Oxygen-Glucose Deprivation in Organotypic Hippocampal Cultures Leads to Cytoskeleton Rearrangement and Immune Activation: Link to the Potential Pathomechanism of Ischaemic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Organotypic Hippocampal Cultures (OHCs)
2.3. Oxygen-Glucose Deprivation (OGD)
2.4. Propidium Iodide Staining
2.5. LDH Assay
2.6. NO Release Assay (Nitrite Ion in Solution)
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. MILLIPLEX® Assay
2.9. Immunofluorescence Staining
2.10. Confocal Fluorescence Imaging
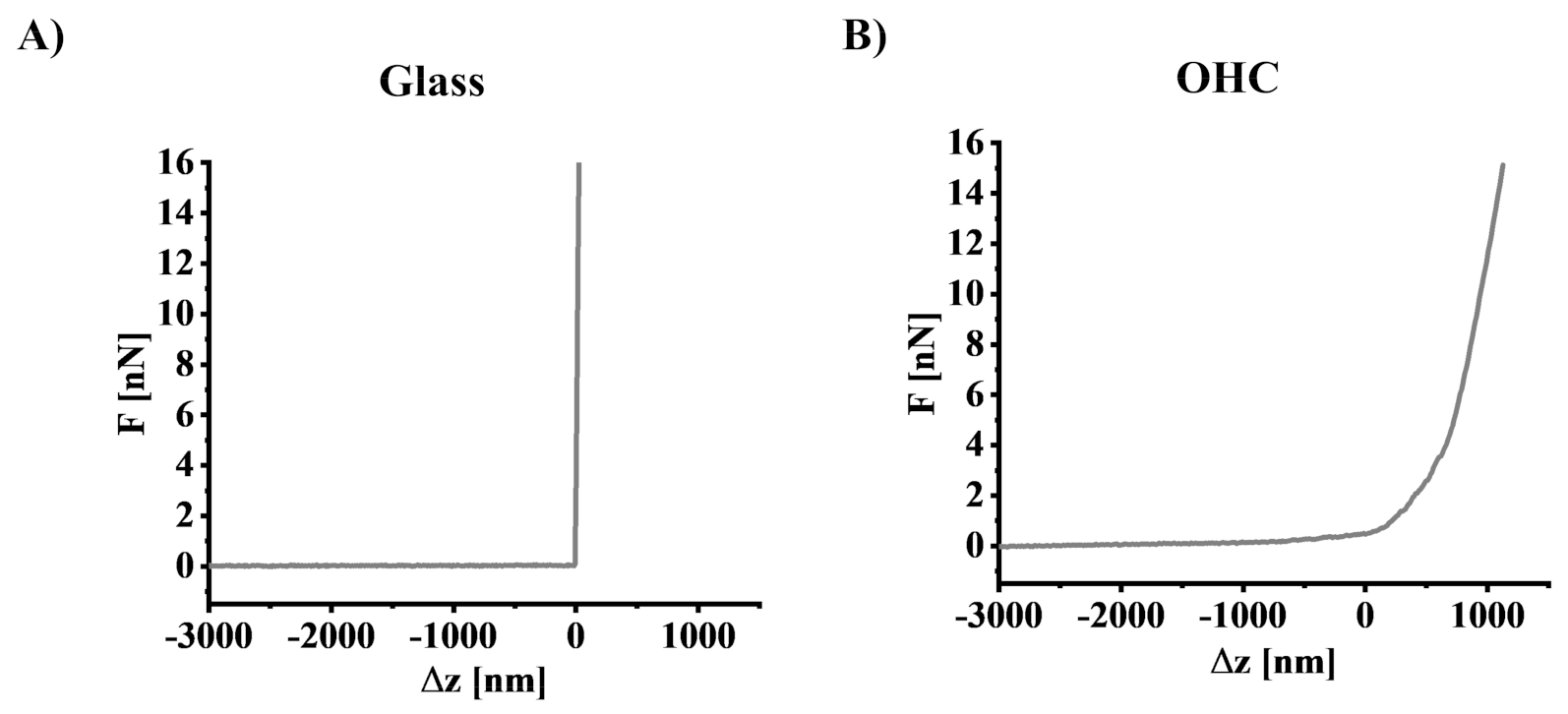
2.11. Determination of Young’s Modulus Using Atomic Force Microscopy
2.12. Quantitative Analysis of Fluorescent Images
2.13. Statistical Analysis
3. Results
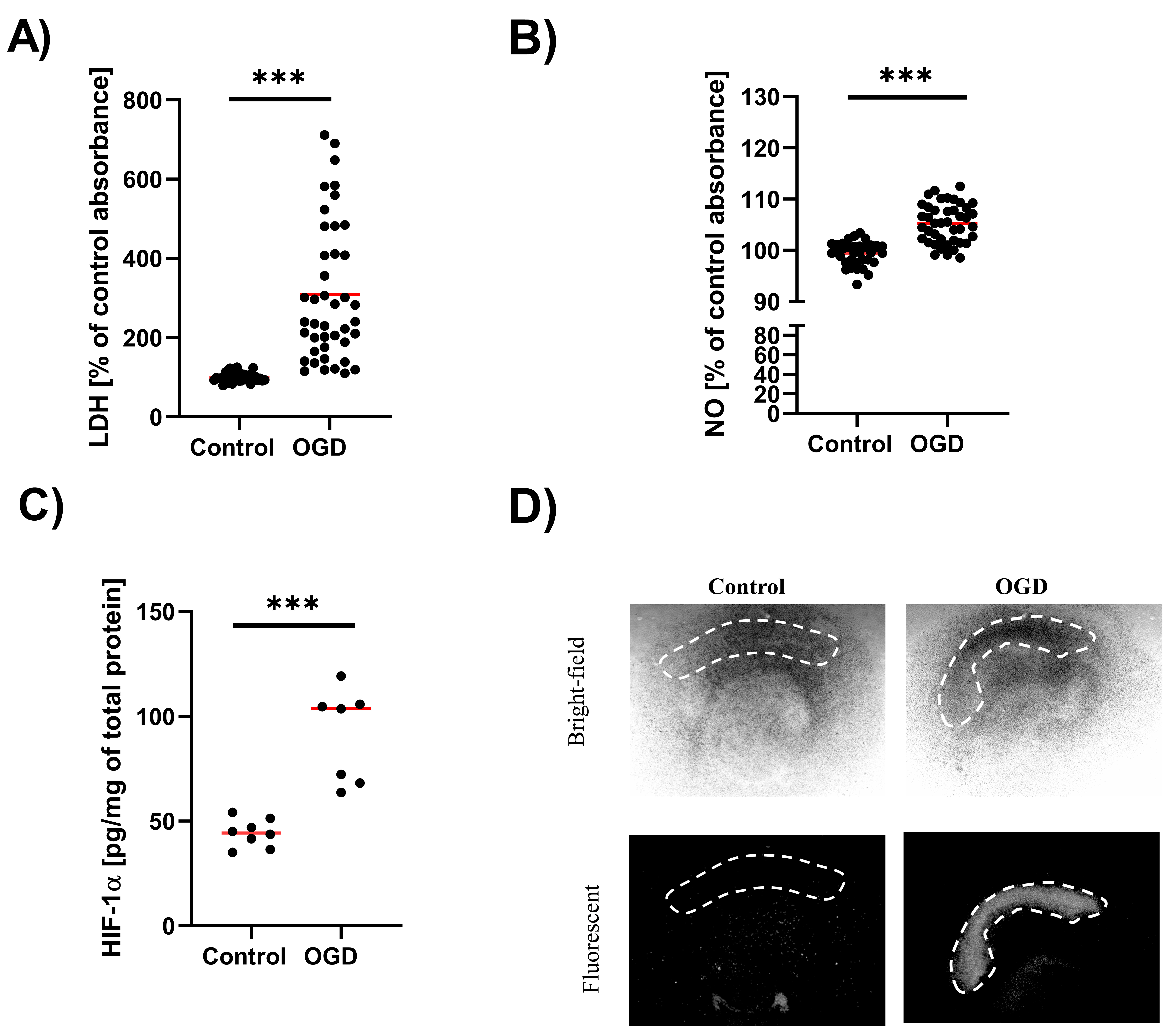
3.1. The Impact of Oxygen-Glucose Deprivation on the OHCs Damage
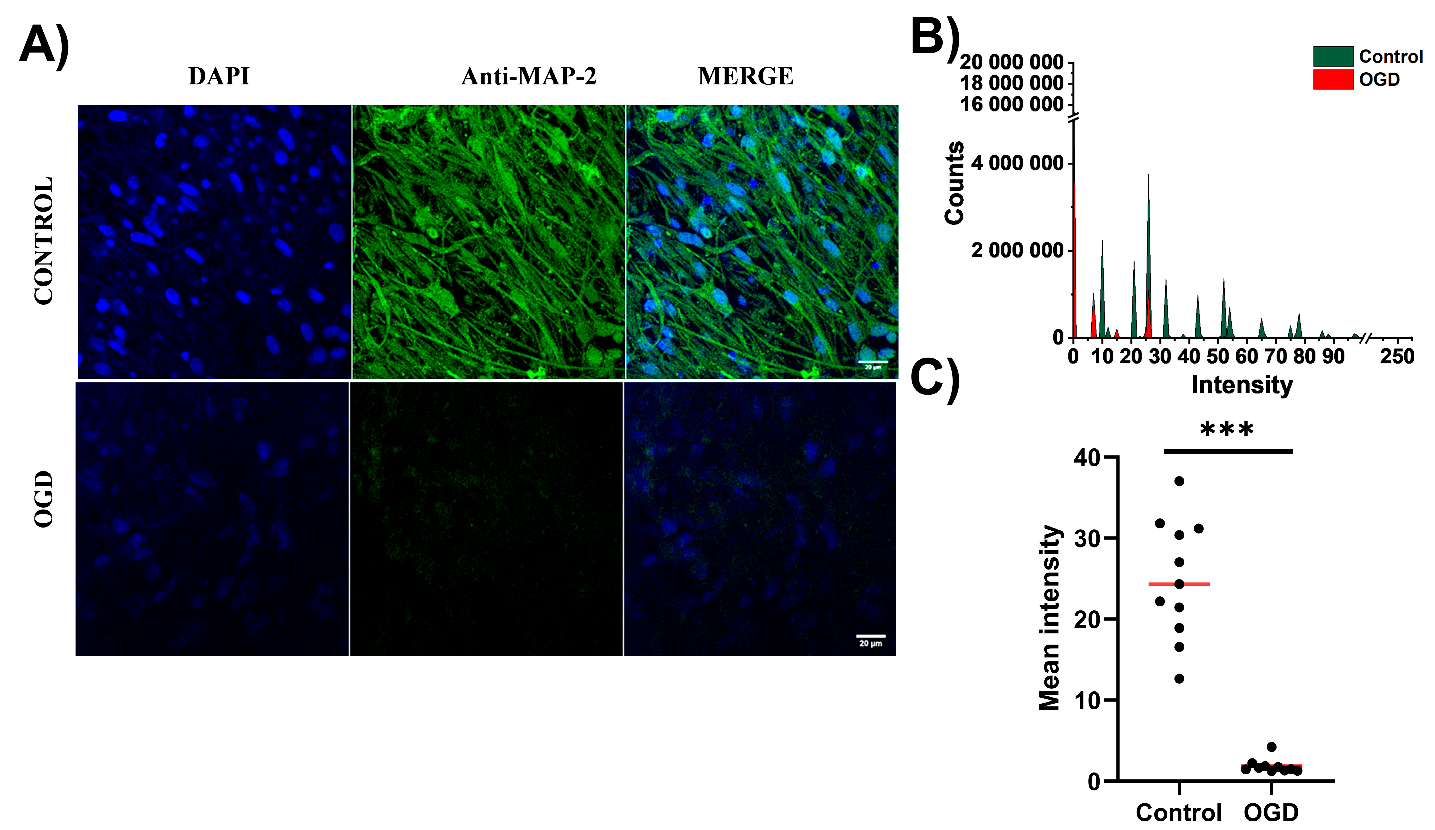
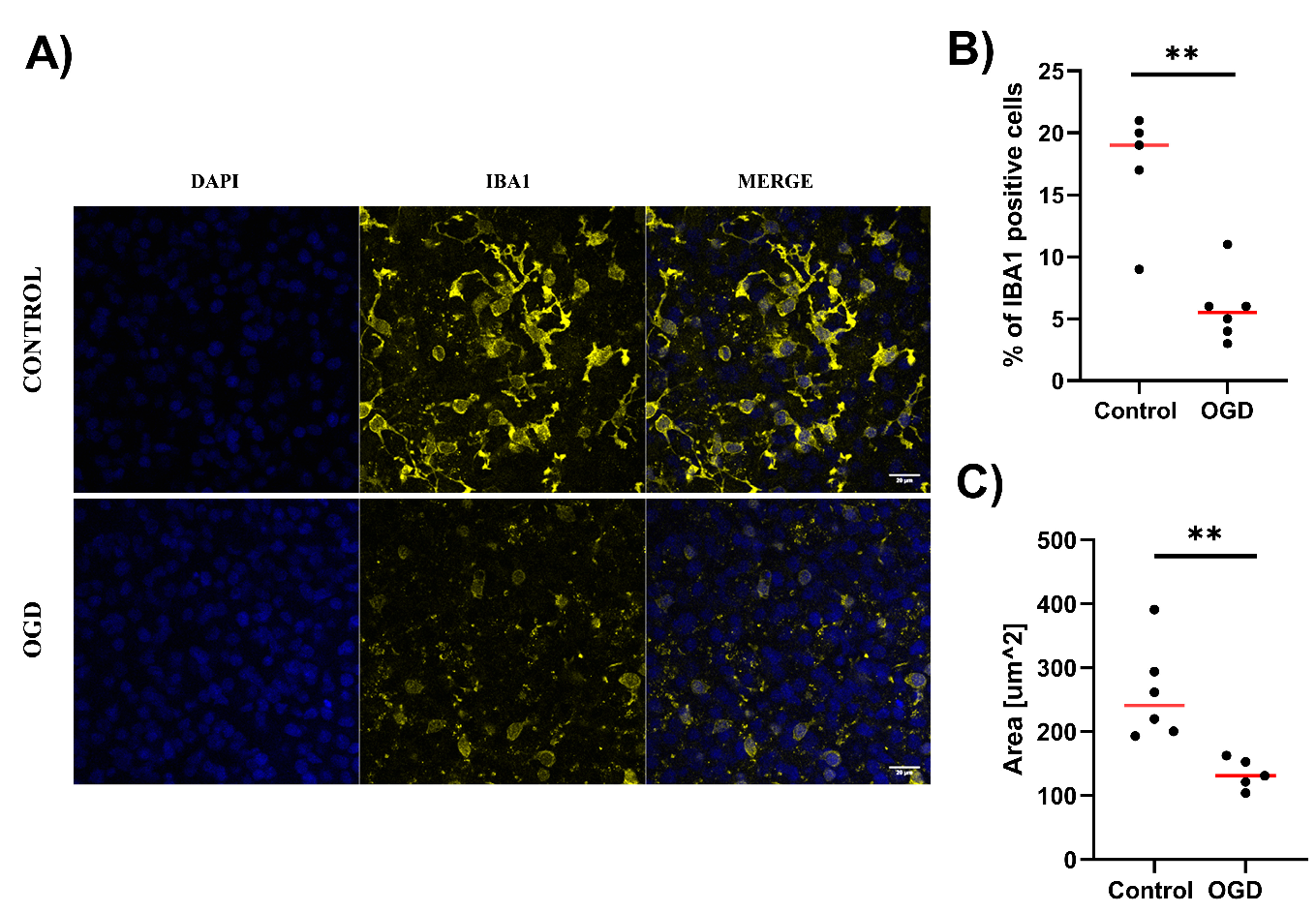
3.2. The Impact of Oxygen-Glucose Deprivation on Neuronal and Microglial Cells in OHCs
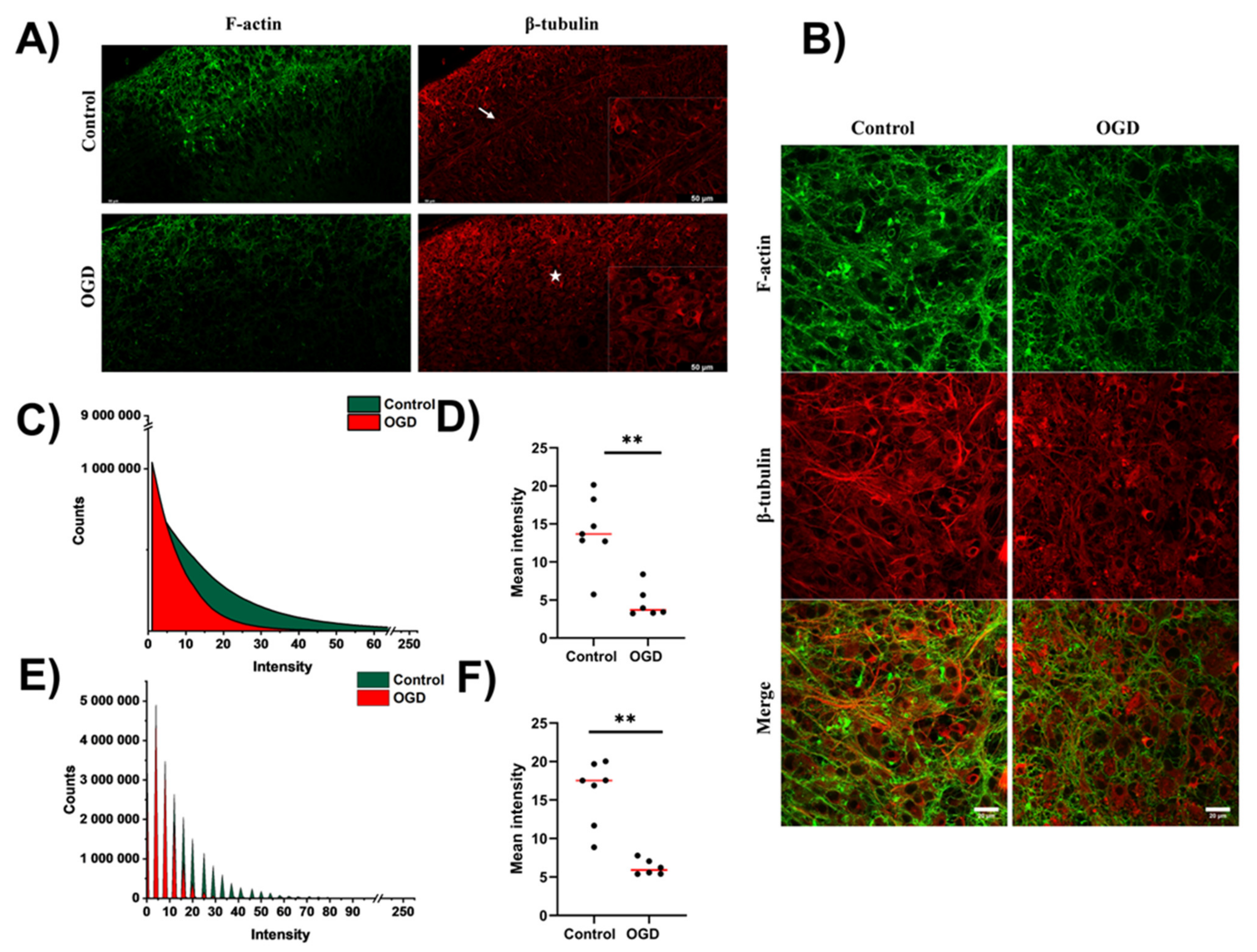
3.3. Impact of Oxygen-Glucose Deprivation on the Architecture of the Microtubular and Actin Cytoskeleton of OHCs
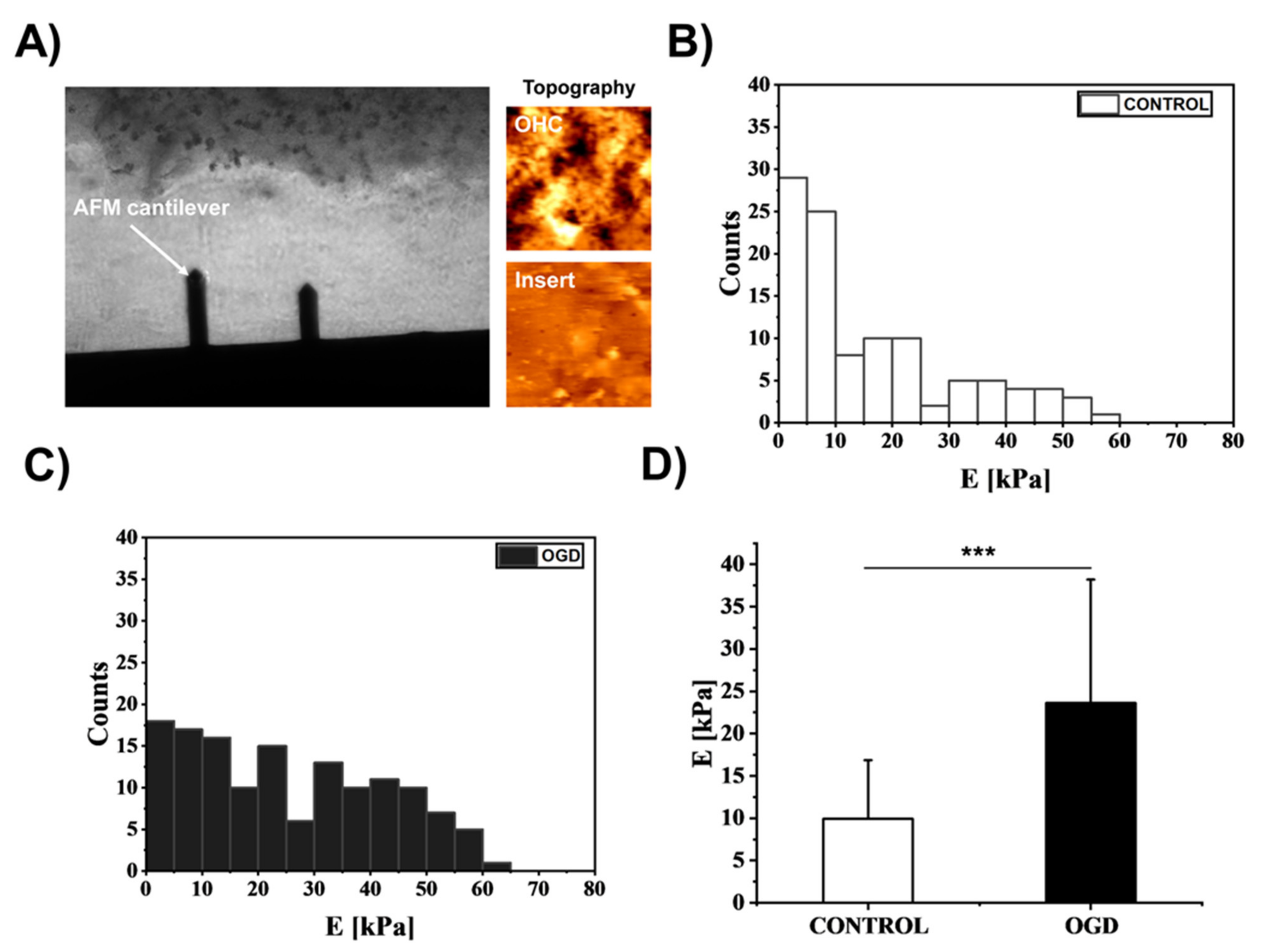
3.4. The Impact of Oxygen-Glucose Deprivation on the Mechanical Properties of OHCs
3.5. The Impact of Oxygen-Glucose Deprivation on the Levels of Pro- and Anti-Inflammatory Factors in OHCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, S.T.; MacManus, J.P. Molecular mechanisms of cerebral ischemia-induced neuronal death. Int. Rev. Cytol. 2002, 221, 93–148. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Q.; Zhang, S.; Lei, P. Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med. Res. Rev. 2021, 42, 259–305. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Nam, S.; Chaudhuri, O. Mitotic cells generate protrusive extracellular forces to divide in three-dimensional microenvironments. Nat. Phys. 2018, 14, 621–628. [Google Scholar] [CrossRef]
- Vining, K.H.; Mooney, D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.-J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef]
- Lekka, M. Atomic force microscopy: A tip for diagnosing cancer. Nat. Nanotechnol. 2012, 7, 691–692. [Google Scholar] [CrossRef]
- Wirtz, D.; Konstantopoulos, K.; Searson, P.C. The physics of cancer: The role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 2011, 11, 512–522. [Google Scholar] [CrossRef]
- Martinac, B.; Nikolaev, Y.A.; Silvani, G.; Bavi, N.; Romanov, V.; Nakayama, Y.; Martinac, A.D.; Rohde, P.; Bavi, O.; Cox, C.D. Cell membrane mechanics and mechanosensory transduction. Curr. Top. Membr. 2020, 86, 83–141. [Google Scholar] [CrossRef]
