Hyperglycosylated-hCG: Its Role in Trophoblast Invasion and Intrauterine Growth Restriction
Abstract
1. Introduction
2. Structure and Variants of hCG
3. hCG and H-hCG Production
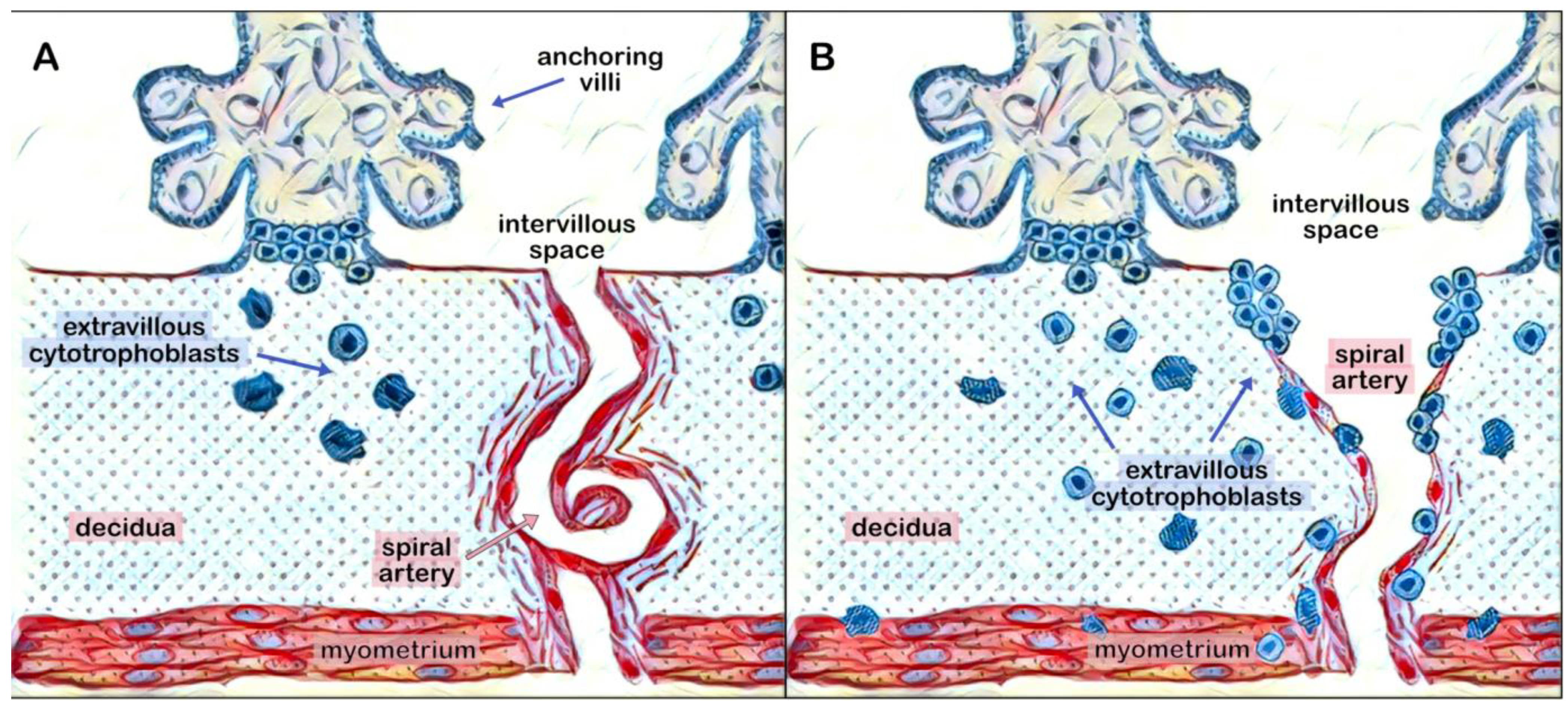
4. Role of H-hCG in Trophoblast Invasion
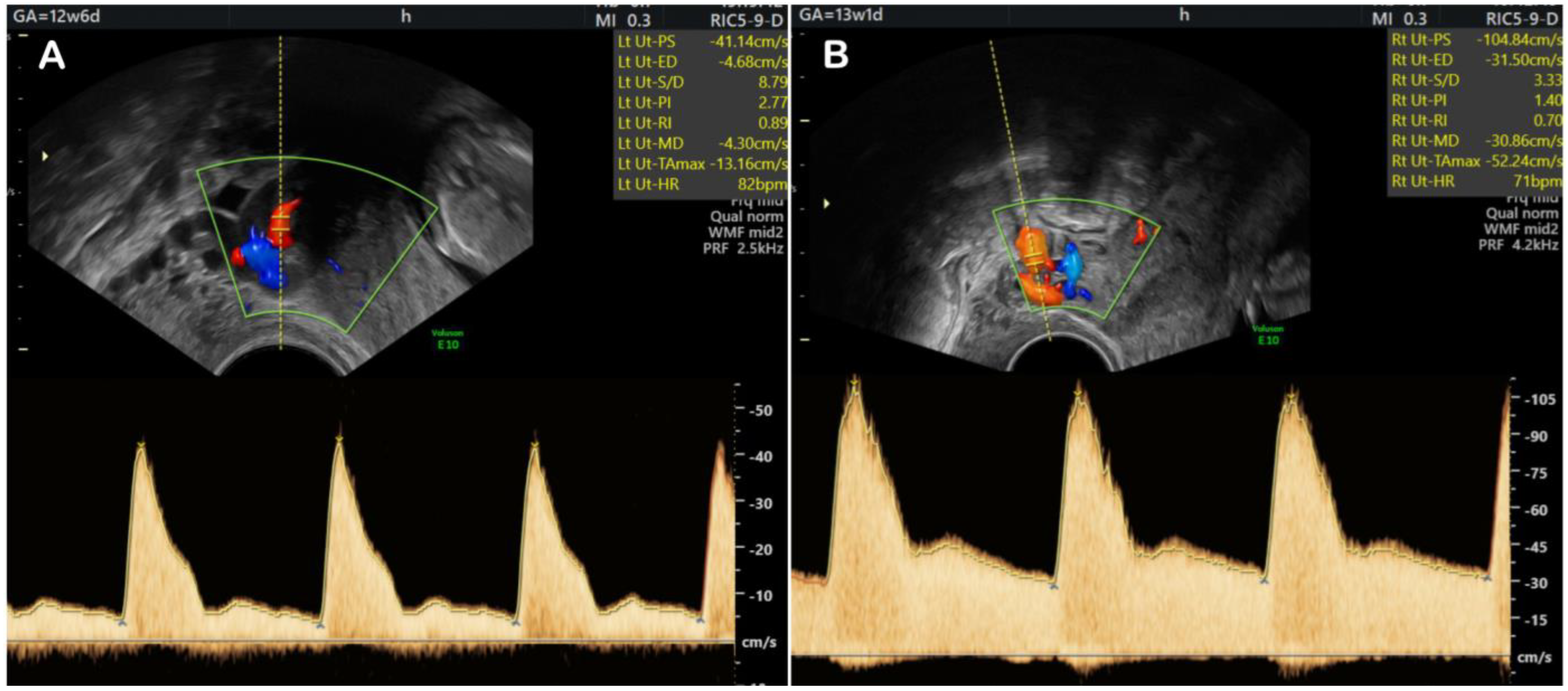
5. Fetal Growth Restriction
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirose, T. Experimentalle Histologische Studie Zur Genese Corpus Luteum. Mitt. Med. Fak. Univ. ZU 1919, 23, 63–70. [Google Scholar]
- Zondek, B.; Aschheim, S. Das Hormon Des Hypophysenvorderlappens: I. Testobjekt Zum Nachweis Des Hormons. Klin. Wochenschr. 1927, 6, 248–252. [Google Scholar] [CrossRef]
- Cole, L.A. HCG, Five Independent Molecules. Clin. Chim. Acta 2012, 413, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Lapthorn, A.J.; Harris, D.C.; Littlejohn, A.; Lustbader, J.W.; Canfield, R.E.; Machin, K.J.; Morgan, F.J.; Isaacs, N.W. Crystal Structure of Human Chorionic Gonadotropin. Nature 1994, 369, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Nwabuobi, C.; Arlier, S.; Schatz, F.; Guzeloglu-Kayisli, O.; Lockwood, C.J.; Kayisli, U.A. HCG: Biological Functions and Clinical Applications. Int. J. Mol. Sci. 2017, 18, 2037. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.; Paus, E.; Hemken, P.M.; Sturgeon, C.; Stewart, W.W.; Skinner, J.P.; Harwick, L.C.; Saldana, S.C.; Ramsay, C.S.; Rupprecht, K.R.; et al. Candidate Epitopes for Measurement of HCG and Related Molecules: The Second ISOBM TD-7 Workshop. Tumor Biol. 2013, 34, 4033. [Google Scholar] [CrossRef]
- Cole, L.A. Biological Functions of HCG and HCG-Related Molecules. Reprod. Biol. Endocrinol. 2010, 8, 102. [Google Scholar] [CrossRef]
- Iles, R.K. Ectopic HCGbeta Expression by Epithelial Cancer: Malignant Behaviour, Metastasis and Inhibition of Tumor Cell Apoptosis. Mol. Cell. Endocrinol. 2007, 260–262, 264–270. [Google Scholar] [CrossRef]
- Valmu, L.; Alfthan, H.; Hotakainen, K.; Birken, S.; Stenman, U.H. Site-Specific Glycan Analysis of Human Chorionic Gonadotropin Beta-Subunit from Malignancies and Pregnancy by Liquid Chromatography--Electrospray Mass Spectrometry. Glycobiology 2006, 16, 1207–1218. [Google Scholar] [CrossRef]
- Cole, L.A.; Butler, S. Hyperglycosylated HCG, HCGβ and Hyperglycosylated HCGβ: Interchangeable Cancer Promoters. Mol. Cell. Endocrinol. 2012, 349, 232–238. [Google Scholar] [CrossRef]
