Inverse Regulation of Cartilage Neogenesis at Physiologically Relevant Calcium Conditions by Human Articular Chondrocytes and Mesenchymal Stromal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Expansion of AC and MSC
2.2. Maturation and Stimulation of Engineered Cartilage
2.3. Glycosaminoglycan and DNA Quantification
2.4. Radiolabel Incorporation
2.5. Total RNA Isolation and mRNA Expression Analysis
2.6. Histology and Immunohistochemistry
2.7. Western Blotting
2.8. ALP Enzyme Activity
2.9. PTHrP Protein Quantification
2.10. Statistical Analysis
3. Results
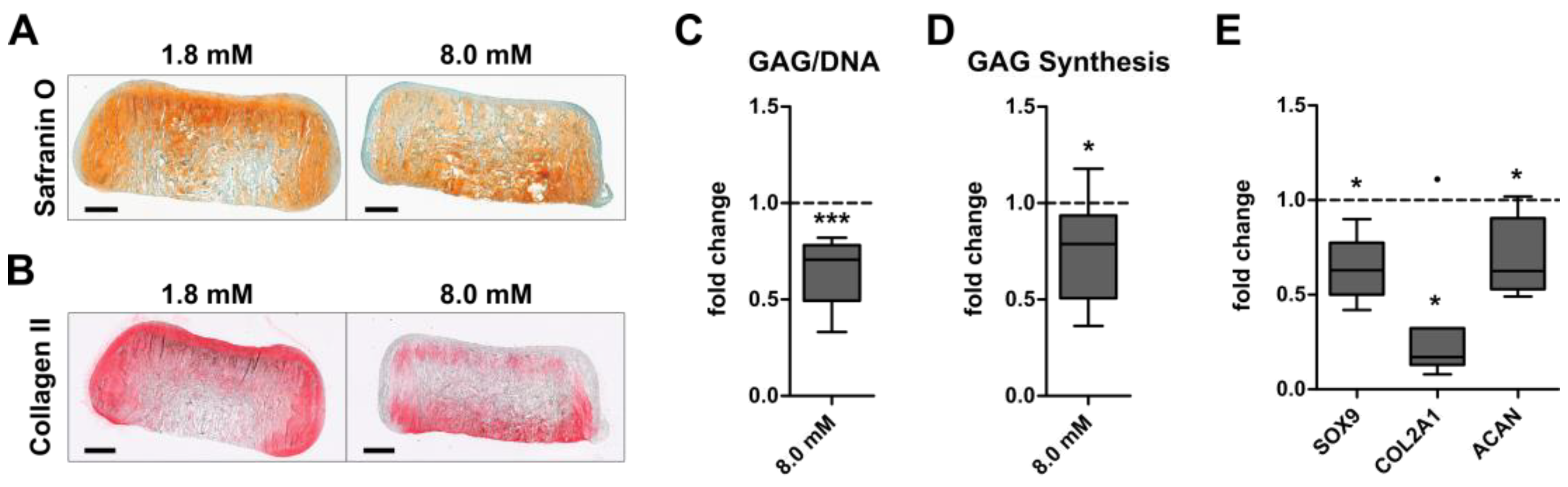
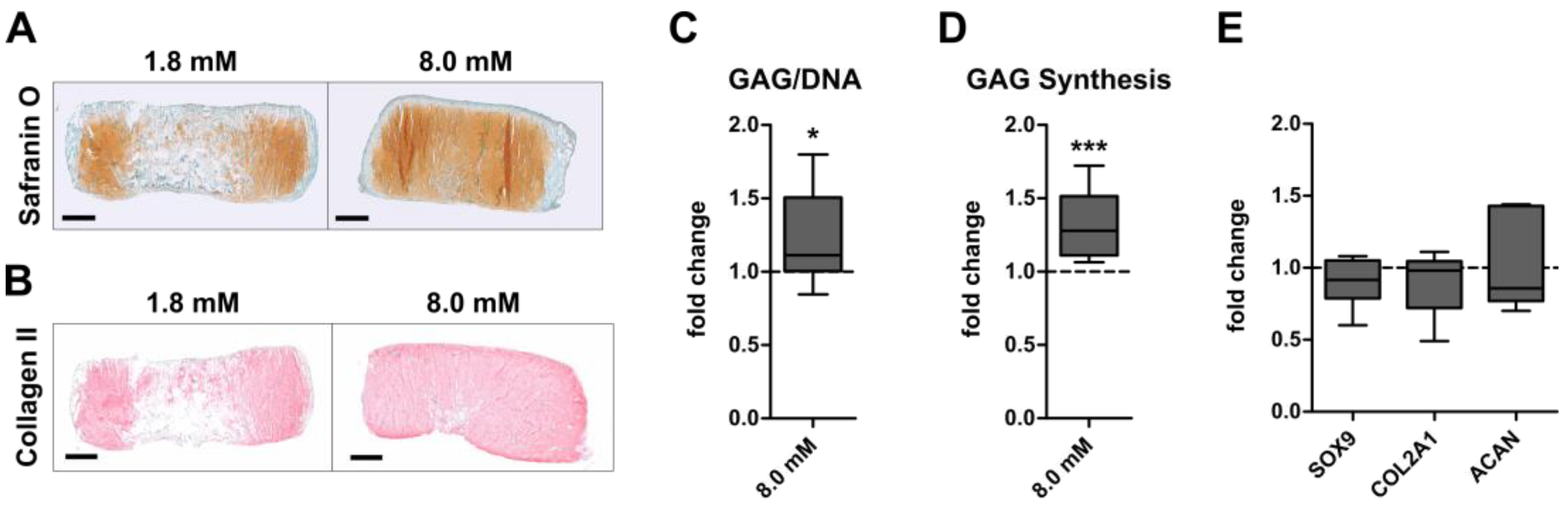
3.1. Inverse Regulation of Cartilage Formation by AC and BMSC-Derived Chondrocytes at Physiologically Relevant Extracellular Calcium Levels
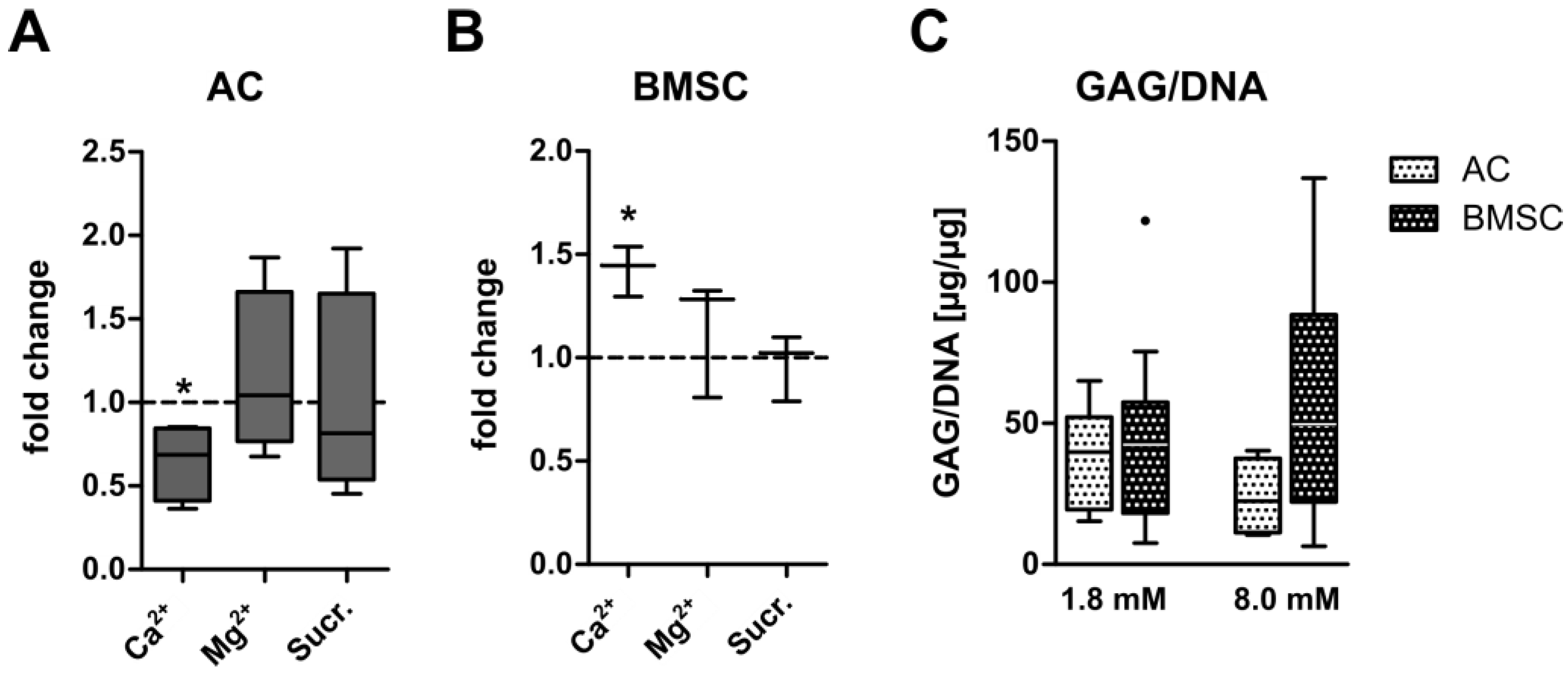
3.2. Ionic Versus Osmotic Effects on GAG Synthesis of AC- and BMSC-Derived Neocartilage
3.3. No Unwanted Hypertrophic Side Effects by Physiologically Relevant Extracellular Calcium Levels
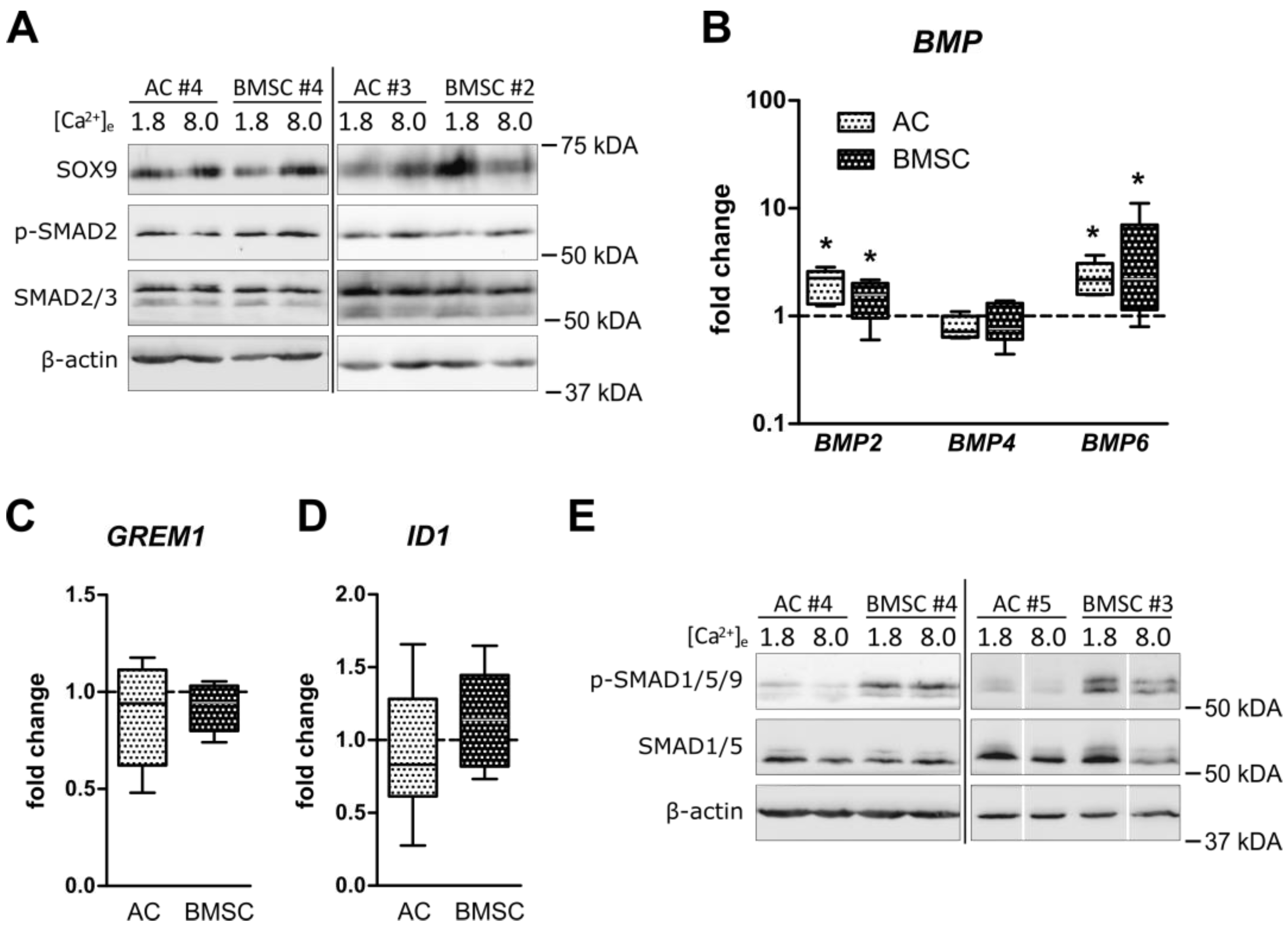
3.4. Unchanged Pro-Chondrogenic Signaling Activity in AC and BMSC-Derived Chondrocytes
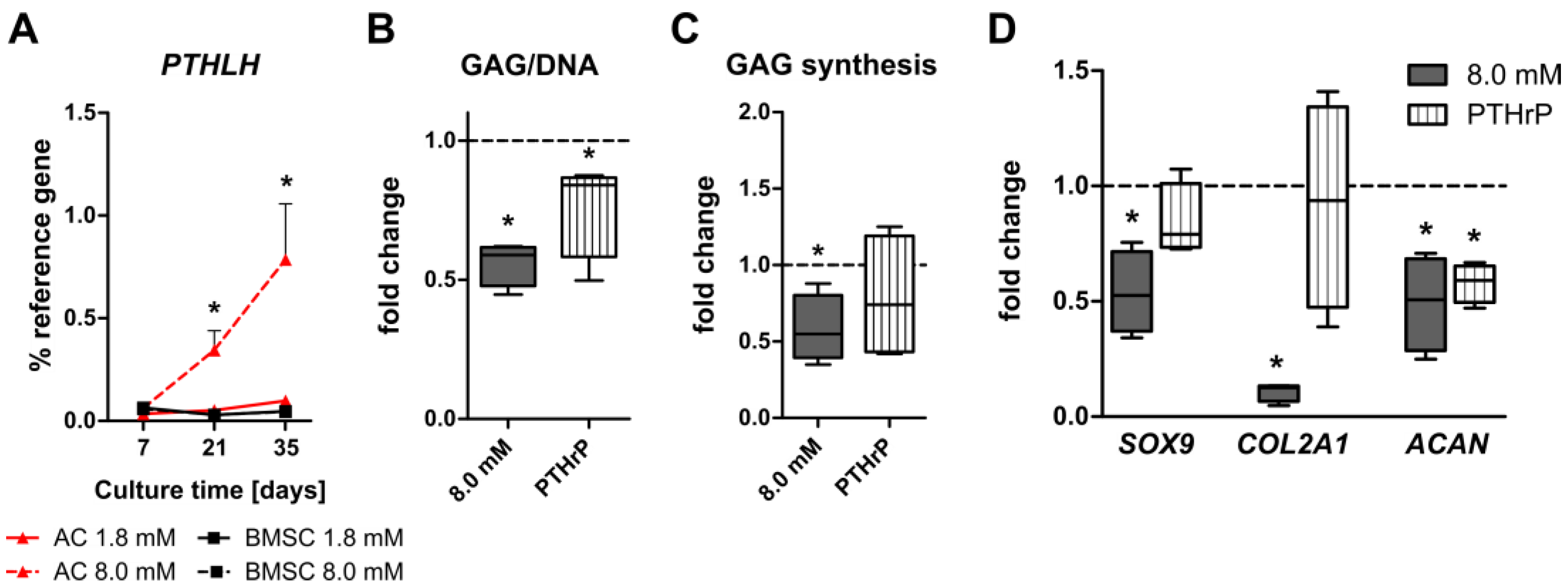
3.5. Induction of PTHrP at Physiologically Relevant Calcium Levels in AC but Not BMSC-Derived Chondrocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Houck, D.A.; Kraeutler, M.J.; Belk, J.W.; Frank, R.M.; McCarty, E.C.; Bravman, J.T. Do Focal Chondral Defects of the Knee Increase the Risk for Progression to Osteoarthritis? A Review of the Literature. Orthop. J. Sports Med. 2018, 6, 2325967118801931. [Google Scholar] [CrossRef] [PubMed]
