Role of Pellino-1 in Inflammation and Cardioprotection following Severe Sepsis: A Novel Mechanism in a Murine Severe Sepsis Model † †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.1.1. Generation of Peli1fl/fl and Peli1KO mice
2.1.2. Generation of Cardiomyocyte-Specific Peli1 (CP1KO) Knockout Mice
2.1.3. Generation of AMPEL1Tg/+ (Cardiomyocytes Overexpressing Peli1)
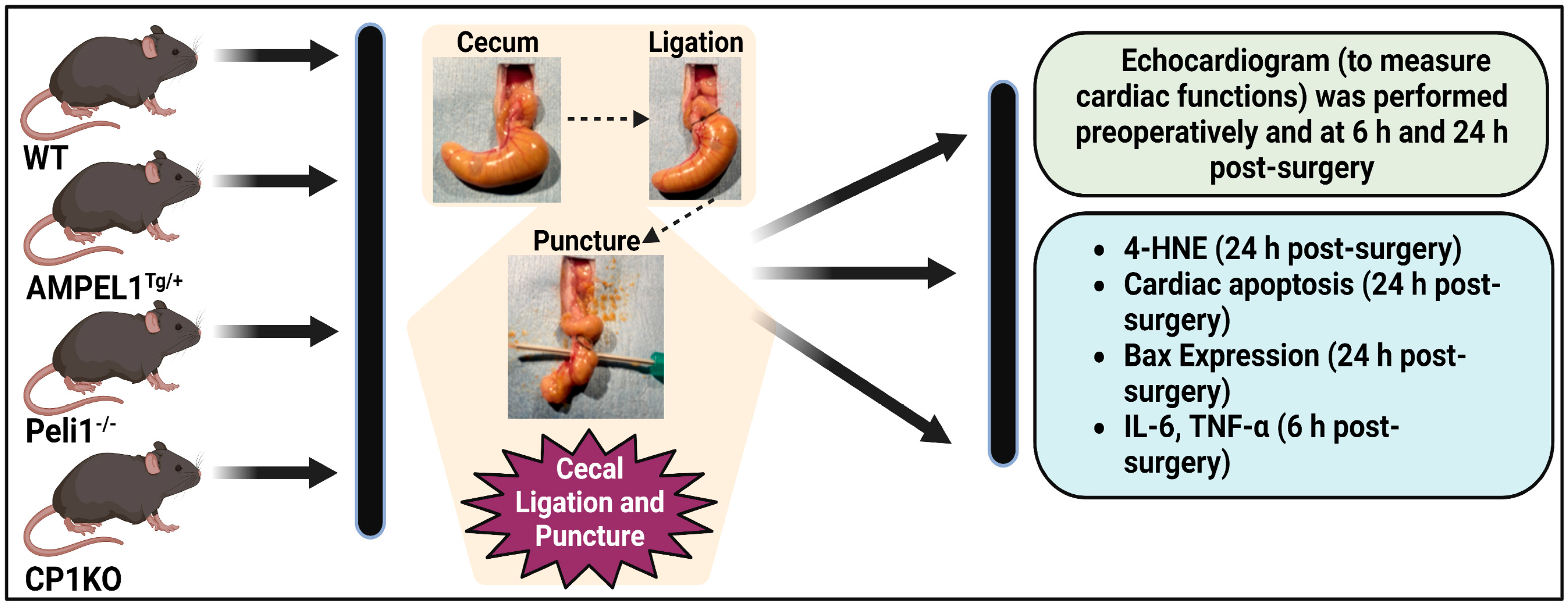
2.2. Study Design
2.2.1. Experiment 1: Effect of Cardiomyocyte Overexpression of Peli1 (AMPELlTg/+) in Mice Subjected to CLP
2.2.2. Experiment 2: Effect of Peli1 Gene Knockout in Mice Subjected to CLP
2.2.3. Experiment 3: Effect of Cardiomyocyte-Specific Peli1 (CP1KO) Knockout in Mice Subjected to CLP
2.2.4. Murine Cecal Ligation and Puncture Model (CLP Model)
2.3. Echocardiography
2.3.1. Immunohistochemical Analysis of 4-Hydroxynonenal (4-HNE) Expression
2.3.2. Immunofluorescence Analysis of Bax Expression
2.3.3. TUNEL Apoptosis Assay
2.3.4. ELISA to Measure Serum Cytokines
3. Results
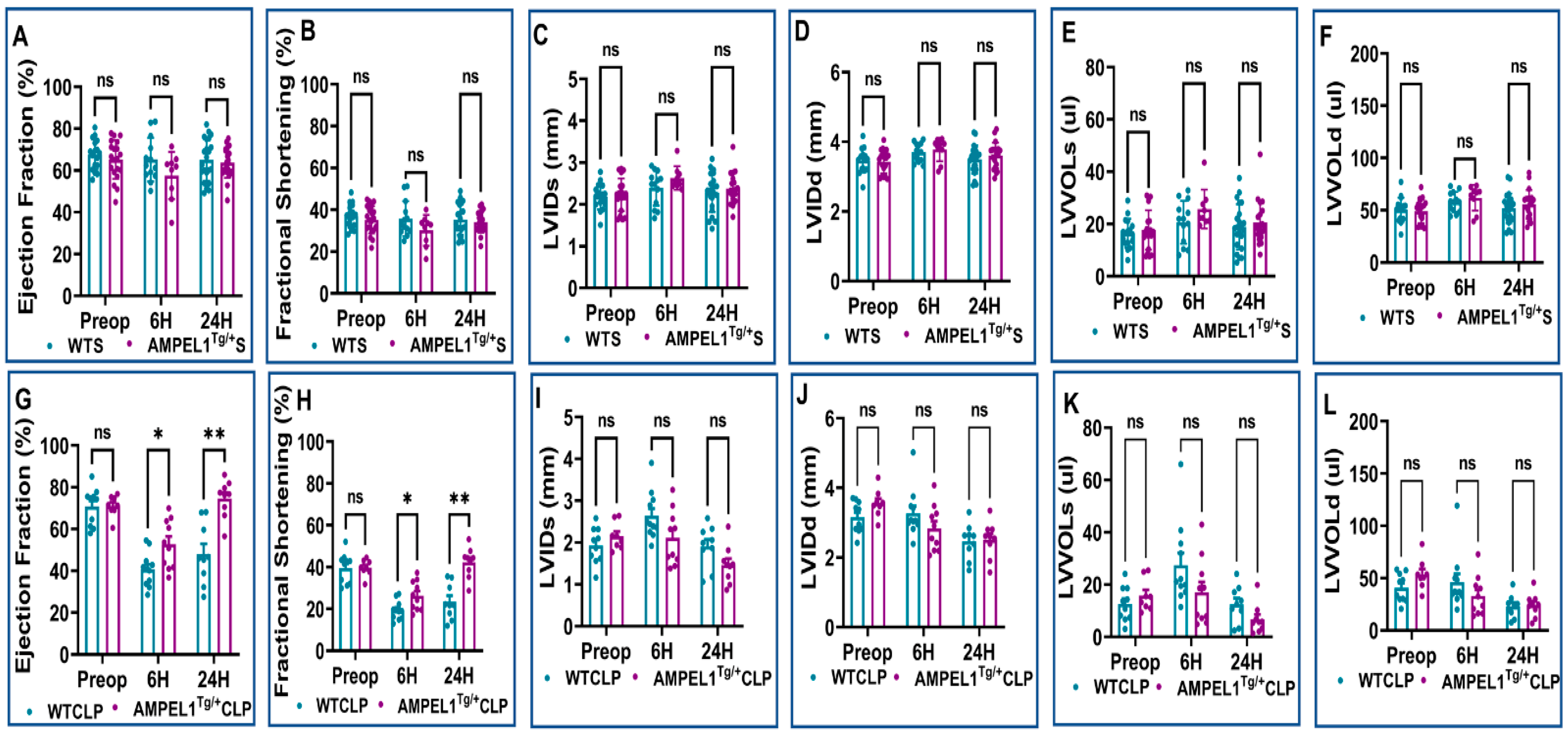
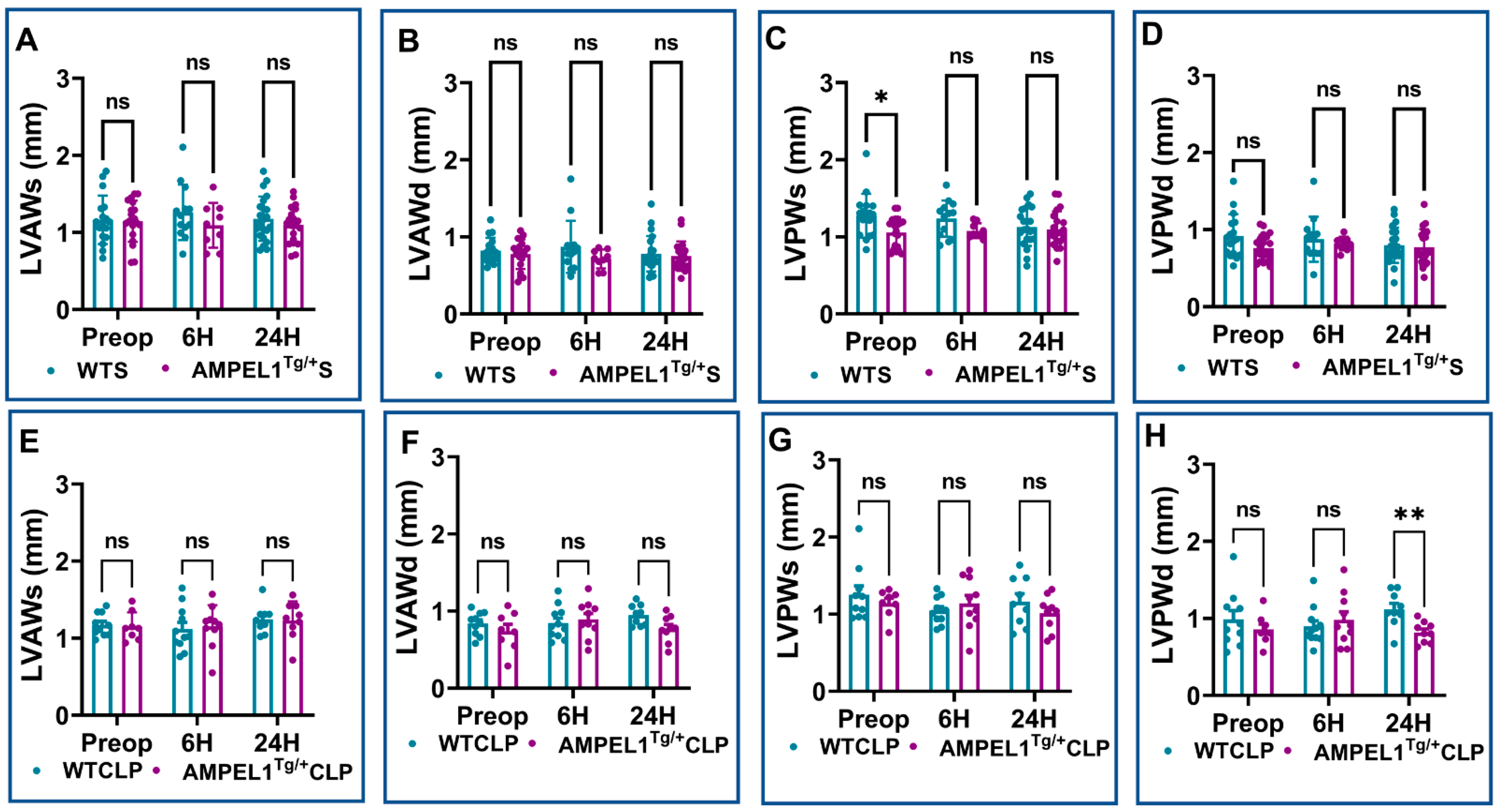
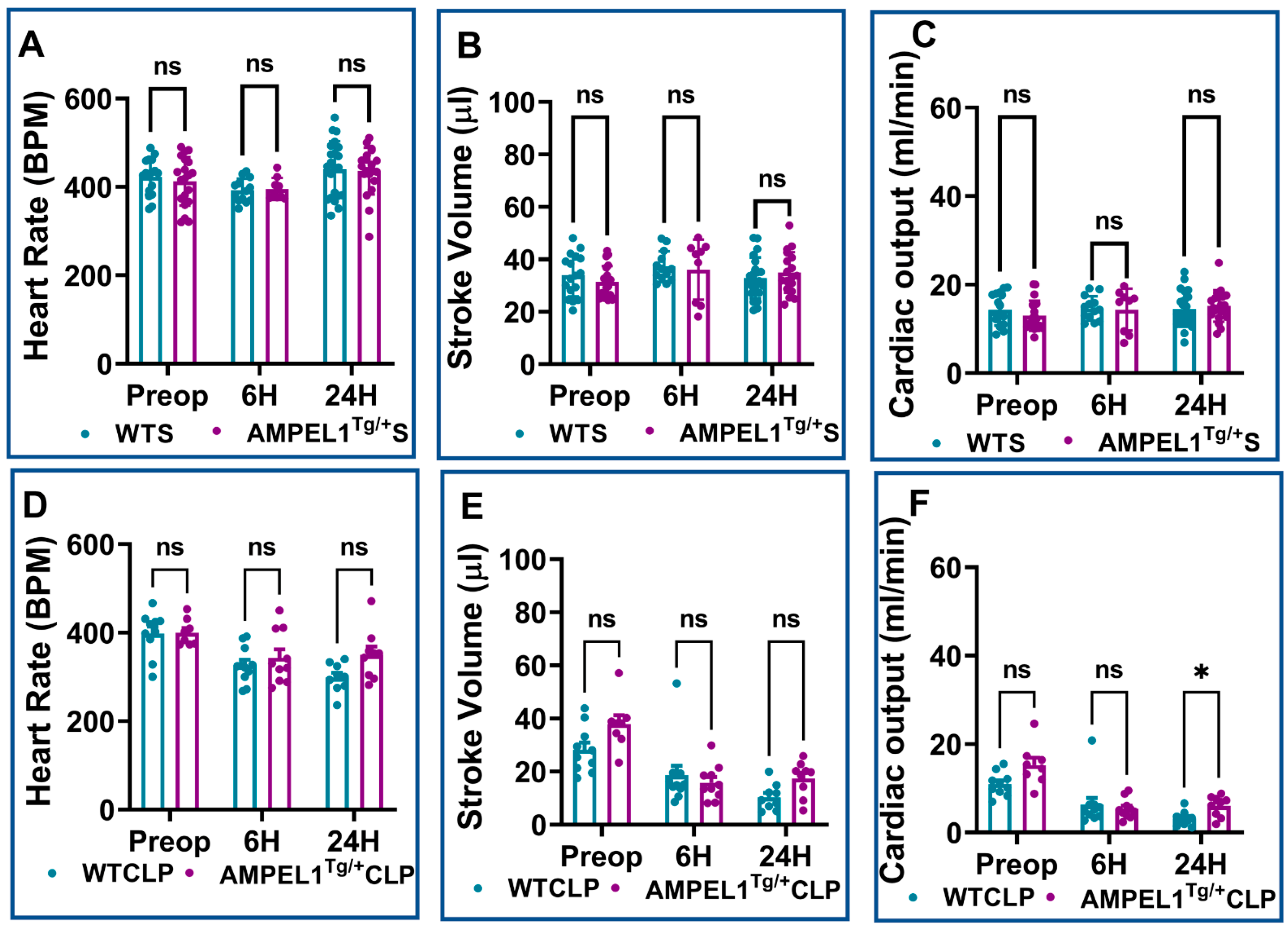
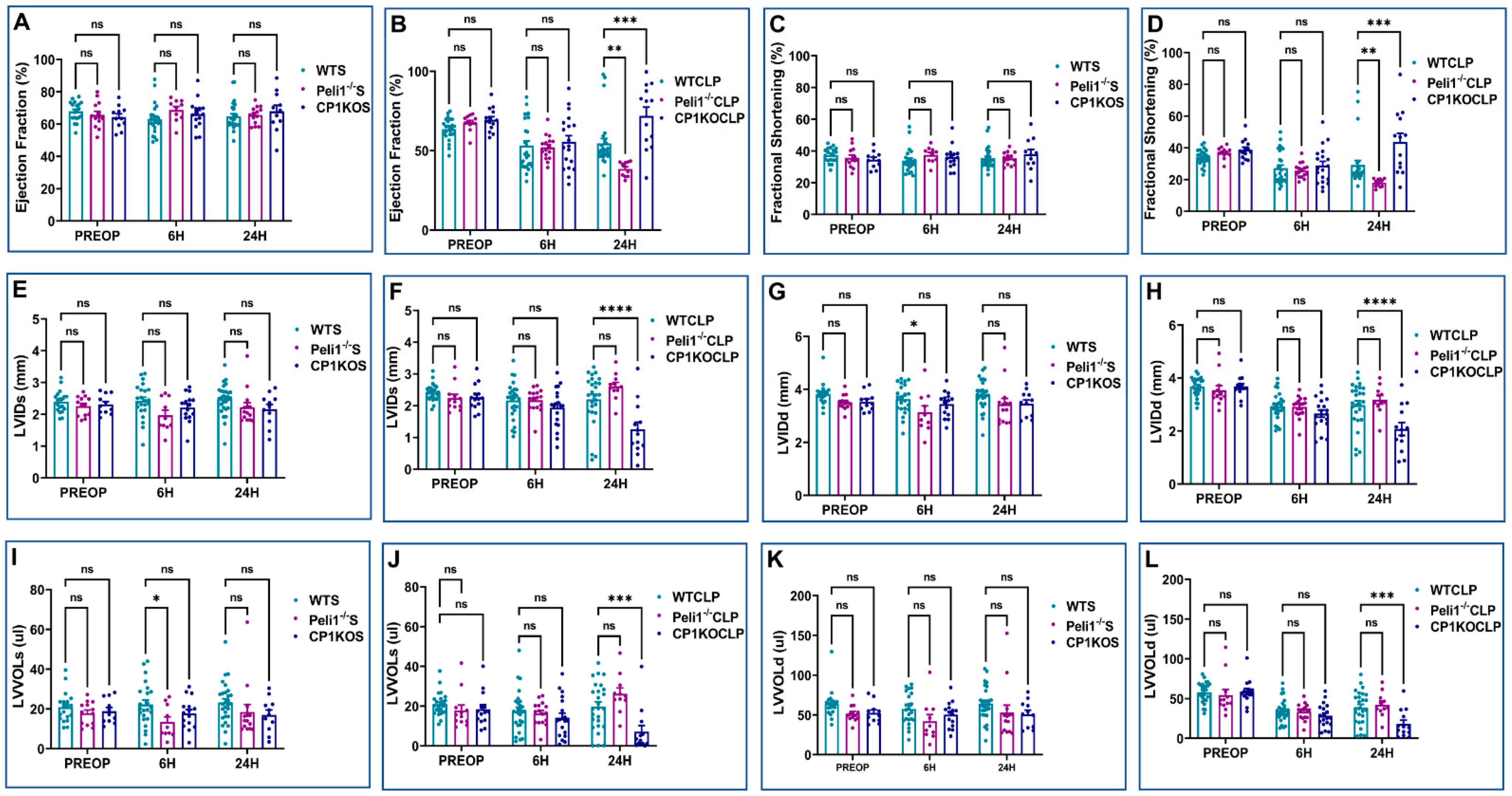
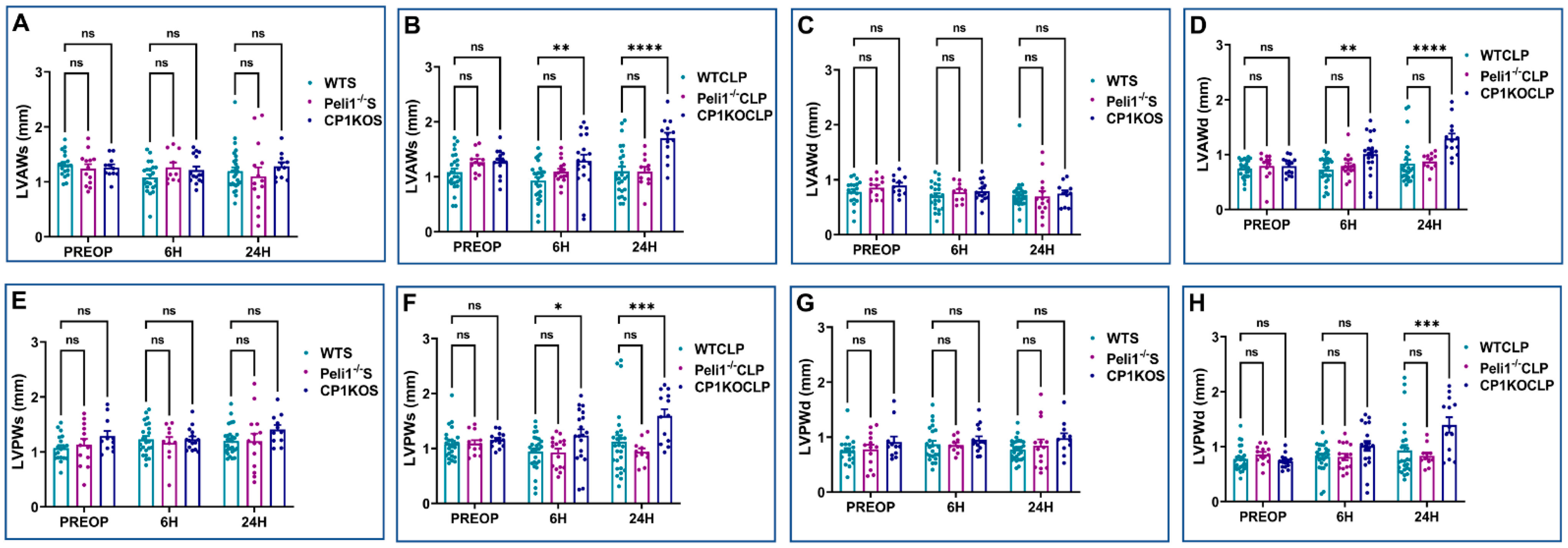
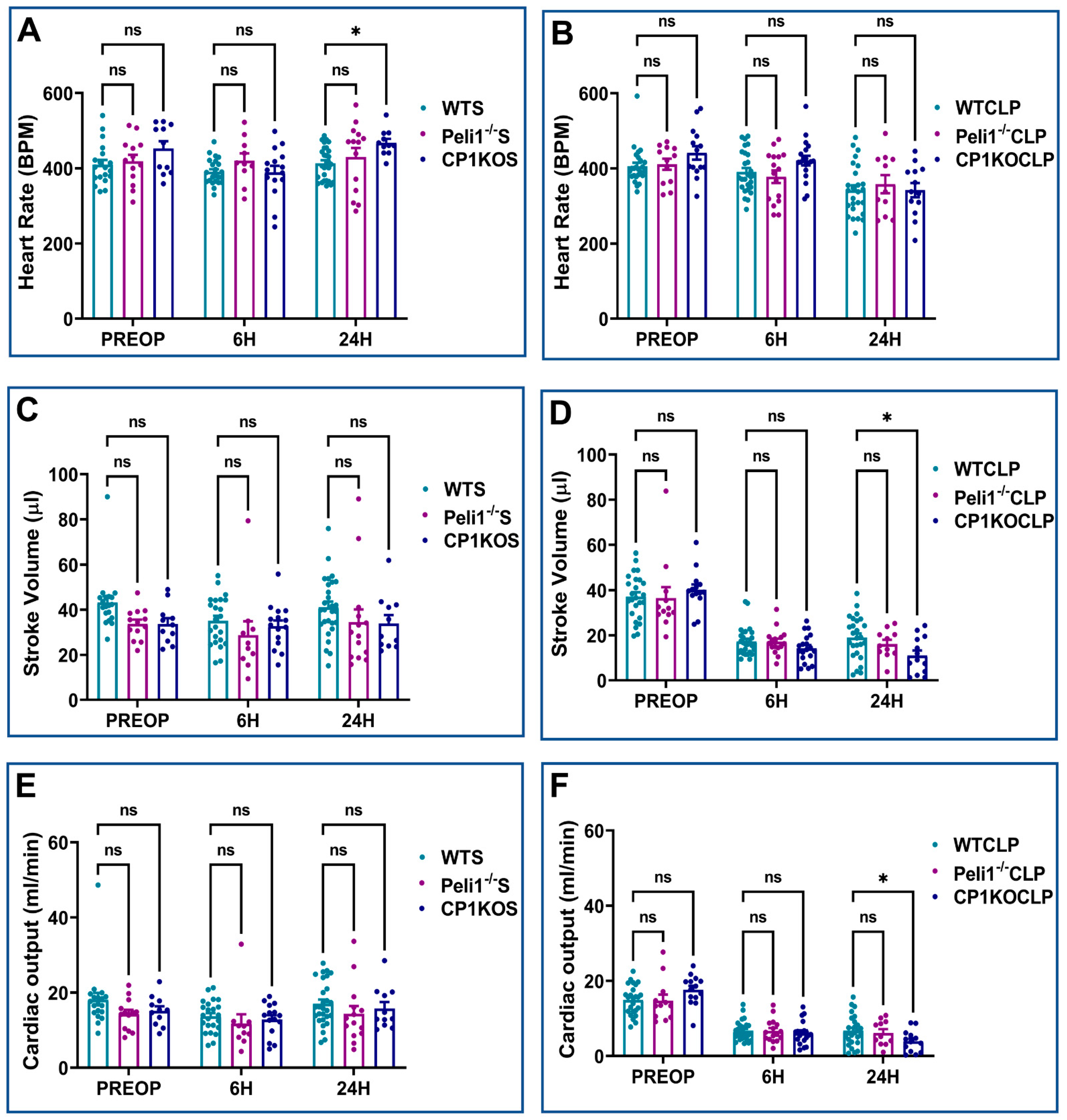
3.1. Overexpression of Cardiomyocyte-Specific Pellino-1 (AMPEL1Tg/+) Allows Preservation of Ejection Fraction and Other Echocardiographic Parameters following Severe Sepsis
3.2. Effect of Pellino-1 Global Knockout (Peli1−/−) and Cardiomyocyte-Specific Pellino-1 Knockout (CP1KO) Mice Subjected to Severe Sepsis on Cardiac Functions
