Updates on the Physiopathology of Group I Metabotropic Glutamate Receptors (mGluRI)-Dependent Long-Term Depression
Abstract
:1. Group 1 Metabotropic Glutamate Receptors (mGluRI)
2. mGluRI-Dependent LTD in Physiology
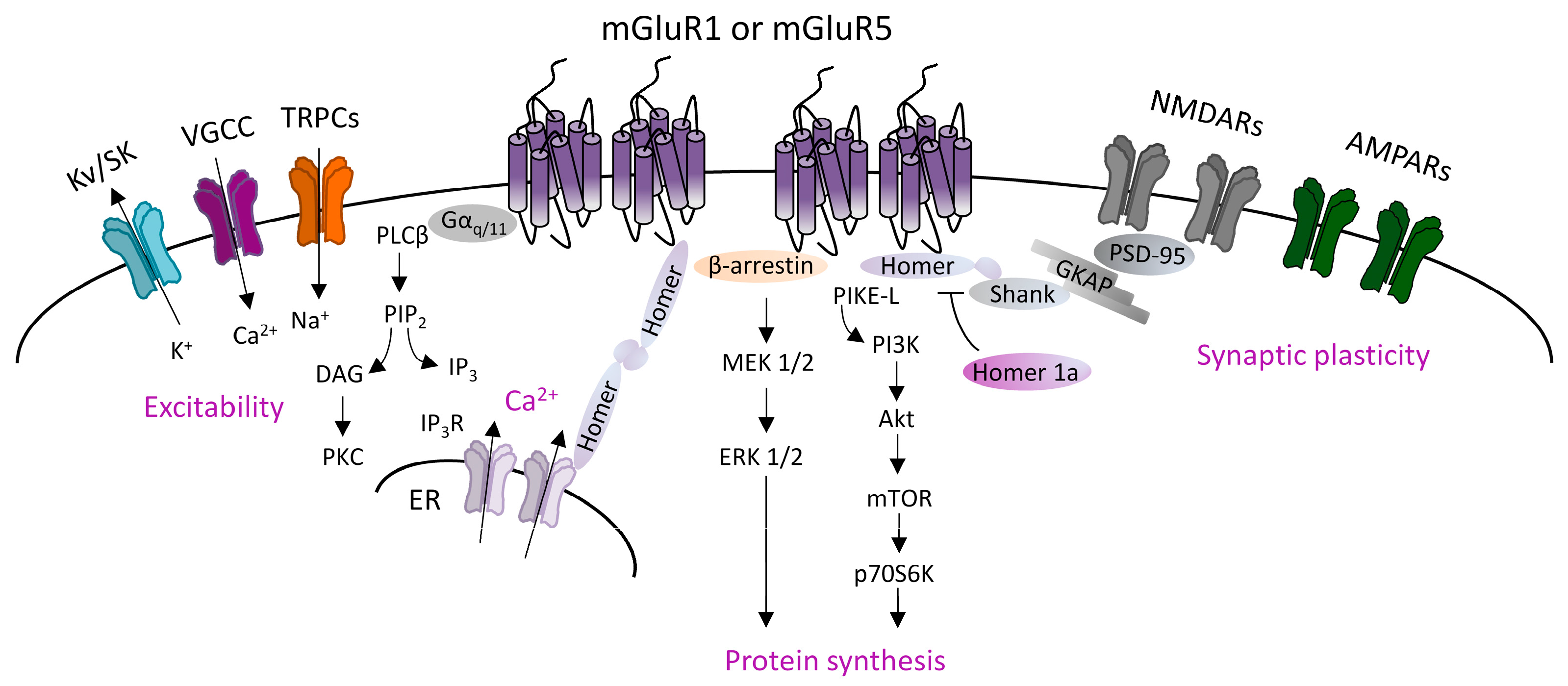
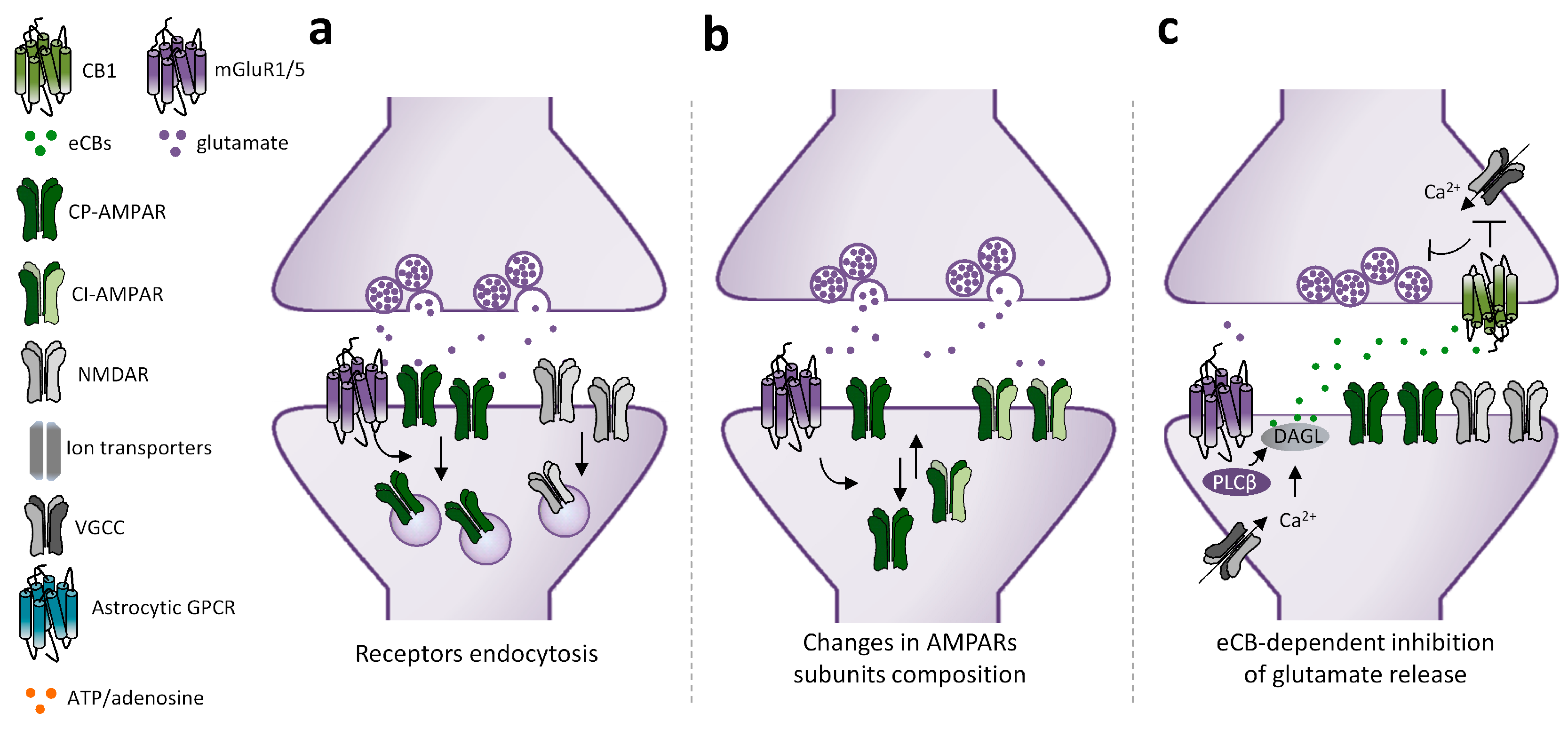
2.1. Mechanisms Underlying mGluRI-LTD
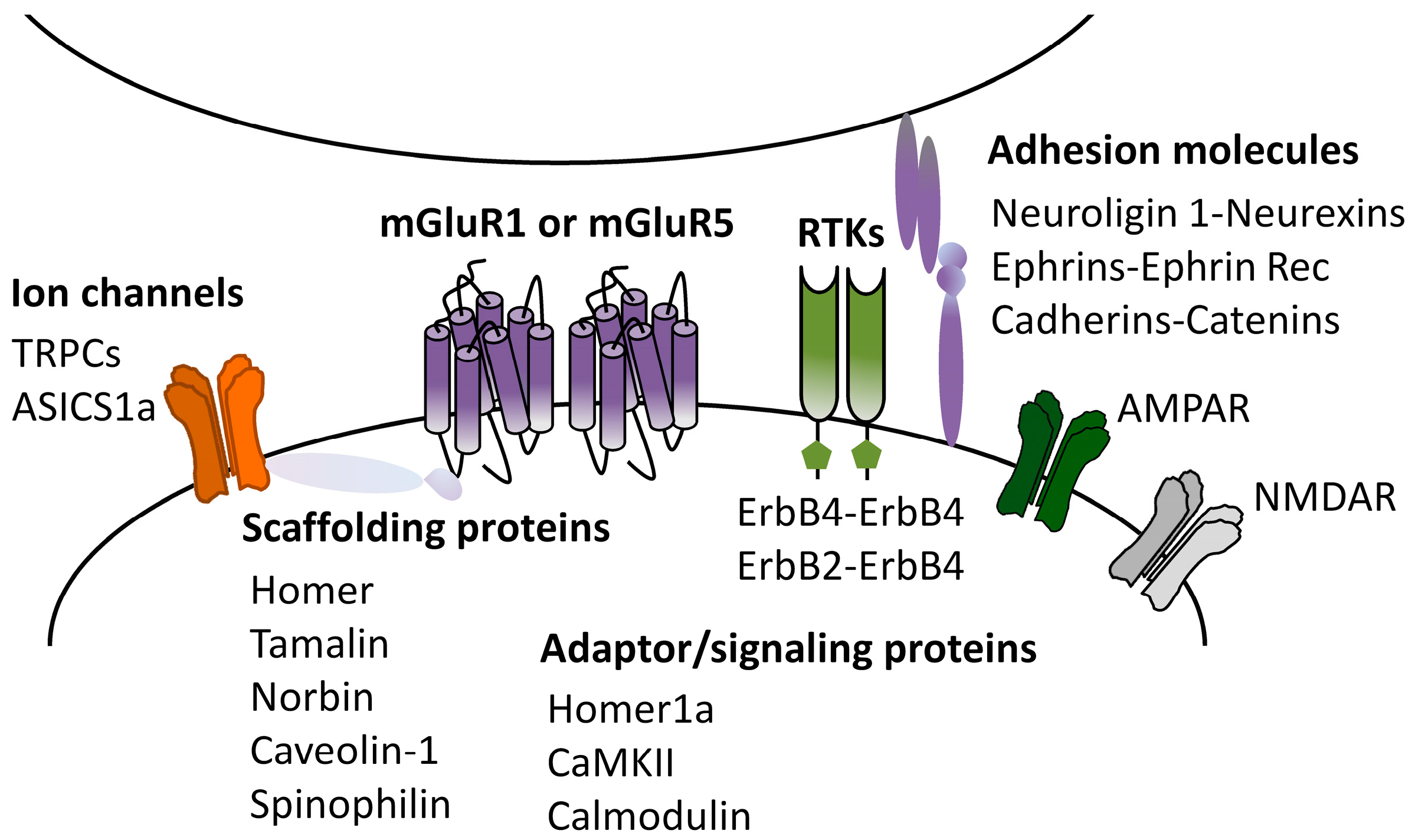
2.2. mGluRI Partners Affecting mGluRI-LTD
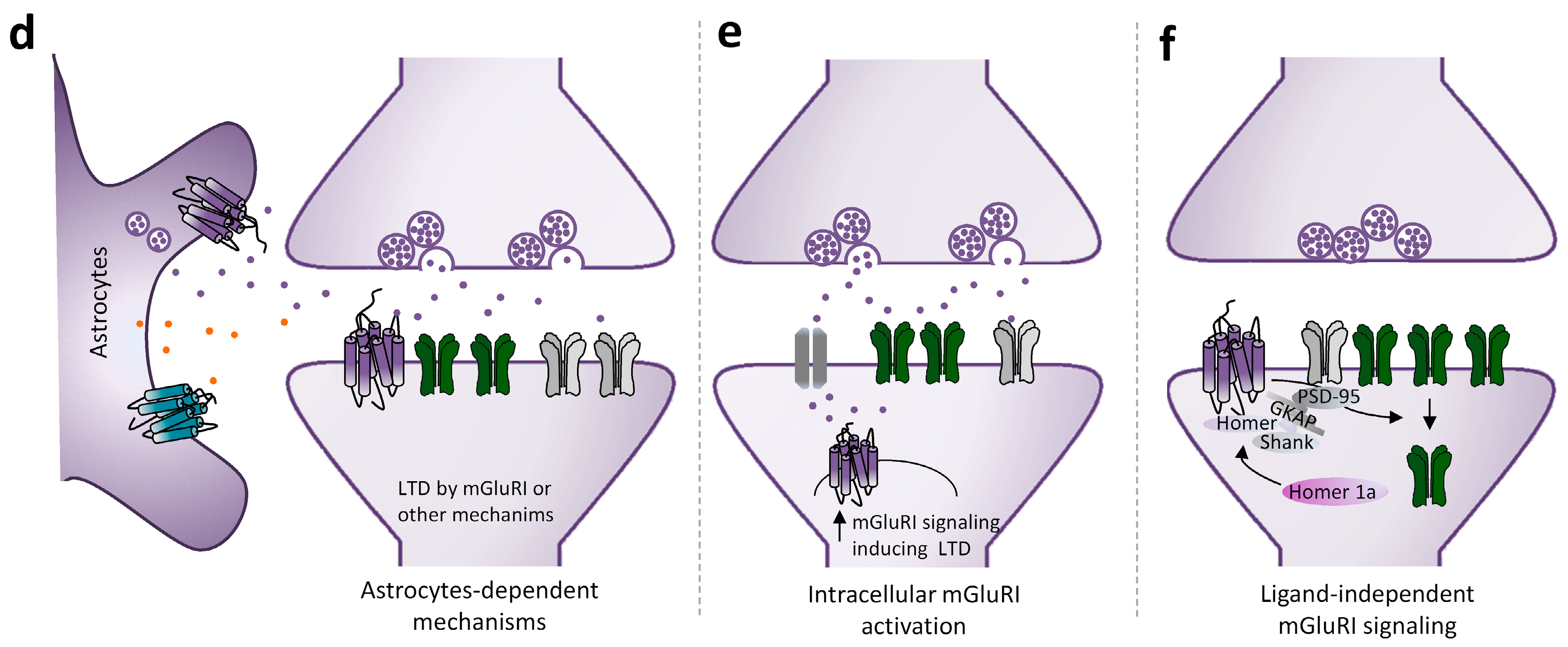
2.3. mGluRI-LTD Types and Their Functional Significance
3. mGluRI-Dependent LTD in Pathology
3.1. Autism Spectrum Disorders, Genetic Intellectual Disabilities, and Schizophrenia
3.2. Alzheimer’s Disease
3.3. Stress-Induced Depressive Phenotypes
3.4. Addiction
3.5. Parkinson’s Disease
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Ferraguti, F.; Crepaldi, L.; Nicoletti, F. Metabotropic Glutamate 1 Receptor: Current Concepts and Perspectives. Pharmacol. Rev. 2008, 60, 536–581. [Google Scholar] [CrossRef] [PubMed]
- Niswender, C.M.; Conn, P.J. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baude, A.; Nusser, Z.; Roberts, J.B.; Mulvihill, E.; Mcllhinney, R.J.; Somogyi, P. The metabotropic glutamate receptor (mGluR1) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron 1993, 11, 771–787. [Google Scholar] [CrossRef]
- Lujan, R.; Nusser, Z.; Roberts, J.D.; Shigemoto, R.; Somogyi, P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur. J. Neurosci. 1996, 8, 1488–1500. [Google Scholar] [CrossRef]
- Scheefhals, N.; Westra, M.; Harold, D.; MacGillavry, H.D. mGluR5 is transiently confined in perisynaptic nanodomains to shape synaptic function. Nat. Commun. 2023, 14, 244. [Google Scholar] [CrossRef]
- Brakeman, P.R.; Lanahan, A.A.; O’Brien, R.; Roche, K.; Barnes, C.A.; Huganir, R.L.; Worley, P.F. Homer: A protein that selectively binds metabotropic glutamate receptors. Nat. Cell. Biol. 1997, 386, 284–288. [Google Scholar] [CrossRef]
- Xiao, B.; Tu, J.C.; Petralia, R.S.; Yuan, J.P.; Doan, A.; Breder, C.D.; Ruggiero, A.; Lanahan, A.A.; Wenthold, R.J.; Worley, P.F. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron 1998, 21, 707–716. [Google Scholar] [CrossRef] [Green Version]
- Fagni, L.; Ango, F.; Perroy, J.; Bockaert, J. Identification and functional roles of metabotropic glutamate receptor-interacting proteins. Semin. Cell Dev. Biol. 2004, 15, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Fagni, L. Diversity of Metabotropic Glutamate Receptor–Interacting Proteins and Pathophysiological Functions. Adv. Exp. Med. Biol. 2012, 970, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Enz, R. Metabotropic glutamate receptors and interacting proteins: Evolving drug targets. Curr. Drug Targets 2012, 13, 145–156. [Google Scholar] [CrossRef]
- Dale, L.B.; Bhattacharya, M.; Anborgh, P.H.; Murdoch, B.; Bhatia, M.; Nakanishi, S.; Ferguson, S.S.G. G Protein-coupled Receptor Kinase-mediated Desensitization of Metabotropic Glutamate Receptor 1A Protects against Cell Death. J. Biol. Chem. 2000, 275, 38213–38220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallese, M.; Mariggiò, S.; D’Urbano, E.; Iacovelli, L.; De Blasi, A. Selective regulation of Gq signaling by G protein-coupled receptor kinase 2: Direct interaction of kinase N terminus with activated galphaq. Mol. Pharmacol. 2000, 57, 826–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacovelli, L.; Salvatore, L.; Capobianco, L.; Picascia, A.; Barletta, E.; Storto, M.; Mariggiò, S.; Sallese, M.; Porcellini, A.; Nicoletti, F.; et al. Role of G protein-coupled receptor kinase 4 and beta-arrestin 1 in agonist-stimulated metabotropic glutamate receptor 1 internalization and activation of mitogen-activated protein kinases. J. Biol. Chem. 2003, 278, 12433–12442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorensen, S.D.; Conn, P.J. G protein-coupled receptor kinases regulate metabotropic glutamate receptor 5 function and expression. Neuropharmacology 2003, 44, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, J.A.; Marino, M.J.; Folk, J.A.; Hepler, J.R.; Conn, P.J. RGS4 Inhibits Signaling by Group I Metabotropic Glutamate Receptors. J. Neurosci. 1998, 18, 905–913. [Google Scholar] [CrossRef] [Green Version]
- Mannaioni, G.; Marino, M.J.; Valenti, O.; Traynelis, S.F.; Conn, P.J. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J. Neurosci. 2001, 21, 5925–5934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenti, O.; Conn, P.J.; Marino, M.J. Distinct physiological roles of the Gq-coupled metabotropic glutamate receptors co-expressed in the same neuronal populations. Rev. J. Cell. Physiol. 2002, 191, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Volk, L.J.; Daly, C.A.; Huber, K.M. Differential roles for group 1 mGluR subtypes in induction and expression of chemically induced hippocampal long-term depression. J. Neurophysiol. 2006, 95, 2427–2438. [Google Scholar] [CrossRef] [Green Version]
- Poisik, O.V.; Mannaioni, G.; Traynelis, S.; Smith, Y.; Conn, P.J. Distinct Functional Roles of the Metabotropic Glutamate Receptors 1 and 5 in the Rat Globus Pallidus. J. Neurosci. 2003, 23, 122–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, P.F.; Williams, J.T. Cocaine Decreases Metabotropic Glutamate Receptor mGluR1 Currents in Dopamine Neurons by Activating mGluR5. Neuropsychopharmacology 2015, 40, 2418–2424. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Tarakanov, A.O.; Brito, I.; Fuxe, K. Glutamate heteroreceptor complexes in the brain. Pharmacol. Rep. 2018, 70, 936–950. [Google Scholar] [CrossRef]
- Prézeau, L.; Rives, M.-L.; Comps-Agrar, L.; Maurel, D.; Kniazeff, J.; Pin, J.-P. Functional crosstalk between GPCRs: With or without oligomerization. Curr. Opin. Pharmacol. 2010, 10, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Ciruela, F.; Escriche, M.; Burgueño, J.; Angulo-Pueyo, E.; Casadó, V.; Soloviev, M.M.; Franco, R.; Mallol, J.; Chan, W.-Y.; Lluis, C.; et al. Metabotropic Glutamate 1_ and Adenosine A1 Receptors Assemble into Functionally Interacting Complexes. J. Biol. Chem. 2001, 276, 18345–18351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakairi, H.; Kamikubo, Y.; Abe, M.; Ikeda, K.; Ichiki, A.; Tabata, T.; Kano, M.; Sakurai, T. G Protein-Coupled Glutamate and GABA Receptors form Complexes and Mutually Modulate Their Signals. ACS Chem. Neurosci. 2020, 11, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Sebastianutto, I.; Goyet, E.; Andreoli, L.; Font-Ingles, J.; Moreno-Delgado, D.; Bouquier, N.; Jahannault-Talignani, C.; Moutin, E.; Di Menna, L.; Maslava, N.; et al. D1-mGlu5 heteromers mediate noncanonical dopamine signaling in Parkinson’s disease. J. Clin. Investig. 2020, 130, 1168–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferré, S.; Popoli, P.; Rimondini, R.; Reggio, R.; Kehr, J.; Fuxe, K. Adenosine A2A and group I metabotropic glutamate receptors synergistically modulate the binding characteristics of dopamine D2 receptors in the rat striatum. Neuropharmacology 1999, 38, 129–140. [Google Scholar] [CrossRef]
- Lai, T.K.Y.; Zhai, D.; Su, P.; Jiang, A.; Boychuk, J.; Liu, F. The receptor-receptor interaction between mGluR1 receptor and NMDA receptor: A potential therapeutic target for protection against ischemic stroke. FASEB J. 2019, 33, 14423–14439. [Google Scholar] [CrossRef] [Green Version]
