Multi-Drug Cocktail Therapy Improves Survival and Neurological Function after Asphyxial Cardiac Arrest in Rodents
Abstract
:1. Introduction
2. Methods
2.1. Experimental Design
2.1.1. Scientific Rationale for the Use of a Multi-Drug Cocktail
2.1.2. Selection and Formulation of the Multi-Drug Cocktail
| Group | Agent | Dose, mg/kg | Mechanism(s) of Action | Reference |
|---|---|---|---|---|
| Antioxidants | CoQ10 | 30 | Increases oxidative stress resistance to, improves mitochondrial bioenergetics | [42] |
| Edaravone | 3 | Reactive oxygen species scavenger: reduces free radical-induced inflammation, reduces oxidative damage and lipid peroxidation to cells minimizes | [21] | |
| N-acetyl cysteine | 150 | Antioxidant, anti-inflammatory | [22] | |
| Vitamin C | 50 | Protects against oxidative stress, cofactor in catecholamine synthesis | [43] | |
| Calcium and ionic control | Zoniporide | 3 | NHE-1 inhibitor: protects from ionic gradients, decreases calcium overload from ischemic reperfusion injury | [44] |
| Lipid membrane regulation | Poloxamer 188 | 150 | Lipid bilayer stabilizer: reduces BBB damage, cerebral edema, and cell death | [45] |
| SS-31 | 0.5 | Mitochondrial protectant: protects the mitochondrial membrane by stabilizing cardiolipin | [46] | |
| Mitochondrial protectant and energy production | Cyclosporine A | 10 | mPTP inhibitor: reduces cytochrome c release, elevate superoxide dismutase activity | [16] |
| Metformin | 100 | Mitochondrial protectant: promotes neurogenesis, protects the BBB, and reduces ROS and inflammation | [38,41] | |
| Sulbutiamine | 12.5 | Metabolic supplement for brain energetics: increases thiamine level by easily crossing the BBB | [47] |
2.2. Preparation of Cocktail and Vehicle
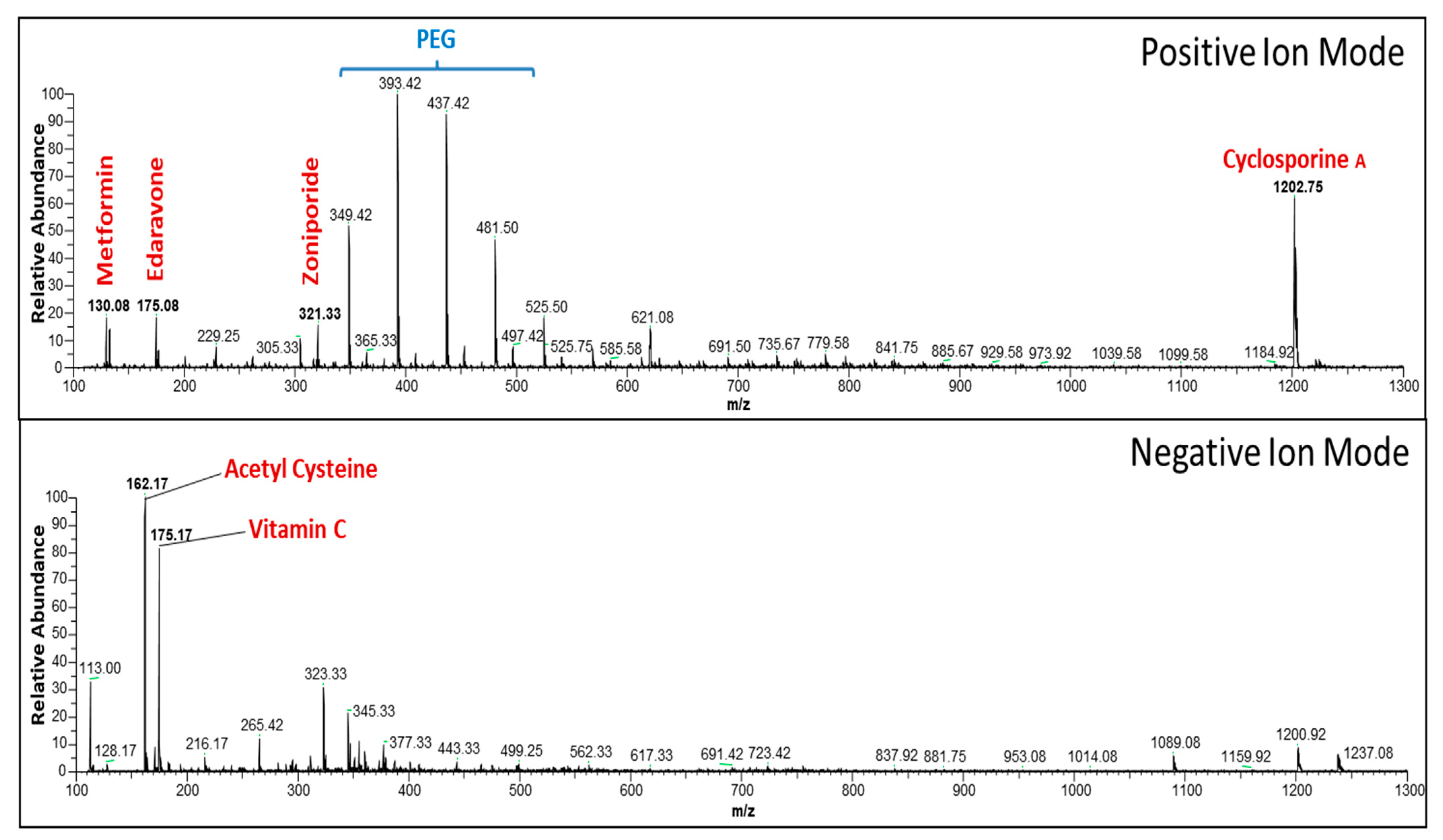
2.3. Evaluation of Active Components in Multi-Drug Cocktail Using Mass Spectrometry
2.4. Experimental Animal Procedure for Inducing 20 Min of Asphyxial Cardiac Arrest and 30 Min of Cardiopulmonary Bypass Resuscitation
2.5. Experimental Animal Procedure for Inducing 12 Min of Asphyxial Cardiac Arrest
2.6. Blinded, Randomized, Placebo-Control Study
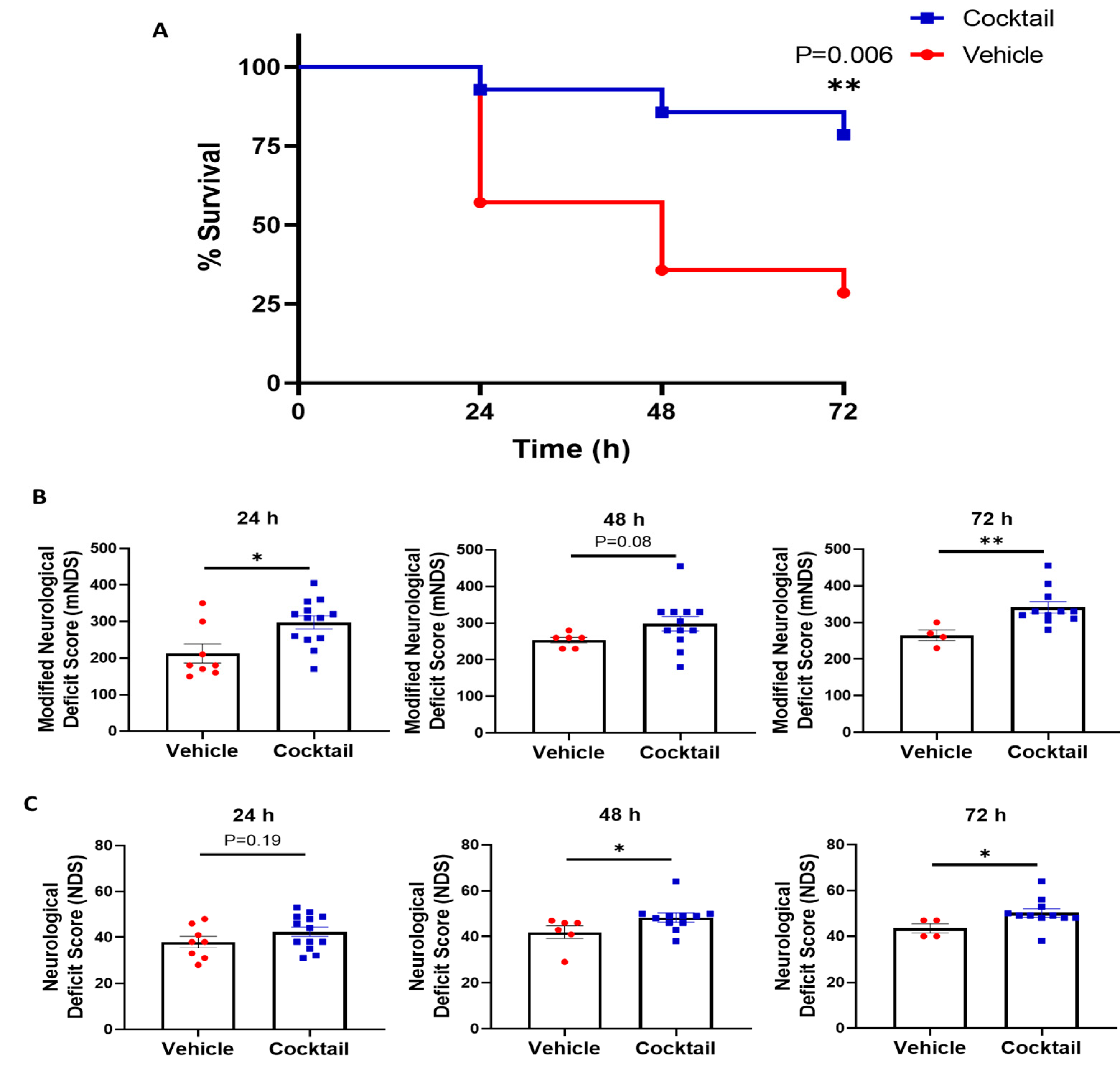
2.7. Neurological Deficit Scores at 24, 48, and 72 h Post-Resuscitation
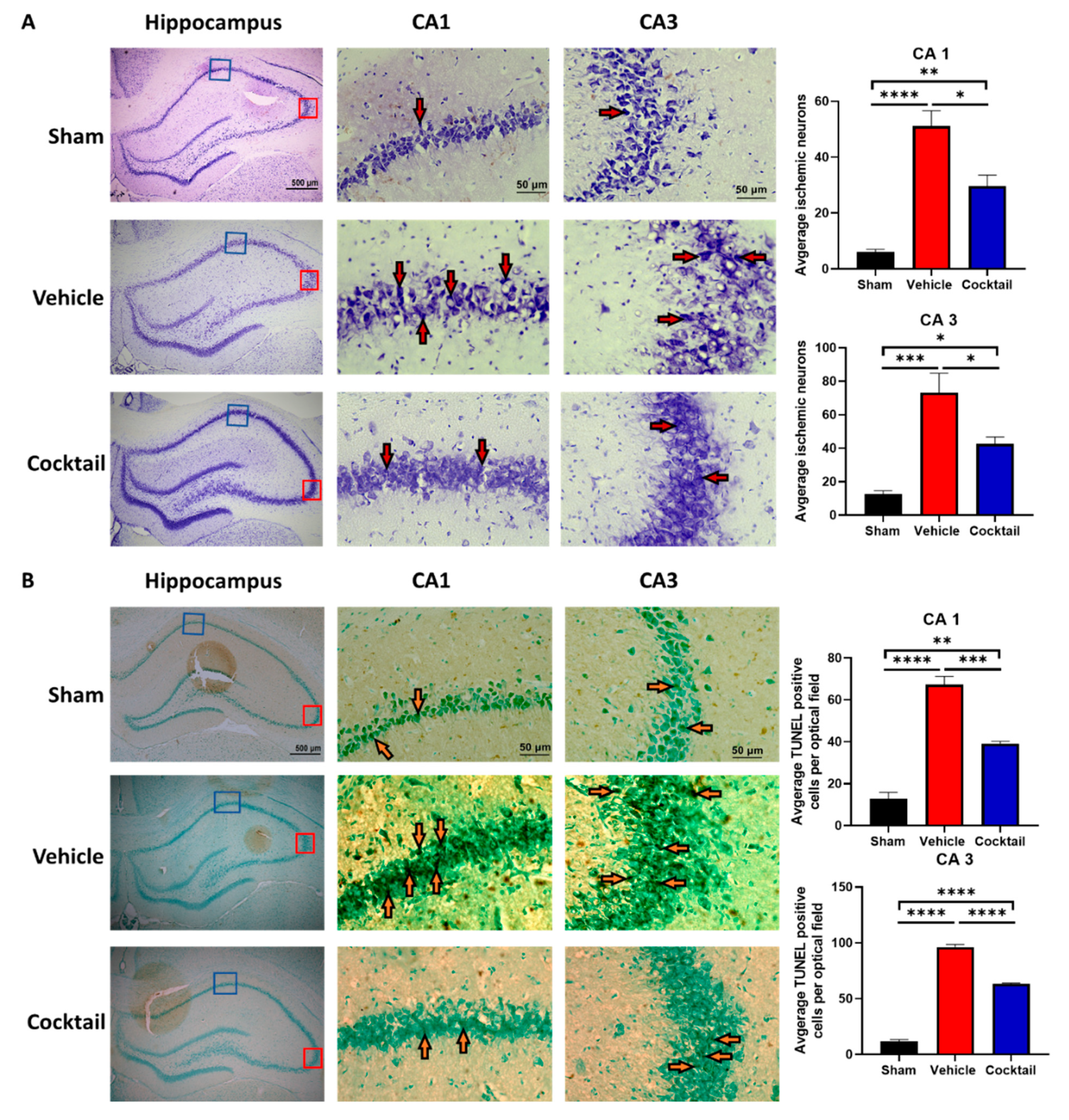
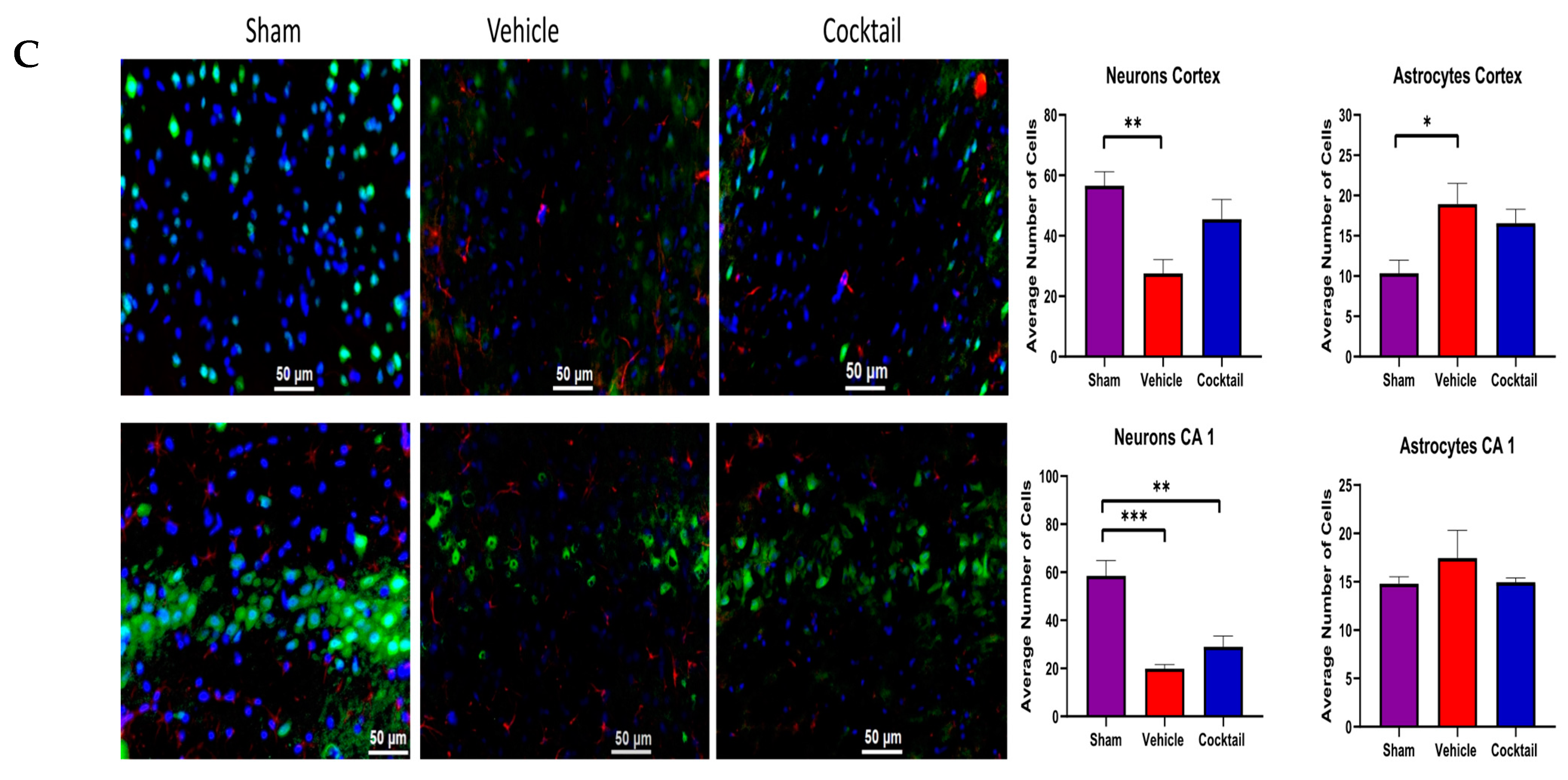
2.8. Histology and Staining
2.9. Biochemical Analysis
2.10. Statistical Analyses
3. Results
3.1. Preliminary Analysis of the Multi-Drug Cocktail
3.1.1. Pilot Study 1: Severe 12 Min of Asphyxial Cardiac Arrest and Cardiopulmonary Resuscitation
3.1.2. Pilot Study 2: Highly Lethal 20 Min Model of Asphyxial Cardiac Arrest and Cardiopulmonary Bypass Resuscitation
3.2. Multi-Drug Cocktail Improves Survival and Mitigates Neurological Dysfunction and Brain Damage after Asphyxial Cardiac Arrest
3.3. Cocktail Reduces Inflammation and Promotes Anti-Inflammatory Activity after Asphyxial Cardiac Arrest
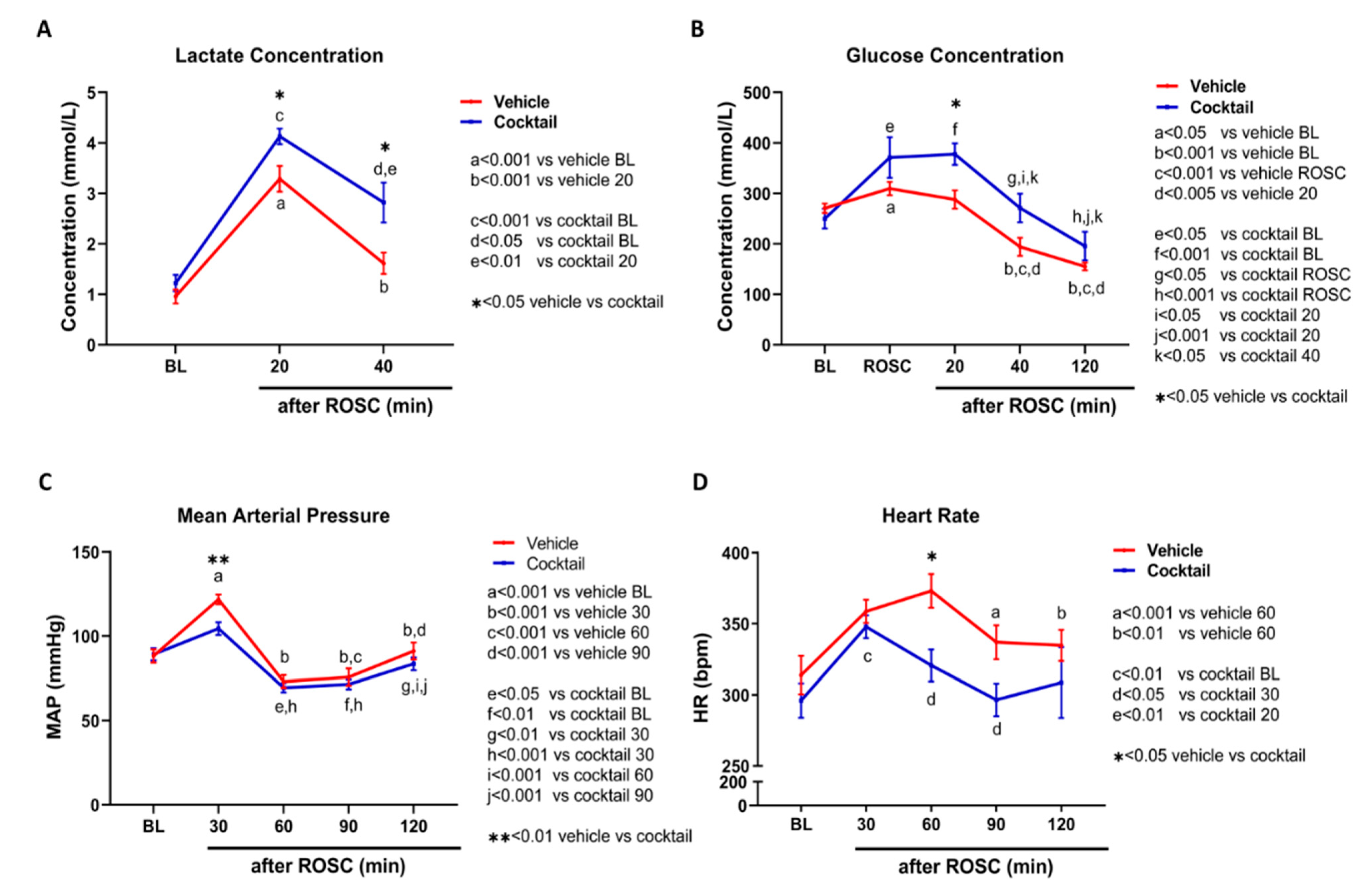
