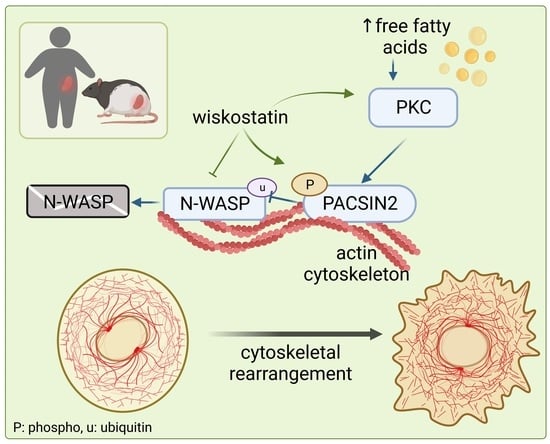
Phosphorylation of PACSIN2 at S313 Regulates Podocyte Architecture in Coordination with N-WASP
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ZDF Rat and Human Glomerular Lysates
2.2. Western Blotting
2.3. Cell Culture and Preparation of Cell Lysates
2.4. FFA Measurements
2.5. Apoptosis Assay
2.6. Adhesion Assay
2.7. Ubiquitination Assay
2.8. Immunofluorescence Analyses
2.9. Cell Classification
2.10. Statistical Analyses
3. Results
3.1. Phosphorylation of PACSIN2 at S313 Is Increased in the Glomeruli of Obese ZDF Rats
3.2. Phosphorylation of PACSIN2 at S313 Associates with DKD Rather Than with Diabetes
3.3. Phosphorylation of PACSIN2 at S313 Is Induced by Palmitate in a PKC-Dependent Manner
3.4. PACSIN2 Overexpression Reduces Apoptosis and Enhances Adhesion Independently of the Phosphorylation Status at S313
3.5. Phosphorylation of PACSIN2 at S313 Associates with Increased N-WASP Expression
3.6. Phosphorylation of PACSIN2 at S313 Is Regulated by N-WASP Activity
3.7. Dynamic Phosphorylation of PACSIN2 at S313 Is Required for Cell Spreading In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukasawa, R.; Kanda, A.; Hara, S. Anti-oxidative effects of rooibos tea extract on autoxidation and thermal oxidation of lipids. J. Oleo. Sci. 2009, 58, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Reiser, J.; Kriz, W.; Kretzler, M.; Mundel, P. The glomerular slit diaphragm is a modified adherens junction. J. Am. Soc. Nephrol. 2000, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kestila, M.; Lenkkeri, U.; Mannikko, M.; Lamerdin, J.; McCready, P.; Putaala, H.; Ruotsalainen, V.; Morita, T.; Nissinen, M.; Herva, R.; et al. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol. Cell 1998, 1, 575–582. [Google Scholar] [CrossRef]
- Yu, S.M.; Nissaisorakarn, P.; Husain, I.; Jim, B. Proteinuric Kidney Diseases: A Podocyte’s Slit Diaphragm and Cytoskeleton Approach. Front. Med. 2018, 5, 221. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Susztak, K. Podocytes: The Weakest Link in Diabetic Kidney Disease? Curr. Diab. Rep. 2016, 16, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinova, T.S.; Angulo-Urarte, A.; Nuchel, J.; Tauber, M.; van der Stoel, M.M.; Janssen, V.; de Haan, A.; Groenen, A.G.; Tebbens, M.; Graupera, M.; et al. A junctional PACSIN2/EHD4/MICAL-L1 complex coordinates VE-cadherin trafficking for endothelial migration and angiogenesis. Nat. Commun. 2021, 12, 2610. [Google Scholar] [CrossRef]
- Postema, M.M.; Grega-Larson, N.E.; Meenderink, L.M.; Tyska, M.J. PACSIN2-dependent apical endocytosis regulates the morphology of epithelial microvilli. Mol. Biol. Cell 2019, 30, 2515–2526. [Google Scholar] [CrossRef]
- Yao, G.; Luyten, A.; Takakura, A.; Plomann, M.; Zhou, J. The cytoplasmic protein Pacsin 2 in kidney development and injury repair. Kidney Int. 2013, 83, 426–437. [Google Scholar] [CrossRef] [Green Version]