- García-Negredo, G.; Soto, D.; Llorente, J.; Morató, X.; Galenkamp, K.M.O.; Gómez-Soler, M.; Fernández-Dueñas, V.; Watanabe, M.; Adelman, J.P.; Shigemoto, R.; et al. Coassembly and Coupling of SK2 Channels and mGlu5 Receptors. J. Neurosci. 2014, 34, 14793–14802. [Google Scholar] [CrossRef] [Green Version]
- Luján, R.; Aguado, C.; Ciruela, F.; Morató Arus, X.; Martín-Belmonte, A.; Alfaro-Ruiz, R.; Martínez-Gómez, J.; de la Ossa, L.; Watanabe, M.; Adelman, J.P.; et al. SK2 Channels Associate With mGlu1α Receptors and CaV2.1 Channels in Purkinje Cells. Front. Cell. Neurosci. 2018, 12, 311. [Google Scholar] [CrossRef] [Green Version]
- Mango, D.; Braksator, E.; Battaglia, G.; Marcelli, S.; Mercuri, N.B.; Feligioni, M.; Nicoletti, F.; Bashir, Z.I.; Nisticò, R. Acid-sensing ion channel 1a is required for mGlu receptor dependent long-term depression in the hippocampus. Pharmacol. Res. 2017, 119, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Ledonne, A.; Mercuri, N.B. Insights on the Functional Interaction between Group 1 Metabotropic Glutamate Receptors (mGluRI) and ErbB Receptors. Int. J. Mol. Sci. 2020, 21, 7913. [Google Scholar] [CrossRef]
- Shigemoto, R.; Nomura, S.; Ohishi, H.; Sugihara, H.; Nakanishi, S.; Mizuno, N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci. Lett. 1993, 163, 53–57. [Google Scholar] [CrossRef]
- Romano, C.; Sesma, M.A.; McDonald, C.T.; O’Malley, K.; van den Pol, A.N.; Olney, J.W. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J. Comp. Neurol. 1995, 355, 455–469. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Copani, A.; Nicoletti, F.; Sortino, M.A.; Caraci, F. Metabotropic Glutamate Receptors in Glial Cells: A New Potential Target for Neuroprotection? Front. Mol. Neurosci. 2018, 11, 414. [Google Scholar] [CrossRef] [Green Version]
- Jong, Y.J.I.; Kumar, V.; Kingston, A.E.; Romano, C.; O’Malley, K.L. Functional metabotropic glutamate receptors on nuclei from brain and primary cultured striatal neurons: Role of transporters in delivering ligand. J. Biol. Chem. 2005, 280, 30469–30480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jong, Y.J.; Kumar, V.; O’Malley, K.L. Intracellular metabotropic glutamate receptor 5 (mGluR5) activates signaling cascades distinct from cell surface counterparts. J. Biol. Chem. 2009, 284, 35827–35838. [Google Scholar] [CrossRef] [Green Version]
- Jong, Y.J.I.; O’Malley, K.L. Mechanisms Associated with Activation of Intracellular Metabotropic Glutamate Receptor, mGluR5. Neurochem. Res. 2017, 42, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Palmer, M.J.; Irving, A.J.; Seabrook, G.R.; Jane, D.E.; Collingridge, G.L. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology 1997, 36, 1517–1532. [Google Scholar] [CrossRef] [PubMed]
- Oliet, S.H.; Malenka, R.C.; Nicoll, R.A. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron 1997, 6, 969–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, N.; Bashir, Z.I. Induction of LTD in the adult hippocampus by the synaptic activation of AMPA/kainite and metabotropic glutamate receptors. Neuropharmacology 1999, 38, 495–504. [Google Scholar] [CrossRef]
- Calabresi, P.; Maj, R.; Pisani, A.; Mercuri, N.B.; Bernardi, G. Long-term synaptic depression in the striatum: Physiological and pharmacological characterization. J. Neurosci. 1992, 12, 4224–4233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surdin, T.; Preissing, B.; Rohr, L.; Grömmke, M.; Böke, H.; Barcik, M.; Azimi, Z.; Jancke, D.; Herlitze, S.; Mark, M.D.; et al. Optogenetic activation of mGluR1 signaling in the cerebellum induces synaptic plasticity. iScience 2023, 26, 105828. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Peineau, S.; Howland, J.G.; Wang, Y.T. Long-term depression in the CNS. Nat. Rev. Neurosci. 2010, 11, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Luscher, C.; Huber, K.M. Group 1 mGluR-dependent synaptic long-term depression: Mechanisms and implications for circuitry and disease. Neuron 2010, 65, 445–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, H.J.; Steinberg, J.P.; Huganir, R.L.; Linden, D.J. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science 2003, 5626, 1751–1755. [Google Scholar] [CrossRef]
- Steinberg, J.P.; Takamiya, K.; Shen, Y.; Xia, J.; Rubio, M.E.; Yu, S.; Jin, W.; Thomas, G.M.; Linden, D.J.; Huganir, R.L. Targeted In Vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron 2006, 49, 845–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiels, E.; Kanterewicz, B.I.; Norman, E.D.; Trzaskos, J.M.; Klann, E. Long-term depression in the adult hippocampus In Vivo involves activation of extracellular signal-regulated kinase and phosphorylation of Elk-1. J. Neurosci. 2002, 22, 2054–2062. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, S.M.; Daly, C.A.; Bear, M.F.; Huber, K.M. Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor dependent long-term depression in hippocampal area CA1. J. Neurosci. 2004, 24, 4859–4864. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Klann, E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor dependent long-term depression. J. Neurosci. 2004, 24, 6352–6361. [Google Scholar] [CrossRef] [Green Version]
- Snyder, E.M.; Philpot, B.D.; Huber, K.M.; Dong, X.; Fallon, J.R.; Bear, M.F. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat. Neurosci. 2001, 4, 1079–1085. [Google Scholar] [CrossRef]
- Ireland, D.R.; Abraham, W.C. Mechanisms of group I mGluR-dependent long-term depression of NMDA receptor-mediated transmission at Schaffer collateral-CA1 synapses. J. Neurophysiol. 2009, 101, 1375–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellone, C.; Lüscher, C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur. J. Neurosci. 2005, 21, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Loweth, J.A.; Scheyer, A.F.; Milovanovic, M.; LaCrosse, A.L.; Flores-Barrera, E.; Werner, C.T.; Li, X.; Ford, K.A.; Le, T.; Olive, M.F.; et al. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat. Neurosci. 2014, 17, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerdeman, G.L.; Ronesi, J.; Lovinger, D.M. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat. Neurosci. 2002, 5, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.L.; Knopfel, T. Presynaptically expressed long-term depression at cerebellar parallel fiber synapses. Pflug. Arch. 2009, 457, 865–875. [Google Scholar] [CrossRef] [Green Version]
- Nevian, T.; Sakmann, B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J. Neurosci. 2006, 26, 11001–11013. [Google Scholar] [CrossRef] [Green Version]
- Hubert, G.W.; Paquet, M.; Smith, Y. Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey Substantia nigra. J. Neurosci. 2001, 21, 1838–1847. [Google Scholar] [CrossRef] [Green Version]
- O’Malley, K.L.; Jong, Y.J.; Gonchar, Y.; Burkhalter, A.; Romano, C. Activation of metabotropic glutamate receptor mGlu5 on nuclear membranes mediates intranuclear Ca2+ changes in heterologous cell types and neurons. J. Biol. Chem. 2003, 278, 28210–28219. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Fahey, P.G.; Jong, Y.J.; Ramanan, N.; O’Malley, K.L. Activation of intracellular metabotropic glutamate receptor 5 in striatal neurons leads to up-regulation of genes associated with sustained synaptic transmission including Arc/Arg3.1 protein. J. Biol. Chem. 2012, 287, 5412–5425. [Google Scholar] [CrossRef] [Green Version]
