Performance of the Photosynthetic Apparatus under Glass with a Luminophore Modifying Red-To-Far-Red-Light Ratio—A Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Cultivation Conditions
2.3. Evaluation of Photosynthetic Apparatus Performance
2.3.1. Photosynthetic Pigment Concentration Assessment
2.3.2. Chl a Fluorescence Measurements
2.3.3. Gas Exchange Measurements
2.3.4. Structural and Functional Photosynthetic Protein Content Determination
2.3.5. Transmission Electron Microscopy Observation
2.4. Evaluation of Leaf Antioxidant Activity
2.4.1. Guaiacol Peroxidase Activity Evaluation
2.4.2. Glutathione Content Evaluation
2.5. Statistical Analyses
3. Results
3.1. Photosynthetic Apparatus Response to Red Light
3.1.1. Photosynthetic Pigment Concentration
3.1.2. Chl a Fluorescence
3.1.3. Gas Exchange
3.1.4. Structural and Functional Photosynthetic Proteins
3.1.5. Chloroplast Ultrastructure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABS/CSo | Absorption flux per CS at t = 0; ABS/CSo ≈ F0 |
| ABS/RC | Absorption flux per RC; ABS/RC = Mo/VJ = 4(F300 μs − F0)/(Fm − F0)/VJ |
| Area | Total complementary area between fluorescence induction curve and F = Fm |
| DIo/CSo | Dissipated energy flux per CS at t = 0; DIo/CSo = ABS CSo − TRo/CSo |
| DIo/RC | Dissipated energy flux per RC at t = 0; DIo/RC = ABS/RC − TRo/RC |
| ETo/CSo | Electron transport flux per CS at t = 0; ETo/CSo = (ABS/CSo)φEo |
| ETo/RC | Electron transport flux per RC at t = 0; ETo/RC = (Mo/VJ)ψEo |
| F0 | Minimum fluorescence, when all PSII reaction centers (RCs) are open |
| F50μs | Fluorescence intensities at 50 μs |
| F100μs | Fluorescence intensities at 100 μs |
| F300μs | Fluorescence intensities at 300 μs |
| F2ms | Fluorescence intensities at 2 ms |
| F30ms | Fluorescence intensities at 30 ms |
| Fm | Maximum fluorescence, when all PSII reaction centers are closed |
| Fv | Variable fluorescence |
| RC/CS | Amount of active PSII RCs per CS at t = 0; RC/CSo = φPo(ABS/CSo)(VJ/Mo) |