- Senju, Y.; Rosenbaum, E.; Shah, C.; Hamada-Nakahara, S.; Itoh, Y.; Yamamoto, K.; Hanawa-Suetsugu, K.; Daumke, O.; Suetsugu, S. Phosphorylation of PACSIN2 by protein kinase C triggers the removal of caveolae from the plasma membrane. J. Cell Sci. 2015, 128, 2766–2780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumont, V.; Tolvanen, T.A.; Kuusela, S.; Wang, H.; Nyman, T.A.; Lindfors, S.; Tienari, J.; Nisen, H.; Suetsugu, S.; Plomann, M.; et al. PACSIN2 accelerates nephrin trafficking and is up-regulated in diabetic kidney disease. FASEB J. 2017, 31, 3978–3990. [Google Scholar] [CrossRef] [Green Version]
- Wasik, A.A.; Dumont, V.; Tienari, J.; Nyman, T.A.; Fogarty, C.L.; Forsblom, C.; Lehto, M.; Lehtonen, E.; Groop, P.H.; Lehtonen, S. Septin 7 reduces nonmuscle myosin IIA activity in the SNAP23 complex and hinders GLUT4 storage vesicle docking and fusion. Exp. Cell Res. 2017, 350, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Polianskyte-Prause, Z.; Tolvanen, T.A.; Lindfors, S.; Dumont, V.; Van, M.; Wang, H.; Dash, S.N.; Berg, M.; Naams, J.B.; Hautala, L.C.; et al. Metformin increases glucose uptake and acts renoprotectively by reducing SHIP2 activity. FASEB J. 2019, 33, 2858–2869. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Eckardt, K.U.; Dorman, N.M.; Christiansen, S.L.; Cheung, M.; Jadoul, M.; Winkelmayer, W.C. Nomenclature for kidney function and disease: Executive summary and glossary from a Kidney Disease: Improving Global Outcomes (KDIGO) consensus conference. Clin. Exp. Nephrol. 2020, 24, 737–747. [Google Scholar] [CrossRef]
- Suetsugu, S.; Hattori, M.; Miki, H.; Tezuka, T.; Yamamoto, T.; Mikoshiba, K.; Takenawa, T. Sustained activation of N-WASP through phosphorylation is essential for neurite extension. Dev. Cell 2002, 3, 645–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heikkila, E.; Ristola, M.; Havana, M.; Jones, N.; Holthofer, H.; Lehtonen, S. Trans-interaction of nephrin and Neph1/Neph3 induces cell adhesion that associates with decreased tyrosine phosphorylation of nephrin. Biochem. J. 2011, 435, 619–628. [Google Scholar] [CrossRef] [Green Version]
- McQuin, C.; Goodman, A.; Chernyshev, V.; Kamentsky, L.; Cimini, B.A.; Karhohs, K.W.; Doan, M.; Ding, L.; Rafelski, S.M.; Thirstrup, D.; et al. CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol. 2018, 16, e2005970. [Google Scholar] [CrossRef] [Green Version]
- Piccinini, F.; Balassa, T.; Szkalisity, A.; Molnar, C.; Paavolainen, L.; Kujala, K.; Buzas, K.; Sarazova, M.; Pietiainen, V.; Kutay, U.; et al. Advanced Cell Classifier: User-Friendly Machine-Learning-Based Software for Discovering Phenotypes in High-Content Imaging Data. Cell Syst. 2017, 4, 651–655. [Google Scholar] [CrossRef] [Green Version]
- Rutledge, J.C.; Ng, K.F.; Aung, H.H.; Wilson, D.W. Role of triglyceride-rich lipoproteins in diabetic nephropathy. Nat. Rev. Nephrol. 2010, 6, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Yu, L.; He, J.C.; Chen, A. Controversies in Podocyte Loss: Death or Detachment? Front. Cell Dev. Biol. 2021, 9, 771931. [Google Scholar] [CrossRef]
- Vogelmann, S.U.; Nelson, W.J.; Myers, B.D.; Lemley, K.V. Urinary excretion of viable podocytes in health and renal disease. Am. J. Physiol. Ren. Physiol. 2003, 285, F40–F48. [Google Scholar] [CrossRef] [Green Version]