- Ohashi, K.; Fujiwara, S.; Mizuno, K. Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J. Biochem. 2017, 161, 245–254. [Google Scholar] [CrossRef]
- Na, S.; Collin, O.; Chowdhury, F.; Tay, B.; Ouyang, M.; Wang, Y.; Wang, N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc. Natl. Acad. Sci. USA 2008, 105, 6626–6631. [Google Scholar] [CrossRef] [PubMed]
- Chighizola, M.; Dini, T.; Lenardi, C.; Milani, P.; Podestà, A.; Schulte, C. Mechanotransduction in neuronal cell development and functioning. Biophys. Rev. 2019, 11, 701–720. [Google Scholar] [CrossRef] [PubMed]
- Seano, G.; Nia, H.T.; Emblem, K.E.; Datta, M.; Ren, J.; Krishnan, S.; Kloepper, J.; Pinho, M.C.; Ho, W.W.; Ghosh, M.; et al. Solid stress in brain tumours causes neuronal loss and neurological dysfunction and can be reversed by lithium. Nat. Biomed. Eng. 2019, 3, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.S.; Lee, R.J.; Bs, S.S.C.; Yao, A.; Paun, M.K.; Murphy, S.P.; Mourad, P.D.; Bs, Z.S.X.; Bs, R.J.L.; Bs, A.Y.; et al. Evidence of changes in brain tissue stiffness after ischemic stroke derived from ultrasound-based elastography. J. Ultrasound Med. 2013, 32, 485–494. [Google Scholar] [CrossRef]
- Stoppini, L.; Buchs, P.-A.; Muller, D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods 1991, 37, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Basta-Kaim, A.; Ślusarczyk, J.; Szczepanowicz, K.; Warszyński, P.; Leśkiewicz, M.; Regulska, M.; Trojan, E.; Lasoń, W. Protective effects of polydatin in free and nanocapsulated form on changes caused by lipopolysaccharide in hippocampal organotypic cultures. Pharmacol. Rep. 2019, 71, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Sarnowska, A.; Braun, H.; Sauerzweig, S.; Reymann, K.G.; Minhas, G. The neuroprotective effect of bone marrow stem cells is not dependent on direct cell contact with hypoxic injured tissue: Experimental Neurology 2009; 215: 317–327. Ann. Neurosci. 2011, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Barron, S.; Mulholland, P.J.; Littleton, J.M.; Prendergast, M.A. Age and gender differences in response to neonatal ethanol withdrawal and polyamine challenge in organotypic hippocampal cultures. Alcohol. Clin. Exp. Res. 2008, 32, 929–936. [Google Scholar] [CrossRef]
- Xapelli, S.; Bernardino, L.; Ferreira, R.; Grade, S.; Silva, A.P.; Salgado, J.R.; Cavadas, C.; Grouzmann, E.; Poulsen, F.R.; Jakobsen, B.; et al. Interaction between neuropeptide Y (NPY) and brain-derived neurotrophic factor in NPY-mediated neuroprotection against excitotoxicity: A role for microglia. Eur. J. Neurosci. 2008, 27, 2089–2102. [Google Scholar] [CrossRef]
