Histone Deacetylase GiSRT2 Negatively Regulates Flavonoid Biosynthesis in Glycyrrhiza inflata
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and NIC Treatment
2.2. RNA Extraction and qRT-PCR Analysis
2.3. Vector Construction
2.4. Generation of Transgenic Hairy Roots of G. inflata
2.5. Compound Extraction and Determination
2.6. Transcriptomic and Metabolomic Analysis
2.7. Enzyme Assay of GiLMT1
2.8. Subcellular Localization Analysis of GiSRT2
3. Results
3.1. Effects of NIC Treatment on Seedling Growth and LCA Accumulation in G. inflata
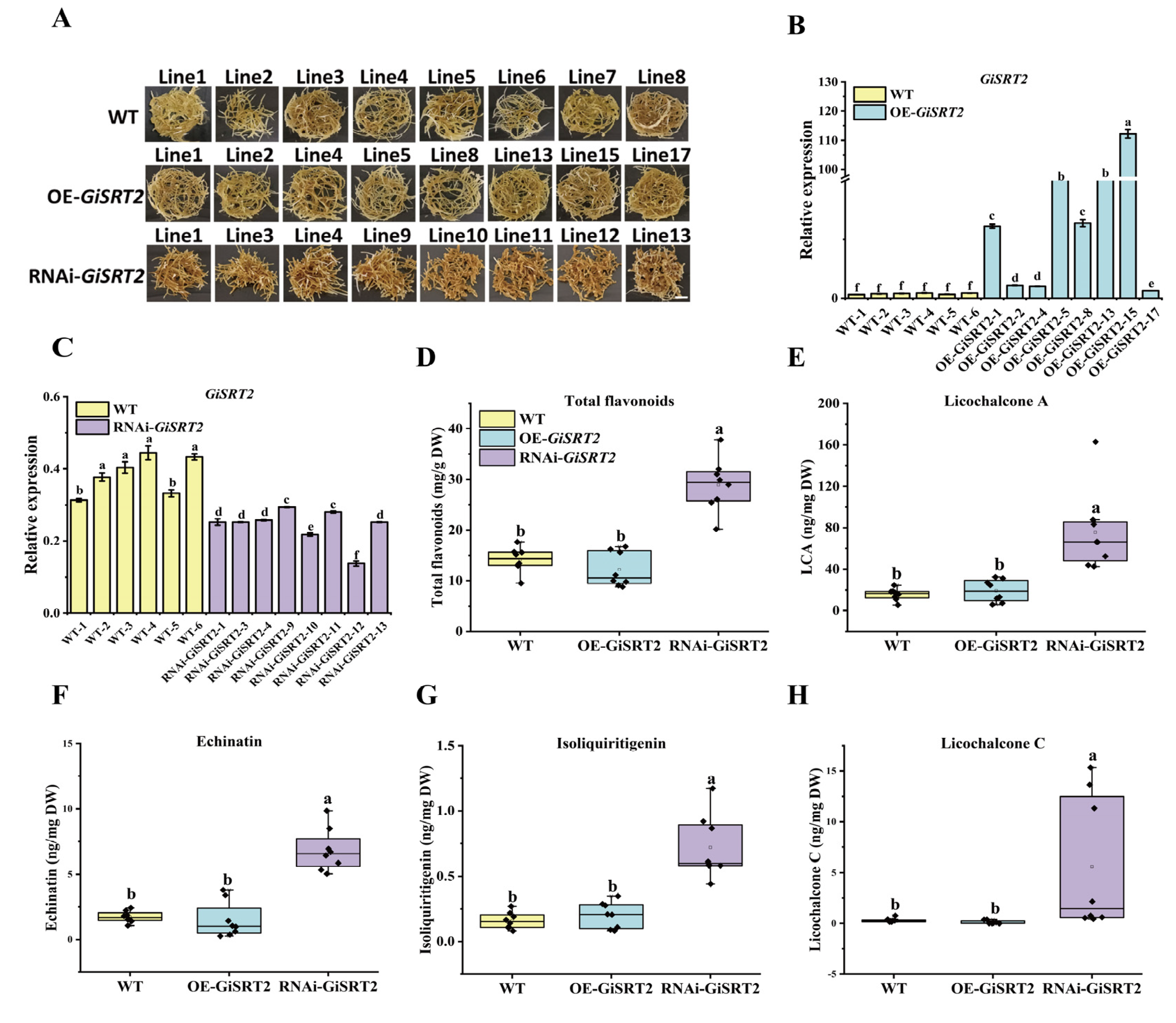
3.2. GiSRT2 Negatively Regulates LCA Accumulation
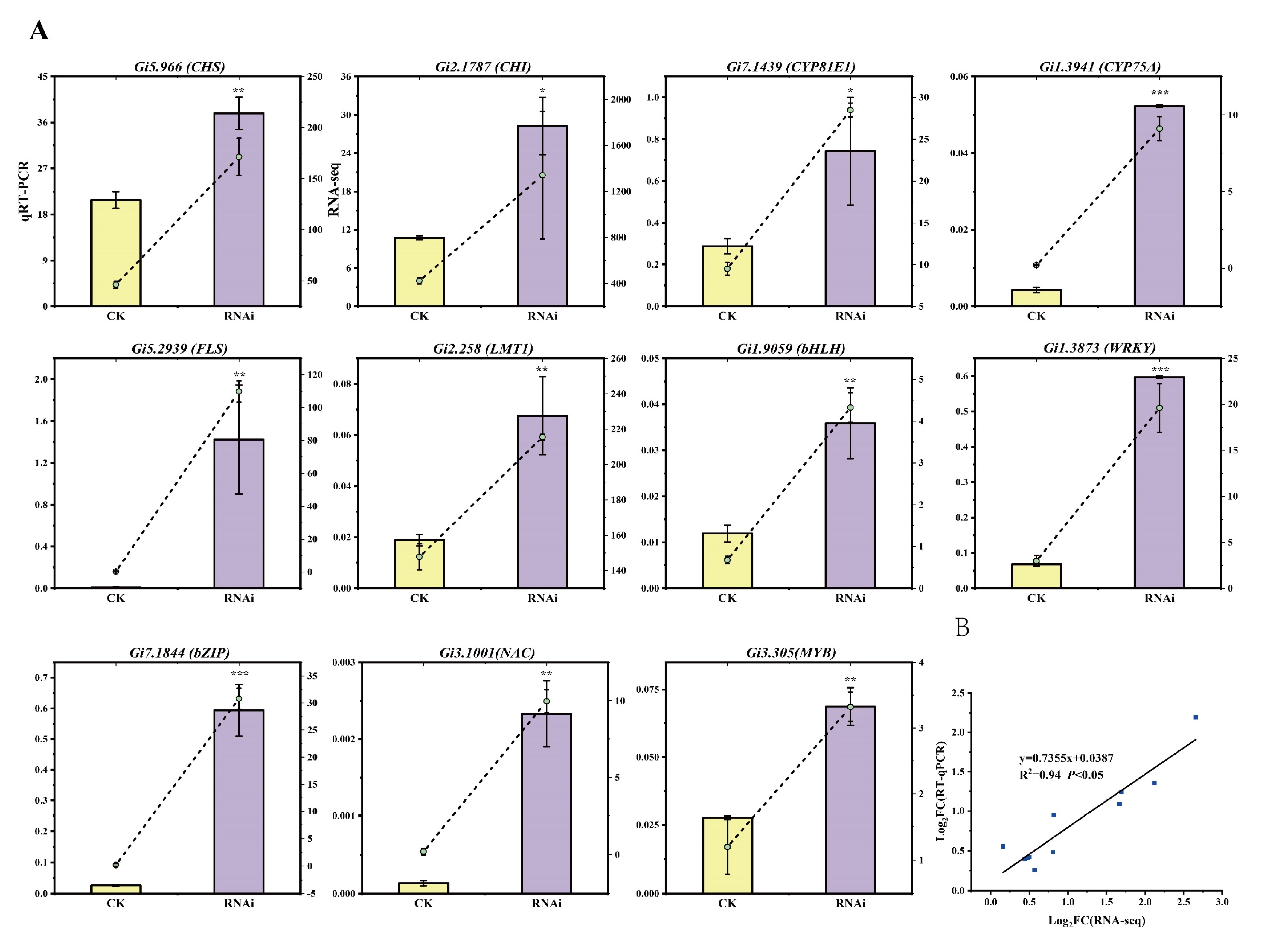
3.3. Transcriptome Sequencing (RNA-Seq) Analysis of RNAi-GiSRT2 Lines
3.4. Metabolome Analysis of RNAi-GiSRT2 Lines
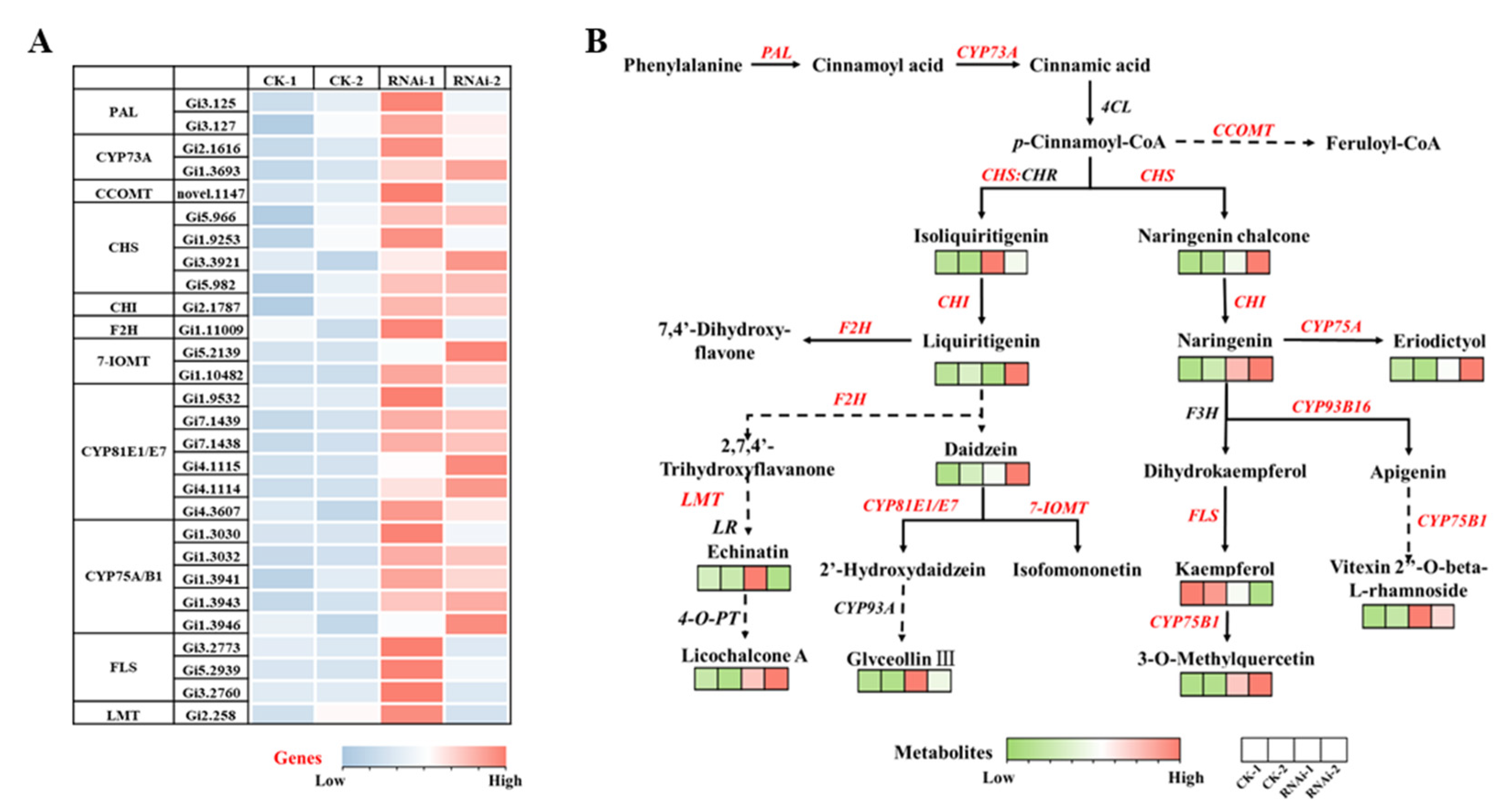
3.5. Combined Analysis of Transcriptome and Metabolome
3.6. Analysis of UDEGs Involved in Flavonoid Biosynthetic Pathways in RNAi-GiSRT2 Hairy Roots
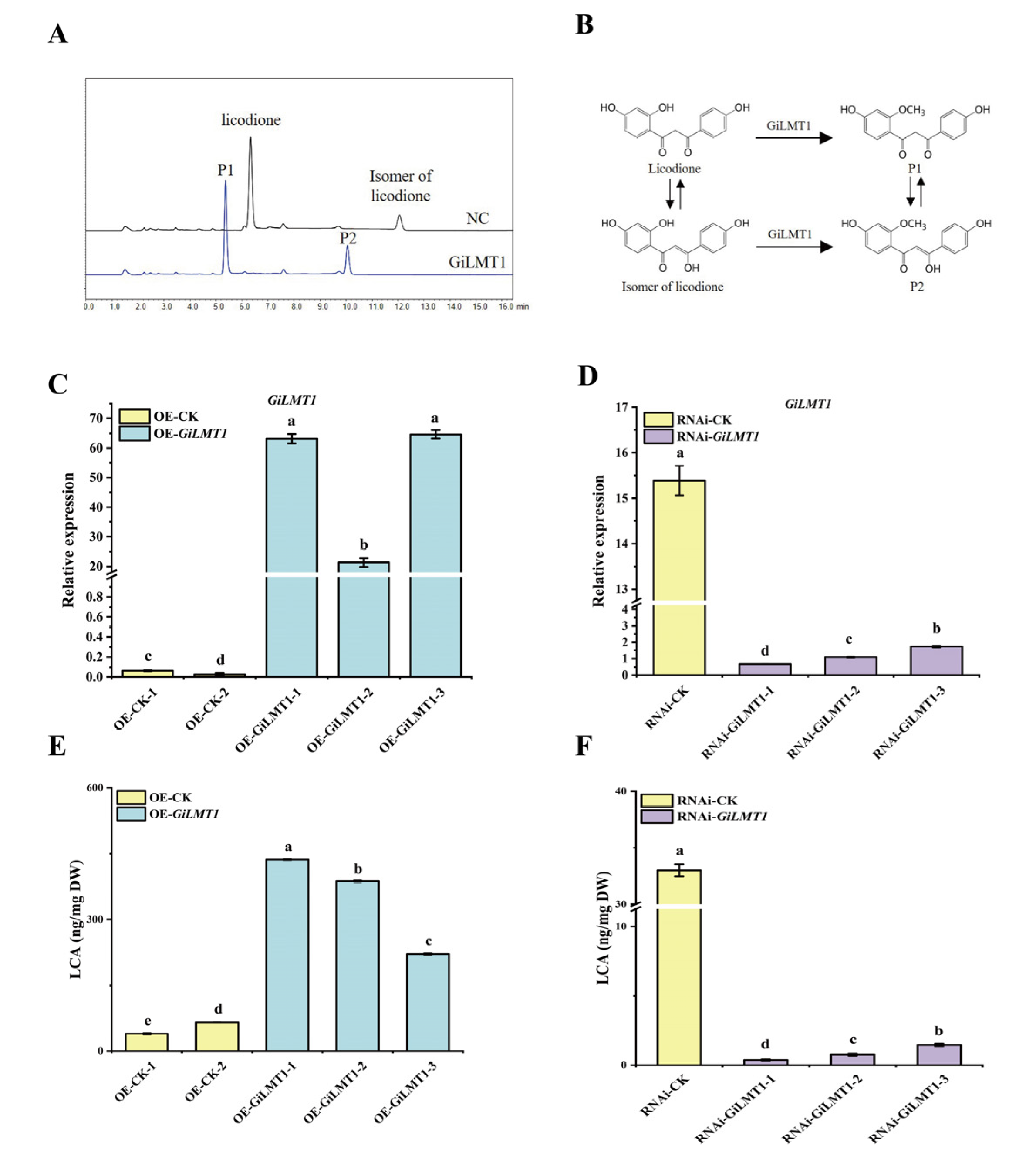
3.7. GiLMT1 Is Involved in the Biosynthesis of LCA in G. inflata
4. Discussion
4.1. The Role of GiSRT2 in Balancing Growth and Resistance of G. inflata
4.2. Determination of Gene Expression and Metabolic Changes in RNAi-GiSRT2 Hairy Roots through Transcriptomic and Metabolomic Techniques
