Therapeutic Strategies Targeting Respiratory Recovery after Spinal Cord Injury: From Preclinical Development to Clinical Translation
Abstract
1. Introduction
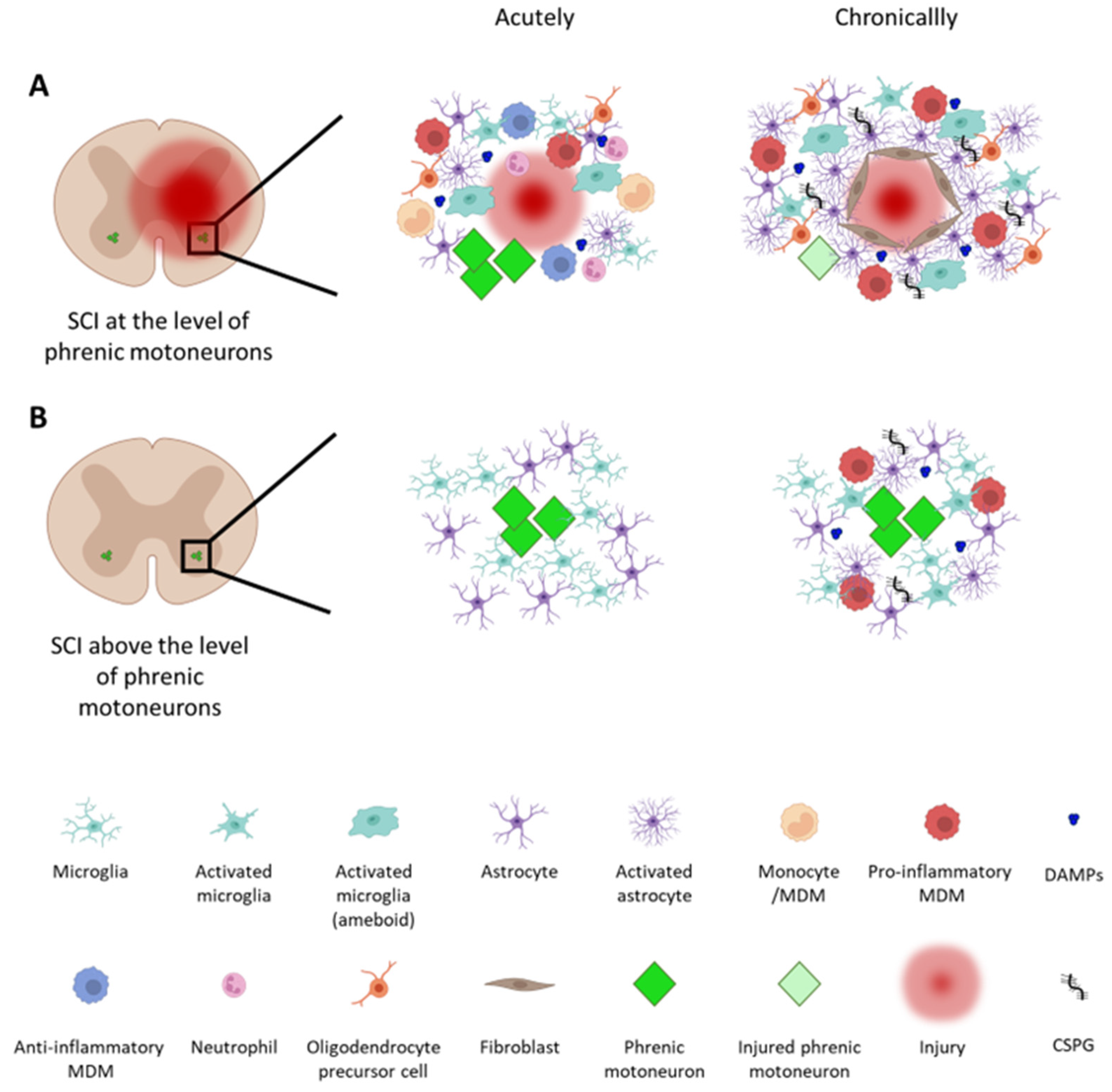
1.1. Inflammation
1.2. Plasticity-Supporting and Inhibitory Factors
1.2.1. Plasticity-Supporting Molecules
1.2.2. Inhibitory Molecules
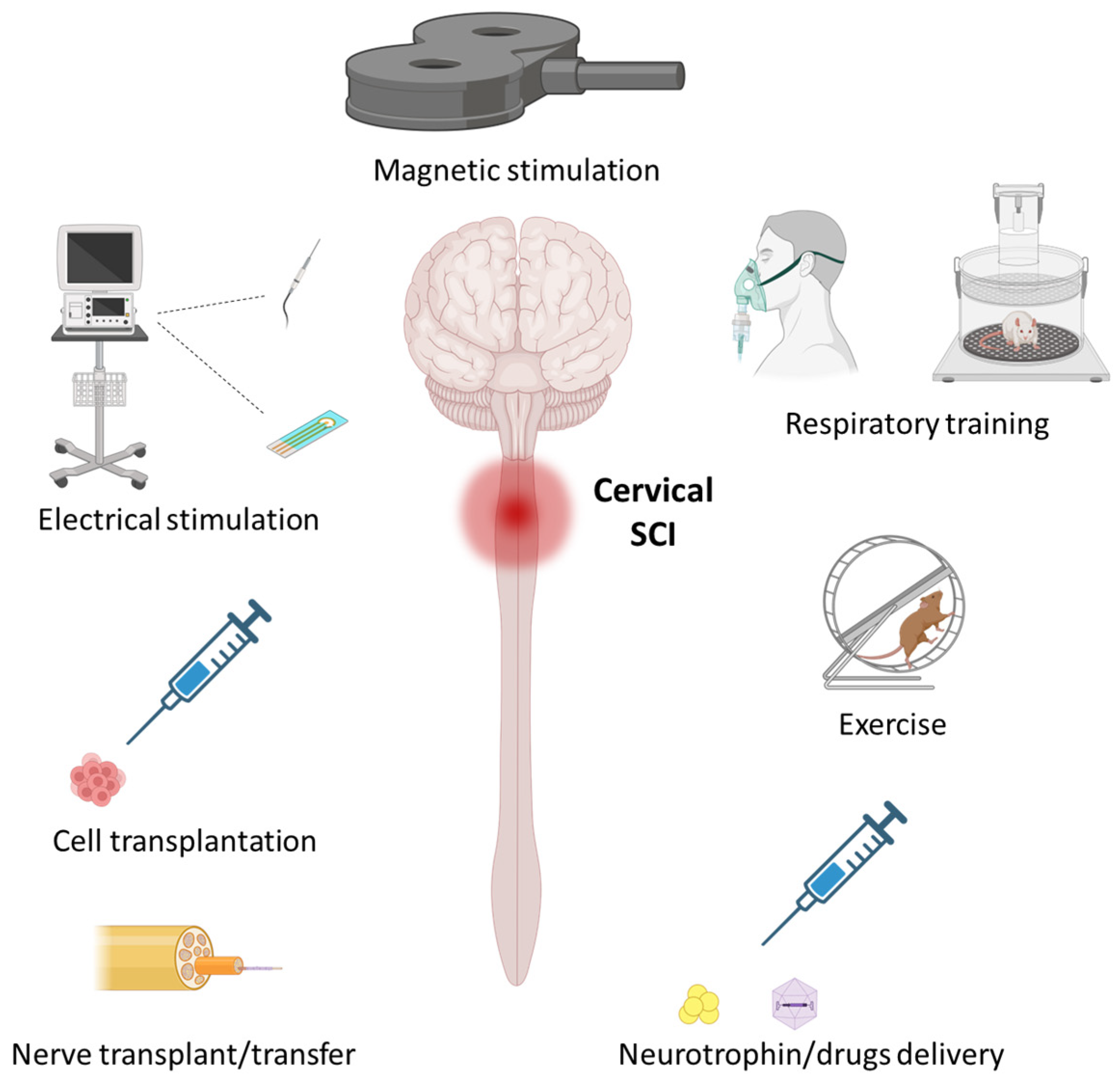
1.3. From Preclinical Models to Humans
2. Activity-Based Therapeutics
2.1. Intermittent Hypoxia
2.2. Exercise
3. Stimulation-Based Therapeutics
3.1. Invasive Stimulation
3.2. Non-Invasive Stimulation
4. Therapeutics for Inducing Regeneration/Reorganization of Neural Pathways
4.1. Cell Transplantation
4.2. Nerve Grafts/Nerve Transfers
4.3. Harnessing the Extracellular Environment
4.4. Neurotrophin Delivery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, R.; Lim, J.; Mekary, R.A.; Rattani, A.; Dewan, M.C.; Sharif, S.Y.; Osorio-Fonseca, E.; Park, K.B. Traumatic Spinal Injury: Global Epidemiology and Worldwide Volume. World Neurosurg. 2018, 113, e345–e363. [Google Scholar] [CrossRef]
- Hou, S.; Rabchevsky, A.G. Autonomic consequences of spinal cord injury. Compr. Physiol. 2014, 4, 1419–1453. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017, 3, 17018. [Google Scholar] [CrossRef]
- Burns, S.P. Acute respiratory infections in persons with spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 2007, 18, 203–216. [Google Scholar] [CrossRef]
- Raab, A.M.; Mueller, G.; Elsig, S.; Gandevia, S.C.; Zwahlen, M.; Hopman, M.T.E.; Hilfiker, R. Systematic Review of Incidence Studies of Pneumonia in Persons with Spinal Cord Injury. J. Clin. Med. 2021, 11, 211. [Google Scholar] [CrossRef]
- Locke, K.C.; Randelman, M.L.; Hoh, D.J.; Zholudeva, L.V.; Lane, M.A. Respiratory plasticity following spinal cord injury: Perspectives from mouse to man. Neural Regen. Res. 2022, 17, 2141–2148. [Google Scholar] [CrossRef]
- Charsar, B.A.; Urban, M.W.; Lepore, A.C. Harnessing the power of cell transplantation to target respiratory dysfunction following spinal cord injury. Exp. Neurol. 2017, 287, 268–275. [Google Scholar] [CrossRef]
- Vose, A.K.; Welch, J.F.; Nair, J.; Dale, E.A.; Fox, E.J.; Muir, G.D.; Trumbower, R.D.; Mitchell, G.S. Therapeutic acute intermittent hypoxia: A translational roadmap for spinal cord injury and neuromuscular disease. Exp. Neurol. 2022, 347, 113891. [Google Scholar] [CrossRef]
- Haggerty, A.E.; Marlow, M.M.; Oudega, M. Extracellular matrix components as therapeutics for spinal cord injury. Neurosci. Lett. 2017, 652, 50–55. [Google Scholar] [CrossRef]
- Ghali, M.G.Z. The crossed phrenic phenomenon. Neural Regen. Res. 2017, 12, 845–864. [Google Scholar] [CrossRef]
- Beattie, M.S.; Hermann, G.E.; Rogers, R.C.; Bresnahan, J.C. Cell death in models of spinal cord injury. Prog. Brain Res. 2002, 137, 37–47. [Google Scholar] [CrossRef]
- Mautes, A.E.; Weinzierl, M.R.; Donovan, F.; Noble, L.J. Vascular Events after Spinal Cord Injury: Contribution to Secondary Pathogenesis. Phys. Ther. 2000, 80, 673–687. [Google Scholar] [CrossRef]
- Tator, C.H.; Fehlings, M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 1991, 75, 15–26. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G. Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp. Neurol. 2014, 258, 24–34. [Google Scholar] [CrossRef]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.E.; Vernoux, N.; Tremblay, M.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef]
- Illes, P.; Rubini, P.; Ulrich, H.; Zhao, Y.; Tang, Y. Regulation of Microglial Functions by Purinergic Mechanisms in the Healthy and Diseased CNS. Cells 2020, 9, 1108. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef]
- James, G.; Butt, A.M. P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. Eur. J. Pharm. 2002, 447, 247–260. [Google Scholar] [CrossRef]
- Park, E.; Velumian, A.A.; Fehlings, M.G. The role of excitotoxicity in secondary mechanisms of spinal cord injury: A review with an emphasis on the implications for white matter degeneration. J. Neurotrauma 2004, 21, 754–774. [Google Scholar] [CrossRef]
- Choi, D.W. Excitotoxicity: Still Hammering the Ischemic Brain in 2020. Front. Neurosci. 2020, 14, 579953. [Google Scholar] [CrossRef]
- Jia, M.; Njapo, S.A.; Rastogi, V.; Hedna, V.S. Taming glutamate excitotoxicity: Strategic pathway modulation for neuroprotection. CNS Drugs 2015, 29, 153–162. [Google Scholar] [CrossRef]
- Brockie, S.; Hong, J.; Fehlings, M.G. The Role of Microglia in Modulating Neuroinflammation after Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 9706. [Google Scholar] [CrossRef]
- Kroner, A.; Rosas Almanza, J. Role of microglia in spinal cord injury. Neurosci. Lett. 2019, 709, 134370. [Google Scholar] [CrossRef]
- Zivkovic, S.; Ayazi, M.; Hammel, G.; Ren, Y. For Better or for Worse: A Look Into Neutrophils in Traumatic Spinal Cord Injury. Front. Cell Neurosci. 2021, 15, 648076. [Google Scholar] [CrossRef]
- Popovich, P.G.; Stokes, B.T.; Whitacre, C.C. Concept of autoimmunity following spinal cord injury: Possible roles for T lymphocytes in the traumatized central nervous system. J. Neurosci. Res. 1996, 45, 349–363. [Google Scholar] [CrossRef]
- Ankeny, D.P.; Guan, Z.; Popovich, P.G. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J. Clin. Investig. 2009, 119, 2990–2999. [Google Scholar] [CrossRef]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef]
- Donnelly, D.J.; Popovich, P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Cregg, J.M.; DePaul, M.A.; Filous, A.R.; Lang, B.T.; Tran, A.; Silver, J. Functional regeneration beyond the glial scar. Exp. Neurol. 2014, 253, 197–207. [Google Scholar] [CrossRef]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success after Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. New insights into glial scar formation after spinal cord injury. Cell Tissue Res. 2022, 387, 319–336. [Google Scholar] [CrossRef]
- Rowland, J.W.; Hawryluk, G.W.; Kwon, B.; Fehlings, M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef]
- Keefe, K.M.; Sheikh, I.S.; Smith, G.M. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef]
- Sieck, G.C.; Mantilla, C.B. Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respir. Physiol. Neurobiol. 2009, 169, 218–225. [Google Scholar] [CrossRef]