3.4. The Cocktail Therapy Resulted in Improved Physiological Characteristics, Hemodynamics, and Arterial Blood Gas Chemistry following Asphyxial Cardiac Arrest
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics-2019 update: A report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics-2021 update: A report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Popp, E.; Böttiger, B.W. Cerebral Resuscitation: State of the Art, Experimental Approaches and Clinical Perspectives. Neurol. Clin. 2006, 24, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.C.; Shoaib, M.; Sohnen, S.; Rolston, D.M.; Jafari, D.; Miyara, S.J.; Hayashida, K.; Molmenti, E.P.; Kim, J.; Becker, L.B. Pharmacological Approach for Neuroprotection After Cardiac Arrest—A Narrative Review of Current Therapies and Future Neuroprotective Cocktail. Front. Med. 2021, 8, 636651. [Google Scholar] [CrossRef] [PubMed]
- Nakka, V.P.; Gusain, A.; Raghubir, R. Endoplasmic Reticulum Stress Plays Critical Role in Brain Damage After Cerebral Ischemia/Reperfusion in Rats. Neurotox. Res. 2010, 17, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Da, T.; Riobo, N.A.; Becker, L.B. Early mitochondrial dysfunction in electron transfer activity and reactive oxygen species generation after cardiac arrest. Crit. Care Med. 2008, 36, S447–S453. [Google Scholar] [CrossRef]
- Vereczki, V.; Martin, E.; Rosenthal, R.E.; Hof, P.R.; Hoffman, G.E.; Fiskum, G. Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J. Cereb. Blood Flow Metab. 2006, 26, 821–835. [Google Scholar] [CrossRef]
- Jiang, J.; Fang, X.; Fu, Y.; Xu, W.; Jiang, L.; Huang, Z. Impaired Cerebral Mitochondrial Oxidative Phosphorylation Function in a Rat Model of Ventricular Fibrillation and Cardiopulmonary Resuscitation. BioMed. Res. Int. 2014, 2014, 192769. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Zhao, H.; Wang, J.; Zhang, L.; Liu, A.; Chen, Y. Inflammatory mechanisms involved in brain injury following cardiac arrest and cardiopulmonary resuscitation. Biomed. Rep. 2016, 5, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Shoaib, M.; Yin, T.; Nayyar, G.; Shinozaki, K.; Stevens, J.F.; Becker, L.B.; Kim, J. Tissue-Specific Metabolic Profiles After Prolonged Cardiac Arrest Reveal Brain Metabolome Dysfunction Predominantly After Resuscitation. J. Am. Heart Assoc. 2019, 8, e012809. [Google Scholar] [CrossRef] [Green Version]
- Shoaib, M.; Choudhary, R.C.; Choi, J.; Kim, N.; Hayashida, K.; Yagi, T.; Yin, T.; Nishikimi, M.; Stevens, J.F.; Becker, L.B.; et al. Plasma metabolomics supports the use of long-duration cardiac arrest rodent model to study human disease by demonstrating similar metabolic alterations. Sci. Rep. 2020, 10, 19707. [Google Scholar] [CrossRef]
- Lundin, A.; Djärv, T.; Engdahl, J.; Hollenberg, J.; Nordberg, P.; Ravn-Fischer, A.; Ringh, M.; Rysz, S.; Svensson, L.; Herlitz, J.; et al. Drug therapy in cardiac arrest: A review of the literature. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 54–75. [Google Scholar] [CrossRef] [Green Version]