- Ślusarczyk, J.; Trojan, E.; Wydra, K.; Głombik, K.; Chamera, K.; Kucharczyk, M.; Budziszewska, B.; Kubera, M.; Lasoń, W.; Filip, M.; et al. Beneficial impact of intracerebroventricular fractalkine administration on behavioral and biochemical changes induced by prenatal stress in adult rats: Possible role of NLRP3 inflammasome pathway. Biochem. Pharmacol. 2016, 113, 45–56. [Google Scholar] [CrossRef]
- Tylek, K.; Trojan, E.; Leśkiewicz, M.; Regulska, M.; Bryniarska, N.; Curzytek, K.; Lacivita, E.; Leopoldo, M.; Basta-Kaim, A. Time-Dependent Protective and Pro-Resolving Effects of FPR2 Agonists on Lipopolysaccharide-Exposed Microglia Cells Involve Inhibition of NF-κB and MAPKs Pathways. Cells 2021, 10, 2373. [Google Scholar] [CrossRef] [PubMed]
- Gogolla, N.; Galimberti, I.; De Paola, V.; Caroni, P. Staining protocol for organotypic hippocampal slice cultures. Nat. Protoc. 2006, 1, 2452–2456. [Google Scholar] [CrossRef] [PubMed]
- Butt, H.-J.; Jaschke, M. Calculation of thermal noise in atomic force microscopy. Nanotechnology 1995, 6, 1. [Google Scholar] [CrossRef]
- Targosz-Korecka, M.; Daniel Brzezinka, G.; Danilkiewicz, J.; Rajfur, Z.; Szymonski, M. Glutaraldehyde fixation preserves the trend of elasticity alterations for endothelial cells exposed to TNF-α. Cytoskeleton 2015, 72, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, I.N. The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int. J. Eng. Sci. 1965, 3, 47–57. [Google Scholar] [CrossRef]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef]
- Bryniarska, N.; Kubiak, A.; Łabędź-Masłowska, A.; Zuba-Surma, E. Impact of developmental origin, niche mechanics and oxygen availability on osteogenic differentiation capacity of mesenchymal stem/stromal cells. Acta Biochim. Pol. 2019, 66, 491–498. [Google Scholar] [CrossRef]
- Nosi, D.; Lana, D.; Giovannini, M.G.; Delfino, G.; Zecchi-Orlandini, S. Neuroinflammation: Integrated Nervous Tissue Response through Intercellular Interactions at the “Whole System” Scale. Cells 2021, 10, 1195. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS Pro-Inflammatory Response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef]
- Li, Q.; Han, X.; Wang, J. Organotypic Hippocampal Slices as Models for Stroke and Traumatic Brain Injury. Mol. Neurobiol. 2015, 53, 4226–4237. [Google Scholar] [CrossRef]
- De Simoni, A.; Yu, L.M. Preparation of organotypic hippocampal slice cultures: Interface method. Nat. Protoc. 2006, 1, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zunich, S.M.; O’Connor, J.C.; Kavelaars, A.; Dantzer, R.; Kelley, K.W. Central administration of lipopolysaccharide induces depressive-like behavior in vivo and activates brain indoleamine 2,3 dioxygenase in murine organotypic hippocampal slice cultures. J. Neuroinflamm. 2010, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kim, H.; Kim, H.-J.; Lee, S.K.; Kim, E.W.; Namkoong, K.; Kim, E. Long-Term Culture of Organotypic Hippocampal Slice from Old 3xTg-AD Mouse: An ex vivo Model of Alzheimer’s Disease. Psychiatry Investig. 2018, 15, 205–213. [Google Scholar] [CrossRef]