- Logan, A.; Berry, M. Transforming growth factor-beta 1 and basic fibroblast growth factor in the injured CNS. Trends Pharm. Sci. 1993, 14, 337–342. [Google Scholar] [CrossRef]
- Ip, N.Y.; Stitt, T.N.; Tapley, P.; Klein, R.; Glass, D.J.; Fandl, J.; Greene, L.A.; Barbacid, M.; Yancopoulos, G.D. Similarities and differences in the way neurotrophins interact with the Trk receptors in neuronal and nonneuronal cells. Neuron 1993, 10, 137–149. [Google Scholar] [CrossRef]
- Blum, R.; Konnerth, A. Neurotrophin-mediated rapid signaling in the central nervous system: Mechanisms and functions. Physiology 2005, 20, 70–78. [Google Scholar] [CrossRef]
- Romero, M.I.; Rangappa, N.; Li, L.; Lightfoot, E.; Garry, M.G.; Smith, G.M. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 4435–4445. [Google Scholar] [CrossRef]
- Rosenthal, A.; Goeddel, D.V.; Nguyen, T.; Lewis, M.; Shih, A.; Laramee, G.R.; Nikolics, K.; Winslow, J.W. Primary structure and biological activity of a novel human neurotrophic factor. Neuron 1990, 4, 767–773. [Google Scholar] [CrossRef]
- Almeida, R.D.; Manadas, B.J.; Melo, C.V.; Gomes, J.R.; Mendes, C.S.; Grãos, M.M.; Carvalho, R.F.; Carvalho, A.P.; Duarte, C.B. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005, 12, 1329–1343. [Google Scholar] [CrossRef]
- Fernandes, K.J.; Fan, D.P.; Tsui, B.J.; Cassar, S.L.; Tetzlaff, W. Influence of the axotomy to cell body distance in rat rubrospinal and spinal motoneurons: Differential regulation of GAP-43, tubulins, and neurofilament-M. J. Comp. Neurol. 1999, 414, 495–510. [Google Scholar] [CrossRef]
- Mason, M.R.; Lieberman, A.R.; Anderson, P.N. Corticospinal neurons up-regulate a range of growth-associated genes following intracortical, but not spinal, axotomy. Eur. J. Neurosci. 2003, 18, 789–802. [Google Scholar] [CrossRef]
- Swieck, K.; Conta-Steencken, A.; Middleton, F.A.; Siebert, J.R.; Osterhout, D.J.; Stelzner, D.J. Effect of lesion proximity on the regenerative response of long descending propriospinal neurons after spinal transection injury. BMC Neurosci. 2019, 20, 10. [Google Scholar] [CrossRef]
- Skene, J.H.; Willard, M. Changes in axonally transported proteins during axon regeneration in toad retinal ganglion cells. J. Cell Biol. 1981, 89, 86–95. [Google Scholar] [CrossRef]
- Oestreicher, A.B.; De Graan, P.N.; Gispen, W.H.; Verhaagen, J.; Schrama, L.H. B-50, the growth associated protein-43: Modulation of cell morphology and communication in the nervous system. Prog. Neurobiol. 1997, 53, 627–686. [Google Scholar] [CrossRef]
- Tetzlaff, W.; Alexander, S.W.; Miller, F.D.; Bisby, M.A. Response of facial and rubrospinal neurons to axotomy: Changes in mRNA expression for cytoskeletal proteins and GAP-43. J. Neurosci. Off. J. Soc. Neurosci. 1991, 11, 2528–2544. [Google Scholar] [CrossRef]
- Vinit, S.; Darlot, F.; Stamegna, J.C.; Gauthier, P.; Kastner, A. Effect of cervical spinal cord hemisection on the expression of axon growth markers. Neurosci. Lett. 2009, 462, 276–280. [Google Scholar] [CrossRef]
- Mantilla, C.B.; Gransee, H.M.; Zhan, W.Z.; Sieck, G.C. Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp. Neurol. 2013, 247, 101–109. [Google Scholar] [CrossRef]
- Vinit, S.; Darlot, F.; Aoulaïche, H.; Boulenguez, P.; Kastner, A. Distinct expression of c-Jun and HSP27 in axotomized and spared bulbospinal neurons after cervical spinal cord injury. J. Mol. Neurosci. 2011, 45, 119–133. [Google Scholar] [CrossRef]
- Bartus, K.; James, N.D.; Bosch, K.D.; Bradbury, E.J. Chondroitin sulphate proteoglycans: Key modulators of spinal cord and brain plasticity. Exp. Neurol. 2012, 235, 5–17. [Google Scholar] [CrossRef]
- Sánchez-Ventura, J.; Lane, M.A.; Udina, E. The Role and Modulation of Spinal Perineuronal Nets in the Healthy and Injured Spinal Cord. Front. Cell. Neurosci. 2022, 16, 893857. [Google Scholar] [CrossRef]
- Rhodes, K.E.; Fawcett, J.W. Chondroitin sulphate proteoglycans: Preventing plasticity or protecting the CNS? J. Anat. 2004, 204, 33–48. [Google Scholar] [CrossRef]
- Fitch, M.T.; Silver, J. Activated macrophages and the blood-brain barrier: Inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp. Neurol. 1997, 148, 587–603. [Google Scholar] [CrossRef]
- Jones, L.L.; Margolis, R.U.; Tuszynski, M.H. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp. Neurol. 2003, 182, 399–411. [Google Scholar] [CrossRef]
- Shen, Y.; Tenney, A.P.; Busch, S.A.; Horn, K.P.; Cuascut, F.X.; Liu, K.; He, Z.; Silver, J.; Flanagan, J.G. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 2009, 326, 592–596. [Google Scholar] [CrossRef]
- Lang, B.T.; Cregg, J.M.; DePaul, M.A.; Tran, A.P.; Xu, K.; Dyck, S.M.; Madalena, K.M.; Brown, B.P.; Weng, Y.L.; Li, S.; et al. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature 2015, 518, 404–408. [Google Scholar] [CrossRef]
- Smedfors, G.; Olson, L.; Karlsson, T.E. A Nogo-Like Signaling Perspective from Birth to Adulthood and in Old Age: Brain Expression Patterns of Ligands, Receptors and Modulators. Front. Mol. Neurosci. 2018, 11, 42. [Google Scholar] [CrossRef]
- Ohtake, Y.; Wong, D.; Abdul-Muneer, P.M.; Selzer, M.E.; Li, S. Two PTP receptors mediate CSPG inhibition by convergent and divergent signaling pathways in neurons. Sci. Rep. 2016, 6, 37152. [Google Scholar] [CrossRef]
- Niederöst, B.; Oertle, T.; Fritsche, J.; McKinney, R.A.; Bandtlow, C.E. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 10368–10376. [Google Scholar] [CrossRef]
- Cafferty, W.B.J.; Duffy, P.; Huebner, E.; Strittmatter, S.M. MAG and OMgp Synergize with Nogo-A to Restrict Axonal Growth and Neurological Recovery after Spinal Cord Trauma. J. Neurosci. 2010, 30, 6825–6837. [Google Scholar] [CrossRef]
- De Winter, F.; Oudega, M.; Lankhorst, A.J.; Hamers, F.P.; Blits, B.; Ruitenberg, M.J.; Pasterkamp, R.J.; Gispen, W.H.; Verhaagen, J. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp. Neurol. 2002, 175, 61–75. [Google Scholar] [CrossRef]
- Hashimoto, M.; Ino, H.; Koda, M.; Murakami, M.; Yoshinaga, K.; Yamazaki, M.; Moriya, H. Regulation of semaphorin 3A expression in neurons of the rat spinal cord and cerebral cortex after transection injury. Acta Neuropathol. 2004, 107, 250–256. [Google Scholar] [CrossRef]