4.3. GiLMT1 Is Required for LCA Biosynthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 7-IOMT | isoflavone 7-O-methyltransferase |
| CCOMT | caffeoyl CoA O-methyltransferase |
| CDS | coding sequence |
| CHI | chalcone isomerase |
| CHR | chalcone reductase |
| CHS | chalcone synthase |
| CYP73A | trans-cinnamate 4-monooxygenase |
| CYP75A | flavonoid 3′,5′-hydroxylase |
| CYP75B1 | flavonoid 3′-monooxygenase |
| DAM | differentially accumulated metabolite |
| DEG | differentially expressed gene |
| F2H | flavanone 2-hydroxylase |
| FLS | flavonol synthase |
| HDAC | histone deacetylase |
| HPLC | high performance liquid chromatography |
| LCA | licochalcone A |
| LCC | licochalcone C |
| LMT | licodione 2′-O-methyltransferase |
| NIC | nicotinamide |
| PAL | phenylalanine ammonia lyase |
| SAM | S-adenosyl methionine |
References
- Kondo, K.; Shiba, M.; Nakamura, R.; Morota, T.; Shoyama, Y. Constituent properties of licorices derived from Glycyrrhiza uralensis, G. glabra, or G. inflata identified by genetic information. Biol. Pharm. Bull. 2007, 30, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Li, M.T.; Xie, L.; Jiang, H.M.; Huang, Q.; Tong, R.S.; Li, X.; Xie, X.; Liu, H.M. Role of licochalcone A in potential pharmacological therapy: A review. Front. Pharmacol. 2022, 13, 878776. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.Y.; Lee, C.H.; Lee, H.L.; Su, C.Y.; Kao, C.Y.; Tsai, J.P.; Hsieh, Y.H. Licochalcone A suppresses renal cancer cell proliferation and metastasis by engagement of Sp1-mediated LC3 expression. Pharmaceutics 2023, 15, 684. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Xu, Y.C.; Deng, B.; Chen, J.B.; Chen, T.F.; Zeng, K.F.; Chen, S.; Deng, S.H.; Tan, Z.B.; Ding, W.J.; et al. Radix Glycyrrhizae extract and licochalcone a exert an anti-inflammatory action by direct suppression of toll like receptor 4. J. Ethnopharmacol. 2023, 302, 115869. [Google Scholar] [CrossRef]
- Chen, W.P.; Hu, Z.N.; Jin, L.B.; Wu, L.D. Licochalcone A inhibits MMPs and ADAMTSs via the NF-κB and wnt/β-catenin signaling pathways in rat chondrocytes. Cell Physiol. Biochem. 2017, 43, 937–944. [Google Scholar] [CrossRef]
- Liang, M.; Li, X.; Ouyang, X.; Xie, H.; Chen, D. Antioxidant Mechanisms of Echinatin and Licochalcone A. Molecules 2018, 24, 3. [Google Scholar] [CrossRef]
- Li, P.; Yu, C.; Zeng, F.S.; Fu, X.; Yuan, X.J.; Wang, Q.; Fan, C.; Sun, B.L.; Sun, Q.S. Licochalcone A attenuates chronic neuropathic pain in rats by inhibiting microglia activation and inflammation. Neurochem. Res. 2021, 46, 1112–1118. [Google Scholar] [CrossRef]
- Jeon, J.H.; Kim, M.R.; Jun, J.G. Concise synthesis of licochalcone A through water-accelerated [3,3]-sigmatropic rearrangement of an aryl prenyl ether. Synthesis 2011, 3, 370–376. [Google Scholar] [CrossRef]