- Purgert, C.A.; Izumi, Y.; Jong, Y.J.; Kumar, V.; Zorumski, C.F.; O’Malley, K.L. Intracellular mGluR5 can mediate synaptic plasticity in the hippocampus. J. Neurosci. 2014, 34, 4589–4598. [Google Scholar] [CrossRef] [Green Version]
- Ango, F.; Prézeau, L.; Muller, T.; Tu, J.C.; Xiao, B.; Worley, P.F.; Pin, J.P.; Bockaert, J.; Fagni, L. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature 2001, 411, 962–965. [Google Scholar] [CrossRef]
- Hu, J.H.; Park, J.M.; Park, S.; Xiao, B.; Dehoff, M.H.; Kim, S.; Hayashi, T.; Schwarz, M.K.; Huganir, R.L.; Seeburg, P.H.; et al. Homeostatic scaling requires group I mGluR activation mediated by Homer1a. Neuron 2010, 68, 1128–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.; Robitaille, R.; Volterra, A. Gliotransmitters travel in time and space. Neuron 2014, 81, 728–739. [Google Scholar] [CrossRef] [Green Version]
- Durkee, C.; Kofuji, P.; Navarrete, M.; Araque, A. Astrocyte and neuron cooperation in long-term depression. Trends Neurosci. 2021, 44, 837–848. [Google Scholar] [CrossRef]
- Cavaccini, A.; Durkee, C.; Kofuji, P.; Tonini, R.; Araque, A. Astrocyte Signaling Gates Long-Term Depression at Corticostriatal Synapses of the Direct Pathway. J. Neurosci. 2020, 40, 5757–5768. [Google Scholar] [CrossRef] [PubMed]
- Lalo, U.; Pankratov, Y. Role for Astrocytes in mGluR-Dependent LTD in the Neocortex and Hippocampus. Brain Sci. 2022, 12, 1718. [Google Scholar] [CrossRef] [PubMed]
- Craven, S.E.; Bredt, D.S. PDZ proteins organize synaptic signaling pathways. Cell 1998, 93, 495–498. [Google Scholar] [CrossRef] [Green Version]
- Kitano, J.; Yamazaki, Y.; Kimura, K.; Masukado, T.; Nakajima, Y.; Nakanishi, S. Tamalin is a scaffold protein that interacts with multiple neuronal proteins in distinct modes of protein-protein association. J. Biol. Chem. 2003, 278, 14762–14768. [Google Scholar] [CrossRef] [Green Version]
- Sugi, T.; Oyama, T.; Muto, T.; Nakanishi, S.; Morikawa, K.; Jingami, H. Crystal structures of autoinhibitory PDZ domain of Tamalin: Implications for metabotropic glutamate receptor trafficking regulation. EMBO J. 2007, 26, 2192–2205. [Google Scholar] [CrossRef] [Green Version]
- Neyman, S.; Braunewell, K.; O’Connell, K.E.; Dev, K.K.; Manahan-Vaughan, D. Inhibition of the Interaction Between Group I Metabotropic Glutamate Receptors and PDZ-Domain Proteins Prevents Hippocampal Long-Term Depression, but Not Long-Term Potentiation. Front. Synaptic Neurosci. 2019, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Westin, L.; Nong, Y.; Birnbaum, S.; Bendor, J.; Brismar, H.; Nestler, E.; Aperia, A.; Flajolet, M.; Greengard, P. Norbin is an endogenous regulator of metabotropic glutamate receptor 5 signaling. Science 2009, 326, 1554–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Z.; Storm, D.R. The role of calmodulin as a signal integrator for synaptic plasticity. Nat. Rev. Neurosci. 2005, 6, 267–276. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.; Choi, K.Y.; Hepp, R.; Lee, J.Y.; Lim, M.K.; Chatani-Hinze, M.; Roche, P.A.; Kim, D.G.; Ahn, Y.S.; et al. Calmodulin dynamically regulates the trafficking of the metabotropic glutamate receptor mGluR5. Proc. Natl. Acad. Sci. USA 2008, 105, 12575–12580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.H.; Lee, J.; Lee, J.Y.; Roche, K.W. Metabotropic glutamate receptors: Phosphorylation and receptor signaling. J. Neurosci. Res. 2008, 86, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sethna, F.; Zhang, M.; Kaphzan, H.; Klann, E.; Autio, D.; Cox, C.L.; Wang, H. Calmodulin activity regulates group I metabotropic glutamate receptor-mediated signal transduction and synaptic depression. J. Neurosci. Res. 2016, 94, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Raka, F.; Di Sebastiano, A.; Kulhawy, S.C.; Ribeiro, F.M.; Godin, C.M.; Caetano, F.A.; Angers, S.; Ferguson, S.S.G. Ca(2+)/calmodulin-dependent protein kinase II interacts with group I metabotropic glutamate and facilitates receptor endocytosis and ERK1/2 signaling: Role of β-amyloid. Mol. Brain. 2015, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Mockett, B.G.; Guévremont, D.; Wutte, M.; Hulme, S.R.; Williams, J.M.; Abraham, W.C. Calcium/calmodulin-dependent protein kinase II mediates group I metabotropic glutamate receptor-dependent protein synthesis and long-term depression in rat hippocampus. J. Neurosci. 2011, 31, 7380–7391. [Google Scholar] [CrossRef] [Green Version]
- Cook, S.G.; Rumian, N.L.; Bayer, K.U. CaMKII T286 phosphorylation has distinct essential functions in three forms of long-term plasticity. J. Biol. Chem. 2022, 298, 102299. [Google Scholar] [CrossRef]
- Francesconi, A.; Kumari, R.; Zukin, R.S. Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. J. Neurosci. 2009, 29, 3590–3602. [Google Scholar] [CrossRef] [Green Version]
- Takayasu, Y.; Takeuchi, K.; Kumari, R.; Bennett, M.V.L.; Zukin, R.S.; Francesconi, A. Caveolin-1 knockout mice exhibit impaired induction of mGluR-dependent long-term depression at CA3-CA1 synapses. Proc. Natl. Acad. Sci. USA 2010, 107, 21778–21783. [Google Scholar] [CrossRef] [Green Version]
- Di Sebastiano, A.R.; Fahim, S.; Dunn, H.A.; Walther, C.; Ribeiro, F.M.; Cregan, S.P.; Angers, S.; Schmid, S.; Ferguson, S.S.G. Role of Spinophilin in Group I Metabotropic Glutamate Receptor Endocytosis, Signaling, and Synaptic Plasticity. J. Biol. Chem. 2016, 291, 17602–17615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yerna, X.; Schakman, O.; Ratbi, I.; Kreis, A.; Lepannetier, S.; de Clippele, M.; Achouri, Y.; Tajeddine, N.; Tissir, F.; Gualdani, R.; et al. Role of the TRPC1 Channel in Hippocampal Long-Term Depression and in Spatial Memory Extinction. Int. J. Mol. Sci. 2020, 21, 1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mango, D.; Nisticò, R. Role of ASIC1a in Normal and Pathological Synaptic Plasticity. Rev. Physiol. Biochem. Pharmacol. 2020, 177, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Ledonne, A.; Nobili, A.; Latagliata, E.C.; Cavallucci, V.; Guatteo, E.; Puglisi-Allegra, S.; D’Amelio, M.; Mercuri, N.B. Neuregulin 1 signalling modulates mGluR1 function in mesencephalic dopaminergic neurons. Mol. Psychiatry 2015, 20, 959–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledonne, A.; Mango, D.; Latagliata, E.C.; Chiacchierini, G.; Nobili, A.; Nisticò, R.; D’Amelio, M.; Puglisi-Allegra, S.; Mercuri, N.B. Neuregulin 1/ErbB signalling modulates hippocampal mGluRI-dependent LTD and object recognition memory. Pharmacol. Res. 2018, 130, 12–24. [Google Scholar] [CrossRef]
- Ledonne, A.; Mercuri, N.B. mGluR1-Dependent Long Term Depression in Rodent Midbrain Dopamine Neurons Is Regulated by Neuregulin 1/ErbB Signaling. Front. Mol. Neurosci. 2018, 11, 346. [Google Scholar] [CrossRef] [Green Version]
- Ledonne, A.; Mercuri, N.B. On the Modulatory Roles of Neuregulins/ErbB Signaling on Synaptic Plasticity. Int. J. Mol. Sci. 2019, 21, 275. [Google Scholar] [CrossRef] [Green Version]
- Peavy, R.D.; Chang, M.S.S.; Sanders-Bush, E.; Conn, P.J. Metabotropic Glutamate Receptor 5-Induced Phosphorylation of Extracellular Signal-Regulated Kinase in Astrocytes Depends on Transactivation of the Epidermal Growth Factor Receptor. J. Neurosci. 2001, 21, 9619–9628. [Google Scholar] [CrossRef] [Green Version]
- Sitcheran, R.; Comb, W.C.; Cogswell, P.C.; Baldwin, A.S. Essential Role for Epidermal Growth Factor Receptor in Glutamate Receptor Signaling to NF-B. Mol. Cell. Biol. 2008, 28, 5061–5070. [Google Scholar] [CrossRef] [Green Version]