- Loy, K.; Fourneau, J.; Meng, N.; Denecke, C.; Locatelli, G.; Bareyre, F.M. Semaphorin 7A restricts serotonergic innervation and ensures recovery after spinal cord injury. Cell Mol. Life Sci. 2021, 78, 2911–2927. [Google Scholar] [CrossRef]
- Kidd, T.; Brose, K.; Mitchell, K.J.; Fetter, R.D.; Tessier-Lavigne, M.; Goodman, C.S.; Tear, G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 1998, 92, 205–215. [Google Scholar] [CrossRef]
- Hollis, E.R. Axon Guidance Molecules and Neural Circuit Remodeling after Spinal Cord Injury. Neurotherapeutics 2016, 13, 360–369. [Google Scholar] [CrossRef]
- Russell, S.A.; Bashaw, G.J. Axon guidance pathways and the control of gene expression. Dev. Dyn. 2018, 247, 571–580. [Google Scholar] [CrossRef]
- Wehrle, R.; Camand, E.; Chedotal, A.; Sotelo, C.; Dusart, I. Expression of netrin-1, slit-1 and slit-3 but not of slit-2 after cerebellar and spinal cord lesions. Eur. J. Neurosci. 2005, 22, 2134–2144. [Google Scholar] [CrossRef]
- Keniry, M.; Parsons, R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene 2008, 27, 5477–5485. [Google Scholar] [CrossRef]
- Park, K.K.; Liu, K.; Hu, Y.; Kanter, J.L.; He, Z. PTEN/mTOR and axon regeneration. Exp. Neurol. 2010, 223, 45–50. [Google Scholar] [CrossRef]
- Allen, L.L.; Seven, Y.B.; Baker, T.L.; Mitchell, G.S. Cervical spinal contusion alters Na(+)-K(+)-2Cl- and K(+)-Cl- cation-chloride cotransporter expression in phrenic motor neurons. Respir. Physiol. Neurobiol. 2019, 261, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.J.; Vinit, S.; Chen, C.L.; Lee, K.Z. 5-HT7 Receptor Inhibition Transiently Improves Respiratory Function Following Daily Acute Intermittent Hypercapnic-Hypoxia in Rats with Chronic Midcervical Spinal Cord Contusion. Neurorehabilit. Neural Repair. 2020, 34, 333–343. [Google Scholar] [CrossRef]
- Wen, M.-H.; Wu, M.-J.; Vinit, S.; Lee, K.-Z. Modulation of Serotonin and Adenosine 2A Receptors on Intermittent Hypoxia-Induced Respiratory Recovery following Mid-Cervical Contusion in the Rat. J. Neurotrauma 2019, 36, 2991–3004. [Google Scholar] [CrossRef]
- Nicaise, C.; Putatunda, R.; Hala, T.J.; Regan, K.A.; Frank, D.M.; Brion, J.-P.; Leroy, K.; Pochet, R.; Wright, M.C.; Lepore, A.C. Degeneration of phrenic motor neurons induces long-term diaphragm deficits following mid-cervical spinal contusion in mice. J. Neurotrauma 2012, 29, 2748–2760. [Google Scholar] [CrossRef]
- Baussart, B.; Stamegna, J.C.; Polentes, J.; Tadié, M.; Gauthier, P. A new model of upper cervical spinal contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol. Dis. 2006, 22, 562–574. [Google Scholar] [CrossRef]
- Golder, F.J.; Fuller, D.D.; Lovett-Barr, M.R.; Vinit, S.; Resnick, D.K.; Mitchell, G.S. Breathing patterns after mid-cervical spinal contusion in rats. Exp. Neurol. 2011, 231, 97–103. [Google Scholar] [CrossRef]
- Lee, K.Z. Neuropathology of distinct diaphragm areas following mid-cervical spinal cord contusion in the rat. Spine J. 2022, 22, 1726–1741. [Google Scholar] [CrossRef] [PubMed]
- Bajjig, A.; Michel-Flutot, P.; Migevent, T.; Cayetanot, F.; Bodineau, L.; Vinit, S.; Vivodtzev, I. Diaphragmatic Activity and Respiratory Function Following C3 or C6 Unilateral Spinal Cord Contusion in Mice. Biology 2022, 11, 558. [Google Scholar] [CrossRef]
- Vinit, S.; Gauthier, P.; Stamegna, J.C.; Kastner, A. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J. Neurotrauma 2006, 23, 1137–1146. [Google Scholar] [CrossRef]
- Lee, K.Z.; Huang, Y.J.; Tsai, I.L. Respiratory motor outputs following unilateral midcervical spinal cord injury in the adult rat. J. Appl. Physiol. 2014, 116, 395–405. [Google Scholar] [CrossRef]
- Bezdudnaya, T.; Lane, M.A.; Marchenko, V. Paced breathing and phrenic nerve responses evoked by epidural stimulation following complete high cervical spinal cord injury in rats. J. Appl. Physiol. 2018, 125, 687–696. [Google Scholar] [CrossRef]
- Cheng, L.; Sami, A.; Ghosh, B.; Goudsward, H.J.; Smith, G.M.; Wright, M.C.; Li, S.; Lepore, A.C. Respiratory axon regeneration in the chronically injured spinal cord. Neurobiol. Dis. 2021, 155, 105389. [Google Scholar] [CrossRef]
- Cheng, L.; Sami, A.; Ghosh, B.; Urban, M.W.; Heinsinger, N.M.; Liang, S.S.; Smith, G.M.; Wright, M.C.; Li, S.; Lepore, A.C. LAR inhibitory peptide promotes recovery of diaphragm function and multiple forms of respiratory neural circuit plasticity after cervical spinal cord injury. Neurobiol. Dis. 2021, 147, 105153. [Google Scholar] [CrossRef]
- Michel-Flutot, P.; Mansart, A.; Deramaudt, B.T.; Jesus, I.; Lee, K.; Bonay, M.; Vinit, S. Permanent diaphragmatic deficits and spontaneous respiratory plasticity in a mouse model of incomplete cervical spinal cord injury. Respir. Physiol. Neurobiol. 2021, 284, 103568. [Google Scholar] [CrossRef]
- Michel-Flutot, P.; Zholudeva, L.V.; Randelman, M.L.; Deramaudt, T.B.; Mansart, A.; Alvarez, J.-C.; Lee, K.-Z.; Petitjean, M.; Bonay, M.; Lane, M.A.; et al. High frequency repetitive Transcranial Magnetic Stimulation promotes long lasting phrenic motoneuron excitability via GABAergic networks. Respir. Physiol. Neurobiol. 2021, 284, 103704. [Google Scholar] [CrossRef]
- Rana, S.; Zhan, W.-Z.; Sieck, G.C.; Mantilla, C.B. Cervical spinal hemisection alters phrenic motor neuron glutamatergic mRNA receptor expression. Exp. Neurol. 2022, 353, 114030. [Google Scholar] [CrossRef]
- Allen, L.L.; Nichols, N.L.; Asa, Z.A.; Emery, A.T.; Ciesla, M.C.; Santiago, J.V.; Holland, A.E.; Mitchell, G.S.; Gonzalez-Rothi, E.J. Phrenic motor neuron survival below cervical spinal cord hemisection. Exp. Neurol. 2021, 346, 113832. [Google Scholar] [CrossRef] [PubMed]
- Ghali, M.G.; Marchenko, V. Dynamic changes in phrenic motor output following high cervical hemisection in the decerebrate rat. Exp. Neurol. 2015, 271, 379–389. [Google Scholar] [CrossRef]
- Minor, K.H.; Akison, L.K.; Goshgarian, H.G.; Seeds, N.W. Spinal cord injury-induced plasticity in the mouse—The crossed phrenic phenomenon. Exp. Neurol. 2006, 200, 486–495. [Google Scholar] [CrossRef]
- Goshgarian, H.G. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir. Physiol. Neurobiol. 2009, 169, 85–93. [Google Scholar] [CrossRef]