- Susztak, K.; Raff, A.C.; Schiffer, M.; Bottinger, E.P. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 2006, 55, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Schell, C.; Baumhakl, L.; Salou, S.; Conzelmann, A.C.; Meyer, C.; Helmstaedter, M.; Wrede, C.; Grahamnner, F.; Eimer, S.; Kerjaschki, D.; et al. N-WASP Is Required for Stabilization of Podocyte Foot Processes. J. Am. Soc. Nephrol. 2013, 24, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.C.; et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 2017, 17, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Xu, B.; Li, J.Y.; Lu, H. LY294002 inhibits leukemia cell invasion and migration through early growth response gene 1 induction independent of phosphatidylinositol 3-kinase-Akt pathway. Biochem. Biophys. Res. Commun. 2008, 377, 187–190. [Google Scholar] [CrossRef]
- Hut, E.F.; Radulescu, M.; Pilut, N.; Macasoi, I.; Berceanu, D.; Coricovac, D.; Pinzaru, I.; Cretu, O.; Dehelean, C. Two Antibiotics, Ampicillin and Tetracycline, Exert Different Effects in HT-29 Colorectal Adenocarcinoma Cells in Terms of Cell Viability and Migration Capacity. Curr. Oncol. 2021, 28, 2466–2480. [Google Scholar] [CrossRef]
- Gokulan, K.; Cerniglia, C.E.; Thomas, C.; Pineiro, S.A.; Khare, S. Effects of residual levels of tetracycline on the barrier functions of human intestinal epithelial cells. Food Chem. Toxicol. 2017, 109, 253–263. [Google Scholar] [CrossRef]
- Dufour, A.; Zucker, S.; Sampson, N.S.; Kuscu, C.; Cao, J. Role of matrix metalloproteinase-9 dimers in cell migration: Design of inhibitory peptides. J. Biol. Chem. 2010, 285, 35944–35956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, A.; Silveira, G.G.; Soave, D.F.; Costa, J.P.O.; Silva, A.R. The Role of the LY294002 - A Non-Selective Inhibitor of Phosphatidylinositol 3-Kinase (PI3K) Pathway- in Cell Survival and Proliferation in Cell Line SCC-25. Asian Pac. J. Cancer Prev. 2019, 20, 3377–3383. [Google Scholar] [CrossRef]
- Haralick, R.M. Statistical and Structural Approaches to Texture. Proc. IEEE 1979, 67, 786–804. [Google Scholar] [CrossRef]
- Kessels, M.M.; Qualmann, B. Syndapins integrate N-WASP in receptor-mediated endocytosis. EMBO J. 2002, 21, 6083–6094. [Google Scholar] [CrossRef] [Green Version]
- Taunton, J.; Rowning, B.A.; Coughlin, M.L.; Wu, M.; Moon, R.T.; Mitchison, T.J.; Larabell, C.A. Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J. Cell Biol. 2000, 148, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Qualmann, B.; Kelly, R.B. Syndapin isoforms participate in receptor-mediated endocytosis and actin organization. J. Cell Biol. 2000, 148, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Kostan, J.; Salzer, U.; Orlova, A.; Toro, I.; Hodnik, V.; Senju, Y.; Zou, J.; Schreiner, C.; Steiner, J.; Merilainen, J.; et al. Direct interaction of actin filaments with F-BAR protein pacsin2. EMBO Rep. 2014, 15, 1154–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, J.; Rikhy, R.; Lippincott-Schwartz, J. Dynamin 2 orchestrates the global actomyosin cytoskeleton for epithelial maintenance and apical constriction. Proc. Natl. Acad. Sci. USA 2009, 106, 20770–20775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Kreu, B.J.; Nethe, M.; Fernandez-Borja, M.; Anthony, E.C.; Hensbergen, P.J.; Deelder, A.M.; Plomann, M.; Hordijk, P.L. The F-BAR domain protein PACSIN2 associates with Rac1 and regulates cell spreading and migration. J. Cell Sci. 2011, 124, 2375–2388. [Google Scholar] [CrossRef] [Green Version]