- Otani, K.; Takahashi, T.; Furuya, T.; Ayabe, S. Licodione synthase, a cytochrome P450 monooxygenase catalyzing 2-hydroxylation of 5-deoxyflavanone, in cultured Glycyrrhiza echinata L. cells. Plant Physiol. 1994, 105, 1427–1432. [Google Scholar] [CrossRef]
- Ayabe, S.; Yoshikawa, T.; Kobayashi, M.; Furuya, T. Biosynthesis of a retrochalcone, echinatin: Involvement of O-methyltransferase to licodione. Phytochemistry 1980, 19, 2331–2336. [Google Scholar] [CrossRef]
- Wu, Z.G.; Singh, S.K.; Lyu, R.; Pattanaik, S.; Wang, Y.; Li, Y.Q.; Yuan, L.; Liu, Y.L. Metabolic engineering to enhance the accumulation of bioactive flavonoids licochalcone A and echinatin in Glycyrrhiza inflata (Licorice) hairy roots. Front. Plant Sci. 2022, 13, 932594. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.E.; Hu, Z.; Li, F.; Zhang, L.; Yu, X.; Tang, B.; Chen, G. Silencing of histone deacetylase SlHDT3 delays fruit ripening and suppresses carotenoid accumulation in tomato. Plant Sci. 2017, 265, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Liu, S.; Liu, X.; Zhang, B.; Li, M.; Zeng, L.; Li, L. Transcriptome profiling reveals histone deacetylase 1 gene overexpression improves flavonoid, isoflavonoid, and phenylpropanoid metabolism in Arachis hypogaea hairy roots. PeerJ 2021, 9, e10976. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.F.; Hyun, T.K. Histone deacetylase inhibitors improve MeJA-induced ginsenoside production in ginseng adventitious roots. Ind. Crops Prod. 2021, 171, 113909. [Google Scholar] [CrossRef]