- Porter, W.T. The Path of the Respiratory Impulse from the Bulb to the Phrenic Nuclei. J. Physiol. 1895, 17, 455–485. [Google Scholar] [CrossRef]
- Bach, K.B.; Mitchell, G.S. Hypercapnia-induced long-term depression of respiratory activity requires alpha2-adrenergic receptors. J. Appl. Physiol. 1998, 84, 2099–2105. [Google Scholar] [CrossRef]
- Nichols, N.L.; Mitchell, G.S. Mechanisms of severe acute intermittent hypoxia-induced phrenic long-term facilitation. J. Neurophysiol. 2021, 125, 1146–1156. [Google Scholar] [CrossRef]
- Powell, F.L.; Milsom, W.K.; Mitchell, G.S. Time domains of the hypoxic ventilatory response. Respir. Physiol. 1998, 112, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Fuller, D.D.; Mitchell, G.S. Respiratory neuroplasticity—Overview, significance and future directions. Exp. Neurol. 2017, 287, 144–152. [Google Scholar] [CrossRef]
- Mitchell, G.S.; Baker, T.L.; Nanda, S.A.; Fuller, D.D.; Zabka, A.G.; Hodgeman, B.A.; Bavis, R.W.; Mack, K.J.; Olson, E.B., Jr. Invited review: Intermittent hypoxia and respiratory plasticity. J. Appl. Physiol. 2001, 90, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Fuller, D.D.; Johnson, S.M.; Olson, E.B., Jr.; Mitchell, G.S. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J. Neurosci. 2003, 23, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.C.; Lesske, J.; Qian, W.; Miller, C.C., 3rd; Unger, T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension 1992, 19, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Daniel, J.M.; Dohanich, G.P. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 2442–2450. [Google Scholar] [CrossRef]
- Navarrete-Opazo, A.; Mitchell, G.S. Therapeutic potential of intermittent hypoxia: A matter of dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1181–R1197. [Google Scholar] [CrossRef]
- Golder, F.J.; Mitchell, G.S. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 2925–2932. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, B.J.; Terada, J.; Springborn, S.R.; Vinit, S.; MacFarlane, P.M.; Mitchell, G.S. Daily acute intermittent hypoxia improves breathing function with acute and chronic spinal injury via distinct mechanisms. Respir. Physiol. Neurobiol. 2018, 256, 50–57. [Google Scholar] [CrossRef]
- Lovett-Barr, M.R.; Satriotomo, I.; Muir, G.D.; Wilkerson, J.E.; Hoffman, M.S.; Vinit, S.; Mitchell, G.S. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J. Neurosci. 2012, 32, 3591–3600. [Google Scholar] [CrossRef] [PubMed]
- Doperalski, N.J.; Fuller, D.D. Long-term facilitation of ipsilateral but not contralateral phrenic output after cervical spinal cord hemisection. Exp. Neurol. 2006, 200, 74–81. [Google Scholar] [CrossRef]
- Navarrete-Opazo, A.; Vinit, S.; Dougherty, B.J.; Mitchell, G.S. Daily acute intermittent hypoxia elicits functional recovery of diaphragm and inspiratory intercostal muscle activity after acute cervical spinal injury. Exp. Neurol. 2015, 266, 1–10. [Google Scholar] [CrossRef]
- Navarrete-Opazo, A.A.; Vinit, S.; Mitchell, G.S. Adenosine 2A Receptor Inhibition Enhances Intermittent Hypoxia-Induced Diaphragm but Not Intercostal Long-Term Facilitation. J. Neurotrauma 2014, 31, 1975–1984. [Google Scholar] [CrossRef]
- Gonzalez-Rothi, E.J.; Tadjalli, A.; Allen, L.L.; Ciesla, M.C.; Chami, M.E.; Mitchell, G.S. Protocol-Specific Effects of Intermittent Hypoxia Pre-Conditioning on Phrenic Motor Plasticity in Rats with Chronic Cervical Spinal Cord Injury. J. Neurotrauma 2021, 38, 1292–1305. [Google Scholar] [CrossRef]
- Navarrete-Opazo, A.; Dougherty, B.J.; Mitchell, G.S. Enhanced recovery of breathing capacity from combined adenosine 2A receptor inhibition and daily acute intermittent hypoxia after chronic cervical spinal injury. Exp. Neurol. 2017, 287, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Komnenov, D.; Solarewicz, J.Z.; Afzal, F.; Nantwi, K.D.; Kuhn, D.M.; Mateika, J.H. Intermittent hypoxia promotes recovery of respiratory motor function in spinal cord-injured mice depleted of serotonin in the central nervous system. J. Appl. Physiol. 2016, 121, 545–557. [Google Scholar] [CrossRef]
- Lee, K.Z.; Chiang, S.C.; Li, Y.J. Mild Acute Intermittent Hypoxia Improves Respiratory Function in Unanesthetized Rats with Midcervical Contusion. Neurorehabilit. Neural Repair. 2017, 31, 364–375. [Google Scholar] [CrossRef]
- Lin, M.-T.; Vinit, S.; Lee, K.-Z. Functional role of carbon dioxide on intermittent hypoxia induced respiratory response following mid-cervical contusion in the rat. Exp. Neurol. 2021, 339, 113610. [Google Scholar] [CrossRef]
- Gutierrez, D.V.; Clark, M.; Nwanna, O.; Alilain, W.J. Intermittent hypoxia training after C2 hemisection modifies the expression of PTEN and mTOR. Exp. Neurol. 2013, 248, 45–52. [Google Scholar] [CrossRef]
- Ciesla, M.C.; Seven, Y.B.; Allen, L.L.; Smith, K.N.; Asa, Z.A.; Simon, A.K.; Holland, A.E.; Santiago, J.V.; Stefan, K.; Ross, A.; et al. Serotonergic innervation of respiratory motor nuclei after cervical spinal injury: Impact of intermittent hypoxia. Exp. Neurol. 2021, 338, 113609. [Google Scholar] [CrossRef]
- Gonzalez-Rothi, E.J.; Lee, K.-Z.; Dale, E.A.; Reier, P.J.; Mitchell, G.S.; Fuller, D.D. Intermittent hypoxia and neurorehabilitation. J. Appl. Physiol. 2015, 119, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Dale, E.A.; Mabrouk, F.B.; Mitchell, G.S. Unexpected Benefits of Intermittent Hypoxia: Enhanced Respiratory and Nonrespiratory Motor Function. Physiology 2014, 29, 39–48. [Google Scholar] [CrossRef]
- Gonzalez-Rothi, E.J.; Lee, K.Z. Intermittent hypoxia and respiratory recovery in pre-clinical rodent models of incomplete cervical spinal cord injury. Exp. Neurol. 2021, 342, 113751. [Google Scholar] [CrossRef]
- Tester, N.J.; Fuller, D.D.; Fromm, J.S.; Spiess, M.R.; Behrman, A.L.; Mateika, J.H. Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am. J. Respir. Crit. Care Med. 2014, 189, 57–65. [Google Scholar] [CrossRef]
- Jaiswal, P.B.; Tester, N.J.; Davenport, P.W. Effect of acute intermittent hypoxia treatment on ventilatory load compensation and magnitude estimation of inspiratory resistive loads in an individual with chronic incomplete cervical spinal cord injury. J. Spinal Cord Med. 2016, 39, 103–110. [Google Scholar] [CrossRef]