- Arts, E.J.; Hazuda, D.J. HIV-1 Antiretroviral Drug Therapy. Cold Spring Harb. Perspect. Med. 2012, 2, a007161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preissner, S.; Dunkel, M.; Hoffmann, M.F.; Preissner, S.C.; Genov, N.; Rong, W.W.; Preissner, R.; Seeger, K. Drug Cocktail Optimization in Chemotherapy of Cancer. PLoS ONE 2012, 7, e51020. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.M.; Frank, J.E.; Glickman, L.T.; McGwin, G., Jr.; Lambert, B.H.; Gordon, C.J. Effect of a pharmacologically induced decrease in core temperature in rats resuscitated from cardiac arrest. Resuscitation 2015, 92, 26–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wang, Y.; Zhuang, Q.; Chen, M.; Wang, Y.; Hou, L.; Han, F. Protective effects of cyclosporine A and hypothermia on neuronal mitochondria in a rat asphyxial cardiac arrest model. Am. J. Emerg. Med. 2016, 34, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, R.; Li, P.; Lu, H.; Hao, J.; Li, L.; Tucker, D.; Zhang, Q. Combination Treatment with Methylene Blue and Hypothermia in Global Cerebral Ischemia. Mol. Neurobiol. 2018, 55, 2042–2055. [Google Scholar] [CrossRef]
- Damian, M.S.; Ellenberg, D.; Gildemeister, R.; Lauermann, J.; Simonis, G.; Sauter, W.; Georgi, C. Coenzyme Q10 combined with mild hypothermia after cardiac arrest: A preliminary study. Circulation 2004, 110, 3011–3016. [Google Scholar] [CrossRef]
- Matthews, R.T.; Yang, L.; Browne, S.; Baik, M.; Beal, M.F. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc. Natl. Acad. Sci. USA 1998, 95, 8892–8897. [Google Scholar] [CrossRef] [Green Version]
- Cruz, M.P. Edaravone (Radicava): A Novel Neuroprotective Agent for the Treatment of Amyotrophic Lateral Sclerosis. P T 2018, 43, 25–28. [Google Scholar]
- Qin, T.; Lei, L.-Y.; Li, N.; Shi, F.R.; Chen, M.-H.; Xie, L. Edaravone improves survival and neurological outcomes after CPR in a ventricular fibrillation model of rats. Am. J. Emerg. Med. 2016, 34, 1944–1949. [Google Scholar] [CrossRef]
- He, F.; Zheng, G.; Hou, J.; Hu, Q.; Ling, Q.; Wu, G.; Zhao, H.; Yang, J.; Wang, Y.; Jiang, L.; et al. N-acetylcysteine alleviates post-resuscitation myocardial dysfunction and improves survival outcomes via partly inhibiting NLRP3 inflammasome induced-pyroptosis. J. Inflamm. 2020, 17, 25. [Google Scholar] [CrossRef]
- Arakawa, M.; Ito, Y. N-acetylcysteine and neurodegenerative diseases: Basic and clinical pharmacology. Cerebellum 2007, 6, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Su, C.; Zhang, G.; Liang, L.; Jin, T.; Bradley, J.; Ornato, J.P.; Tang, W. Vitamin C Improves the Outcomes of Cardiopulmonary Resuscitation and Alters Shedding of Syndecan-1 and p38/MAPK Phosphorylation in a Rat Model. J. Am. Heart Assoc. 2022, 11, e023787. [Google Scholar] [CrossRef]
