.; Zhang, J.; Duan, L.; Zhang, M.; Li, Z. Increased abscisic acid levels in transgenic maize overexpressing atlos5 mediated root ion fluxes and leaf water status under salt stress. J. Exp. Bot. 2016, 67, 1339–1355. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, Q.; Zhang, Z.; Wang, Y.; Zheng, X. The role of respiratory burst oxidase homologues in elicitor-induced stomatal closure and hypersensitive response in nicotiana benthamiana. J. Exp. Bot. 2009, 60, 3109–3122. [Google Scholar] [CrossRef]
- Niu, M.; Huang, Y.; Sun, S.; Sun, J.; Cao, H.; Shabala, S.; Bie, Z. Root respiratory burst oxidase homologue-dependent h2o2 production confers salt tolerance on a grafted cucumber by controlling na+ exclusion and stomatal closure. J. Exp. Bot. 2018, 69, 3465–3476. [Google Scholar] [CrossRef] [PubMed]
- Gaur, A.; Jindal, Y.; Singh, V.; Tiwari, R.; Kumar, D.; Kaushik, D.; Singh, J.; Narwal, S.; Jaiswal, S.; Iquebal, M.A. Gwas to identify novel qtns for wscs accumulation in wheat peduncle under different water regimes. Front. Plant Sci. 2022, 13, 825687. [Google Scholar] [CrossRef] [PubMed]
- Huynh, B.-L.; Mather, D.E.; Schreiber, A.W.; Toubia, J.; Baumann, U.; Shoaei, Z.; Stein, N.; Ariyadasa, R.; Stangoulis, J.C.; Edwards, J. Clusters of genes encoding fructan biosynthesizing enzymes in wheat and barley. Plant Mol. Biol. 2012, 80, 299–314. [Google Scholar] [CrossRef]
- Kooiker, M.; Drenth, J.; Glassop, D.; McIntyre, C.L.; Xue, G.-P. Tamyb13-1, a r2r3 myb transcription factor, regulates the fructan synthetic pathway and contributes to enhanced fructan accumulation in bread wheat. J. Exp. Bot. 2013, 64, 3681–3696. [Google Scholar] [CrossRef]
- Gao, F.; Yao, H.; Zhao, H.; Zhou, J.; Luo, X.; Huang, Y.; Li, C.; Chen, H. Tartary buckwheat ftmyb10 encodes an r2r3-myb transcription factor that acts as a novel negative regulator of salt and drought response in transgenic arabidopsis. Plant Physiol. Biochem. 2016, 109, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, H.; Gao, F.; Yao, P.; Deng, R.; Li, C.; Chen, H.; Wu, Q. A r2r3-myb transcription factor gene, ftmyb13, from tartary buckwheat improves salt/drought tolerance in arabidopsis. Plant Physiol. Biochem. 2018, 132, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niu, Y.; Zheng, Y. Multiple functions of myb transcription factors in abiotic stress responses. Int. J. Mol. Sci. 2021, 22, 6125. [Google Scholar] [CrossRef] [PubMed]
- Kasinathan, V.; Wingler, A. Effect of reduced arginine decarboxylase activity on salt tolerance and on polyamine formation during salt stress in arabidopsis thaliana. Physiol. Plant. 2004, 121, 101–107. [Google Scholar] [CrossRef]
- Saijo, Y.; Kinoshita, N.; Ishiyama, K.; Hata, S.; Kyozuka, J.; Hayakawa, T.; Nakamura, T.; Shimamoto, K.; Yamaya, T.; Izui, K. A ca2+-dependent protein kinase that endows rice plants with cold-and salt-stress tolerance functions in vascular bundles. Plant Cell Physiol. 2001, 42, 1228–1233. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Lu, Z.; Pang, S.; Wang, L.; Wang, L.; Li, W. Gene expression profiles and flavonoid accumulation during salt stress in ginkgo biloba seedlings. Plants 2020, 9, 1162. [Google Scholar] [CrossRef]