| Sm | Normalized total complementary area above the OJIP transient (reflecting multiple-turnover QA reduction events) or total electron carriers r RC; Sm = Area/(Fm − F0) |
| TRo/CSo | Trapped energy flux per CS at t = 0; TRo/CSo = (ABS/CSo)φPo |
| TRo/RC | Trapped energy flux per RC at t = 0; TRo/RC = Mo/VJ |
| VI | Relative variable fluorescence at 30 ms (I-step); VI = (F30ms − F0)/(Fm − F0) |
| VJ | Relative variable fluorescence at 2 ms (J-step); VJ = (F2ms − F0)/(Fm − F0) |
| δRo | Efficiency with which an electron can move from the reduced intersystem electron acceptors to the PSI end electron acceptors; δRo = REo/ETo = (1 − VI)/(1 − VJ) |
| ρRo | Efficiency with which a trapped exciton can move an electron into the electron transport chain from QA − to the PSI and electron acceptors; ρRo = ψEoδRo = (1 − VJ)(1 − VI)/(1 − VJ) |
| φEo | Quantum yield for electron transport at t = 0; φEo = (Fv/Fm)(1 − VJ) |
| φPo | Maximum quantum yield of primary photochemistry at t = 0; φPo = 1 − F0/Fm = Fv/Fm |
| φRo | Quantum yield for the reduction of end acceptors of PSI per photon absorbed; φRo = REo/ABS = φPoψEoδRo |
| ψEo | Probability (at time 0) that a trapped exciton moves an electron into the electron transport chain beyond; ψEo = 1 − VJ |
References
- Shafiq, I.; Hussain, S.; Raza, M.A.; Iqbal, N.; Asghar, M.A.; Raza, A.; Fan, Y.; Mumtaz, M.; Shoaib, M.; Ansar, M.; et al. Crop photosynthetic response to light quality and light intensity. J. Integr. Agric. 2021, 20, 4–23. [Google Scholar] [CrossRef]
- Belkov, V.; Garnik, E.Y.; Konstantinov, Y.M. Mechanism of plant adaptation to changing illumination by rearrangements of their photosynthetic apparatus. In Proceedings of the Fifth International Scientific Conference PlantGen2019: Current Challenges in Plant Genetics, Genomics, Bioinformatics, and Biotechnology, Novosibirsk, Russia, 24–29 June 2019; pp. 101–103. [Google Scholar]