- Sutor, T.; Cavka, K.; Vose, A.K.; Welch, J.F.; Davenport, P.; Fuller, D.D.; Mitchell, G.S.; Fox, E.J. Single-session effects of acute intermittent hypoxia on breathing function after human spinal cord injury. Exp. Neurol. 2021, 342, 113735. [Google Scholar] [CrossRef]
- Warren, P.M.; Steiger, S.C.; Dick, T.E.; MacFarlane, P.M.; Alilain, W.J.; Silver, J. Rapid and robust restoration of breathing long after spinal cord injury. Nat. Commun. 2018, 9, 4843. [Google Scholar] [CrossRef]
- Sandhu, M.S.; Gray, E.; Kocherginsky, M.; Jayaraman, A.; Mitchell, G.S.; Rymer, W.Z. Prednisolone Pretreatment Enhances Intermittent Hypoxia-Induced Plasticity in Persons with Chronic Incomplete Spinal Cord Injury. Neurorehabilit. Neural Repair. 2019, 33, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Opazo, A.; Alcayaga, J.; Sepúlveda, O.; Rojas, E.; Astudillo, C. Repetitive Intermittent Hypoxia and Locomotor Training Enhances Walking Function in Incomplete Spinal Cord Injury Subjects: A Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. J. Neurotrauma 2017, 34, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.Q.; Sohn, W.J.; Naidu, A.; Trumbower, R.D. Daily acute intermittent hypoxia combined with walking practice enhances walking performance but not intralimb motor coordination in persons with chronic incomplete spinal cord injury. Exp. Neurol. 2021, 340, 113669. [Google Scholar] [CrossRef]
- Tan, A.Q.; Barth, S.; Trumbower, R.D. Acute intermittent hypoxia as a potential adjuvant to improve walking following spinal cord injury: Evidence, challenges, and future directions. Curr. Phys. Med. Rehabil. Rep. 2020, 8, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Trumbower, R.D.; Hayes, H.B.; Mitchell, G.S.; Wolf, S.L.; Stahl, V.A. Effects of acute intermittent hypoxia on hand use after spinal cord trauma: A preliminary study. Neurology 2017, 89, 1904–1907. [Google Scholar] [CrossRef] [PubMed]
- Hérent, C.; Diem, S.; Fortin, G.; Bouvier, J. Upregulation of breathing rate during running exercise by central locomotor circuits. bioRxiv 2021. [Google Scholar] [CrossRef]
- Jesus, I.; Michel-Flutot, P.; Deramaudt, T.B.; Paucard, A.; Vanhee, V.; Vinit, S.; Bonay, M. Effects of aerobic exercise training on muscle plasticity in a mouse model of cervical spinal cord injury. Sci. Rep. 2021, 11, 112. [Google Scholar] [CrossRef]
- Lemos, J.R.; da Cunha, F.A.; Lopes, A.J.; Guimarães, F.S.; do Amaral Vasconcellos, F.V.; Dos Santos Vigário, P. Respiratory muscle training in non-athletes and athletes with spinal cord injury: A systematic review of the effects on pulmonary function, respiratory muscle strength and endurance, and cardiorespiratory fitness based on the FITT principle of exercise prescription. J. Back Musculoskelet. Rehabil. 2020, 33, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Boswell-Ruys, C.L.; Lewis, C.R.H.; Wijeysuriya, N.S.; McBain, R.A.; Lee, B.B.; McKenzie, D.K.; Gandevia, S.C.; Butler, J.E. Impact of respiratory muscle training on respiratory muscle strength, respiratory function and quality of life in individuals with tetraplegia: A randomised clinical trial. Thorax 2020, 75, 279–288. [Google Scholar] [CrossRef]
- Kang, D.; Park, J.; Eun, S.D. A preliminary study on the feasibility of community game-based respiratory muscle training for individuals with high cervical spinal cord injury levels: A novel approach. BMC Sport Sci. Med. Rehabil. 2022, 14, 137. [Google Scholar] [CrossRef]
- Gee, C.M.; Williams, A.M.; Sheel, A.W.; Eves, N.D.; West, C.R. Respiratory muscle training in athletes with cervical spinal cord injury: Effects on cardiopulmonary function and exercise capacity. J. Physiol. 2019, 597, 3673–3685. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.A.; Chadwick, M.R.; Davies, M.J. Mechanisms of improved exercise capacity following respiratory muscle training in athletes with cervical spinal cord injury. J. Physiol. 2019, 597, 5531–5532. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Hori, S.; Momosaki, R. Effect of Vocal Exercise on Respiratory Function and Voice Quality in Patients with Cervical Spinal Cord Injury: A Mini-review. Prog. Rehabil. Med. 2022, 7, 20220041. [Google Scholar] [CrossRef]
- Terson de Paleville, D.; McKay, W.; Aslan, S.; Folz, R.; Sayenko, D.; Ovechkin, A. Locomotor step training with body weight support improves respiratory motor function in individuals with chronic spinal cord injury. Respir. Physiol. Neurobiol. 2013, 189, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Nath, R.; Wyant, G.M. Treatment of chronic pain by epidural spinal cord stimulation: A 10-year experience. J. Neurosurg. 1991, 75, 402–407. [Google Scholar] [CrossRef]
- Moreno-Duarte, I.; Morse, L.R.; Alam, M.; Bikson, M.; Zafonte, R.; Fregni, F. Targeted therapies using electrical and magnetic neural stimulation for the treatment of chronic pain in spinal cord injury. Neuroimage 2014, 85, 1003–1013. [Google Scholar] [CrossRef]
- Gerasimenko, Y.P.; Avelev, V.D.; Nikitin, O.A.; Lavrov, I.A. Initiation of locomotor activity in spinal cats by epidural stimulation of the spinal cord. Neurosci. Behav. Physiol. 2003, 33, 247–254. [Google Scholar] [CrossRef]
- Gerasimenko, Y.P.; Lavrov, I.A.; Courtine, G.; Ichiyama, R.M.; Dy, C.J.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J. Neurosci. Methods 2006, 157, 253–263. [Google Scholar] [CrossRef]
- Ichiyama, R.M.; Gerasimenko, Y.P.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci. Lett. 2005, 383, 339–344. [Google Scholar] [CrossRef]
- Herman, R.; He, J.; D’Luzansky, S.; Willis, W.; Dilli, S. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord 2002, 40, 65–68. [Google Scholar] [CrossRef]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef]
- Kowalski, K.E.; Hsieh, Y.H.; Dick, T.E.; DiMarco, A.F. Diaphragm activation via high frequency spinal cord stimulation in a rodent model of spinal cord injury. Exp. Neurol. 2013, 247, 689–693. [Google Scholar] [CrossRef]
- Gonzalez-Rothi, E.J.; Streeter, K.A.; Hanna, M.H.; Stamas, A.C.; Reier, P.J.; Baekey, D.M.; Fuller, D.D. High-frequency epidural stimulation across the respiratory cycle evokes phrenic short-term potentiation after incomplete cervical spinal cord injury. J. Neurophysiol. 2017, 118, 2344–2357. [Google Scholar] [CrossRef]