3.3. Assessment of 4-HNE Expression following Sepsis
3.4. Assessment of Cardiac Myocyte Apoptosis at 24 Hours after Severe Sepsis
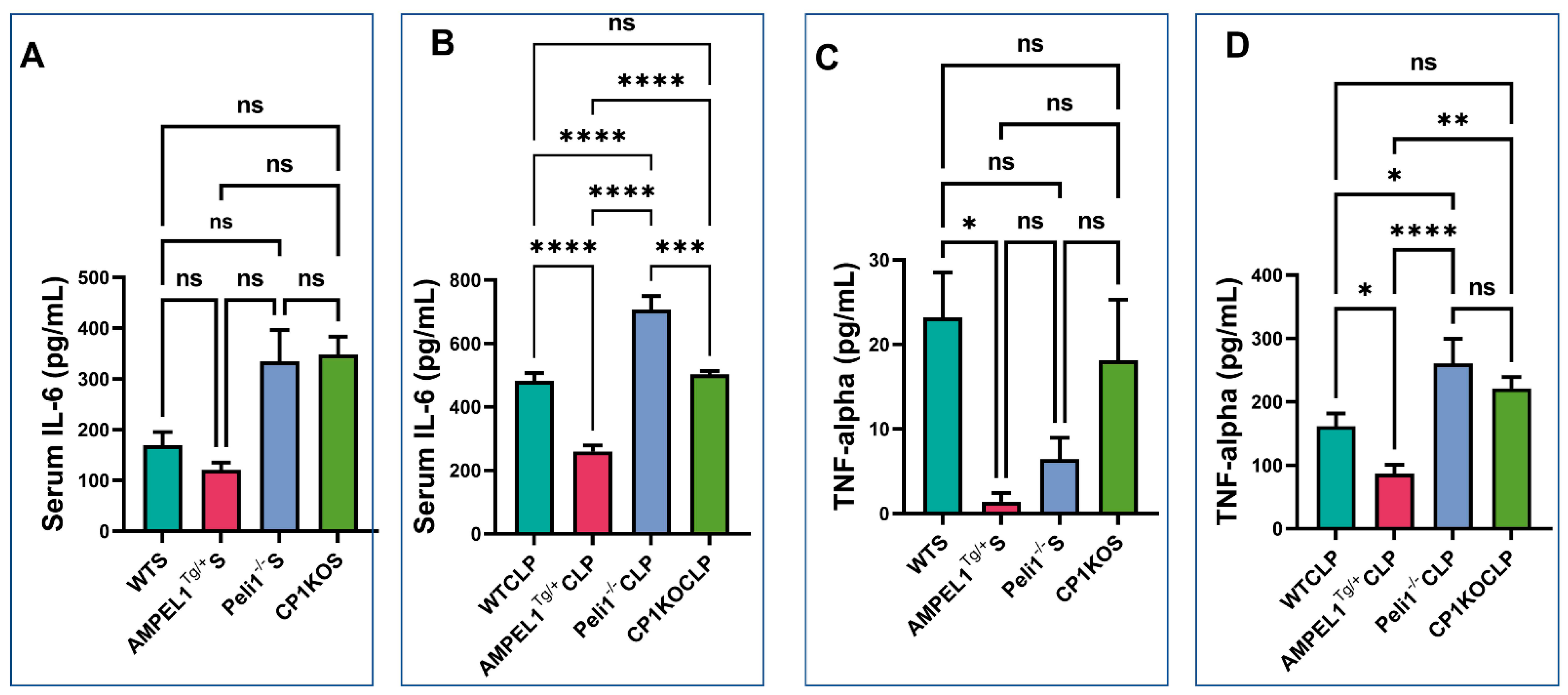
3.5. Assessment of Pro-Inflammatory Cytokines following Severe Sepsis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef]
- Levy, M.M.; Dellinger, R.P.; Townsend, S.R.; Linde-Zwirble, W.T.; Marshall, J.C.; Bion, J.; Schorr, C.; Artigas, A.; Ramsay, G.; Beale, R.; et al. The Surviving Sepsis Campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010, 36, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Landesberg, G.; Gilon, D.; Meroz, Y.; Georgieva, M.; Levin, P.D.; Goodman, S.; Avidan, A.; Beeri, R.; Weissman, C.; Jaffe, A.S.; et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur. Heart J. 2012, 33, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Walley, K.R. Sepsis-induced myocardial dysfunction. Curr. Opin. Crit. Care 2018, 24, 292–299. [Google Scholar] [CrossRef]
- Grosshans, J.; Schnorrer, F.; Nüsslein-Volhard, C. Oligomerisation of Tube and Pelle leads to nuclear localisation of dorsal. Mech. Dev. 1999, 81, 127–138. [Google Scholar] [CrossRef]
- Haghayeghi, A.; Sarac, A.; Czerniecki, S.; Grosshans, J.; Schöck, F. Pellino enhances innate immunity in Drosophila. Mech. Dev. 2010, 127, 301–307. [Google Scholar] [CrossRef]