- Xue, X.; Wang, Q.; Qu, Y.; Wu, H.; Dong, F.; Cao, H.; Wang, H.-L.; Xiao, J.; Shen, J.; Wan, Y. Development of the photosynthetic apparatus of Cunninghamia lanceolata in light and darkness. New Phytol. 2017, 213, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.D.; Barman, M.; Mitra, A. Photosynthetic apparatus plays a central role in photosensitive physiological acclimations affecting spinach (Spinacia oleracea L.) growth in response to blue and red photon flux ratios. Environ. Exp. Bot. 2018, 156, 170–182. [Google Scholar] [CrossRef]
- Kochetova, G.V.; Avercheva, O.V.; Bassarskaya, E.M.; Zhigalova, T.V. Light quality as a driver of photosynthetic apparatus development. Biophys. Rev. 2022, 14, 779–803. [Google Scholar] [CrossRef] [PubMed]
- Galvão, V.C.; Fankhauser, C. Sensing the light environment in plants: Photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Szechyńska-Hebda, M.; Karpiński, S. Light intensity-dependent retrograde signalling in higher plants. J. Plant Physiol. 2013, 170, 1501–1516. [Google Scholar] [CrossRef]
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants response to light stress. J. Gen. Genom. 2022, 49, 735–747. [Google Scholar] [CrossRef]
- Miernicka, K.; Tokarz, B.; Makowski, W.; Mazur, S.; Banasiuk, R.; Tokarz, K.M. The Adjustment Strategy of Venus Flytrap Photosynthetic Apparatus to UV-A Radiation. Cells 2022, 11, 3030. [Google Scholar] [CrossRef]
- Tokarz, K.M.; Wesołowski, W.; Tokarz, B.; Makowski, W.; Wysocka, A.; Jędrzejczyk, R.J.; Chrabaszcz, K.; Malek, K.; Kostecka-Gugała, A. Stem photosynthesis—A key element of grass pea (Lathyrus sativus L.) acclimatisation to salinity. Int. J. Mol. Sci. 2021, 22, 685. [Google Scholar] [CrossRef]
- Nevo, R.; Charuvi, D.; Tsabari, O.; Reich, Z. Composition, architecture and dynamics of the photosynthetic apparatus in higher plants. Plant J. 2012, 70, 157–176. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Guo, Y.; Govindjee, G. Photosynthesis: Basics, history and modelling. Ann. Bot. 2020, 126, 511–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurrieri, L.; Fermani, S.; Zaffagnini, M.; Sparla, F.; Trost, P. Calvin–Benson cycle regulation is getting complex. Trends Plant Sci. 2021, 26, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Solymosi, K.; Mysliwa-Kurdziel, B. The role of membranes and lipid-protein interactions in the Mg-branch of tetrapyrrole biosynthesis. Front. Plant Sci. 2021, 12, 663309. [Google Scholar] [CrossRef]
- Yuan, M.; Zhao, Y.Q.; Zhang, Z.W.; Chen, Y.E.; Ding, C.B.; Yuan, S. Light regulates transcription of chlorophyll biosynthetic genes during chloroplast biogenesis. CRC Crit. Rev. Plant Sci. 2017, 36, 35–54. [Google Scholar] [CrossRef]
- Cope, K.R.; Snowden, M.C.; Bugbee, B. Photobiological interactions of blue light and photosynthetic photon flux: Effects of monochromatic and broad-spectrum light sources. Photochem. Photobiol. 2014, 90, 574–584. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Wientjes, E.; Douwstra, P.; Trouwborst, G.; Van Ieperen, W.; Croce, R.; Harbinson, J. Photosynthetic quantum yield dynamics: From photosystems to leaves. Plant Cell 2012, 24, 1921–1935. [Google Scholar] [CrossRef] [Green Version]
- Rehman, M.; Ullah, S.; Bao, Y.; Wang, B.; Peng, D.; Liu, L. Light-emitting diodes: Whether an efficient source of light for indoor plants? Environ. Sci. Pollut. Res. 2017, 24, 24743–24752. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue light alleviates ‘red light syndrome’ by regulating chloroplast ultrastructure, photosynthetic traits and nutrient accumulation in cucumber plants. Sci. Hort. 2019, 257, 108680. [Google Scholar] [CrossRef]
- Trouwborst, G.; Hogewoning, S.W.; van Kooten, O.; Harbinson, J.; van Ieperen, W. Plasticity of photosynthesis after the ‘red light syndrome’in cucumber. Environ. Exp. Bot. 2016, 121, 75–82. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Q.; Xin, Y.; Mei, Z.; Gao, A.; Liu, W.; Yu, L.; Chen, X.; Chen, Z.; Wang, N. Analyses of the photosynthetic characteristics, chloroplast ultrastructure, and transcriptome of apple (Malus domestica) grown under red and blue lights. BMC Plant Biol. 2021, 21, 483. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, S.; van Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, T.; Li, S.; Fan, Y.; Wang, Z.; Raza, M.A.; Shafiq, I.; Wang, B.; Wu, X.; Yong, T.; Wang, X.; et al. Far-red light: A regulator of plant morphology and photosynthetic capacity. Crop. J. 2021, 10, 300–309. [Google Scholar] [CrossRef]
- Hang, T.; Lu, N.; Takagaki, M.; Mao, H. Leaf area model based on thermal effectiveness and photosynthetically active radiation in lettuce grown in mini-plant factories under different light cycles. Sci. Hort. 2019, 252, 113–120. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J.; Fu, W. Growth, photosynthesis, and nutrient uptake at different light intensities and temperatures in lettuce. HortScience 2019, 54, 1925–1933. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Li, P.; Wang, J. Effects of Light Intensity and Temperature on the Photosynthesis Characteristics and Yield of Lettuce. Horticulturae 2022, 8, 178. [Google Scholar] [CrossRef]
- Jeremiasz, O.; Sobik, P.; Sala, A.; Pluta, A.; Szendera, F. Photoluminescent Dye, in Particular for Photovoltaic Modules and Method of Producing a Photoluminescent Dye, in Particular for Photovoltaic Module. PL Patent 240248 B1, 29 July 2019. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Advances in Photosynthesis and Respiration; Papageorgiou, G., Govindjee, Eds.; Springer: Dordrecht, Germany, 2004; Volume 19, pp. 321–336. [Google Scholar]
- Jiang, H.X.; Chen, L.S.; Zheng, J.G.; Han, S.; Tang, N.; Smith, B.R. Aluminum-induced effects on photosystem II photochemistry in Citrus leaves assessed by the chlorophyll a fluorescence transient. Tree Physiol. 2008, 28, 1863–1871. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Govindjee Bosa, K.; Kościelniak, J.; Zuk-Gołaszewska, K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Goltsev, V.N.; Kalaji, H.M.