- Foley, C.M.; Mott, J.A.; Hay, M.; Hasser, E.M. Glutamate in the nucleus of the solitary tract activates both ionotropic and metabotropic glutamate receptors. Am. J. Physiol. Content. 1998, 275, R1858–R1866. [Google Scholar] [CrossRef]
- Piccinin, S.; Cinque, C.; Calò, L.; Molinaro, G.; Battaglia, G.; Maggi, L.; Nicoletti, F.; Melchiorri, D.; Eusebi, F.; Massey, P.V.; et al. Interaction between Ephrins and mGlu5 metabotropic glutamate receptors in the induction of long-term synaptic depression in the hippocampus. J. Neurosci. 2010, 30, 2835–2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Hu, J.; Passafaro, M.; Xie, W.; Jia, Z. GluA2 (GluR2) Regulates Metabotropic Glutamate Receptor-Dependent Long-Term Depression through N-Cadherin-Dependent and Cofilin-Mediated Actin Reorganization. J. Neurosci. 2011, 31, 819–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, R.; Qi, J.; Liu, A.; Ren, Q.; Lv, D.; Han, L.; Zhou, Z.; Cao, F.; Xie, W.; Jia, Z. Regulation of hippocampal long term depression by Neuroligin 1. Neuropharmacology 2018, 143, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Sakurai, M.; Tongroach, P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J. Physiol. 1982, 324, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Kato, M. Quisqualate receptors are specifically involved in cerebellar synaptic plasticity. Nature 1987, 325, 276–279. [Google Scholar] [CrossRef]
- Aiba, A.; Kano, M.; Chen, C.; Stanton, M.E.; Fox, G.D.; Herrup, K.; Zwingman, T.A.; Tonegawa, S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell 1994, 79, 377–388. [Google Scholar]
- Conquet, F.; Bashir, Z.I.; Davies, C.H.; Daniel, H.; Ferraguti, F.; Bordi, F.; Franz-Bacon, K.; Reggiani, A.; Matarese, V.; Condé, F. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature 1994, 372, 237–243. [Google Scholar] [CrossRef]
- Crepel, F.; Daniel, H.; Hemart, N.; Jaillard, D. Effects of ACPD and AP3 on parallel-fibre mediated EPSPs of Purkinje cells in cerebellar slices In Vitro. Exp. Brain Res. 1991, 86, 402–406. [Google Scholar] [CrossRef]
- Matsuda, S.; Launey, T.; Mikawa, S.; Hirai, H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO J. 2000, 19, 2765–2774. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.T.; Linden, D.J. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron 2000, 25, 635–647. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Chung, H.J.; Wihler, C.; Huganir, R.L.; Linden, D.J. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron 2000, 28, 499–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safo, P.K.; Regehr, W.G. Endocannabinoids control the induction of cerebellar LTD. Neuron 2005, 48, 647–659. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, Y.; Kano, M. Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning. J. Neurosci. 2006, 26, 8829–8837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kano, M.; Hashimoto, K.; Tabata, T. Type-1 metabotropic glutamate receptor in cerebellar Purkinje cells: A key molecule responsible for long-term depression, endocannabinoid signalling and synapse elimination. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 2173–2186. [Google Scholar] [CrossRef] [PubMed]
- Ichise, T.; Kano, M.; Hashimoto, K.; Yanagihara, D.; Nakao, K.; Shigemoto, R.; Katsuki, M.; Aiba, A. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science 2000, 288, 1832–1835. [Google Scholar] [CrossRef]
- Heysieattalab, S.; Lee, K.H.; Liu, Y.; Wang, Y.; Foy, M.R.; Bi, X.; Baudry, M. Impaired cerebellar plasticity and eye-blink conditioning in calpain-1 knock-out mice. Neurobiol. Learn. Mem. 2020, 170, 106995. [Google Scholar] [CrossRef]
- Huber, K.M.; Kayser, M.S.; Bear, M.F. Role for rapid dendritic protein synthesis in hippocampal mGluR dependent long-term depression. Science 2000, 288, 1254–1257. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, T.M.; Hogg, E.L.; Collingridge, G.L.; Corrêa, S.A.L. Hippocampal metabotropic glutamate receptor long-term depression in health and disease: Focus on mitogen-activated protein kinase pathways. J. Neurochem. 2016, 139, 200–214. [Google Scholar] [CrossRef] [Green Version]
- Nosyreva, E.D.; Huber, K.M. Developmental switch in synaptic mechanisms of hippocampal metabotropic glutamate receptor-dependent long-term depression. J. Neurosci. 2005, 25, 2992–3001. [Google Scholar] [CrossRef] [Green Version]
- O’Mara, S.M.; Rowan, M.J.; Anwyl, R. Metabotropic glutamate receptor-induced homosynaptic long-term depression and depotentiation in the dentate gyrus of the rat hippocampus In Vitro. Neuropharmacology 1995, 34, 983–989. [Google Scholar] [CrossRef]
- Camodeca, N.; Breakwell, N.A.; Rowan, M.J.; Anwyl, R. Induction of LTD by activation of group I mGluR in the dentate gyrus In Vitro. Neuropharmacology 1999, 38, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Naie, K.; Tsanov, M.; Manahan-Vaughan, D. Group I metabotropic glutamate receptors enable two distinct forms of long-term depression in the rat dentate gyrus In Vivo. Eur. J. Neurosci. 2007, 25, 3264–3275. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.M.; Wu, J.; Rowan, M.J.; Anwyl, R. Group I metabotropic glutamate receptor (mGluR)-dependent long-term depression mediated via p38 mitogen-activated protein kinase is inhibited by previous high-frequency stimulation and activation of mGluRs and protein kinase C in the rat dentate gyrus In Vitro. J. Neurosci. 2002, 22, 6121–6128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Chang, L.; Rowan, M.J.; Anwyl, R. Developmental dependence, the role of the kinases p38 MAPK and PKC, and the involvement of tumor necrosis factor-R1 in the induction of mGlu-5 LTD in the dentate gyrus. Neuroscience 2007, 144, 110–118. [Google Scholar] [CrossRef]
- Matta, J.A.; Ashby, M.C.; Sanz-Clemente, A.; Roche, K.W.; Isaac, J.T.R. mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron 2011, 70, 339–351. [Google Scholar] [CrossRef] [Green Version]
- Manahan-Vaughan, D.; Braunewell, K.H. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc. Natl. Acad. Sci. USA 1999, 96, 8739–8744. [Google Scholar] [CrossRef] [Green Version]
- Popkirov, S.G.; Manahan-Vaughan, D. Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses. Cereb. Cortex 2011, 21, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goh, J.J.; Manahan-Vaughan, D. Endogenous hippocampal LTD that is enabled by spatial object recognition requires activation of NMDA receptors and the metabotropic glutamate receptor, mGlu5. Hippocampus 2013, 23, 129–138. [Google Scholar] [CrossRef]
- Dong, Z.; Gong, B.; Li, H.; Bai, Y.; Wu, X.; Huang, Y.; He, W.; Li, T.; Yu Wang, T. Mechanisms of hippocampal long-term depression are required for memory enhancement by novelty exploration. J. Neurosci. 2012, 32, 11980–11990. [Google Scholar] [CrossRef] [Green Version]
- Di Prisco, G.V.; Huang, W.; Buffington, S.A.; Hsu, C.C.; Bonnen, P.E.; Placzek, A.N.; Sidrauski, C.; Krnjević, K.; Kaufman, R.J.; Walter, P.; et al. Translational control of mGluR-dependent long-term depression and object-place learning by eIF2α. Nat. Neurosci. 2014, 17, 1073–1082. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, S.K.; Ryan, R.T.; Wong, T.P.; Srivastava, L.K. Loss of dysbindin-1, a risk gene for schizophrenia, leads to impaired group 1 metabotropic glutamate receptor function in mice. Front. Behav. Neurosci. 2015, 9, 72. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhu, Y.; Contractor, A.; Heinemann, S.F. mGluR5 Has a Critical Role in Inhibitory Learning. J. Neurosci. 2009, 29, 3676–3684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ménard, C.; Quirion, R. Group 1 metabotropic glutamate receptor function and its regulation of learning and memory in the aging brain. Front. Pharmacol. 2012, 3, 182. [Google Scholar] [CrossRef] [Green Version]