- Chen, Q.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Reversible Blockade of Electron Transport during Ischemia Protects Mitochondria and Decreases Myocardial Injury following Reperfusion. Experiment 2006, 319, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Aldakkak, M.; Stowe, D.F.; Chen, Q.; Lesnefsky, E.J.; Camara, A.K. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc. Res. 2008, 77, 406–415. [Google Scholar]
- Gazmuri, R.J.; Radhakrishnan, J.; Ayoub, I.M. Sodium-Hydrogen Exchanger Isoform-1 Inhibition: A Promising Pharmacological Intervention for Resuscitation from Cardiac Arrest. Molecules 2019, 24, 1765. [Google Scholar] [CrossRef] [Green Version]
- Clements-Jewery, H.; Sutherland, F.J.; Allen, M.C.; Tracey, W.R.; Avkiran, M. Cardioprotective efficacy of zoniporide, a potent and selective inhibitor of Na+/H+ exchanger isoform 1, in an experimental model of cardiopulmonary bypass. Br. J. Pharmacol. 2004, 142, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pille, J.A.; Riess, M.L. Potential Effects of Poloxamer 188 on Rat Isolated Brain Mitochondria after Oxidative Stress In Vivo and In Vitro. Brain Sci. 2021, 11, 122. [Google Scholar] [CrossRef]
- Bartos, J.; Matsuura, T.R.; Tsangaris, A.; Olson, M.; McKnite, S.H.; Rees, J.N.; Haman, K.; Shekar, K.C.; Riess, M.L.; Bates, F.S.; et al. Intracoronary Poloxamer 188 Prevents Reperfusion Injury in a Porcine Model of ST-Segment Elevation Myocardial Infarction. JACC Basic Transl. Sci. 2016, 1, 224–234. [Google Scholar] [CrossRef] [Green Version]
- Szeto, H.H.; Liu, S. Cardiolipin-targeted peptides rejuvenate mitochondrial function, remodel mitochondria, and promote tissue regeneration during aging. Arch. Biochem. Biophys. 2018, 660, 137–148. [Google Scholar] [CrossRef]
- Zhang, W.; Tam, J.; Shinozaki, K.; Yin, T.; Lampe, J.W.; Becker, L.B.; Kim, J. Increased Survival Time With SS-31 After Prolonged Cardiac Arrest in Rats. Heart Lung Circ. 2019, 28, 505–508. [Google Scholar] [CrossRef]
- Szeto, H.H. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br. J. Pharmacol. 2014, 171, 2029–2050. [Google Scholar] [CrossRef] [Green Version]
- Paskitti, M.; Reid, K.H. Use of an adenosine triphosphate-based ‘cocktail’ early in reperfusion substantially improves brain protein synthesis after global ischemia in rats. Neurosci. Lett. 2002, 331, 147–150. [Google Scholar] [CrossRef]
- Cour, M.; Loufouat, J.; Paillard, M.; Augeul, L.; Goudable, J.; Ovize, M.; Argaud, L. Inhibition of mitochondrial permeability transition to prevent the post-cardiac arrest syndrome: A pre-clinical study. Eur. Heart J. 2011, 32, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, M.; Pukenas, B.; Chawla, S.; Ehinger, J.K.; Plyler, R.; Stolow, M.; Gabello, M.; Hugerth, M.; Elmér, E.; Hansson, M.J.; et al. Neuroprotective Effects of Cyclosporine in a Porcine Pre-Clinical Trial of Focal Traumatic Brain Injury. J. Neurotrauma 2018, 36, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasri, H.; Rafieian-Kopaei, M. Metformin: Current knowledge. J. Res. Med. Sci. 2014, 19, 658–664. [Google Scholar] [PubMed]