- Daviaud, N.; Garbayo, E.; Lautram, N.; Franconi, F.; Lemaire, L.; Perez-Pinzon, M.; Montero-Menei, C. Modeling nigrostriatal degeneration in organotypic cultures, a new ex vivo model of Parkinson’s disease. Neuroscience 2013, 256, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Pringle, A.; Benham, C.; Sim, L.; Kennedy, J.; Iannotti, F.; Sundstrom, L. Selective N-type calcium channel antagonist omega conotoxin MVIIA is neuroprotective against hypoxic neurodegeneration in organotypic hippocampal-slice cultures. Stroke 1996, 27, 2124–2130. [Google Scholar] [CrossRef]
- Liu, J.-J.; Ding, X.-Y.; Xiang, L.; Zhao, F.; Huang, S.-L. A novel method for oxygen glucose deprivation model in organotypic spinal cord slices. Brain Res. Bull. 2017, 135, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Figiel-Dabrowska, A.; Radoszkiewicz, K.; Rybkowska, P.; Krzesniak, N.E.; Sulejczak, D.; Sarnowska, A. Neurogenic and Neuroprotective Potential of Stem/Stromal Cells Derived from Adipose Tissue. Cells 2021, 10, 1475. [Google Scholar] [CrossRef]
- Chip, S.; Nitsch, C.; Wellmann, S.; Kapfhammer, J.P. Subfield-specific neurovascular remodeling in the entorhino-hippocampal-organotypic slice culture as a response to oxygen-glucose deprivation and excitotoxic cell death. J. Cereb. Blood Flow Metab. 2012, 33, 508–518. [Google Scholar] [CrossRef]
- Van Kanegan, M.J.; He, D.N.; Dunn, D.E.; Yang, P.; Newman, R.A.; West, A.E.; Lo, D.C. BDNF mediates neuroprotection against oxygen-glucose deprivation by the cardiac glycoside oleandrin. J. Neurosci. 2014, 34, 963–968. [Google Scholar] [CrossRef]
- Lamprecht, M.R.; Morrison, B. GPR30 activation is neither necessary nor sufficient for acute neuroprotection by 17β-estradiol after an ischemic injury in organotypic hippocampal slice cultures. Brain Res. 2014, 1563, 131–137. [Google Scholar] [CrossRef]
- Trojan, E.; Tylek, K.; Leśkiewicz, M.; Lasoń, W.; Brandenburg, L.-O.; Leopoldo, M.; Lacivita, E.; Basta-Kaim, A. The N-Formyl Peptide Receptor 2 (FPR2) Agonist MR-39 Exhibits Anti-Inflammatory Activity in LPS-Stimulated Organotypic Hippocampal Cultures. Cells 2021, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Strasser, U.; Fischer, G. Quantitative measurement of neuronal degeneration in organotypic hippocampal cultures after combined oxygen/glucose deprivation. J. Neurosci. Methods 1995, 57, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, J.-J.; Li, S.-N.; Feng, J.; Liu, T.; Wang, F.; Dai, W.-Q.; Xia, Y.-J.; Lu, J.; Zhou, Y.-Q.; et al. 15-Deoxy-Δ12,14-prostaglandin J2 alleviates hepatic ischemia-reperfusion injury in mice via inducing antioxidant response and inhibiting apoptosis and autophagy. Acta Pharmacol. Sin. 2017, 38, 672–687. [Google Scholar] [CrossRef] [PubMed]