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S.I. Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- Laureau, C.; De Paepe, R.; Latouche, G.; Moreno-Chacon, M.; Finazzi, G.; Kuntz, M.; Cornic, G.; Streb, P. Plastid terminal oxidase (PTOX) has the potential to act as a safety valve for excess excitation energy in the alpine plant species Ranunculus glacialis L. Plant Cell Environ. 2013, 36, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Lűck, H. Methoden der Enzymatischen Analyse; Verlag Chemie GmbH: Weinheim, Germany, 1962. [Google Scholar]
- Guri, A. Variation in glutathione and ascorbic acid content among selected cultivars of Phaseolus vulgaris prior to and after exposure to ozone. Can. J. Plant Sci. 1983, 63, 733–737. [Google Scholar] [CrossRef]
- Avercheva, O.V.; Berkovich, Y.A.; Erokhin, A.N.; Zhigalova, T.V.; Pogosyan, S.I.; Smolyanina, S.O. Growth and photosynthesis of Chinese cabbage plants grown under light-emitting diode-based light source. Russ. J. Plant Physiol. 2009, 56, 14–21. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- XiaoYing, L.; ShiRong, G.; ZhiGang, X.; XueLei, J.; Tezuka, T. Regulation of chloroplast ultrastructure, cross-section anatomy of leaves, and morphology of stomata of cherry tomato by different light irradiations of light-emitting diodes. HortScience 2011, 46, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Izzo, L.G.; Mele, B.H.; Vitale, L.; Vitale, E.; Arena, C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- Bantis, F.; Radoglou, K. Morphology, development, and transplant potential of Prunus avium and Cornus sanguinea seedlings growing under different LED lights. Turk. J. Biol. 2017, 41, 314–321. [Google Scholar] [CrossRef]
- Shibuya, T.; Endo, R.; Kitamura, Y.; Kitaya, Y.; Hayashi, N. Potential photosynthetic advantages of cucumber (Cucumis sativus L.) seedlings grown under fluorescent lamps with high red:far-red light. HortScience 2010, 45, 553–558. [Google Scholar] [CrossRef] [Green Version]