- Shoaib, M.; Choudhary, R.C.; Chillale, R.K.; Kim, N.; Miyara, S.J.; Haque, S.; Yin, T.; Frankfurt, M.; Molmenti, E.P.; Zanos, S.; et al. Metformin-mediated mitochondrial protection post-cardiac arrest improves EEG activity and confers neuroprotection and survival benefit. FASEB J. 2022, 36, e22307. [Google Scholar] [CrossRef] [PubMed]
- Starling-Soares, B.; Carrera-Bastos, P.; Bettendorff, L. Role of the Synthetic B1 Vitamin Sulbutiamine on Health. J. Nutr. Metab. 2020, 2020, 9349063. [Google Scholar] [CrossRef]
- Kwag, J.; Majid, A.S.A.; Kang, K.D. Evidence for Neuroprotective Effect of Sulbutiamine against Oxygen-Glucose Deprivation in Rat Hippocampal CA1 Pyramidal Neurons. Biol. Pharm. Bull. 2011, 34, 1759–1764. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.H.; Kim, S.G.; Lee, M.G. Dose-Independent Pharmacokinetics of Metformin in Rats: Hepatic and Gastrointestinal First-Pass Effects. J. Pharm. Sci. 2006, 95, 2543–2552. [Google Scholar] [CrossRef] [PubMed]
- Belousova, M.; Tokareva, O.G.; Gorodetskaya, E.; Kalenikova, E.I.; Medvedev, O.S. Intravenous Treatment With Coenzyme Q10 Improves Neurological Outcome and Reduces Infarct Volume After Transient Focal Brain Ischemia in Rats. J. Cardiovasc. Pharmacol. 2016, 67, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-S.; Huang, C.-H.; Tsai, C.-Y.; Cheng, H.-J.; Hsu, C.-Y.; Chang, W.-T.; Chen, W.-J. Combination of Intravenous Ascorbic Acid Administration and Hypothermia After Resuscitation Improves Myocardial Function and Survival in a Ventricular Fibrillation Cardiac Arrest Model in the Rat. Acad. Emerg. Med. 2014, 21, 257–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamoureux, L.; Whitehouse, H.K.; Radhakrishnan, J.; Gazmuri, R.J. Abstract 148: Zoniporide Combined with α-Methylnorepinephrine Appears Highly Effective for Resuscitation from Cardiac Arrest in a Rat Model of Ventricular Fibrillation. Circulation 2014, 130, A148. [Google Scholar] [CrossRef]
- Walters, T.J.; Mase, V.J.; Roe, J.L.; Dubick, M.A.; Christy, R.J. Poloxamer-188 Reduces Muscular Edema After Tourniquet-Induced Ischemia-Reperfusion Injury in Rats. J. Trauma: Inj. Infect. Crit. Care 2011, 70, 1192–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbah, H.N.; Gupta, R.C.; Kohli, S.; Wang, M.; Hachem, S.; Zhang, K. Chronic Therapy With Elamipretide (MTP-131), a Novel Mitochondria-Targeting Peptide, Improves Left Ventricular and Mitochondrial Function in Dogs With Advanced Heart Failure. Circ. Heart Fail. 2016, 9, e002206. [Google Scholar] [CrossRef] [Green Version]
- Trovero, F.; Gobbi, M.; Weil-Fuggaza, J.; Besson, M.-J.; Brochet, D.; Pirot, S. Evidence for a modulatory effect of sulbutiamine on glutamatergic and dopaminergic cortical transmissions in the rat brain. Neurosci. Lett. 2000, 292, 49–53. [Google Scholar] [CrossRef]