- Cole, L.A. Hyperglycosylated HCG, a Review. Placenta 2010, 31, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Ibeto, L.; Antonopoulos, A.; Grassi, P.; Pang, P.C.; Panico, M.; Bobdiwala, S.; Al-Memar, M.; Davis, P.; Davis, M.; Taylor, J.N.; et al. Insights into the Hyperglycosylation of Human Chorionic Gonadotropin Revealed by Glycomics Analysis. PLoS ONE 2020, 15, e0228507. [Google Scholar] [CrossRef] [PubMed]
- Berndt, S.; Blacher, S.; Munaut, C.; Detilleux, J.; D’hauterive, S.P.; Huhtaniemi, I.; Evain-Brion, D.; Noël, A.; Fournier, T.; Foidart, J.M. Hyperglycosylated Human Chorionic Gonadotropin Stimulates Angiogenesis through TGF-β Receptor Activation. FASEB J. 2013, 27, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Evans, J. Hyperglycosylated HCG: A Unique Human Implantation and Invasion Factor. Am. J. Reprod. Immunol. 2016, 75, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Handschuh, K.; Guibourdenche, J.; Tsatsaris, V.; Guesnon, M.; Laurendeau, I.; Evain-Brion, D.; Fournier, T. Human Chorionic Gonadotropin Produced by the Invasive Trophoblast but Not the Villous Trophoblast Promotes Cell Invasion and Is Down-Regulated by Peroxisome Proliferator-Activated Receptor-Gamma. Endocrinology 2007, 148, 5011–5019. [Google Scholar] [CrossRef]
- Jurisicova, A.; Antenos, M.; Kapasi, K.; Meriano, J.; Casper, R.F. Variability in the Expression of Trophectodermal Markers β-Human Chorionic Gonadotrophin, Human Leukocyte Antigen-G and Pregnancy Specific β-1 Glycoprotein by the Human Blastocyst. Hum. Reprod. 1999, 14, 1852–1858. [Google Scholar] [CrossRef]
- Woodward, B.J.; Lenton, E.A.; Turner, K. Human Chorionic Gonadotrophin: Embryonic Secretion Is a Time-Dependent Phenomenon. Hum. Reprod. 1993, 8, 1463–1468. [Google Scholar] [CrossRef]
- Gridelet, V.; d’Hauterive, S.P.; Polese, B.; Foidart, J.M.; Nisolle, M.; Geenen, V. Human Chorionic Gonadotrophin: New Pleiotropic Functions for an “Old” Hormone During Pregnancy. Front. Immunol. 2020, 11, 343. [Google Scholar] [CrossRef]
- Hay, D.L.; Lopata, A. Chorionic Gonadotropin Secretion by Human Embryos in Vitro. J. Clin. Endocrinol. Metab. 1988, 67, 1322–1324. [Google Scholar] [CrossRef]
- Hoshina, M.; Hussa, R.; Pattillo, R.; Boime, I. Cytological Distribution of Chorionic Gonadotropin Subunit and Placental Lactogen Messenger RNA in Neoplasms Derived from Human Placenta. J. Cell Biol. 1983, 97, 1200–1206. [Google Scholar] [CrossRef]