- Ślusarczyk, J.; Trojan, E.; Głombik, K.; Budziszewska, B.; Kubera, M.; Lasoń, W.; Popiołek-Barczyk, K.; Mika, J.; Wędzony, K.; Basta-Kaim, A. Prenatal stress is a vulnerability factor for altered morphology and biological activity of microglia cells. Front. Cell. Neurosci. 2015, 9, 82. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Araujo, I.M.; Carvalho, C.M. Role of nitric oxide and calpain activation in neuronal death and survival. Curr. Drug Target-CNS Neurol. Disord. 2005, 4, 319–324. [Google Scholar] [CrossRef]
- Hellwig-Bürgel, T.; Stiehl, D.P.; Wagner, A.E.; Metzen, E.; Jelkmann, W. Review: Hypoxia-inducible factor-1 (HIF-1): A novel transcription factor in immune reactions. J. Interf. Cytokine Res. 2005, 25, 297–310. [Google Scholar] [CrossRef]
- Ruscher, K.; Isaev, N.; Trendelenburg, G.; Weih, M.; Iurato, L.; Meisel, A.; Dirnagl, U. Induction of hypoxia inducible factor 1 by oxygen glucose deprivation is attenuated by hypoxic preconditioning in rat cultured neurons. Neurosci. Lett. 1998, 254, 117–120. [Google Scholar] [CrossRef]
- Liang, X.; Liu, X.; Lu, F.; Zhang, Y.; Jiang, X.; Ferriero, D.M. HIF1α Signaling in the Endogenous Protective Responses after Neonatal Brain Hypoxia-Ischemia. Dev. Neurosci. 2018, 40, 617–626. [Google Scholar] [CrossRef]
- Chen, W.; Jadhav, V.; Tang, J.; Zhang, J.H. HIF-1 alpha inhibition ameliorates neonatal brain damage after hypoxic-ischemic injury. Acta Neurochir. Suppl. 2008, 102, 395–399. [Google Scholar] [CrossRef]
- Shihan, M.H.; Novo, S.G.; Le Marchand, S.J.; Wang, Y.; Duncan, M.K. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 2021, 25, 100916. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-M.; Lin, L.; Wei, C.; Guo, Y.; Qin, Y.; Li, Z.-S.; Kent, T.A.; McCoy, C.E.; Wang, Z.-X.; Ni, Y.; et al. The Key Regulator of Necroptosis, RIP1 Kinase, Contributes to the Formation of Astrogliosis and Glial Scar in Ischemic Stroke. Transl. Stroke Res. 2021, 12, 991–1017. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.-X.; Yao, P.-S.; Chen, P.-P.; Guan, J.-H.; Zhuang, J.-H.; Zhu, J.-B.; Wu, G.; Yang, J.-S. Neuronal EphA4 Regulates OGD/R-Induced Apoptosis by Promoting Alternative Activation of Microglia. Inflammation 2018, 42, 572–585. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, S.; Song, Z.; Zhang, Q.; Guo, Y.; Yu, Y.; Wang, Y.; Li, T.; Megahed, F.A.K.; Addissouky, T.A.; et al. MLIF Modulates Microglia Polarization in Ischemic Stroke by Targeting eEF1A1. Front. Pharmacol. 2021, 12, 725268. [Google Scholar] [CrossRef] [PubMed]