- Ferslew, K. Specimen Preparation/Extraction. In Principles of Forensic Toxicology, 5th ed.; Levine, B.S., Kerrigan, S., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2020; pp. 109–125. [Google Scholar]
- Han, F.; Boller, M.; Guo, W.; Merchant, R.M.; Lampe, J.W.; Smith, T.M.; Becker, L.B. A rodent model of emergency cardiopulmonary bypass resuscitation with different temperatures after asphyxial cardiac arrest. Resuscitation 2010, 81, 93–99. [Google Scholar] [CrossRef]
- Neumar, R.W.; Bircher, N.G.; Sim, K.M.; Xiao, F.; Zadach, K.S.; Radovsky, A.; Katz, L.; Ebmeyer, E.; Safar, P. Epinephrine and sodium bicarbonate during CPR following asphyxial cardiac arrest in rats. Resuscitation 1995, 29, 249–263. [Google Scholar] [CrossRef]
- Jia, X.; Koenig, M.A.; Shin, H.-C.; Zhen, G.; Pardo, C.A.; Hanley, D.F.; Thakor, N.V.; Geocadin, R.G. Improving neurological outcomes post-cardiac arrest in a rat model: Immediate hypothermia and quantitative EEG monitoring. Resuscitation 2008, 76, 431–442. [Google Scholar] [CrossRef] [Green Version]
- Ascierto, P.A.; Marincola, F.M. Combination therapy: The next opportunity and challenge of medicine. J. Transl. Med. 2011, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Rationalizing combination therapies. Nat. Med. 2017, 23, 1113. [CrossRef] [PubMed] [Green Version]
- Shoaib, M.; Becker, L.B. A walk through the progression of resuscitation medicine. Ann. N. Y. Acad. Sci. 2020, 1507, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhou, G.-M.; Wang, L.-H.; Zhu, L.; Liu, J.-M.; Wang, X.-D.; Li, H.-T.; Chen, L. Inhibiting High-Mobility Group Box 1 (HMGB1) Attenuates Inflammatory Cytokine Expression and Neurological Deficit in Ischemic Brain Injury Following Cardiac Arrest in Rats. Inflammation 2016, 39, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Ju, M.; Song, J.; Zheng, Y.; Lin, S.; Zhu, D.; Wen, L.; Zhong, M.; Pan, S.; et al. Neuroprotective Effect of the Inhibitor Salubrinal after Cardiac Arrest in a Rodent Model. Oxidat. Med. Cell Longev. 2020, 2020, 7468738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashida, K.; Miyara, S.J.; Shinozaki, K.; Takegawa, R.; Yin, T.; Rolston, D.M.; Choudhary, R.C.; Guevara, S.; Molmenti, E.P.; Becker, L.B. Inhaled Gases as Therapies for Post–Cardiac Arrest Syndrome: A Narrative Review of Recent Developments. Front. Med. 2020, 7, 586229. [Google Scholar] [CrossRef]
- Mader, T.J.; Coute, R.A.; Kellogg, A.R.; Nathanson, B.H. Blinded Evaluation of Combination Drug Therapy for Prolonged Ventricular Fibrillation Using a Swine Model of Sudden Cardiac Arrest. Prehospital Emerg. Care 2016, 20, 390–398. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, K.; Huang, K.; Gu, Y.; Hu, Y.; Pan, S.; Ji, Z. Metformin Improves Neurologic Outcome via AMP-Activated Protein Kinase–Mediated Autophagy Activation in a Rat Model of Cardiac Arrest and Resuscitation. J. Am. Heart Assoc. 2018, 7, e008389. [Google Scholar] [CrossRef] [Green Version]