- Jiang, Z.; Johnson, H.J.; Nie, H.; Qin, J.; Bird, T.A.; Li, X. Pellino 1 is required for interleukin-1 (IL-1)-mediated signaling through its interaction with the IL-1 receptor-associated kinase 4 (IRAK4)-IRAK-tumor necrosis factor receptor-associated factor 6 (TRAF6) complex. J. Biol. Chem. 2003, 278, 10952–10956. [Google Scholar] [CrossRef]
- Jiang, Z.; Ninomiya-Tsuji, J.; Qian, Y.; Matsumoto, K.; Li, X. Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol. Cell. Biol. 2002, 22, 7158–7167. [Google Scholar] [CrossRef]
- Schauvliege, R.; Janssens, S.; Beyaert, R. Pellino proteins: Novel players in TLR and IL-1R signalling. J. Cell. Mol. Med. 2007, 11, 453–461. [Google Scholar] [CrossRef]
- Zhang, E.; Li, X. The Emerging Roles of Pellino Family in Pattern Recognition Receptor Signaling. Front. Immunol. 2022, 13, 728794. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, V.; Thirunavukkarasu, M.; Joshi, M.; Oriowo, B.; Shaikh, I.A.; Rishi, M.T.; Tapias, L.; Coca-Soliz, V.; Saad, I.; Campbell, J.; et al. Deletion of newly described pro-survival molecule Pellino-1 increases oxidative stress, downregulates cIAP2/NF-κB cell survival pathway, reduces angiogenic response, and thereby aggravates tissue function in mouse ischemic models. Basic. Res. Cardiol. 2020, 115, 45. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, M.; Selvaraju, V.; Joshi, M.; Coca-Soliz, V.; Tapias, L.; Saad, I.; Fournier, C.; Husain, A.; Campbell, J.; Yee, S.P.; et al. Disruption of VEGF Mediated Flk-1 Signaling Leads to a Gradual Loss of Vessel Health and Cardiac Function during Myocardial Infarction: Potential Therapy with Pellino-1. J. Am. Heart Assoc. 2018, 7, e007601. [Google Scholar] [CrossRef] [PubMed]