- Kasperbauer, M.J.; Hamilton, J.L. Chloroplast structure and starch grain accumulation in leaves that received different red and far-red levels during development. Plant Physiol. 1984, 74, 967–970. [Google Scholar] [CrossRef] [Green Version]
- Schuerger, A.C.; Brown, C.S.; Stryjewski, E.C. Anatomical features of pepper plants (Capsicum annuum L.) grown under red light-emitting diodes supplemented with blue or far-red light. Ann. Bot. 1997, 79, 273–282. [Google Scholar] [CrossRef]
- Kirchhoff, H. Chloroplast ultrastructure in plants. New Phytol. 2019, 223, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Scheller, H.V.; Jensen, P.E.; Haldrup, A.; Lunde, C.; Knoetzel, J. Role of subunits in eukaryotic Photosystem I. Biochim. Biophys. Acta (BBA)-Bioenergy 2001, 1507, 41–60. [Google Scholar] [CrossRef] [Green Version]
- Ernstsen, J.; Woodrow, I.E.; Mott, K.A. Effects of growth-light quantity, growth-light quality and CO2 concentration on Rubisco deactivation during low PFD or darkness. Photosynth. Res. 1999, 61, 65–75. [Google Scholar] [CrossRef]
- Yamazaki, J.Y. Is light quality involved in the regulation of the photosynthetic apparatus in attached rice leaves? Photosynth. Res. 2010, 105, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Kramer, D.M.; Fisher, N.; Fu, X. Flexibility in the energy balancing network of photosynthesis enables safe operation under changing environmental conditions. Plants 2020, 9, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Feng, L.; Liu, Q.; Wu, X.; Fan, Y.; Raza, M.A.; Cheng, J.; Wang, X.; Yong, T.; Liu, W.; et al. Effect of interactions between light intensity and red-to-far-red ratio on the photosynthesis of soybean leaves under shade condition. Environ. Exp. Bot. 2018, 150, 79–87. [Google Scholar] [CrossRef]