- Knapp, J.; Roewer, J.; Bruckner, T.; Böttiger, B.W.; Popp, E. Evaluation of Cyclosporine a as a Cardio- and Neuroprotective Agent after Cardiopulmonary Resuscitation in a Rat Model. Shock 2015, 43, 576–581. [Google Scholar] [CrossRef]
- Ikeda, K.; Liu, X.; Kida, K.; Marutani, E.; Hirai, S.; Sakaguchi, M.; Andersen, L.W.; Bagchi, A.; Cocchi, M.N.; Berg, K.M.; et al. Thiamine as a neuroprotective agent after cardiac arrest. Resuscitation 2016, 105, 138–144. [Google Scholar] [CrossRef]
- Marcos, M.A.; Cabaleiro, D.; Guimarey, M.J.G.; Comuñas, M.J.P.; Fedele, L.; Fernández, J.; Lugo, L. PEG 400-Based Phase Change Materials Nano-Enhanced with Functionalized Graphene Nanoplatelets. Nanomaterials 2017, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Cui, D.; Shang, H.; Zhang, X.; Jiang, W.; Jia, X. Cardiac arrest triggers hippocampal neuronal death through autophagic and apoptotic pathways. Sci. Rep. 2016, 6, 27642. [Google Scholar] [CrossRef] [Green Version]
- Kriz, J. Inflammation in Ischemic Brain Injury: Timing Is Important. Crit. Rev. Neurobiol. 2006, 18, 145–157. [Google Scholar] [CrossRef]
- Liu, F.; Mccullough, L.D. Inflammatory responses in hypoxic ischemic encephalopathy. Acta Pharmacol. Sin. 2013, 34, 1121–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, S.-M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S232–S240. [Google Scholar] [CrossRef] [Green Version]
- Swanson, R.A.; Ying, W.; Kauppinen, T. Astrocyte Influences on Ischemic Neuronal Death. Curr. Mol. Med. 2004, 4, 193–205. [Google Scholar] [CrossRef]
- Wong, C.H.; Crack, P.J. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr. Med. Chem. 2008, 15, 1–14. [Google Scholar]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Lambertsen, K.L.; Clausen, B.H.; Babcock, A.A.; Gregersen, R.; Fenger, C.; Nielsen, H.H.; Haugaard, L.S.; Wirenfeldt, M.; Nielsen, M.; Dagnaes-Hansen, F.; et al. Microglia Protect Neurons against Ischemia by Synthesis of Tumor Necrosis Factor. J. Neurosci. 2009, 29, 1319–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doll, D.N.; Rellick, S.L.; Barr, T.L.; Ren, X.; Simpkins, J.W. Rapid mitochondrial dysfunction mediates TNF-alpha-induced neurotoxicity. J. Neurochem. 2015, 132, 443–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strle, K.; Zhou, J.H.; Shen, W.H.; Broussard, S.R.; Johnson, R.W.; Freund, G.G.; Dantzer, R.; Kelley, K.W. Interleukin-10 in the brain. Crit. Rev. Immunol. 2001, 21, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Grilli, M.; Barbieri, I.; Basudev, H.; Brusa, R.; Casati, C.; Lozza, G.; Ongini, E. Interleukin-10 modulates neuronal threshold of vulnerability to ischaemic damage. Eur. J. Neurosci. 2000, 12, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Ooboshi, H.; Ibayashi, S.; Shichita, T.; Kumai, Y.; Takada, J.; Ago, T.; Arakawa, S.; Sugimori, H.; Kamouchi, M.; Kitazono, T.; et al. Postischemic Gene Transfer of Interleukin-10 Protects Against Both Focal and Global Brain Ischemia. Circulation 2005, 111, 913–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraljevic, S.; Stambrook, P.J.; Pavelic, K. Accelerating drug discovery. EMBO Rep. 2004, 5, 837–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Vehicle (n = 14) | Cocktail (n = 14) | p Value | |