- Cole, L.A.; Butler, S.A. The Biological Function of Hyperglycosylated HCG. Asian Pac. J. Reprod. 2012, 1, 7–12. [Google Scholar] [CrossRef]
- Kovalevskaya, G.; Genbacevl, O.; Fisher, S.J.; Caceres, E.; O’Connor, J.F. Trophoblast Origin of HCG Isoforms: Cytotrophoblasts Are the Primary Source of Choriocarcinoma-like HCG. Mol. Cell Endocrinol. 2002, 194, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Black, S.; Huppertz, B. Endovascular Trophoblast Invasion: Implications for the Pathogenesis of Intrauterine Growth Retardation and Preeclampsia. Biol. Reprod. 2003, 69, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lyall, F.; Bulmer, J.N.; Duffie, E.; Cousins, F.; Theriault, A.; Robson, S.C. Human Trophoblast Invasion and Spiral Artery Transformation: The Role of PECAM-1 in Normal Pregnancy, Preeclampsia, and Fetal Growth Restriction. Am. J. Pathol. 2001, 158, 1713. [Google Scholar] [CrossRef] [PubMed]
- Moser, G.; Weiss, G.; Sundl, M.; Gauster, M.; Siwetz, M.; Lang-Olip, I.; Huppertz, B. Extravillous Trophoblasts Invade More than Uterine Arteries: Evidence for the Invasion of Uterine Veins. Histochem. Cell Biol. 2017, 147, 353. [Google Scholar] [CrossRef]
- de Medeiros, S.F.; Norman, R.J. Human Choriogonadotrophin Protein Core and Sugar Branches Heterogeneity: Basic and Clinical Insights. Hum. Reprod. Update 2009, 15, 69–95. [Google Scholar] [CrossRef]
- Nakamura, K.; Niimi, K.; Yamamoto, E.; Ikeda, Y.; Nishino, K.; Suzuki, S.; Kajiyama, H.; Kikkawa, F. Core 2 Β1,6-N-Acetylglucosaminyltransferases Accelerate the Escape of Choriocarcinoma from Natural Killer Cell Immunity. Biochem. Biophys. Rep. 2021, 26, 100951. [Google Scholar] [CrossRef]
- Tomiie, M.; Isaka, S.; Miyoshi, E.; Taniguchi, N.; Kimura, T.; Ogita, K.; Tsutsui, T.; Shimoya, K.; Nakagawa, T.; Kondo, A.; et al. Elevated Expression of N-Acetylglucosaminyltransferase V in First Trimester Human Placenta. Biochem. Biophys. Res. Commun. 2005, 330, 999–1004. [Google Scholar] [CrossRef]
- Niimi, K.; Yamamoto, E.; Fujiwara, S.; Shinjo, K.; Kotani, T.; Umezu, T.; Kajiyama, H.; Shibata, K.; Ino, K.; Kikkawa, F. High Expression of N-Acetylglucosaminyltransferase IVa Promotes Invasion of Choriocarcinoma. Br. J. Cancer 2012, 107, 1969. [Google Scholar] [CrossRef]
- Yamamoto, E.; Ino, K.; Miyoshi, E.; Inamori, K.I.; Abe, A.; Sumigama, S.; Iwase, A.; Kajiyama, H.; Shibata, K.; Nawa, A.; et al. N-Acetylglucosaminyltransferase V Regulates Extravillous Trophoblast Invasion through Glycosylation of A5β1 Integrin. Endocrinology 2009, 150, 990–999. [Google Scholar] [CrossRef]