- Eales, K.L.; Palygin, O.; O’Loughlin, T.; Rasooli-Nejad, S.; Gaestel, M.; Muller, J.; Collins, D.R.; Pankratov, Y.; Correa, S.A. The MK2/3 cascade regulates AMPAR trafficking and cognitive flexibility. Nat. Commun. 2014, 5, 4701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wall, M.J.; Collins, D.R.; Chery, S.L.; Allen, Z.D.; Pastuzyn, E.D.; George, A.J.; Nikolova, V.D.; Moy, S.S.; Philpot, B.D.; Shepherd, J.D.; et al. The temporal dynamics of arc expression regulate cognitive flexibility. Neuron 2018, 98, 1124–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabb, A.M.; Je, H.S.; Wall, M.J.; Robinson, C.G.; Larsen, R.S.; Qiang, Y.; Correa, S.A.; Ehlers, M.D. Triad3A regulates synaptic strength by ubiquitination of Arc. Neuron 2014, 82, 1299–1316. [Google Scholar] [CrossRef] [Green Version]
- Privitera, L.; Hogg, E.L.; Gaestel, M.; Wall, M.J.; Corrêa, S.A.L. The MK2 cascade regulates mGluR-dependent synaptic plasticity and reversal learning. Neuropharmacology 2019, 155, 121–130. [Google Scholar] [CrossRef]
- Braunewell, K.H.; Manahan-Vaughan, D. Long-term depression: A cellular basis for learning? Rev. Neurosci. 2001, 12, 121–140. [Google Scholar] [CrossRef]
- Nakao, K.; Ikegaya, Y.; Yamada, M.K.; Nishiyama, N.; Matsuki, N. Hippocampal long-term depression as an index of spatial working memory. Eur. J. Neurosci. 2002, 16, 970–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, A.; Manahan-Vaughan, D. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb. Cortex 2008, 18, 968–977. [Google Scholar] [CrossRef] [Green Version]
- Gubellini, P.; Saulle, E.; Centonze, D.; Bonsi, P.; Pisani, A.; Bernardi, G.; Conquet, F.; Calabresi, P. Selective involvement of mGlu1 receptors in corticostriatal LTD. Neuropharmacology 2001, 40, 839–846. [Google Scholar] [CrossRef]
- Sung, K.W.; Choi, S.; Lovinger, D.M. Activation of group I mGluRs is necessary for induction of long-term depression at striatal synapses. J. Neurophysiol. 2001, 86, 2405–2412. [Google Scholar] [CrossRef]
- Calabresi, P.; Pisani, A.; Mercuri, N.B.; Bernardi, G. Post-receptor mechanisms underlying striatal long-term depression. J. Neurosci. 1994, 14, 4871–4881. [Google Scholar] [CrossRef] [Green Version]
- Kreitzer, A.C.; Malenka, R.C. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature 2007, 445, 643–647. [Google Scholar] [CrossRef]
- Adermark, L.; Lovinger, D.M. Retrograde endocannabinoid signaling at striatal synapses requires a regulated postsynaptic release step. Proc. Natl. Acad. Sci. USA 2007, 104, 20564–20569. [Google Scholar] [CrossRef] [Green Version]
- Lovinger, D.M. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology 2010, 58, 951–961. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.H.; Knowlton, B.J. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 2006, 7, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Grahn, J.A.; Parkinson, J.A.; Owen, A.M. The cognitive functions of the caudate nucleus. Prog. Neurobiol. 2008, 86, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Augustin, S.M.; Lovinger, D.M. Functional Relevance of Endocannabinoid-Dependent Synaptic Plasticity in the Central Nervous System. ACS Chem. Neurosci. 2018, 9, 2146–2161. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Ma, Y.Y.; Huang, Y.H.; Wang, X.; Otaka, M.; Ishikawa, M.; Neumann, P.A.; Graziane, N.M.; Brown, T.E.; Suska, A.; et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat. Neurosci. 2013, 16, 1644–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.Y.; Lee, B.R.; Wang, X.; Guo, C.; Liu, L.; Cui, R.; Lan, Y.; Balcita-Pedicino, J.J.; Wolf, M.E.; Sesack, S.R.; et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 2014, 83, 1453–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbe, D.; Kopf, M.; Remaury, A.; Bockaert, J.; Manzoni, O.J. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 2002, 99, 8384–8388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourgeaud, L.; Mato, S.; Bouchet, D.; Hemar, A.; Worley, P.F.; Manzoni, O.J. A single In Vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J. Neurosci. 2004, 24, 6939–6945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCutcheon, J.E.; Loweth, J.A.; Ford, K.A.; Marinelli, M.; Wolf, M.E.; Tseng, K.Y. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C dependent mechanism. J. Neurosci. 2011, 31, 14536–14541. [Google Scholar] [CrossRef] [Green Version]
- Russo, S.J.; Dietz, D.M.; Dumitriu, D.; Morrison, J.H.; Malenka, R.C.; Nestler, E.J. The addicted synapse: Mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010, 33, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Luscher, C.; Malenka, R.C. Drug-evoked synaptic plasticity in addiction: From molecular changes to circuit remodeling. Neuron 2011, 69, 650–663. [Google Scholar] [CrossRef] [Green Version]
- Bellone, C.; Lüscher, C. Cocaine triggered AMPA receptor redistribution is reversed In Vivo by mGluR-dependent long-term depression. Nat. Neurosci. 2006, 9, 636–641. [Google Scholar] [CrossRef]
- Mameli, M.; Balland, B.; Luján, R.; Lüscher, C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science 2007, 317, 530–533. [Google Scholar] [CrossRef] [Green Version]
- Da Cunha, C.; Wietzikoski, S.; Wietzikoski, E.C.; Miyoshi, E.; Ferro, M.M.; Anselmo-Franci, J.A.; Canteras, N.S. Evidence for the substantia nigra pars compacta as an essential component of a memory system independent of the hippocampal memory system. Neurobiol. Learn. Mem. 2003, 79, 236–242. [Google Scholar] [CrossRef]
- Palmiter, R.D. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: Lessons from dopamine-deficient mice. Ann. N. Y. Acad. Sci. 2008, 1129, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Wise, R.A. Roles for nigrostriatal—Not justmesocorticolimbic—Dopamine in reward and addiction. Trends Neurosci. 2009, 32, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Haber, S.N. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience 2014, 282, 248–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilango, A.; Kesner, A.J.; Keller, K.L.; Stuber, G.D.; Bonci, A.; Ikemoto, S. Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. J. Neurosci. 2014, 34, 817–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledonne, A.; Mercuri, N.B. Current Concepts on the Physiopathological Relevance of Dopaminergic Receptors. Front. Cell. Neurosci. 2017, 11, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentinova, K.; Mameli, M. mGluR-LTD at Excitatory and Inhibitory Synapses in the Lateral Habenula Tunes Neuronal Output. Cell. Rep. 2016, 16, 2298–2307. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.J.; Liu, M.G.; Chen, T.; Ko, H.G.; Baek, G.C.; Lee, H.R.; Lee, K.; Collingridge, G.L.; Kaang, B.K.; Zhuo, M. Plasticity of metabotropic glutamate receptor-dependent long-term depression in the anterior cingulate cortex after amputation. J. Neurosci. 2012, 32, 11318–11329. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, M. Cortical excitation and chronic pain. Trends Neurosci. 2008, 31, 199–207. [Google Scholar] [CrossRef]