- Zheng, W. Review: The plant sirtuins. Plant Sci. 2020, 293, 110434. [Google Scholar] [CrossRef] [PubMed]
- König, A.C.; Hartl, M.; Pham, P.A.; Laxa, M.; Boersema, P.J.; Orwat, A.; Kalitventseva, I.; Plöchinger, M.; Braun, H.P.; Leister, D.; et al. The Arabidopsis class II sirtuin is a lysine deacetylase and interacts with mitochondrial energy metabolism. Plant. Physiol. 2014, 164, 1401–1414. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.; Ko, E.E.; Shao, K.; Qiao, H. Histone deacetylases SRT1 and SRT2 interact with ENAP1 to mediate ethylene-induced transcriptional repression. Plant Cell 2018, 30, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y.; Zhou, D.X. Rice NAD+-dependent histone deacetylase OsSRT1 represses glycolysis and regulates the moonlighting function of GAPDH as a transcriptional activator of glycolytic genes. Nucleic Acids Res. 2017, 45, 12241–12255. [Google Scholar] [CrossRef]
- Hu, J.; He, B.; Bhargava, S.; Lin, H. A fluorogenic assay for screening Sirt6 modulators. Org. Biomol. Chem. 2013, 11, 5213–5216. [Google Scholar] [CrossRef]
- Li, Y.P.; Zhuang, F.; Zeng, J.Y.; Yang, C.; Li, Y.Q.; Luo, M.; Wang, Y. Identification of the histone demethylases gene family in Glycyrrhiza inflata reveals genes responding to abiotic stresses. J. Cell Biochem. 2022, 123, 1780–1792. [Google Scholar] [CrossRef]