- Birken, S.; Krichevsky, A.; O’Connor, J.; Schlatterer, J.; Cole, L.; Kardana, A.; Canfield, R. Development and Characterization of Antibodies to a Nicked and Hyperglycosylated Form of HCG from a Choriocarcinoma Patient: Generation of Antibodies That Differentiate between Pregnancy HCG and Choriocarcinoma HCG. Endocrine 1999, 10, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.A.; Omrani, A.; Cermik, D.; Singh, R.O.B.; Mahoney, M.J. Hyperglycosylated Hcg, a Potential Alternative to Hcg in Down Syndrome Screening. Prenat. Diagn. 1998, 18, 926–933. [Google Scholar] [CrossRef]
- Caniggia, I.; Grisaru-Gravnosky, S.; Kuliszewsky, M.; Post, M.; Lye, S.J. Inhibition of TGF-Beta 3 Restores the Invasive Capability of Extravillous Trophoblasts in Preeclamptic Pregnancies. J. Clin. Investig. 1999, 103, 1641–1650. [Google Scholar] [CrossRef]
- Aplin, J.D. Implantation, Trophoblast Differentiation and Haemochorial Placentation: Mechanistic Evidence in Vivo and in Vitro. J. Cell Sci. 1991, 99 Pt 4, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; How, T.; Kirkbride, K.C.; Gordon, K.J.; Lee, J.D.; Hempel, N.; Kelly, P.; Moeller, B.J.; Marks, J.R.; Blobe, G.C. The Type III TGF-Beta Receptor Suppresses Breast Cancer Progression. J. Clin. Investig. 2007, 117, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, J.B.; Blobe, G.C. TGF-β Superfamily Co-Receptors in Cancer. Dev. Dyn. 2022, 251, 117–143. [Google Scholar] [CrossRef]
- Cole, L.A.; Dai, D.; Butler, S.A.; Leslie, K.K.; Kohorn, E.I. Gestational Trophoblastic Diseases: 1. Pathophysiology of Hyperglycosylated HCG. Gynecol. Oncol. 2006, 102, 145–150. [Google Scholar] [CrossRef]
- Melamed, N.; Baschat, A.; Yinon, Y.; Athanasiadis, A.; Mecacci, F.; Figueras, F.; Berghella, V.; Nazareth, A.; Tahlak, M.; McIntyre, H.D.; et al. FIGO (International Federation of Gynecology and Obstetrics) Initiative on Fetal Growth: Best Practice Advice for Screening, Diagnosis, and Management of Fetal Growth Restriction. Int. J. Gynecol. Obstet. 2021, 152, 3–57. [Google Scholar] [CrossRef]
- Salomon, L.J.; Alfirevic, Z.; Da Silva Costa, F.; Deter, R.L.; Figueras, F.; Ghi, T.; Glanc, P.; Khalil, A.; Lee, W.; Napolitano, R.; et al. ISUOG Practice Guidelines: Ultrasound Assessment of Fetal Biometry and Growth. Ultrasound Obstet. Gynecol. 2019, 53, 715–723. [Google Scholar] [CrossRef]