- Kudoh, M.; Sakai, M.; Shibuki, K. Differential dependence of LTD on glutamate receptors in the auditory cortical synapses of cortical and thalamic inputs. J. Neurophysiol. 2002, 88, 3167–3174. [Google Scholar] [CrossRef]
- Cho, K.; Kemp, N.; Noel, J.; Aggleton, J.P.; Brown, M.W.; Bashir, Z.I. A new form of long-term depression in the perirhinal cortex. Nat. Neurosci. 2000, 3, 150–156. [Google Scholar] [CrossRef]
- Bagni, C.; Tassone, F.; Neri, G.; Hagerman, R. Fragile X syndrome: Causes, diagnosis, mechanisms, and therapeutics. J. Clin. Investig. 2012, 122, 4314–4322. [Google Scholar] [CrossRef] [Green Version]
- Bagni, C.; Zukin, R.S. A Synaptic Perspective of Fragile X Syndrome and Autism Spectrum Disorders. Neuron 2019, 101, 1070–1088. [Google Scholar] [CrossRef] [Green Version]
- Bear, M.F.; Huber, K.M.; Warren, S.T. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004, 27, 370–377. [Google Scholar] [CrossRef]
- Dölen, G.; Osterweil, E.; Rao, B.S.; Smith, G.B.; Auerbach, B.D.; Chattarji, S.; Bear, M.F. Correction of fragile X syndrome in mice. Neuron 2007, 56, 955–962. [Google Scholar] [CrossRef] [Green Version]
- Michalon, A.; Sidorov, M.; Ballard, T.M.; Ozmen, L.; Spooren, W.; Wettstein, J.G.; Jaeschke, G.; Bear, M.F.; Lindemann, L. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron 2012, 74, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Michalon, A.; Bruns, A.; Risterucci, C.; Honer, M.; Ballard, T.M.; Ozmen, L.; Jaeschke, G.; Wettstein, J.G.; von Kienlin, M.; Künnecke, B.; et al. Chronic metabotropic glutamate receptor 5 inhibition corrects local alterations of brain activity and improves cognitive performance in fragile X mice. Biol. Psychiatry 2014, 75, 189–197. [Google Scholar] [CrossRef]
- Huber, K.M.; Gallagher, S.M.; Warren, S.T.; Bear, M.F. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. USA 2002, 99, 7746–7750. [Google Scholar] [CrossRef] [Green Version]
- Nosyreva, E.D.; Huber, K.M. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J. Neurophysiol. 2006, 95, 3291–3295. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Antion, M.D.; Hu, D.; Spencer, C.M.; Paylor, R.; Klann, E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron 2006, 51, 441–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, J.B.; Nosyreva, E.D.; Spencer, C.M.; Volk, L.J.; Musunuru, K.; Zhong, R.; Stone, E.F.; Yuva-Paylor, L.A.; Huber, K.M.; Paylor, R.; et al. A mouse model of the human Fragile X syndrome I304N mutation. PLoS Genet. 2009, 5, e1000758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Till, S.M.; Asiminas, A.; Jackson, A.D.; Katsanevaki, D.; Barnes, S.A.; Osterweil, E.K.; Bear, M.F.; Chattarji, S.; Wood, E.R.; Wyllie, D.J.; et al. Conserved hippocampal cellular pathophysiology but distinct behavioural deficits in a new rat model of FXS. Hum. Mol. Genet. 2015, 24, 5977–5984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waung, M.W.; Huber, K.M. Protein translation in synaptic plasticity: mGluR-LTD, Fragile X. Curr. Opin. Neurobiol. 2009, 19, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Molinaro, G.; Collins, K.A.; Hays, S.A.; Paylor, R.; Worley, P.F.; Szumlinski, K.K.; Huber, K.M. Selective Disruption of Metabotropic Glutamate Receptor 5-Homer Interactions Mimics Phenotypes of Fragile X Syndrome in Mice. J. Neurosci. 2016, 36, 2131–2147. [Google Scholar] [CrossRef] [Green Version]
- Gross, C.; Chang, C.W.; Kelly, S.M.; Bhattacharya, A.; McBride, S.M.J.; Danielson, S.W.; Jiang, M.Q.; Chan, C.B.; Ye, K.; Gibson, J.R.; et al. Increased expression of the PI3K enhancer PIKE mediates deficits in synaptic plasticity and behavior in fragile X syndrome. Cell. Rep. 2015, 11, 727–736. [Google Scholar] [CrossRef] [Green Version]
- Koekkoek, S.K.E.; Yamaguchi, K.; Milojkovic, B.A.; Dortland, B.R.; Ruigrok, T.J.H.; Maex, R.; De Graaf, W.; Smit, A.E.; VanderWerf, F.; Bakker, C.E.; et al. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron 2005, 47, 339–352. [Google Scholar] [CrossRef] [Green Version]
- Huber, K.M. The fragile X-cerebellum connection. Trends Neurosci. 2006, 29, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Crino, P.B.; Nathanson, K.L.; Henske, E.P. The tuberous sclerosis complex. N. Engl. J. Med. 2006, 355, 1345–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vries, P.J.; Howe, C.J. The tuberous sclerosis complex proteins—A GRIPP on cognition and neurodevelopment. Trends Mol. Med. 2007, 13, 319–326. [Google Scholar] [CrossRef]
- Curatolo, P.; Bombardieri, R.; Jozwiak, S. Tuberous sclerosis. Lancet 2008, 372, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Tee, A.R.; Fingar, D.C.; Manning, B.D.; Kwiatkowski, D.J.; Cantley, L.C.; Blenis, J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)- mediated downstream signaling. Proc. Natl. Acad. Sci. USA 2002, 99, 13571–13576. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Manning, B.D. The TSC1-TSC2 complex: A molecular switchboard controlling cell growth. Biochem. J. 2008, 412, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Auerbach, B.D.; Osterweil, E.K.; Bear, M.F. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature 2011, 480, 63–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bateup, H.S.; Takasaki, K.T.; Saulnier, J.L.; Denefrio, C.L.; Sabatini, B.L. Loss of Tsc1 In Vivo impairs hippocampal mGluR-LTD and increases excitatory synaptic function. J. Neurosci. 2011, 31, 8862–8869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevere-Torres, I.; Kaphzan, H.; Bhattacharya, A.; Kang, A.; Maki, J.M.; Gambello, M.J.; Arbiser, J.L.; Santini, E.; Klann, E. Metabotropic glutamate receptor-dependent long-term depression is impaired due to elevated ERK signaling in the DeltaRG mouse model of tuberous sclerosis complex. Neurobiol. Dis. 2012, 45, 1101–1110. [Google Scholar] [CrossRef] [Green Version]
- Greer, P.L.; Hanayama, R.; Bloodgood, B.L.; Mardinly, A.R.; Lipton, D.M.; Flavell, S.W.; Kim, T.K.; Griffith, E.C.; Waldon, Z.; Maehr, R.; et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell 2010, 140, 704–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Zhu, G.; Liu, Y.; Standley, S.; Ji, A.; Tunuguntla, R.; Wang, Y.; Claus, C.; Luo, Y.; Baudry, M.; et al. UBE3A Regulates Synaptic Plasticity and Learning and Memory by Controlling SK2 Channel Endocytosis. Cell. Rep. 2015, 12, 449–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pignatelli, M.; Piccinin, S.; Molinaro, G.; Di Menna, L.; Riozzi, B.; Cannella, M.; Motolese, M.; Vetere, G.; Catania, M.V.; Battaglia, G.; et al. Changes in mGlu5 receptor-dependent synaptic plasticity and coupling to homer proteins in the hippocampus of Ube3A hemizygous mice modeling angelman syndrome. J. Neurosci. 2014, 34, 4558–4566. [Google Scholar] [CrossRef] [Green Version]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef]
- Moretti, P.; Levenson, J.M.; Battaglia, F.; Atkinson, R.; Teague, R.; Antalffy, B.; Armstrong, D.; Arancio, O.; Sweatt, J.D.; Zoghbi, H.Y. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J. Neurosci. 2006, 26, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Wu, H.; Coronado, A.A.; de Laittre, E.; Osterweil, E.K.; Zhang, Y.; Bear, M.F. Negative allosteric modulation of mGluR5 partially corrects pathophysiology in a mouse model of rett syndrome. J. Neurosci. 2016, 36, 11946–11958. [Google Scholar] [CrossRef] [Green Version]