- Rednam, C.K.; Wilson, R.L.; Selvaraju, V.; Rishi, M.T.; Thirunavukkarasu, M.; Coca-Soliz, V.; Lakshmanan, R.; Palesty, J.A.; McFadden, D.W.; Maulik, N. Increased survivability of ischemic skin flap tissue in Flk-1(+/−) mice by Pellino-1 intervention. Microcirculation 2017, 24, 12362. [Google Scholar] [CrossRef]
- Tidswell, R.; Singer, M. Sepsis-thoughtful management for the non-expert. Clin. Med. 2018, 18, 62–68. [Google Scholar] [CrossRef]
- Liu, P.; Jenkins, N.A.; Copeland, N.G. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003, 13, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.L.; Selvaraju, V.; Lakshmanan, R.; Thirunavukkarasu, M.; Campbell, J.; McFadden, D.W.; Maulik, N. Thioredoxin-1 attenuates sepsis-induced cardiomyopathy after cecal ligation and puncture in mice. J. Surg. Res. 2017, 220, 68–78. [Google Scholar] [CrossRef]
- Campbell, J.D.; Lakshmanan, R.; Selvaraju, V.; Accorsi, D.; McFadden, D.W.; Maulik, N.; Thirunavukkarasu, M. Engineered resveratrol-loaded fibrous scaffolds promotes functional cardiac repair and regeneration through Thioredoxin-1 mediated VEGF pathway. Int. J. Pharm. 2021, 597, 120236. [Google Scholar] [CrossRef]
- Thirunavukkarasu, M.; Tipu Rishi, M.; Pradeep, S.R.; Swaminathan, S.; Accorsi, D.; Palesty, J.A.; Maulik, N. Heat shock protein A12B gene therapy improves perfusion, promotes neovascularization, and decreases fibrosis in a murine model of hind limb ischemia. Surgery 2021, 170, 969–977. [Google Scholar] [CrossRef]