- Li, Y.P.; Liang, X.J.; Zhou, X.G.; An, Y.; Li, M.; Yuan, L.; Li, Y.Q.; Wang, Y. Spatio-temporal selection of reference genes in the two congeneric species of Glycyrrhiza. Sci. Rep. 2021, 11, 1122. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Shen, W.; Gao, X.; Li, X.; Zhou, P.; Duan, J. High-throughput construction of intron-containing hairpin RNA vectors for RNAi in plants. PLoS ONE 2012, 7, e38186. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.Y.; Feng, Y.L.; Zhou, Y.S.; Song, X.M.; Li, H.L.; Ma, Y.; Ye, C.L.; Yu, X.P. Schaftoside interacts with NlCDK1 protein: A mechanism of rice resistance to brown planthopper, Nilaparvata lugens. Front. Plant Sci. 2018, 9, 710. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Guo, K.; Liu, L.; Tian, W.; Xie, X.; Wen, S.; Wen, C. Integrated transcriptomic and metabolomic data reveal the flavonoid biosynthesis metabolic pathway in Perilla frutescens (L.) leaves. Sci. Rep. 2020, 10, 16207. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Qiao, X.; Chen, K.; Wang, Y.; Ji, S.; Feng, J.; Li, K.; Lin, Y.; Ye, M. Biosynthesis-based quantitative analysis of 151 secondary metabolites of licorice to differentiate medicinal Glycyrrhiza species and their hybrids. Anal. Chem. 2017, 89, 3146–3153. [Google Scholar] [CrossRef]