- Dale, E.A.; Zhong, H.; Edgerton, V.R. Cervical Spinal Stimulation and Respiratory Recovery after Upper Cervical Spinal Cord Injury. FASEB J. 2016, 30, 1294–1297. [Google Scholar]
- Dale, E.A.; Sunshine, M.D.; Kelly, M.N.; Mitchell, G.S.; Fuller, D.D.; Reier, P.J. Chronic, closed-loop, cervical epidural stimulation elicits plasticity in diaphragm motor output and upregulates spinal neurotrophic factor gene expression. FASEB J. 2019, 33, 843.10. [Google Scholar] [CrossRef]
- Lin, A.; Shaaya, E.; Calvert, J.S.; Parker, S.R.; Borton, D.A.; Fridley, J.S. A Review of Functional Restoration From Spinal Cord Stimulation in Patients with Spinal Cord Injury. Neurospine 2022, 19, 703–734. [Google Scholar] [CrossRef]
- Karamian, B.A.; Siegel, N.; Nourie, B.; Serruya, M.D.; Heary, R.F.; Harrop, J.S.; Vaccaro, A.R. The role of electrical stimulation for rehabilitation and regeneration after spinal cord injury. J. Orthop. Traumatol. 2022, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.R.; Pereira, M.; Salvador, R.; Miranda, P.C.; de Carvalho, M. Cervical trans-spinal direct current stimulation: A modelling-experimental approach. J. Neuroeng. Rehabil. 2019, 16, 123. [Google Scholar] [CrossRef]
- Martin, D.M.; McClintock, S.M.; Forster, J.J.; Lo, T.Y.; Loo, C.K. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: A systematic review and meta-analysis of individual task effects. Depress. Anxiety 2017, 34, 1029–1039. [Google Scholar] [CrossRef]
- Jassova, K.; Albrecht, J.; Ceresnakova, S.; Papezova, H.; Anders, M. Repetitive transcranial magnetic stimulation significantly influences the eating behavior in depressive patients. Neuropsychiatr. Dis. Treat. 2019, 15, 2579–2586. [Google Scholar] [CrossRef]
- McClintock, S.M.; Reti, I.M.; Carpenter, L.L.; McDonald, W.M.; Dubin, M.; Taylor, S.F.; Cook, I.A.; O’Reardon, J.; Husain, M.M.; Wall, C.; et al. Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression. J. Clin. Psychiatry 2018, 79, 3651. [Google Scholar] [CrossRef]
- Yan, T.; Xie, Q.; Zheng, Z.; Zou, K.; Wang, L. Different frequency repetitive transcranial magnetic stimulation (rTMS) for posttraumatic stress disorder (PTSD): A systematic review and meta-analysis. J. Psychiatr. Res. 2017, 89, 125–135. [Google Scholar] [CrossRef]
- Kozel, F.A. Clinical Repetitive Transcranial Magnetic Stimulation for Posttraumatic Stress Disorder, Generalized Anxiety Disorder, and Bipolar Disorder. Psychiatr. Clin. N. Am. 2018, 41, 433–446. [Google Scholar] [CrossRef]
- Wincek, A.; Huber, J.; Leszczyńska, K.; Fortuna, W.; Okurowski, S.; Chmielak, K.; Tabakow, P. The Long-Term Effect of Treatment Using the Transcranial Magnetic Stimulation rTMS in Patients after Incomplete Cervical or Thoracic Spinal Cord Injury. J. Clin. Med. 2021, 10, 2975. [Google Scholar] [CrossRef]
- Tazoe, T.; Perez, M.A. Effects of repetitive transcranial magnetic stimulation on recovery of function after spinal cord injury. Arch. Phys. Med. Rehabil. 2015, 96, S145–S155. [Google Scholar] [CrossRef] [PubMed]
- Leszczyńska, K.; Wincek, A.; Fortuna, W.; Huber, J.; Łukaszek, J.; Okurowski, S.; Chmielak, K.; Tabakow, P. Treatment of patients with cervical and upper thoracic incomplete spinal cord injury using repetitive transcranial magnetic stimulation. Int. J. Artif. Organs 2020, 43, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, T.; Brito, R.; Luna, P.; Campêlo, M.; Shirahige, L.; Fontes, L.; Dias, R.; Piscitelli, D.; Monte-Silva, K. Repetitive transcranial magnetic stimulation on the modulation of cortical and spinal cord excitability in individuals with spinal cord injury. Restor. Neurol. Neurosci. 2021, 39, 291–301. [Google Scholar] [CrossRef]
- Belci, M.; Catley, M.; Husain, M.; Frankel, H.L.; Davey, N.J. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord 2004, 42, 417–419. [Google Scholar] [CrossRef]
- Similowski, T.; Straus, C.; Coïc, L.; Derenne, J.P. Facilitation-independent response of the diaphragm to cortical magnetic stimulation. Am. J. Respir. Crit. Care Med. 1996, 154, 1771–1777. [Google Scholar] [CrossRef]
- Similowski, T.; Catala, M.; Rancurel, G.; Derenne, J.P. Impairment of central motor conduction to the diaphragm in stroke. Am. J. Respir. Crit. Care Med. 1996, 154, 436–441. [Google Scholar] [CrossRef]
- Sharshar, T.; Ross, E.; Hopkinson, N.S.; Dayer, M.; Nickol, A.; Lofaso, F.; Moxham, J.; Similowski, T.; Polkey, M.I. Effect of voluntary facilitation on the diaphragmatic response to transcranial magnetic stimulation. J. Appl. Physiol. 2003, 95, 26–34. [Google Scholar] [CrossRef]
- Demoule, A.; Verin, E.; Locher, C.; Derenne, J.P.; Similowski, T. Validation of surface recordings of the diaphragm response to transcranial magnetic stimulation in humans. J. Appl. Physiol. 2003, 94, 453–461. [Google Scholar] [CrossRef]
- Welch, J.F.; Argento, P.J.; Mitchell, G.S.; Fox, E.J. Reliability of diaphragmatic motor-evoked potentials induced by transcranial magnetic stimulation. J. Appl. Physiol. 2020, 129, 1393–1404. [Google Scholar] [CrossRef]
- Ren, M.Y.; Liou, L.M.; Vinit, S.; Lee, K.Z. Position effect of trans-spinal magnetic stimulation on diaphragmatic motor evoked potential in healthy humans. J. Appl. Physiol. 2022, 133, 1042–1054. [Google Scholar] [CrossRef]
- Vinit, S.; Keomani, E.; Deramaudt, T.B.; Spruance, V.M.; Bezdudnaya, T.; Lane, M.A.; Bonay, M.; Petitjean, M. Interdisciplinary approaches of transcranial magnetic stimulation applied to a respiratory neuronal circuitry model. PLoS ONE 2014, 9, e113251. [Google Scholar] [CrossRef]
- Vinit, S.; Keomani, E.; Deramaudt, T.B.; Bonay, M.; Petitjean, M. Reorganization of Respiratory Descending Pathways following Cervical Spinal Partial Section Investigated by Transcranial Magnetic Stimulation in the Rat. PLoS ONE 2016, 11, e0148180. [Google Scholar] [CrossRef]