- Handala, L.; Fiore, T.; Rouillé, Y.; Helle, F. QuantIF: An ImageJ Macro to Automatically Determine the Percentage of Infected Cells after Immunofluorescence. Viruses 2019, 11, 165. [Google Scholar] [CrossRef]
- Moynagh, P.N. The Pellino family: IRAK E3 ligases with emerging roles in innate immune signalling. Trends Immunol. 2009, 30, 33–42. [Google Scholar] [CrossRef]
- Resch, K.; Jockusch, H.; Schmitt-John, T. Assignment of homologous genes, Peli1/PELI1 and Peli2/PELI2, for the Pelle adaptor protein Pellino to mouse chromosomes 11 and 14 and human chromosomes 2p13.3 and 14q21, respectively, by physical and radiation hybrid mapping. Cytogenet Cell Genet. 2001, 92, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Rich, T.; Allen, R.L.; Lucas, A.M.; Stewart, A.; Trowsdale, J. Pellino-related sequences from Caenorhabditis elegans and Homo sapiens. Immunogenetics 2000, 52, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Moynagh, P.N. The roles of Pellino E3 ubiquitin ligases in immunity. Nat. Rev. Immunol. 2014, 14, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Marsh, E.K.; Prestwich, E.C.; Williams, L.; Hart, A.R.; Muir, C.F.; Parker, L.C.; Jonker, M.R.; Heijink, I.H.; Timens, W.; Fife, M.; et al. Pellino-1 Regulates the Responses of the Airway to Viral Infection. Front. Cell Infect. Microbiol. 2020, 10, 456. [Google Scholar] [CrossRef]