- Mages, B.; Fuhs, T.; Aleithe, S.; Blietz, A.; Hobusch, C.; Härtig, W.; Schob, S.; Krueger, M.; Michalski, D. The Cytoskeletal Elements MAP2 and NF-L Show Substantial Alterations in Different Stroke Models While Elevated Serum Levels Highlight Especially MAP2 as a Sensitive Biomarker in Stroke Patients. Mol. Neurobiol. 2021, 58, 4051–4069. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Schröder, E.; Schmoll, H.; Walker, L.C.; Kessler, C. Upregulation of MAP1B and MAP2 in the rat brain after middle cerebral artery occlusion: Effect of age. J. Cereb. Blood Flow Metab. 1999, 19, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Nakano, F.; Liu, L.; Kawakita, F.; Kanamaru, H.; Nakatsuka, Y.; Nishikawa, H.; Okada, T.; Shiba, M.; Suzuki, H. Morphological Characteristics of Neuronal Death After Experimental Subarachnoid Hemorrhage in Mice Using Double Immunoenzymatic Technique. J. Histochem. Cytochem. 2019, 67, 919–930. [Google Scholar] [CrossRef]
- Yagita, Y.; Matsumoto, M.; Kitagawa, K.; Mabuchi, T.; Ohtsuki, T.; Hori, M.; Yanagihara, T. DNA cleavage and proteolysis of microtubule-associated protein 2 after cerebral ischemia of different severity. Neuroscience 1999, 92, 1417–1424. [Google Scholar] [CrossRef]
- Härtig, W.; Krueger, M.; Hofmann, S.; Preißler, H.; Märkel, M.; Frydrychowicz, C.; Mueller, W.C.; Bechmann, I.; Michalski, D. Up-regulation of neurofilament light chains is associated with diminished immunoreactivities for MAP2 and tau after ischemic stroke in rodents and in a human case. J. Chem. Neuroanat. 2016, 78, 140–148. [Google Scholar] [CrossRef]
- Pettigrew, L.C.; Holtz, M.L.; Craddock, S.D.; Minger, S.L.; Hall, N.; Geddes, J.W. Microtubular proteolysis in focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1996, 16, 1189–1202. [Google Scholar] [CrossRef]
- Yagensky, O.; Dehaghi, T.K.; Chua, J.J.E. The Roles of Microtubule-Based Transport at Presynaptic Nerve Terminals. Front. Synaptic Neurosci. 2016, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-Y.; Xiong, T.-Q.; Tan, B.-H.; Gui, Y.; Ye, N.; Li, S.-L.; Li, Y.-C. The temporal and spatial changes of actin cytoskeleton in the hippocampal CA1 neurons following transient global ischemia. Brain Res. 2019, 1720, 146297. [Google Scholar] [CrossRef]
- Zieliński, T.; Pabijan, J.; Zapotoczny, B.; Zemła, J.; Wesołowska, J.; Pera, J.; Lekka, M. Changes in nanomechanical properties of single neuroblastoma cells as a model for oxygen and glucose deprivation (OGD). Sci. Rep. 2022, 12, 16276. [Google Scholar] [CrossRef] [PubMed]