- Mow, V.C.; Kuei, S.C.; Lai, W.M.; Armstrong, C.G. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J. Biomech. Eng. 1980, 102, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Kreuz, P.C.; Steinwachs, M.R.; Erggelet, C.; Krause, S.J.; Konrad, G.; Uhl, M.; Sudkamp, N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthr. Cartil. 2006, 14, 1119–1125. [Google Scholar] [CrossRef] [Green Version]
- Mollon, B.; Kandel, R.; Chahal, J.; Theodoropoulos, J. The clinical status of cartilage tissue regeneration in humans. Osteoarthr. Cartil. 2013, 21, 1824–1833. [Google Scholar] [CrossRef] [Green Version]
- Krase, A.; Abedian, R.; Steck, E.; Hurschler, C.; Richter, W. BMP activation and Wnt-signalling affect biochemistry and functional biomechanical properties of cartilage tissue engineering constructs. Osteoarthr. Cartil. 2014, 22, 284–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middendorf, J.M.; Griffin, D.J.; Shortkroff, S.; Dugopolski, C.; Kennedy, S.; Siemiatkoski, J.; Cohen, I.; Bonassar, L.J. Mechanical properties and structure-function relationships of human chondrocyte-seeded cartilage constructs after in vitro culture. J. Orthop. Res. 2017, 35, 2298–2306. [Google Scholar] [CrossRef] [Green Version]
- Griffin, D.J.; Ortved, K.F.; Nixon, A.J.; Bonassar, L.J. Mechanical properties and structure-function relationships in articular cartilage repaired using IGF-I gene-enhanced chondrocytes. J. Orthop. Res. 2016, 34, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Sennett, M.L.; Friedman, J.M.; Ashley, B.S.; Stoeckl, B.D.; Patel, J.M.; Alini, M.; Cucchiarini, M.; Eglin, D.; Madry, H.; Mata, A.; et al. Long term outcomes of biomaterial-mediated repair of focal cartilage defects in a large animal model. Eur. Cell Mater. 