- Chang, M.; Jin, W.; Sun, S.C. Peli1 facilitates TRIF-dependent Toll-like receptor signaling and proinflammatory cytokine production. Nat. Immunol. 2009, 10, 1089–1095. [Google Scholar] [CrossRef]
- Jensen, L.E.; Whitehead, A.S. Pellino3, a novel member of the Pellino protein family, promotes activation of c-Jun and Elk-1 and may act as a scaffolding protein. J. Immunol. 2003, 171, 1500–1506. [Google Scholar] [CrossRef]
- Marquez, R.T.; Wendlandt, E.; Galle, C.S.; Keck, K.; McCaffrey, A.P. MicroRNA-21 is upregulated during the proliferative phase of liver regeneration, targets Pellino-1, and inhibits NF-kappaB signaling. Am. J. Physiol. Gastrointest Liver Physiol. 2010, 298, G535–G541. [Google Scholar] [CrossRef]
- Schauvliege, R.; Janssens, S.; Beyaert, R. Pellino proteins are more than scaffold proteins in TLR/IL-1R signalling: A role as novel RING E3-ubiquitin-ligases. FEBS Lett. 2006, 580, 4697–4702. [Google Scholar] [CrossRef]
- Strelow, A.; Kollewe, C.; Wesche, H. Characterization of Pellino2, a substrate of IRAK1 and IRAK4. FEBS Lett. 2003, 547, 157–161. [Google Scholar] [CrossRef]
- Burger, F.; Baptista, D.; Roth, A.; Brandt, K.J.; Miteva, K. The E3 Ubiquitin Ligase Peli1 Deficiency Promotes Atherosclerosis Progression. Cells 2022, 11, 2014, PMCID:PMC9265341. [Google Scholar] [CrossRef] [PubMed]
- L’Heureux, M.; Sternberg, M.; Brath, L.; Turlington, J.; Kashiouris, M.G. Sepsis-Induced Cardiomyopathy: A Comprehensive Review. Curr. Cardiol. Rep. 2020, 22, 35. [Google Scholar] [CrossRef]
- Merx, M.W.; Weber, C. Sepsis and the heart. Circulation 2007, 116, 793–802. [Google Scholar] [CrossRef]