- Maxwell, C.A.; Harrison, M.J.; Dixon, R.A. Molecular characterization and expression of alfalfa isoliquiritigenin 2′-O-methyltransferase, an enzyme specifically involved in the biosynthesis of an inducer of Rhizobium meliloti nodulation genes. Plant J. 1993, 4, 971–981. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Mathesius, U. Flavonoids induced in cells undergoing nodule organogenesis in white clover are regulators of auxin breakdown by peroxidase. J. Exp. Bot. 2001, 52, 419–426. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Huang, Y.; Zhou, L.; Liang, X.; Yang, C.; Wang, H.; Yuan, L.; Wang, Y.; Li, Y. Histone Deacetylase GiSRT2 Negatively Regulates Flavonoid Biosynthesis in Glycyrrhiza inflata. Cells 2023, 12, 1501. https://doi.org/10.3390/cells12111501
Zeng J, Huang Y, Zhou L, Liang X, Yang C, Wang H, Yuan L, Wang Y, Li Y. Histone Deacetylase GiSRT2 Negatively Regulates Flavonoid Biosynthesis in Glycyrrhiza inflata. Cells. 2023; 12(11):1501. https://doi.org/10.3390/cells12111501
Chicago/Turabian StyleZeng, Jiangyi, Yun Huang, Lijun Zhou, Xiaoju Liang, Chao Yang, Hongxia Wang, Ling Yuan, Ying Wang, and Yongqing Li. 2023. "Histone Deacetylase GiSRT2 Negatively Regulates Flavonoid Biosynthesis in Glycyrrhiza inflata" Cells 12, no. 11: 1501. https://doi.org/10.3390/cells12111501