2021, 41, 40–51. [Google Scholar] [CrossRef]
- Mauck, R.L.; Seyhan, S.L.; Ateshian, G.A.; Hung, C.T. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann. Biomed. Eng. 2002, 30, 1046–1056. [Google Scholar] [CrossRef]
- Steck, E.; Bertram, H.; Walther, A.; Brohm, K.; Mrozik, B.; Rathmann, M.; Merle, C.; Gelinsky, M.; Richter, W. Enhanced biochemical and biomechanical properties of scaffolds generated by flock technology for cartilage tissue engineering. Tissue Eng. Part A 2010, 16, 3697–3707. [Google Scholar] [CrossRef]
- Hunter, C.J.; Mouw, J.K.; Levenston, M.E. Dynamic compression of chondrocyte-seeded fibrin gels: Effects on matrix accumulation and mechanical stiffness. Osteoarthr. Cartil. 2004, 12, 117–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauck, R.L.; Soltz, M.A.; Wang, C.C.; Wong, D.D.; Chao, P.H.; Valhmu, W.B.; Hung, C.T.; Ateshian, G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J. Biomech. Eng. 2000, 122, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennis, J.E.; Whitney, G.A.; Rai, J.; Fernandes, R.J.; Kean, T.J. Physioxia Stimulates Extracellular Matrix Deposition and Increases Mechanical Properties of Human Chondrocyte-Derived Tissue-Engineered Cartilage. Front. Bioeng. Biotechnol. 2020, 8, 590743. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E.; Markway, B.D.; Bond, D.; McCarthy, H.E.; Johnstone, B. Responses to altered oxygen tension are distinct between human stem cells of high and low chondrogenic capacity. Stem. Cell Res. Ther. 2016, 7, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeser, R.F.; Pacione, C.A.; Chubinskaya, S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003, 48, 2188–2196. [Google Scholar] [CrossRef]
- Murphy, M.K.; Huey, D.J.; Hu, J.C.; Athanasiou, K.A. TGF-beta1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem Cells 2015, 33, 762–773. [Google Scholar] [CrossRef] [Green Version]
- Campo, R.D. Effects of cations on cartilage structure: Swelling of growth plate and degradation of proteoglycans induced by chelators of divalent cations. Calcif. Tissue Int. 1988, 43, 108–121. [Google Scholar] [CrossRef]
- Koyano, Y.; Hejna, M.; Flechtenmacher, J.; Schmid, T.M.; Thonar, E.J.; Mollenhauer, J. Collagen and proteoglycan production by bovine fetal and adult chondrocytes under low levels of calcium and zinc ions. Connect. Tissue Res. 1996, 34, 213–225. [Google Scholar] [CrossRef]
- Shulman, H.J.; Opler, A. The stimulatory effect of calcium on the synthesis of cartilage proteoglycan. Biochem. Biophys. Res. Commun. 1974, 59, 914–919. [Google Scholar] [CrossRef]
- Hall, A.C.; Horwitz, E.R.; Wilkins, R.J. The cellular physiology of articular cartilage. Exp. Physiol. 1996, 81, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Nakatani, S.; Mano, H.; Im, R.; Shimizu, J.; Wada, M. Glucosamine regulates differentiation of a chondrogenic cell line, ATDC5. Biol. Pharm. Bull. 2007, 30, 433–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, L.Q.; Jiang, J.; Arnold, D.E.; Guo, X.E.; Lu, H.H.; Mow, V.C. Calcium Concentration Effects on the Mechanical and Biochemical Properties of Chondrocyte-Alginate Constructs. Cell. Mol. Bioeng. 