- Habimana, R.; Choi, I.; Cho, H.J.; Kim, D.; Lee, K.; Jeong, I. Sepsis-induced cardiac dysfunction: A review of pathophysiology. Acute Crit. Care 2020, 35, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, W.; Sun, X.; Xie, L.; Yang, Y.; Sang, M.; Jiao, R. SS31 Ameliorates Sepsis-Induced Heart Injury by Inhibiting Oxidative Stress and Inflammation. Inflammation 2019, 42, 2170–2180. [Google Scholar] [CrossRef] [PubMed]
- Ehrman, R.R.; Sullivan, A.N.; Favot, M.J.; Sherwin, R.L.; Reynolds, C.A.; Abidov, A.; Levy, P.D. Pathophysiology, echocardiographic evaluation, biomarker findings, and prognostic implications of septic cardiomyopathy: A review of the literature. Crit. Care 2018, 22, 112. [Google Scholar] [CrossRef]
- Antonucci, E.; Fiaccadori, E.; Donadello, K.; Taccone, F.S.; Franchi, F.; Scolletta, S. Myocardial depression in sepsis: From pathogenesis to clinical manifestations and treatment. J. Crit. Care 2014, 29, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak, M.; Persichini, R.; Monnet, X.; Teboul, J.L. Management of myocardial dysfunction in severe sepsis. Semin. Respir. Crit. Care Med. 2011, 32, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Derwall, M.; Al Zoubi, S.; Zechendorf, E.; Reuter, D.A.; Thiemermann, C.; Schuerholz, T. The Septic Heart: Current Understanding of Molecular Mechanisms and Clinical Implications. Chest 2019, 155, 427–437. [Google Scholar] [CrossRef]