- Modregger, J.; Ritter, B.; Witter, B.; Paulsson, M.; Plomann, M. All three PACSIN isoforms bind to endocytic proteins and inhibit endocytosis. J. Cell Sci. 2000, 113, 4511–4521. [Google Scholar] [CrossRef]
- Moldovan, L.; Irani, K.; Moldovan, N.I.; Finkel, T.; Goldschmidt-Clermont, P.J. The actin cytoskeleton reorganization induced by Rac1 requires the production of superoxide. Antioxid. Redox Signal. 1999, 1, 29–43. [Google Scholar] [CrossRef]
- Padrick, S.B.; Rosen, M.K. Physical mechanisms of signal integration by WASP family proteins. Annu. Rev. Biochem. 2010, 79, 707–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quack, I.; Woznowski, M.; Potthoff, S.A.; Palmer, R.; Konigshausen, E.; Sivritas, S.; Schiffer, M.; Stegbauer, J.; Vonend, O.; Rump, L.C.; et al. PKC alpha mediates beta-arrestin2-dependent nephrin endocytosis in hyperglycemia. J. Biol. Chem. 2011, 286, 12959–12970. [Google Scholar] [CrossRef] [Green Version]
- Tossidou, I.; Teng, B.; Menne, J.; Shushakova, N.; Park, J.K.; Becker, J.U.; Modde, F.; Leitges, M.; Haller, H.; Schiffer, M. Podocytic PKC-alpha is regulated in murine and human diabetes and mediates nephrin endocytosis. PLoS ONE 2010, 5, e10185. [Google Scholar] [CrossRef] [Green Version]
- Hagenfeldt, L.; Wahren, J.; Pernow, B.; Raf, L. Uptake of individual free fatty acids by skeletal muscle and liver in man. J. Clin. Investig. 1972, 51, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Nam, S.M.; Kim, J.H.; Das, R.; Choi, S.K.; Nguyen, T.T.; Quan, X.; Choi, S.J.; Chung, C.H.; Lee, E.Y.; et al. Palmitate induces ER calcium depletion and apoptosis in mouse podocytes subsequent to mitochondrial oxidative stress. Cell Death Dis. 2015, 6, e1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouslama, R.; Dumont, V.; Lindfors, S.; Paavolainen, L.; Tienari, J.; Nisen, H.; Mirtti, T.; Saleem, M.A.; Gordin, D.; Groop, P.-H.; et al. Phosphorylation of PACSIN2 at S313 Regulates Podocyte Architecture in Coordination with N-WASP. Cells 2023, 12, 1487. https://doi.org/10.3390/cells12111487
Bouslama R, Dumont V, Lindfors S, Paavolainen L, Tienari J, Nisen H, Mirtti T, Saleem MA, Gordin D, Groop P-H, et al. Phosphorylation of PACSIN2 at S313 Regulates Podocyte Architecture in Coordination with N-WASP. Cells. 2023; 12(11):1487. https://doi.org/10.3390/cells12111487
Chicago/Turabian StyleBouslama, Rim, Vincent Dumont, Sonja Lindfors, Lassi Paavolainen, Jukka Tienari, Harry Nisen, Tuomas Mirtti, Moin A. Saleem, Daniel Gordin, Per-Henrik Groop, and et al. 2023. "Phosphorylation of PACSIN2 at S313 Regulates Podocyte Architecture in Coordination with N-WASP" Cells 12, no. 11: 1487. https://doi.org/10.3390/cells12111487