- Galan, H.; Grobman, W. ACOG Practice Bulletin No. 204: Fetal Growth Restriction. Obstet. Gynecol. 2019, 133, E97–E109. [Google Scholar] [CrossRef]
- Nardozza, L.M.M.; Caetano, A.C.R.; Zamarian, A.C.P.; Mazzola, J.B.; Silva, C.P.; Marçal, V.M.G.; Lobo, T.F.; Peixoto, A.B.; Júnior, E.A. Fetal Growth Restriction: Current Knowledge. Arch. Gynecol. Obstet. 2017, 295, 1061–1077. [Google Scholar] [CrossRef]
- Blencowe, H.; Krasevec, J.; de Onis, M.; Black, R.E.; An, X.; Stevens, G.A.; Borghi, E.; Hayashi, C.; Estevez, D.; Cegolon, L.; et al. National, Regional, and Worldwide Estimates of Low Birthweight in 2015, with Trends from 2000: A Systematic Analysis. Lancet Glob. Health 2019, 7, e849–e860. [Google Scholar] [CrossRef] [PubMed]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus Definition of Fetal Growth Restriction: A Delphi Procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, F.P.; Harrist, R.B.; Martinez-Poyer, J. In Utero Analysis of Fetal Growth: A Sonographic Weight Standard. Radiology 1991, 181, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Papageorghiou, A.T.; Ohuma, E.O.; Altman, D.G.; Todros, T.; Ismail, L.C.; Lambert, A.; Jaffer, Y.A.; Bertino, E.; Gravett, M.G.; Purwar, M.; et al. International Standards for Fetal Growth Based on Serial Ultrasound Measurements: The Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Alfirevic, Z.; Berghella, V.; Bilardo, C.; Hernandez-Andrade, E.; Johnsen, S.L.; Kalache, K.; Leung, K.-Y.; Malinger, G.; Munoz, H.; et al. Practice Guidelines for Performance of the Routine Mid-Trimester Fetal Ultrasound Scan. Ultrasound Obs. Gynecol. 2010, 37, 116–126. [Google Scholar] [CrossRef]
- Dudley, N.J. A Systematic Review of the Ultrasound Estimation of Fetal Weight. Ultrasound Obstet. Gynecol. 2005, 25, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Prefumo, F.; Sebire, N.J.; Thilaganathan, B. Decreased Endovascular Trophoblast Invasion in First Trimester Pregnancies with High-Resistance Uterine Artery Doppler Indices. Hum. Reprod. 2004, 19, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The Uterine Spiral Arteries in Human Pregnancy: Facts and Controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Pathophysiology of Placental-Derived Fetal Growth Restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef]
- Zur, R.L.; Kingdom, J.C.; Parks, W.T.; Hobson, S.R. The Placental Basis of Fetal Growth Restriction. Obstet. Gynecol. Clin. N. Am. 2020, 47, 81–98. [Google Scholar] [CrossRef] [PubMed]