- Bryniarska-Kubiak, N.; Kubiak, A.; Lekka, M.; Basta-Kaim, A. The emerging role of mechanical and topographical factors in the development and treatment of nervous system disorders: Dark and light sides of the force. Pharmacol. Rep. 2021, 73, 1626–1641. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Chighizola, M.; Schulte, C.; Bryniarska, N.; Wesołowska, J.; Pudełek, M.; Lasota, M.; Ryszawy, D.; Basta-Kaim, A.; Laidler, P.; et al. Stiffening of DU145 prostate cancer cells driven by actin filaments—Microtubule crosstalk conferring resistance to microtubule-targeting drugs. Nanoscale 2021, 13, 6212–6226. [Google Scholar] [CrossRef]
- Gisselsson, L.; Toresson, H.; Ruscher, K.; Wieloch, T. Rho kinase inhibition protects CA1 cells in organotypic hippocampal slices during in vitro ischemia. Brain Res. 2010, 1316, 92–100. [Google Scholar] [CrossRef]
- Xiao, B.-G.; Ma, C.-G.; Wang, Q.; Song, L.-J.; Ding, Z.-B.; Chai, Z.; Yu, J.-Z. Advantages of Rho-associated kinases and their inhibitor fasudil for the treatment of neurodegenerative diseases. Neural Regen. Res. 2022, 17, 2623. [Google Scholar] [CrossRef]
- Atcha, H.; Jairaman, A.; Holt, J.R.; Meli, V.S.; Nagalla, R.R.; Veerasubramanian, P.K.; Brumm, K.T.; Lim, H.E.; Othy, S.; Cahalan, M.D.; et al. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat. Commun. 2021, 12, 3256. [Google Scholar] [CrossRef]
- Bryniarska-Kubiak, N.; Kubiak, A.; Basta-Kaim, A. Mechanotransductive receptor Piezo1 as a promising target in the treatment of neurological diseases. Curr. Neuropharmacol. 2022. [Google Scholar] [CrossRef]
- Ryu, Y.; Iwashita, M.; Lee, W.; Uchimura, K.; Kosodo, Y. A Shift in Tissue Stiffness During Hippocampal Maturation Correlates to the Pattern of Neurogenesis and Composition of the Extracellular Matrix. Front. Aging Neurosci. 2021, 13, 709620. [Google Scholar] [CrossRef]
- Holtzmann, K.; Gautier, H.O.; Christ, A.F.; Guck, J.; Káradóttir, R.T.; Franze, K. Brain tissue stiffness is a sensitive marker for acidosis. J. Neurosci. Methods 2016, 271, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Haga, H. Matrix Stiffness Contributes to Cancer Progression by Regulating Transcription Factors. Cancers 2022, 14, 1049. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.D.; Tellides, G. Central artery stiffness and thoracic aortopathy. Am. J. Physiol. Circ. Physiol. 2019, 316, H169–H182. [Google Scholar] [CrossRef]
- Mennens, S.F.B.; Bolomini-Vittori, M.; Weiden, J.; Joosten, B.; Cambi, A.; Dries, K.V.D. Substrate stiffness influences phenotype and function of human antigen-presenting dendritic cells. Sci. Rep. 2017, 7, 17511. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; Swartzlander, M.D.; Bryant, S.J. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res. Part A 2012, 100, 1375–1386. [Google Scholar] [CrossRef]