- Lee, K.Z.; Liou, L.M.; Vinit, S.; Ren, M.Y. Rostral-Caudal Effect of Cervical Magnetic Stimulation on the Diaphragm Motor Evoked Potential after Cervical Spinal Cord Contusion in the Rat. J. Neurotrauma 2022, 39, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Michel-Flutot, P.; Jesus, I.; Vanhee, V.; Bourcier, C.H.; Emam, L.; Ouguerroudj, A.; Lee, K.-Z.; Zholudeva, L.V.; Lane, M.A.; Mansart, A.; et al. Effects of Chronic High-Frequency rTMS Protocol on Respiratory Neuroplasticity Following C2 Spinal Cord Hemisection in Rats. Biology 2022, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Fischer, I.; Dulin, J.N.; Lane, M.A. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat. Rev. Neurosci. 2020, 21, 366–383. [Google Scholar] [CrossRef]
- Zholudeva, L.V.; Lane, M.A. Transplanting Cells for Spinal Cord Repair: Who, What, When, Where and Why? Cell Transpl. 2019, 28, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Stokes, B.T.; Reier, P.J. Fetal grafts alter chronic behavioral outcome after contusion damage to the adult rat spinal cord. Exp. Neurol. 1992, 116, 1–12. [Google Scholar] [CrossRef]
- Rosenzweig, E.S.; Brock, J.H.; Lu, P.; Kumamaru, H.; Salegio, E.A.; Kadoya, K.; Weber, J.L.; Liang, J.J.; Moseanko, R.; Hawbecker, S.; et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 2018, 24, 484–490. [Google Scholar] [CrossRef]
- Hou, S.; Tom, V.J.; Graham, L.; Lu, P.; Blesch, A. Partial restoration of cardiovascular function by embryonic neural stem cell grafts after complete spinal cord transection. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 17138–17149. [Google Scholar] [CrossRef]
- Farhy-Tselnicker, I.; Allen, N.J. Astrocytes, neurons, synapses: A tripartite view on cortical circuit development. Neural. Dev. 2018, 13, 7. [Google Scholar] [CrossRef]
- Chu, T.; Zhou, H.; Li, F.; Wang, T.; Lu, L.; Feng, S. Astrocyte transplantation for spinal cord injury: Current status and perspective. Brain Res. Bull. 2014, 107, 18–30. [Google Scholar] [CrossRef]
- Li, K.; Javed, E.; Hala, T.J.; Sannie, D.; Regan, K.A.; Maragakis, N.J.; Wright, M.C.; Poulsen, D.J.; Lepore, A.C. Transplantation of glial progenitors that overexpress glutamate transporter GLT1 preserves diaphragm function following cervical SCI. Mol. Ther. 2015, 23, 533–548. [Google Scholar] [CrossRef]
- Falnikar, A.; Li, K.; Lepore, A.C. Therapeutically targeting astrocytes with stem and progenitor cell transplantation following traumatic spinal cord injury. Brain Res. 2015, 1619, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Javed, E.; Scura, D.; Hala, T.J.; Seetharam, S.; Falnikar, A.; Richard, J.P.; Chorath, A.; Maragakis, N.J.; Wright, M.C.; et al. Human iPS cell-derived astrocyte transplants preserve respiratory function after spinal cord injury. Exp. Neurol. 2015, 271, 479–492. [Google Scholar] [CrossRef]
- Goulão, M.; Ghosh, B.; Urban, M.W.; Sahu, M.; Mercogliano, C.; Charsar, B.A.; Komaravolu, S.; Block, C.G.; Smith, G.M.; Wright, M.C.; et al. Astrocyte progenitor transplantation promotes regeneration of bulbospinal respiratory axons, recovery of diaphragm function, and a reduced macrophage response following cervical spinal cord injury. Glia 2019, 67, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Fortino, T.; Spruance, V.; Niceforo, A.; Harrop, J.S.; Phelps, P.E.; Priest, C.A.; Zholudeva, L.V.; Lane, M.A. Chapter Three—Cell transplantation to repair the injured spinal cord. In International Review of Neurobiology; Lane, E.L., Drew, C.J.G., Lelos, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 166, pp. 79–158. [Google Scholar]
- White, T.E.; Lane, M.A.; Sandhu, M.S.; O’Steen, B.E.; Fuller, D.D.; Reier, P.J. Neuronal progenitor transplantation and respiratory outcomes following upper cervical spinal cord injury in adult rats. Exp. Neurol. 2010, 225, 231–236. [Google Scholar] [CrossRef]
- Lee, K.Z.; Lane, M.A.; Dougherty, B.J.; Mercier, L.M.; Sandhu, M.S.; Sanchez, J.C.; Reier, P.J.; Fuller, D.D. Intraspinal transplantation and modulation of donor neuron electrophysiological activity. Exp. Neurol. 2014, 251, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Zholudeva, L.V.; Qiang, L.; Marchenko, V.; Dougherty, K.J.; Sakiyama-Elbert, S.E.; Lane, M.A. The Neuroplastic and Therapeutic Potential of Spinal Interneurons in the Injured Spinal Cord. Trends. Neurosci. 2018, 41, 625–639. [Google Scholar] [CrossRef]
- Romer, S.H.; Seedle, K.; Turner, S.M.; Li, J.; Baccei, M.L.; Crone, S.A. Accessory respiratory muscles enhance ventilation in ALS model mice and are activated by excitatory V2a neurons. Exp. Neurol. 2017, 287, 192–204. [Google Scholar] [CrossRef]
- Kathe, C.; Skinnider, M.A.; Hutson, T.H.; Regazzi, N.; Gautier, M.; Demesmaeker, R.; Komi, S.; Ceto, S.; James, N.D.; Cho, N.; et al. The neurons that restore walking after paralysis. Nature 2022, 611, 540–547. [Google Scholar] [CrossRef]
- Zholudeva, L.V.; Iyer, N.; Qiang, L.; Spruance, V.M.; Randelman, M.L.; White, N.W.; Bezdudnaya, T.; Fischer, I.; Sakiyama-Elbert, S.E.; Lane, M.A. Transplantation of Neural Progenitors and V2a Interneurons after Spinal Cord Injury. J. Neurotrauma 2018, 35, 2883–2903. [Google Scholar] [CrossRef]
- Stamegna, J.C.; Sadelli, K.; Escoffier, G.; Girard, S.D.; Veron, A.D.; Bonnet, A.; Khrestchatisky, M.; Gauthier, P.; Roman, F.S. Grafts of Olfactory Stem Cells Restore Breathing and Motor Functions after Rat Spinal Cord Injury. J. Neurotrauma 2018, 35, 1765–1780. [Google Scholar] [CrossRef]
- Watson, R.A.; Yeung, T.M. What is the potential of oligodendrocyte progenitor cells to successfully treat human spinal cord injury? BMC Neurol. 2011, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Priest, C.A.; Manley, N.C.; Denham, J.; Wirth, E.D., 3rd; Lebkowski, J.S. Preclinical safety of human embryonic stem cell-derived oligodendrocyte progenitors supporting clinical trials in spinal cord injury. Regen. Med. 2015, 10, 939–958. [Google Scholar] [CrossRef] [PubMed]
- Manley, N.C.; Priest, C.A.; Denham, J.; Wirth, E.D., 3rd; Lebkowski, J.S. Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor Cells: Preclinical Efficacy and Safety in Cervical Spinal Cord Injury. Stem Cells Transl. Med. 2017, 6, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Watzlawick, R.; Rind, J.; Sena, E.S.; Brommer, B.; Zhang, T.; Kopp, M.A.; Dirnagl, U.; Macleod, M.R.; Howells, D.W.; Schwab, J.M. Olfactory Ensheathing Cell Transplantation in Experimental Spinal Cord Injury: Effect size and Reporting Bias of 62 Experimental Treatments: A Systematic Review and Meta-Analysis. PLoS Biol. 2016, 14, e1002468. [Google Scholar] [CrossRef]