- Pi, E.; Xu, J.; Li, H.; Fan, W.; Zhu, C.; Zhang, T.; Jiang, J.; He, L.; Lu, H.; Wang, H. Enhanced salt tolerance of rhizobia-inoculated soybean correlates with decreased phosphorylation of the transcription factor gmmyb183 and altered flavonoid biosynthesis. Mol. Cell. Proteom. 2019, 18, 2225–2243. [Google Scholar] [CrossRef] [PubMed]
- Hussein, Z.; Dryanova, A.; Maret, D.; Gulick, P.J. Gene expression analysis in the roots of salt-stressed wheat and the cytogenetic derivatives of wheat combined with the salt-tolerant wheatgrass, lophopyrum elongatum. Plant Cell Rep. 2014, 33, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Shabani, L.; Hashemi-Shahraki, S. Improving the effects of salt stress by β-carotene and gallic acid using increasing antioxidant activity and regulating ion uptake in Lepidium sativum L. Bot. Stud. 2022, 63, 22. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, Y.; Xie, N.; Guo, Y.; Zhang, F.; Xiang, Z.; Wang, R.; Wang, F.; Gao, Q.; Tian, L. Osnced5, a 9-cis-epoxycarotenoid dioxygenase gene, regulates salt and water stress tolerance and leaf senescence in rice. Plant Sci. 2019, 287, 110188. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Liu, A.; Wang, Y.; Yang, W.; Jin, Y. Mechanism of salt-inhibited early seed germination analysed by transcriptomic sequencing. Seed Sci. Res. 2019, 29, 73–84. [Google Scholar] [CrossRef]
- Consortium, I.W.G.S.; Mayer, K.F.; Rogers, J.; Doležel, J.; Pozniak, C.; Eversole, K.; Feuillet, C.; Gill, B.; Friebe, B.; Lukaszewski, A.J. A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 2014, 345, 1251788. [Google Scholar]


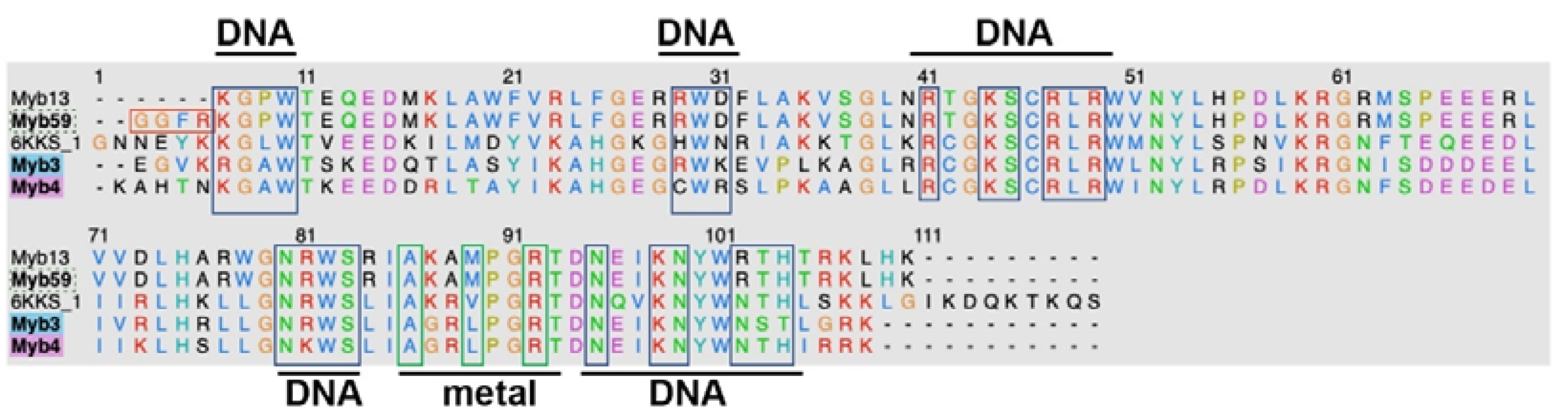


| Protein | Hits-PDBID | Identity (%) | RMSD (Å) |
|---|---|---|---|
| MYB3 | 6KKS:A | 61 | 0.8 |
| MYB4 | 6KKS:A | 65 | 0.9 |
| MYB13 | 6KKS:A | 56 | 1 |
| MYB59 | 6KKS:A | 56 | 1.05 |
| Gene, MYB Regulator | Function | Molecular Function | Biological Process | Cellular Component |
|---|---|---|---|---|
| TraesCS3D02G350100 MYB3, MYB59 | Zinc finger protein ZAT11 (uncharacterized) | DNA-binding transcription factor activity, metal ion binding | Regulation of transcription, DNA-templated, response to chitin, cellular response to nickel ion, regulation of root development | Nucleus |
| TraesCS5D02G411800 MYB3, MYB4 | Transcription factor HHO5 | DNA-binding, DNA-binding transcription factor activity | Regulation of transcription, DNA-templated, negative regulation of gene expression, floral organ formation, specification of plant organ identity | Nucleus, cytosol |
| TraesCS2D02G379300 MYB4 | Casparian strip membrane protein 1 (uncharacterized) | 4 iron, 4 sulfur cluster binding | Cell–cell junction assembly | Plasma membrane, integral component of membrane, Casparian strip |