- Previtera, M.L.; Sengupta, A. Substrate Stiffness Regulates Proinflammatory Mediator Production through TLR4 Activity in Macrophages. PLoS ONE 2015, 10, e0145813. [Google Scholar] [CrossRef]
- Wahl, A.; Dinet, C.; Dillard, P.; Nassereddine, A.; Puech, P.-H.; Limozin, L.; Sengupta, K. Biphasic mechanosensitivity of T cell receptor-mediated spreading of lymphocytes. Proc. Natl. Acad. Sci. USA 2019, 116, 5908–5913. [Google Scholar] [CrossRef]
- Zak, A.; Merino-Cortés, S.V.; Sadoun, A.; Mustapha, F.; Babataheri, A.; Dogniaux, S.; Dupré-Crochet, S.; Hudik, E.; He, H.-T.; Barakat, A.I.; et al. Rapid viscoelastic changes are a hallmark of early leukocyte activation. Biophys. J. 2021, 120, 1692–1704. [Google Scholar] [CrossRef]


| Antibody/Dye | Dilution | Company |
|---|---|---|
| Anti-MAP-2 | 1:300 | Abcam, Cambridge, UK |
| Anti-IBA1 | 1:200 | Abcam, Cambridge, UK |
| Anti-beta-tubulin conjugated with Cy3 | 1:150 | Sigma-Aldrich, Saint Louis, MI, USA |
| Phalloidin conjugated with AlexaFluor488 | 1:200 | Invitrogen, Waltham, MA, USA |
| Antibody/Dye | Dilution | Company |
|---|---|---|
| Donkey anti-goat conjugated with Alexa Fluor 555 | 1:300 | Abcam, Cambridge, UK |
| Goat anti-rabbit conjugated with Alexa Fluor 647 | 1:200 | Abcam, Cambridge, UK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryniarska-Kubiak, N.; Kubiak, A.; Trojan, E.; Wesołowska, J.; Lekka, M.; Basta-Kaim, A. Oxygen-Glucose Deprivation in Organotypic Hippocampal Cultures Leads to Cytoskeleton Rearrangement and Immune Activation: Link to the Potential Pathomechanism of Ischaemic Stroke. Cells 2023, 12, 1465. https://doi.org/10.3390/cells12111465
Bryniarska-Kubiak N, Kubiak A, Trojan E, Wesołowska J, Lekka M, Basta-Kaim A. Oxygen-Glucose Deprivation in Organotypic Hippocampal Cultures Leads to Cytoskeleton Rearrangement and Immune Activation: Link to the Potential Pathomechanism of Ischaemic Stroke. Cells. 2023; 12(11):1465. https://doi.org/10.3390/cells12111465
Chicago/Turabian StyleBryniarska-Kubiak, Natalia, Andrzej Kubiak, Ewa Trojan, Julita Wesołowska, Małgorzata Lekka, and Agnieszka Basta-Kaim. 2023. "Oxygen-Glucose Deprivation in Organotypic Hippocampal Cultures Leads to Cytoskeleton Rearrangement and Immune Activation: Link to the Potential Pathomechanism of Ischaemic Stroke" Cells 12, no. 11: 1465. https://doi.org/10.3390/cells12111465
APA StyleBryniarska-Kubiak, N., Kubiak, A., Trojan, E., Wesołowska, J., Lekka, M., & Basta-Kaim, A. (2023). Oxygen-Glucose Deprivation in Organotypic Hippocampal Cultures Leads to Cytoskeleton Rearrangement and Immune Activation: Link to the Potential Pathomechanism of Ischaemic Stroke. Cells, 12(11), 1465. https://doi.org/10.3390/cells12111465