- Jarocha, D.; Milczarek, O.; Kawecki, Z.; Wendrychowicz, A.; Kwiatkowski, S.; Majka, M. Preliminary study of autologous bone marrow nucleated cells transplantation in children with spinal cord injury. Stem Cells Transl. Med. 2014, 3, 395–404. [Google Scholar] [CrossRef]
- Richardson, P.M.; McGuinness, U.M.; Aguayo, A.J. Axons from CNS neurons regenerate into PNS grafts. Nature 1980, 284, 264–265. [Google Scholar] [CrossRef]
- David, S.; Aguayo, A.J. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 1981, 214, 931–933. [Google Scholar] [CrossRef]
- Decherchi, P.; Lammari-Barreault, N.; Gauthier, P. Regeneration of respiratory pathways within spinal peripheral nerve grafts. Exp. Neurol. 1996, 137, 1–14. [Google Scholar] [CrossRef]
- Decherchi, P.; Gauthier, P. Regeneration of acutely and chronically injured descending respiratory pathways within post-traumatic nerve grafts. Neuroscience 2002, 112, 141–152. [Google Scholar] [CrossRef]
- Senjaya, F.; Midha, R. Nerve transfer strategies for spinal cord injury. World Neurosurg. 2013, 80, e319–e326. [Google Scholar] [CrossRef]
- Krieger, L.M.; Krieger, A.J. The Intercostal to Phrenic Nerve Transfer: An Effective Means of Reanimating the Diaphragm in Patients with High Cervical Spine Injury. Plast. Reconstr. Surg. 2000, 105, 1255–1261. [Google Scholar]
- Kwok, J.C.; Dick, G.; Wang, D.; Fawcett, J.W. Extracellular matrix and perineuronal nets in CNS repair. Dev. Neurobiol. 2011, 71, 1073–1089. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Moon, L.D.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640. [Google Scholar] [CrossRef]
- James, N.D.; Shea, J.; Muir, E.M.; Verhaagen, J.; Schneider, B.L.; Bradbury, E.J. Chondroitinase gene therapy improves upper limb function following cervical contusion injury. Exp. Neurol. 2015, 271, 131–135. [Google Scholar] [CrossRef]
- García-Alías, G.; Barkhuysen, S.; Buckle, M.; Fawcett, J.W. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat. Neurosci. 2009, 12, 1145–1151. [Google Scholar] [CrossRef]
- Kwok, J.C.F.; Heller, J.P.; Zhao, R.-R.; Fawcett, J.W. Targeting Inhibitory Chondroitin Sulphate Proteoglycans to Promote Plasticity after Injury. In Axon Growth and Regeneration: Methods and Protocols; Murray, A.J., Ed.; Springer: New York, NY, USA, 2014; pp. 127–138. [Google Scholar]
- Bartus, K.; James, N.D.; Didangelos, A.; Bosch, K.D.; Verhaagen, J.; Yanez-Munoz, R.J.; Rogers, J.H.; Schneider, B.L.; Muir, E.M.; Bradbury, E.J. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 4822–4836. [Google Scholar] [CrossRef]
- Didangelos, A.; Iberl, M.; Vinsland, E.; Bartus, K.; Bradbury, E.J. Regulation of IL-10 by chondroitinase ABC promotes a distinct immune response following spinal cord injury. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 16424–16432. [Google Scholar] [CrossRef]
- Muir, E.; De Winter, F.; Verhaagen, J.; Fawcett, J. Recent advances in the therapeutic uses of chondroitinase ABC. Exp. Neurol. 2019, 321, 113032. [Google Scholar] [CrossRef]
- Alilain, W.J.; Horn, K.P.; Hu, H.; Dick, T.E.; Silver, J. Functional regeneration of respiratory pathways after spinal cord injury. Nature 2011, 475, 196–200. [Google Scholar] [CrossRef]
- Urban, M.W.; Ghosh, B.; Block, C.G.; Charsar, B.A.; Smith, G.M.; Wright, M.C.; Li, S.; Lepore, A.C. Protein Tyrosine Phosphatase σ Inhibitory Peptide Promotes Recovery of Diaphragm Function and Sprouting of Bulbospinal Respiratory Axons after Cervical Spinal Cord Injury. J. Neurotrauma 2019, 37, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Nong, J.; Wang, Z.; Urban, M.W.; Heinsinger, N.M.; Trovillion, V.A.; Wright, M.C.; Lepore, A.C.; Zhong, Y. A hydrogel engineered to deliver minocycline locally to the injured cervical spinal cord protects respiratory neural circuitry and preserves diaphragm function. Neurobiol. Dis. 2019, 127, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Ordaz, J.D.; Liu, N.K.; Richardson, Z.; Wu, W.; Xia, Y.; Qu, W.; Wang, Y.; Dai, H.; Zhang, Y.P.; et al. Descending motor circuitry required for NT-3 mediated locomotor recovery after spinal cord injury in mice. Nat. Commun. 2019, 10, 5815. [Google Scholar] [CrossRef]
- Ghosh, B.; Wang, Z.; Nong, J.; Urban, M.W.; Zhang, Z.; Trovillion, V.A.; Wright, M.C.; Zhong, Y.; Lepore, A.C. Local BDNF delivery to the injured cervical spinal cord using an engineered hydrogel enhances diaphragmatic respiratory function. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 5982–5995. [Google Scholar] [CrossRef] [PubMed]
- Charsar, B.A.; Brinton, M.A.; Locke, K.; Chen, A.Y.; Ghosh, B.; Urban, M.W.; Komaravolu, S.; Krishnamurthy, K.; Smit, R.; Pasinelli, P.; et al. AAV2-BDNF promotes respiratory axon plasticity and recovery of diaphragm function following spinal cord injury. FASEB J. 2019, 33, 13775–13793. [Google Scholar] [CrossRef] [PubMed]
- Gransee, H.M.; Zhan, W.Z.; Sieck, G.C.; Mantilla, C.B. Targeted delivery of TrkB receptor to phrenic motoneurons enhances functional recovery of rhythmic phrenic activity after cervical spinal hemisection. PLoS ONE 2013, 8, e64755. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel-Flutot, P.; Lane, M.A.; Lepore, A.C.; Vinit, S. Therapeutic Strategies Targeting Respiratory Recovery after Spinal Cord Injury: From Preclinical Development to Clinical Translation. Cells 2023, 12, 1519. https://doi.org/10.3390/cells12111519
Michel-Flutot P, Lane MA, Lepore AC, Vinit S. Therapeutic Strategies Targeting Respiratory Recovery after Spinal Cord Injury: From Preclinical Development to Clinical Translation. Cells. 2023; 12(11):1519. https://doi.org/10.3390/cells12111519
Chicago/Turabian StyleMichel-Flutot, Pauline, Michael A. Lane, Angelo C. Lepore, and Stéphane Vinit. 2023. "Therapeutic Strategies Targeting Respiratory Recovery after Spinal Cord Injury: From Preclinical Development to Clinical Translation" Cells 12, no. 11: 1519. https://doi.org/10.3390/cells12111519
APA StyleMichel-Flutot, P., Lane, M. A., Lepore, A. C., & Vinit, S. (2023). Therapeutic Strategies Targeting Respiratory Recovery after Spinal Cord Injury: From Preclinical Development to Clinical Translation. Cells, 12(11), 1519. https://doi.org/10.3390/cells12111519