|---|---|---|---|
| Baseline characteristics | |||
| Body weight (g) | 450.6 ± 6.9 | 458.1 ± 7.2 | 0.46 |
| Mean arterial pressure (mmHg) | 88.1 ± 4.0 | 89.1 ± 3.6 | 0.86 |
| Heart rate (bpm) | 313.8 ± 13.5 | 295.9 ± 12.1 | 0.33 |
| Esophageal temperature (°C) | 36.8 ± 0.1 | 36.9 ± 0.1 | 0.59 |
| Cardiac arrest characteristics | |||
| Time to cardiac arrest (sec) | 184.0 ± 6.6 | 193.4 ± 6.6 | 0.27 |
| CPR time to ROSC (sec) | 57.4 ± 2.2 | 57.9 ± 2.6 | 0.93 |
| Time after ROSC | |||
|---|---|---|---|
| Baseline | 20 Min Post-ROSC | 40 Min Post-ROSC | |
| pH | |||
| Vehicle | 7.42 ± 0.01 | 7.23 ± 0.01 | 7.34 ± 0.01 |
| Cocktail | 7.40 ± 0.01 | 7.18 ± 0.02 | 7.31 ± 0.02 |
| pCO2 (mmHg) | |||
| Vehicle | 39.91 ± 1.60 | 52.23 ± 2.59 | 41.25 ± 1.64 |
| Cocktail | 43.36 ± 1.16 | 54.84 ± 3.28 | 41.80 ± 2.01 |
| pO2 (mmHg) | |||
| Vehicle | 113.42 ± 7.97 | 384.08 ± 35.93 | 128.08 ± 9.33 |
| Cocktail | 109.71 ± 6.79 | 227.93 ± 30.22 ** | 106.29 ± 17.44 |
| HCO3− (mEq/L) | |||
| Vehicle | 25.86 ± 0.85 | 21.99 ± 0.56 | 22.13 ± 0.43 |
| Cocktail | 27.21 ± 0.53 | 20.62 ± 0.63 | 21.20 ± 0.47 |
| SaO2 (%) | |||
| Vehicle | 98.08 ± 0.42 | 99.33 ± 0.67 | 98.50 ± 0.36 |
| Cocktail | 98.00 ± 0.38 | 97.36 ± 1.42 | 94.86 ± 1.27 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhary, R.C.; Shoaib, M.; Hayashida, K.; Yin, T.; Miyara, S.J.; d’Abramo, C.; Heuser, W.G.; Shinozaki, K.; Kim, N.; Takegawa, R.; et al. Multi-Drug Cocktail Therapy Improves Survival and Neurological Function after Asphyxial Cardiac Arrest in Rodents. Cells 2023, 12, 1548. https://doi.org/10.3390/cells12111548
Choudhary RC, Shoaib M, Hayashida K, Yin T, Miyara SJ, d’Abramo C, Heuser WG, Shinozaki K, Kim N, Takegawa R, et al. Multi-Drug Cocktail Therapy Improves Survival and Neurological Function after Asphyxial Cardiac Arrest in Rodents. Cells. 2023; 12(11):1548. https://doi.org/10.3390/cells12111548
Chicago/Turabian StyleChoudhary, Rishabh C., Muhammad Shoaib, Kei Hayashida, Tai Yin, Santiago J. Miyara, Cristina d’Abramo, William G. Heuser, Koichiro Shinozaki, Nancy Kim, Ryosuke Takegawa, and et al. 2023. "Multi-Drug Cocktail Therapy Improves Survival and Neurological Function after Asphyxial Cardiac Arrest in Rodents" Cells 12, no. 11: 1548. https://doi.org/10.3390/cells12111548
APA StyleChoudhary, R. C., Shoaib, M., Hayashida, K., Yin, T., Miyara, S. J., d’Abramo, C., Heuser, W. G., Shinozaki, K., Kim, N., Takegawa, R., Nishikimi, M., Li, T., Owens, C., Molmenti, E. P., He, M., Vanpatten, S., Al-Abed, Y., Kim, J., & Becker, L. B. (2023). Multi-Drug Cocktail Therapy Improves Survival and Neurological Function after Asphyxial Cardiac Arrest in Rodents. Cells, 12(11), 1548. https://doi.org/10.3390/cells12111548