| TraesCS3B02G314000 MYB4 | Respiratory burst oxidase homolog protein F (uncharacterized) | Peroxidase activity, calcium ion binding, NAD(P)H oxidase activity | Respiratory burst involved in defence response, osmosensory signalling pathway, response to ethylene, abscisic acid-activated signalling pathway, ethylene-activated signalling pathway, regulation of stomatal movement, carbohydrate homeostasis, negative regulation of programmed cell death, hydrogen peroxide biosynthetic process, defence response by callose deposition, oxidation-reduction process | Plasma membrane, integral component of membrane |
| TraesCS7D02G063900 MYB13 | Arginine decarboxylase 1 | Arginine decarboxylase activity, cell wall modification | Arginine catabolic process, spermidine biosynthetic process, response to cold, putrescine biosynthetic process from arginine | NA |
| TraesCS2A02G456100 MYB13 | Calcium-dependent protein kinase 13 (uncharacterized) | Calcium ion binding, calmodulin binding, ATP binding, calcium-dependent protein serine/threonine kinase activity | Response to cold, response to water deprivation, peptidyl-serine phosphorylation, intracellular signal transduction, protein autophosphorylation, positive regulation of response to salt stress | Nucleus |
| TraesCS4B02G19500 MYB59 | Probable 2-oxoglutarate-dependent dioxygenase At3g111800 | Metal ion binding, dioxygenase activity | Flavonoid biosynthetic process | NA |
| TraesCS4D02G236600 MYB59 | Homeobox-leucine zipper protein HOX19 | Sequence-specific DNA binding | Regulation of transcription, DNA-templated | Nucleus |
| TraesCS5B02G029300 MYB59 | 9-cis-Epoxycarotenoid dioxygenase NCED5, chloroplastic (uncharacterized) | Carotenoid dioxygenase activity, 9-cis-epoxycarotenoid dioxygenase activity, metal ion binding | Abscisic acid biosynthetic process, carotene catabolic process | Chloroplast, chloroplast stroma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukumaran, S.; Lethin, J.; Liu, X.; Pelc, J.; Zeng, P.; Hassan, S.; Aronsson, H. Genome-Wide Analysis of MYB Transcription Factors in the Wheat Genome and Their Roles in Salt Stress Response. Cells 2023, 12, 1431. https://doi.org/10.3390/cells12101431
Sukumaran S, Lethin J, Liu X, Pelc J, Zeng P, Hassan S, Aronsson H. Genome-Wide Analysis of MYB Transcription Factors in the Wheat Genome and Their Roles in Salt Stress Response. Cells. 2023; 12(10):1431. https://doi.org/10.3390/cells12101431
Chicago/Turabian StyleSukumaran, Selvakumar, Johanna Lethin, Xin Liu, Justyna Pelc, Peng Zeng, Sameer Hassan, and Henrik Aronsson. 2023. "Genome-Wide Analysis of MYB Transcription Factors in the Wheat Genome and Their Roles in Salt Stress Response" Cells 12, no. 10: 1431. https://doi.org/10.3390/cells12101431
APA StyleSukumaran, S., Lethin, J., Liu, X., Pelc, J., Zeng, P., Hassan, S., & Aronsson, H. (2023). Genome-Wide Analysis of MYB Transcription Factors in the Wheat Genome and Their Roles in Salt Stress Response. Cells, 12(10), 1431. https://doi.org/10.3390/cells12101431