2008, 1, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plumb, M.S.; Aspden, R.M. The response of elderly human articular cartilage to mechanical stimuli in vitro. Osteoarthr. Cartil. 2005, 13, 1084–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.E.; Johnstone, B. Dynamic Mechanical Compression of Chondrocytes for Tissue Engineering: A Critical Review. Front. Bioeng. Biotechnol. 2017, 5, 76. [Google Scholar] [CrossRef] [Green Version]
- Pelttari, K.; Winter, A.; Steck, E.; Goetzke, K.; Hennig, T.; Ochs, B.G.; Aigner, T.; Richter, W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006, 54, 3254–3266. [Google Scholar] [CrossRef]
- Chasan, S.; Hesse, E.; Atallah, P.; Gerstner, M.; Diederichs, S.; Schenker, A.; Grobe, K.; Werner, C.; Richter, W. Sulfation of glycosaminoglycan hydrogels instructs cell fate and chondral versus endochondral lineage decision of skeletal stem cells in vivo. Adv. Funct. Mater. 2022, 32, 2109176. [Google Scholar] [CrossRef]
- Mellor, L.F.; Mohiti-Asli, M.; Williams, J.; Kannan, A.; Dent, M.R.; Guilak, F.; Loboa, E.G. Extracellular calcium modulates chondrogenic and osteogenic differentiation of human adipose-derived stem cells: A novel approach for osteochondral tissue engineering using a single stem cell source. Tissue Eng. Part A 2015, 21, 2323–2333. [Google Scholar] [CrossRef] [Green Version]