- King, V.J.; Bennet, L.; Stone, P.R.; Clark, A.; Gunn, A.J.; Dhillon, S.K. Fetal Growth Restriction and Stillbirth: Biomarkers for Identifying at Risk Fetuses. Front. Physiol. 2022, 13, 959750. [Google Scholar] [CrossRef] [PubMed]
- Ridder, A.; Giorgione, V.; Khalil, A.; Thilaganathan, B. Preeclampsia: The Relationship between Uterine Artery Blood Flow and Trophoblast Function. Int. J. Mol. Sci. 2019, 20, 3263. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, S.; Glinianaia, S.V.; Torrioli, M.G.; Platt, M.J.; Miceli, M.; Jouk, P.S.; Johnson, A.; Hutton, J.; Hemming, K.; Hagberg, G.; et al. Cerebral Palsy and Intrauterine Growth in Single Births: European Collaborative Study. Lancet 2003, 362, 1106–1111. [Google Scholar] [CrossRef]
- Mayer, C.; Joseph, K.S. Fetal Growth: A Review of Terms, Concepts and Issues Relevant to Obstetrics. Ultrasound Obstet. Gynecol. 2013, 41, 136–145. [Google Scholar] [CrossRef]
- Lees, C.C.; Stampalija, T.; Baschat, A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Poon, L.C.; Salomon, L.J.; Unterscheider, J. ISUOG Practice Guidelines: Diagnosis and Management of Small-for-Gestational-Age Fetus and Fetal Growth Restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
- Swanson, A.M.; David, A.L. Animal Models of Fetal Growth Restriction: Considerations for Translational Medicine. Placenta 2015, 36, 623–630. [Google Scholar] [CrossRef]
- Lopez-Tello, J.; Arias-Alvarez, M.; Gonzalez-Bulnes, A.; Sferuzzi-Perri, A.N. Models of Intrauterine Growth Restriction and Fetal Programming in Rabbits. Mol. Reprod. Dev. 2019, 86, 1781–1809. [Google Scholar] [CrossRef]
- Palomaki, G.E.; Neveux, L.M.; Haddow, J.E.; Wyatt, P. Hyperglycosylated-HCG (h-HCG) and Down Syndrome Screening in the First and Second Trimesters of Pregnancy. Prenat. Diagn. 2007, 27, 808–813. [Google Scholar] [CrossRef]
- Nicolaides, K.H. Screening for Fetal Aneuploidies at 11 to 13 Weeks. Prenat. Diagn. 2011, 31, 7–15. [Google Scholar] [CrossRef]
- Birken, S. Specific Measurement of O-Linked Core 2 Sugar-Containing Isoforms of Hyperglycosylated Human Chorionic Gonadotropin by Antibody B152. Tumor Biol. 2005, 26, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Brady, P.C.; Farland, L.V.; Racowsky, C.; Ginsburg, E.S. Hyperglycosylated Human Chorionic Gonadotropin as a Predictor of Ongoing Pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 68.e1–68.e12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Hu, M.; Liu, X.; Qi, H. Timing of Human Chorionic Gonadotropin (HCG) Hormone Administration in IVF/ICSI Protocols Using GnRH Agonist or Antagonists: A Systematic Review and Meta-Analysis. Gynecol. Endocrinol. 