- Gogliotti, R.G.; Senter, R.K.; Rook, J.M.; Ghoshal, A.; Zamorano, R.; Malosh, C.; Stauffer, S.R.; Bridges, T.M.; Bartolome, J.M.; Daniels, J.S.; et al. mGlu5 positive allosteric modulation normalizes synaptic plasticity defects and motor phenotypes in a mouse model of Rett syndrome. Hum. Mol. Genet. 2016, 25, 1990–2004. [Google Scholar] [CrossRef] [Green Version]
- Phelan, K.; McDermid, H.E. The 22q13.3 Deletion Syndrome (Phelan-McDermid Syndrome). Mol. Syndromol. 2012, 2, 186–201. [Google Scholar] [CrossRef] [Green Version]
- Harony-Nicolas, H.; De Rubeis, S.; Buxbaum, J.D. Phelan McDermid syndrome: From genetic discoveries to animal models and treatments. J. Child. Neurol. 2015, 30, 1861–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speed, H.E.; Kouser, M.; Xuan, Z.; Reimers, J.M.; Ochoa, C.F.; Gupta, N.; Liu, S.; Powell, C.M. Autism-Associated Insertion Mutation (InsG) of Shank3 Exon 21 Causes Impaired Synaptic Transmission and Behavioral Deficits. J. Neurosci. 2015, 35, 9648–9665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Vyas, Y.; Garner, C.C.; Montgomery, J.M. Autism-associated Shank3 mutations alter mGluR expression and mGluR-dependent but not NMDA receptor-dependent long-term depression. Synapse 2019, 73, e22097. [Google Scholar] [CrossRef] [PubMed]
- Kouser, M.; Speed, H.E.; Dewey, C.M.; Reimers, J.M.; Widman, A.J.; Gupta, N.; Liu, S.; Jaramillo, T.C.; Bangash, M.; Xiao, B.; et al. Loss of predominant Shank3 isoforms results in hippocampus-dependent impairments in behavior and synaptic transmission. J. Neurosci. 2013, 33, 18448–18468. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Bey, A.L.; Katz, B.M.; Badea, A.; Kim, N.; David, L.K.; Duffney, L.J.; Kumar, S.; Mague, S.D.; Hulbert, S.W.; et al. Altered mGluR5-Homer scaffolds and corticostriatal connectivity in a Shank3 complete knockout model of autism. Nat. Commun. 2016, 7, 11459. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, D.; Sebat, J. CNVs: Harbingers of a rare variant revolution in psychiatric genetics. Cell 2012, 148, 1223–1241. [Google Scholar] [CrossRef] [Green Version]
- Zufferey, F.; Sherr, E.H.; Beckmann, N.D.; Hanson, E.; Maillard, A.M.; Hippolyte, L.; Macé, A.; Ferrari, C.; Kutalik, Z.; Andrieux, J.; et al. A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. J. Med. Genet. 2014, 49, 660–668, Erratum in J. Med. Genet. 2014, 51, 478. [Google Scholar] [CrossRef] [Green Version]
- Tian, D.; Stoppel, L.J.; Heynen, A.J.; Lindemann, L.; Jaeschke, G.; Mills, A.A.; Bear, M.F. Contribution of mGluR5 to pathophysiology in a mouse model of human chromosome 16p11.2 microdeletion. Nat. Neurosci. 2015, 18, 182–184. [Google Scholar] [CrossRef] [Green Version]
- Urbano-Gámez, J.D.; Casañas, J.J.; Benito, I.; Montesinos, M.L. Prenatal treatment with rapamycin restores enhanced hippocampal mGluR-LTD and mushroom spine size in a Down’s syndrome mouse model. Mol. Brain. 2021, 14, 84. [Google Scholar] [CrossRef]
- Li, S.; Selkoe, D.J. A mechanistic hypothesis for the impairment of synaptic plasticity by soluble Aβ oligomers from Alzheimer’s brain. J. Neurochem. 2020, 154, 583–597. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, H.; Boehm, J.; Sato, C.; Iwatsubo, T.; Tomita, T.; Sisodia, S.; Malinow, R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron 2006, 52, 831–843. [Google Scholar] [CrossRef] [Green Version]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Hong, S.; Shepardson, N.E.; Walsh, D.M.; Shankar, G.M.; Selkoe, D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 2009, 62, 788–801. [Google Scholar] [CrossRef] [Green Version]
- Um, J.W.; Kaufman, A.C.; Kostylev, M.; Heiss, J.K.; Stagi, M.; Takahashi, H.; Kerrisk, M.E.; Vortmeyer, A.; Wisniewski, T.; Koleske, A.J.; et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer abeta oligomer bound to cellular prion protein. Neuron 2013, 79, 887–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Lin, R.; Chang, L.; Xu, S.; Wei, X.; Zhang, J.; Wang, C.; Anwyl, R.; Wang, Q. Enhancement of long-term depression by soluble amyloid β protein in rat hippocampus is mediated by metabotropic glutamate receptor and involves activation of p38MAPK, STEP and caspase-3. Neuroscience 2013, 253, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.W.; Nicoll, A.J.; Zhang, D.; Mably, A.J.; O’Malley, T.; Purro, S.A.; Terry, C.; Collinge, J.; Walsh, D.M.; Rowan, M.J. mGlu5 receptors and cellular prion protein mediate amyloid-β-facilitated synaptic long-term depression In Vivo. Nat. Commun. 2014, 5, 3374. [Google Scholar] [CrossRef] [Green Version]
- Rammes, G.; Hasenjager, A.; Sroka-Saidi, K.; Deussing, J.M.; Parsons, C.G. Therapeutic significance of NR2B-containing NMDA receptors and mGluR5 metabotropic glutamate receptors in mediating the synaptotoxic effects of beta-amyloid oligomers on long-term potentiation (LTP) in murine hippocampal slices. Neuropharmacology 2011, 60, 98–990. [Google Scholar] [CrossRef] [PubMed]
- Varga, E.; Juhász, G.; Bozsó, Z.; Penke, B.; Fülöp, L.; Szegedi, V. Amyloid-β1-42 Disrupts Synaptic Plasticity by Altering Glutamate Recycling at the Synapse. J. Alzheimers Dis. 2015, 45, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Mango, D.; Nisticò, R. Role of ASIC1a in Aβ-induced synaptic alterations in the hippocampus. Pharmacol. Res. 2018, 131, 61–65. [Google Scholar] [CrossRef]
- Lanté, F.; Chafai, M.; Raymond, E.F.; Pereira, A.R.; Mouska, X.; Kootar, S.; Barik, J.; Bethus, I.; Marie, H. Subchronic glucocorticoid receptor inhibition rescues early episodic memory and synaptic plasticity deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2015, 40, 1772–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Zhou, X.; Zimmermann, H.R.; Cavener, D.R.; Klann, E.; Ma, T. Repression of the eIF2α kinase PERK alleviates mGluR-LTD impairments in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2016, 41, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaki, S.; Fukumoto, K. mGlu receptors as potential targets for novel antidepressants. Curr. Opin. Pharmacol. 2018, 38, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Deschwanden, A.; Karolewicz, B.; Feyissa, A.M.; Treyer, V.; Ametamey, S.M.; Johayem, A.; Burger, C.; Auberson, Y.P.; Sovago, J.; Stockmeier, C.A.; et al. Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am. J. Psychiatry 2011, 168, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Su, R.; Zhang, X.; Wen, J.; Yao, D.; Gao, X.; Zhu, Z.; Li, H. Hippocampal GR- and CB1-mediated mGluR5 differentially produces susceptibility and resilience to acute and chronic mild stress in rats. Neuroscience 2017, 357, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, M.; Vollmayr, B.; Richter, S.H.; Middei, S.; Matrisciano, F.; Molinaro, G.; Nasca, C.; Battaglia, G.; Ammassari-Teule, M.; Feligioni, M.; et al. Enhanced mGlu5-receptor dependent long-term depression at the Schaffer collateral-CA1 synapse of congenitally learned helpless rats. Neuropharmacology 2013, 66, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.M.; Wang, J.Q. Alterations in mGlu5 receptor expression and function in the striatum in a rat depression model. J. Neurochem. 2018, 145, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Chaouloff, F.; Hémar, A.; Manzoni, O. Acute stress facilitates hippocampal CA1 metabotropic glutamate receptor-dependent long-term depression. J. Neurosci. 2007, 27, 7130–7135. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Lin, W.; Cheng, Y.; Wang, D. mGluR5 Facilitates Long-Term Synaptic Depression in a Stress-Induced Depressive Mouse Model. Can. J. Psychiatry 2019, 65, 347–355. [Google Scholar] [CrossRef]