- Tikkanen, M.; Rantala, S.; Grieco, M.; Aro, E.M. Comparative analysis of mutant plants impaired in the main regulatory mechanisms of photosynthetic light reactions-From biophysical measurements to molecular mechanisms. Plant Physiol. Biochem. 2017, 112, 290–301. [Google Scholar] [CrossRef]
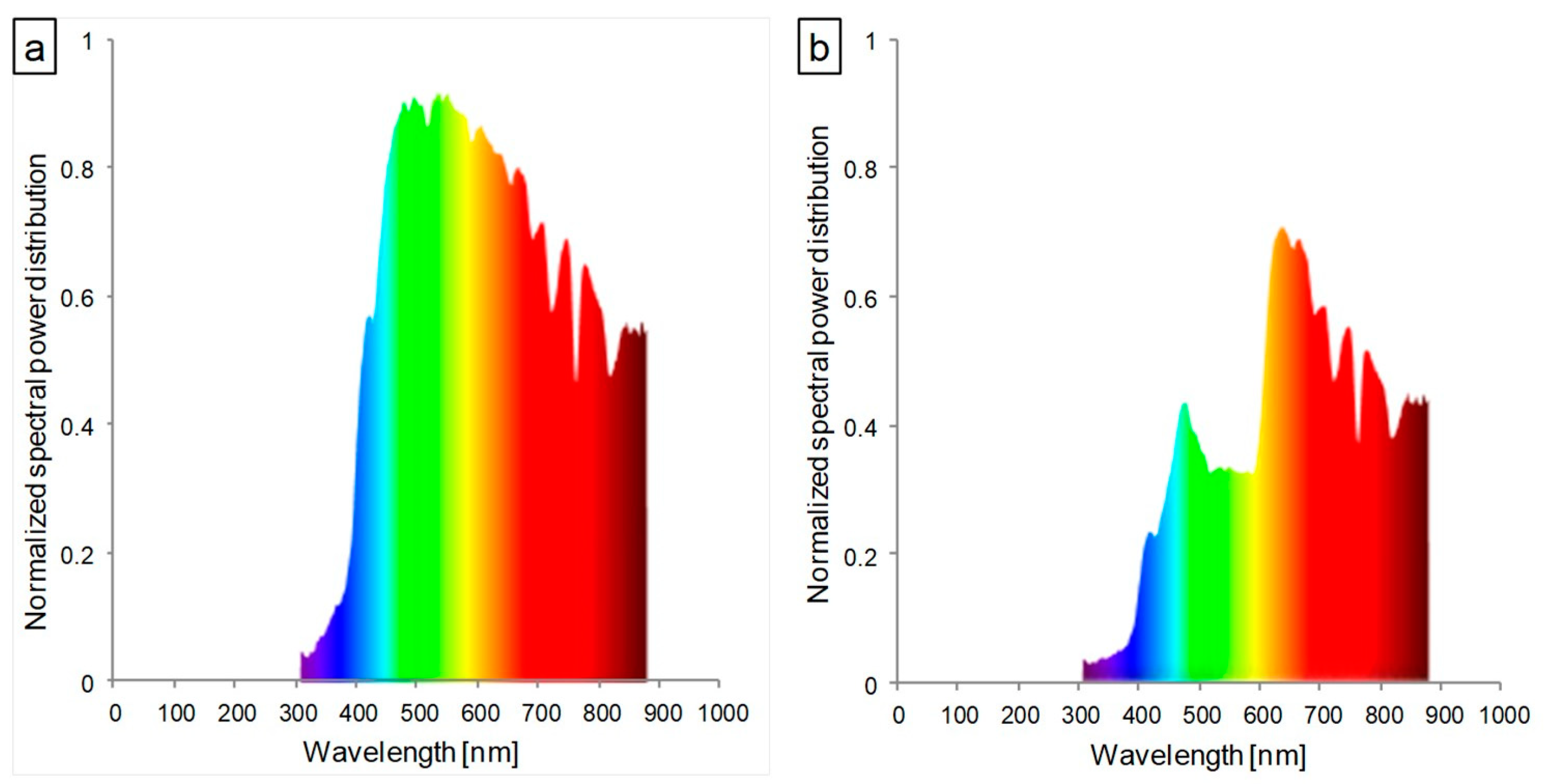
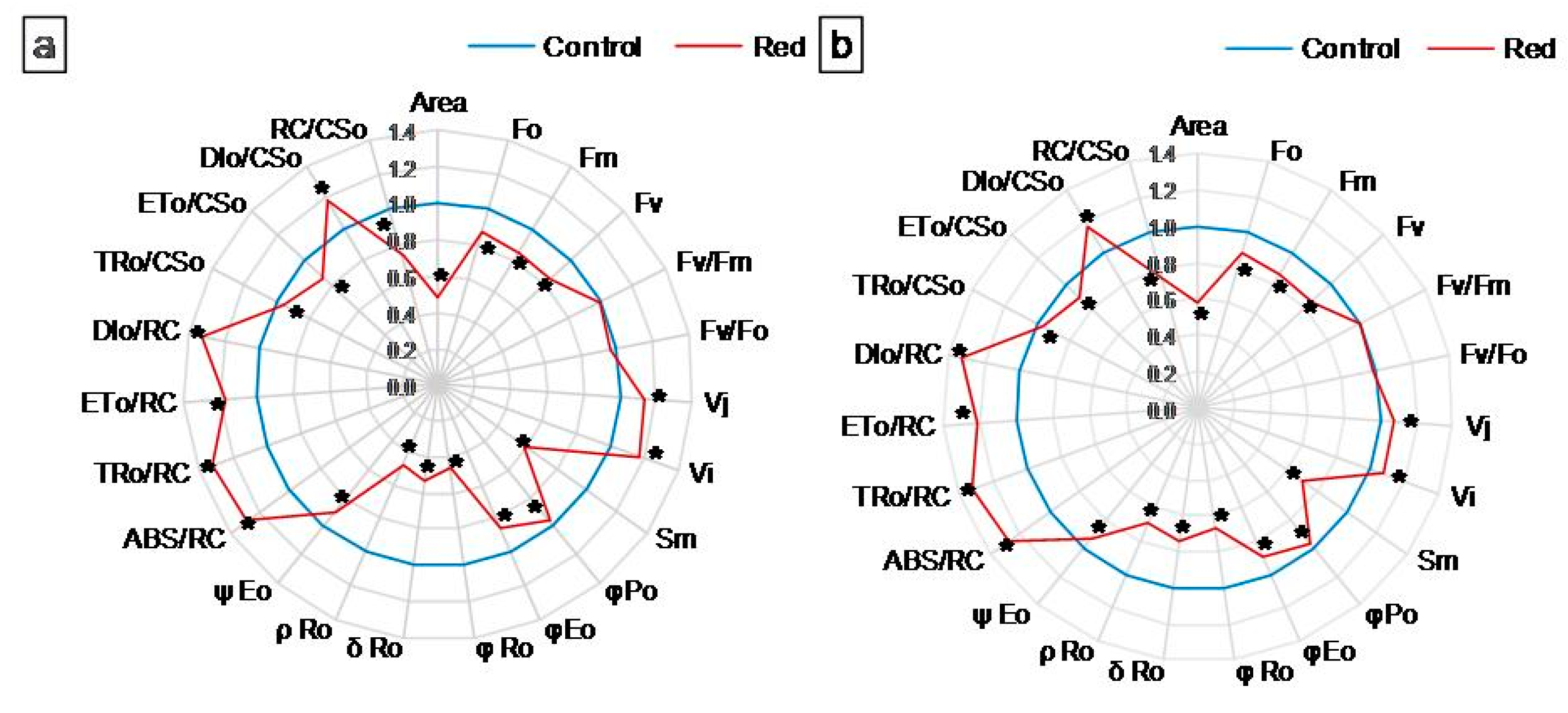
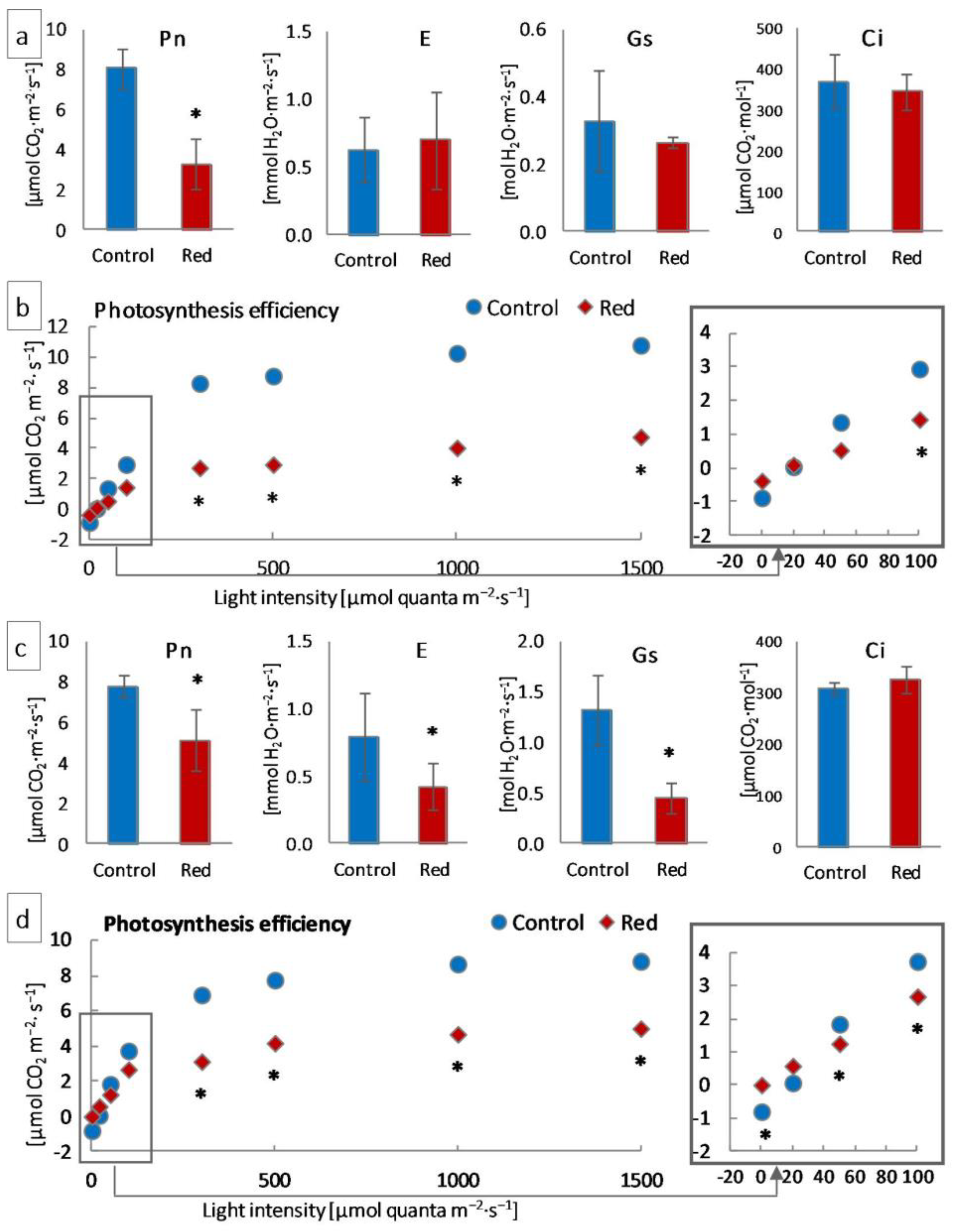


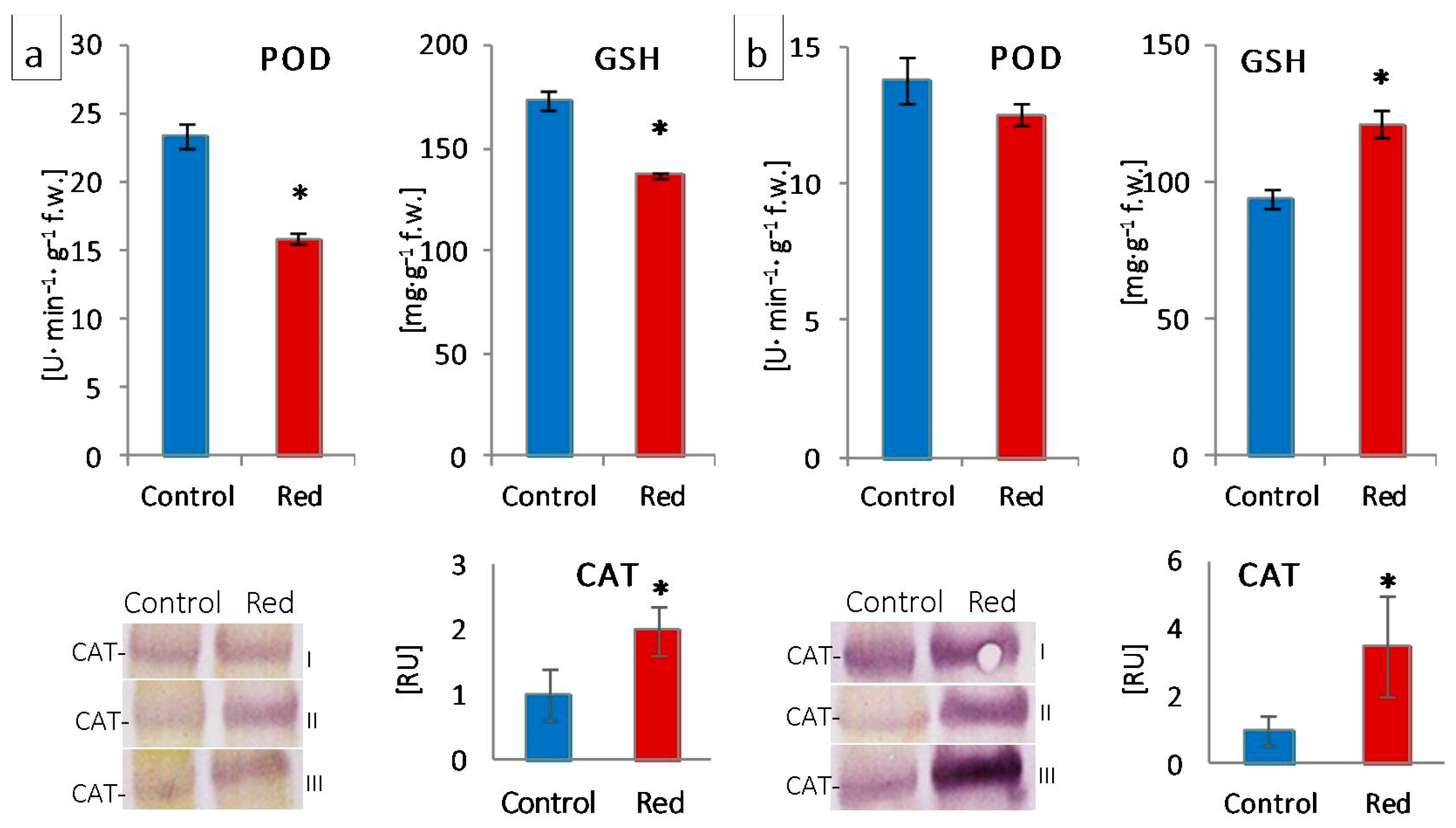
| Lettuce Type | Conditions | Pigment Concentration a (mg·g−1 FW) | Pigment Ratio (RU) | |||
|---|---|---|---|---|---|---|
| Chl a | Chl b | Chl a + b | Car | Chl a/b | ||
| Butterhead | Control | 0.20 ± 0.03 | 0.06 ± 0.01 | 0.27 ± 0.04 | 0.07 ± 0.01 | 3.3 ± 0.1 |
| Red | 0.28 * ± 0.03 | 0.08 * ± 0.01 | 0.35 * ± 0.03 | 0.07 ± 0.00 | 3.6 * ± 0.1 | |
| Iceberg | Control | 0.26 ± 0.16 | 0.10 ± 0.04 | 0.36 ± 0.2 | 0.11 ± 0.05 | 2.4 ± 0.4 |
| Red | 0.29 ± 0.07 | 0.11 ± 0.02 | 0.40 ± 0.09 | 0.09 ± 0.02 | 2.6 ± 0.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokarz, K.M.; Makowski, W.; Tokarz, B.; Muszyńska, E.; Gajewski, Z.; Mazur, S.; Kunicki, E.; Jeremiasz, O.; Sobik, P.; Nowak, P.; et al. Performance of the Photosynthetic Apparatus under Glass with a Luminophore Modifying Red-To-Far-Red-Light Ratio—A Case Study. Cells 2023, 12, 1552. https://doi.org/10.3390/cells12111552
Tokarz KM, Makowski W, Tokarz B, Muszyńska E, Gajewski Z, Mazur S, Kunicki E, Jeremiasz O, Sobik P, Nowak P, et al. Performance of the Photosynthetic Apparatus under Glass with a Luminophore Modifying Red-To-Far-Red-Light Ratio—A Case Study. Cells. 2023; 12(11):1552. https://doi.org/10.3390/cells12111552
Chicago/Turabian StyleTokarz, Krzysztof M., Wojciech Makowski, Barbara Tokarz, Ewa Muszyńska, Zbigniew Gajewski, Stanisław Mazur, Edward Kunicki, Olgierd Jeremiasz, Piotr Sobik, Paweł Nowak, and et al. 2023. "Performance of the Photosynthetic Apparatus under Glass with a Luminophore Modifying Red-To-Far-Red-Light Ratio—A Case Study" Cells 12, no. 11: 1552. https://doi.org/10.3390/cells12111552