2014, 30, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Davar, R.; Miraj, S.; Mojtahedi, M.F. Effect of Adding Human Chorionic Gonadotropin to Frozen Thawed Embryo Transfer Cycles with History of Thin Endometrium. Int. J. Reprod. Biomed. 2016, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, M.; Aleyasin, A.; Aghahosseini, M.; Najafian, A.; Nashtaei, M.S.; Hosseinimousa, S. The Effect of Intrauterine HCG Injection before Embryo Transfer on Pregnancy Rate in Frozen Embryo Transfer Cycles. Ann. Med. Surg. 2022, 79, 104091. [Google Scholar] [CrossRef]
- Drug Approval Package: Ovidrel (Choriogonadotropin Alfa) NDA #021149. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21-149_ovidrel.cfm (accessed on 7 June 2023).
- Pregnyl (Chorionic Gonadotropin for Injection): Uses, Dosage, Side Effects, Interactions, Warning. Available online: https://www.rxlist.com/pregnyl-drug.htm (accessed on 7 June 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herghelegiu, C.G.; Veduta, A.; Stefan, M.F.; Magda, S.L.; Ionascu, I.; Radoi, V.E.; Oprescu, D.N.; Calin, A.M. Hyperglycosylated-hCG: Its Role in Trophoblast Invasion and Intrauterine Growth Restriction. Cells 2023, 12, 1647. https://doi.org/10.3390/cells12121647
Herghelegiu CG, Veduta A, Stefan MF, Magda SL, Ionascu I, Radoi VE, Oprescu DN, Calin AM. Hyperglycosylated-hCG: Its Role in Trophoblast Invasion and Intrauterine Growth Restriction. Cells. 2023; 12(12):1647. https://doi.org/10.3390/cells12121647
Chicago/Turabian StyleHerghelegiu, Catalin Gabriel, Alina Veduta, Miruna Florina Stefan, Stefania Lucia Magda, Iuliana Ionascu, Viorica Elena Radoi, Daniela Nuti Oprescu, and Alina Mihaela Calin. 2023. "Hyperglycosylated-hCG: Its Role in Trophoblast Invasion and Intrauterine Growth Restriction" Cells 12, no. 12: 1647. https://doi.org/10.3390/cells12121647
APA StyleHerghelegiu, C. G., Veduta, A., Stefan, M. F., Magda, S. L., Ionascu, I., Radoi, V. E., Oprescu, D. N., & Calin, A. M. (2023). Hyperglycosylated-hCG: Its Role in Trophoblast Invasion and Intrauterine Growth Restriction. Cells, 12(12), 1647. https://doi.org/10.3390/cells12121647