- Li, M.X.; Li, Q.; Sun, X.J.; Luo, C.; Li, Y.; Wang, Y.N.; Chen, J.; Gong, C.Z.; Li, Y.J.; Shi, L.P.; et al. Increased Homer1-mGluR5 mediates chronic stress-induced depressive-like behaviors and glutamatergic dysregulation via activation of PERK-eIF2α. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 95, 109682. [Google Scholar] [CrossRef]
- Wolf, M.E. Synaptic mechanisms underlying persistent cocaine craving. Nat. Rev. Neurosci. 2016, 17, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Mato, S.; Chevaleyre, V.; Robbe, D.; Pazos, A.; Castillo, P.E.; Manzoni, O.J. A single In-Vivo exposure to delta 9THC blocks endocannabinoid-mediated synaptic plasticity. Nat. Neurosci. 2004, 7, 585–586. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Liang, Y.C.; Lee, C.C.; Hsu, K.S. Cocaine Withdrawal Impairs mGluR5-Dependent Long-Term Depression in Nucleus Accumbens Shell Neurons of Both Direct and Indirect Pathways. Mol. Neurobiol. 2015, 52, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Bilbao, A.; Neuhofer, D.; Sepers, M.; Wei, S.P.; Eisenhardt, M.; Hertle, S.; Lassalle, O.; Ramos-Uriarte, A.; Puente, N.; Lerner, R.; et al. Endocannabinoid LTD in Accumbal D1 Neurons Mediates Reward-Seeking Behavior. iScience 2020, 23, 100951. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, J.E.; Wang, X.; Tseng, K.Y.; Wolf, M.E.; Marinelli, M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J. Neurosci. 2011, 31, 5737–5743. [Google Scholar] [CrossRef] [Green Version]
- Scheyer, A.F.; Loweth, J.A.; Christian, D.T.; Uejima, J.; Rabei, R.; Le, T.; Dolubizno, H.; Stefanik, M.T.; Murray, C.H.; Sakas, C.; et al. AMPA Receptor Plasticity in Accumbens Core Contributes to Incubation of Methamphetamine Craving. Biol. Psychiatry 2016, 80, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Argilli, E.; Sibley, D.R.; Malenka, R.C.; England, P.M.; Bonci, A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J. Neurosci. 2008, 28, 9092–9100. [Google Scholar] [CrossRef] [Green Version]
- Sveinbjornsdottir, S.J. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 168, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Hussein, A.; Guevara, C.A.; Del Valle, P.; Gupta, S.; Benson, D.L.; Huntley, G.W. Non-Motor Symptoms of Parkinson’s Disease: The Neurobiology of Early Psychiatric and Cognitive Dysfunction. Neuroscientist 2023, 29, 97–116. [Google Scholar] [CrossRef]
- Grégoire, L.; Morin, N.; Ouattara, B.; Gasparini, F.; Bilbe, G.; Johns, D.; Vranesic, I.; Sahasranaman, S.; Gomez-Mancilla, B.; Di Paolo, T. The acute antiparkinsonian and antidyskinetic effect of AFQ056, a novel metabotropic glutamate receptor type 5 antagonist, in L-Dopa-treated parkinsonian monkeys. Park. Relat. Disord. 2011, 17, 270–276. [Google Scholar] [CrossRef]
- Yamasaki, T.; Fujinaga, M.; Kawamura, K.; Furutsuka, K.; Nengaki, N.; Shimoda, Y.; Shiomi, S.; Takei, M.; Hashimoto, H.; Yui, J.; et al. Dynamic Changes in Striatal mGluR1 But Not mGluR5 during Pathological Progression of Parkinson’s Disease in Human Alpha-Synuclein A53T Transgenic Rats: A Multi-PET Imaging Study. J. Neurosci. 2016, 36, 375–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tozzi, A.; Sciaccaluga, M.; Loffredo, V.; Megaro, A.; Ledonne, A.; Cardinale, A.; Federici, M.; Bellingacci, L.; Paciotti, S.; Ferrari, E.; et al. Dopamine-dependent early synaptic and motor dysfunctions induced by α-synuclein in the nigrostriatal circuit. Brain 2021, 144, 3477–3491. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, H.; Wu, S.; Cheng, Y.; Li, Q.; Wang, J.; Zhu, G. MPP+ inhibits mGluR1/5-mediated long-term depression in mouse hippocampus by calpain activation. Eur. J. Pharmacol. 2017, 795, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Sgobio, C.; Siliquini, S.; Tozzi, A.; Tantucci, M.; Ghiglieri, V.; DiFilippo, M.; Pendolino, V.; deIure, A.; Marti, M.; et al. Mechanisms underlying the impairment of hippocampal long-term potentiation and memory in experimental Parkinson’s disease. Brain 2012, 135, 1884–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, G.; Chen, Y.; Huang, Y.; Li, Q.; Behnisch, T. MPTP-meditated hippocampal dopamine deprivation modulates synaptic transmission and activity-dependent synaptic plasticity. Toxicol. Appl. Pharmacol. 2011, 254, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.M.; Lindemann, L.; Jønch, A.E.; Apostol, G.; Bear, M.F.; Carpenter, R.L.; Crawley, J.N.; Curie, A.; Des Portes, V.; Hossain, F.; et al. Drug development for neurodevelopmental disorders: Lessons learned from fragile X syndrome. Nat. Rev. Drug. Discov. 2018, 17, 280–299. [Google Scholar] [CrossRef] [Green Version]
| Brain Area | Synapse/ Cellular Type | mGluRI Subtype | Main Mechanism of Induction | Physiological Functions | Former Refs | Dysregulation in Disease Models |
|---|---|---|---|---|---|---|
| Cerebellum | Purkinje cells | mGluR1 | AMPARs endocytosis | Motor learning | [96] | |
| Hippocampus | CA3-CA1 | mGluR1 mGluR5 | AMPARs endocytosis | Novelty detection Object-place recognition learning Cognitive flexibility | [18,38,39,40,107] | Fragile X syndrome ↑ Tuberous sclerosis ↓ Angelman’s syndrome↑ Rett’s syndrome ↓ Down’s syndrome↑ Phelan–McDermid syndrome ↓ Schizophrenia ↓ Stress-induced depression ↑ |
| CA3-CA1 | mGluR1 mGluR5 | NMDA endocytosis | Spatial learning | [50,51] | ||
| PP-DG | mGluR1 mGluR5 | [110,111,112] | ||||
| DG-CA3 | mGluR5 | Changes in NMDARs composition | [115] | |||
| Dorsal striatum | Corticostriatal/ MSNs | mGluR1 mGluR5 (D2R) | eCB-regulated Glu release | Motor-learning Goal-oriented behaviors | [41,54] | Parkinson’s disease ↓ |
| Nucleus accumbens | MSNs | mGluR5 | eCB-regulated Glu release | Rewards-seeking behaviors | [142] | Addiction (reward-seeking) |
| MSNs | mGluR1 | Changes in AMPARs composition | Rewards-seeking behaviors | [53] | Addiction (drug craving) | |
| Ventral tegmental area | DA neurons | mGluR1 | Changes in AMPARs composition | [52,147] | Addiction (reduces addictive drugs-induced plasticity) | |
| Substantia nigra pars compacta | DA neurons | mGluR1 | [86] | |||
| Lateral habenula | LHb neurons | mGluR1 | eCB-regulated Glu release | [155] | ||
| Anterior cingulate cortex | Layer II/III Layer V Pyramidal neurons | mGluR1 | [156] | Chronic pain conditions | ||
| Auditory cortex | Layer IV Pyramidal neurons | mGluR5 | [158] | |||
| Perirhinal cortex | Layer II/III Pyramidal neurons | mGluR1 mGluR5 | Recognition memory | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mango, D.; Ledonne, A. Updates on the Physiopathology of Group I Metabotropic Glutamate Receptors (mGluRI)-Dependent Long-Term Depression. Cells 2023, 12, 1588. https://doi.org/10.3390/cells12121588
Mango D, Ledonne A. Updates on the Physiopathology of Group I Metabotropic Glutamate Receptors (mGluRI)-Dependent Long-Term Depression. Cells. 2023; 12(12):1588. https://doi.org/10.3390/cells12121588
Chicago/Turabian StyleMango, Dalila, and Ada Ledonne. 2023. "Updates on the Physiopathology of Group I Metabotropic Glutamate Receptors (mGluRI)-Dependent Long-Term Depression" Cells 12, no. 12: 1588. https://doi.org/10.3390/cells12121588