- Parker, M.M.; Shelhamer, J.H.; Bacharach, S.L.; Green, M.V.; Natanson, C.; Frederick, T.M.; Damske, B.A.; Parrillo, J.E. Profound but reversible myocardial depression in patients with septic shock. Ann. Intern. Med. 1984, 100, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Beesley, S.J.; Weber, G.; Sarge, T.; Nikravan, S.; Grissom, C.K.; Lanspa, M.J.; Shahul, S.; Brown, S.M. Septic Cardiomyopathy. Crit. Care Med. 2018, 46, 625–634. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thirunavukkarasu, M.; Swaminathan, S.; Kemerley, A.; Pradeep, S.R.; Lim, S.T.; Accorsi, D.; Wilson, R.; Campbell, J.; Saad, I.; Yee, S.-P.; et al. Role of Pellino-1 in Inflammation and Cardioprotection following Severe Sepsis: A Novel Mechanism in a Murine Severe Sepsis Model †. Cells 2023, 12, 1527. https://doi.org/10.3390/cells12111527
Thirunavukkarasu M, Swaminathan S, Kemerley A, Pradeep SR, Lim ST, Accorsi D, Wilson R, Campbell J, Saad I, Yee S-P, et al. Role of Pellino-1 in Inflammation and Cardioprotection following Severe Sepsis: A Novel Mechanism in a Murine Severe Sepsis Model †. Cells. 2023; 12(11):1527. https://doi.org/10.3390/cells12111527
Chicago/Turabian StyleThirunavukkarasu, Mahesh, Santosh Swaminathan, Andrew Kemerley, Seetur R. Pradeep, Sue Ting Lim, Diego Accorsi, Rickesha Wilson, Jacob Campbell, Ibnalwalid Saad, Siu-Pok Yee, and et al. 2023. "Role of Pellino-1 in Inflammation and Cardioprotection following Severe Sepsis: A Novel Mechanism in a Murine Severe Sepsis Model †" Cells 12, no. 11: 1527. https://doi.org/10.3390/cells12111527