- Sarem, M.; Heizmann, M.; Barbero, A.; Martin, I.; Shastri, V.P. Hyperstimulation of CaSR in human MSCs by biomimetic apatite inhibits endochondral ossification via temporal down-regulation of PTH1R. Proc. Natl. Acad. Sci. USA 2018, 115, E6135–E6144. [Google Scholar] [CrossRef] [Green Version]
- Yetley, E.A. Multivitamin and multimineral dietary supplements: Definitions, characterization, bioavailability, and drug interactions. Am. J. Clin. Nutr. 2007, 85, 269S–276S. [Google Scholar] [CrossRef] [Green Version]
- Levy, I.; Attias, S.; Ben-Arye, E.; Goldstein, L.; Schiff, E. Adverse events associated with interactions with dietary and herbal supplements among inpatients. Br. J. Clin. Pharmacol. 2017, 83, 836–845. [Google Scholar] [CrossRef] [Green Version]
- Krebs, J. The Influence of Thyroid Hormone on Ca2+ Signaling Pathways during Embryonal Development. Curr. Top. Med. Chem. 2021, 21, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Martinez, R.; Artigas, N.; Gamez, B.; Rosa, J.L.; Ventura, F. Extracellular calcium promotes bone formation from bone marrow mesenchymal stem cells by amplifying the effects of BMP-2 on SMAD signalling. PLoS ONE 2017, 12, e0178158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brocher, J.; Janicki, P.; Voltz, P.; Seebach, E.; Neumann, E.; Mueller-Ladner, U.; Richter, W. Inferior ectopic bone formation of mesenchymal stromal cells from adipose tissue compared to bone marrow: Rescue by chondrogenic pre-induction. Stem. Cell. Res. 2013, 11, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Scholtes, S.; Krämer, E.; Weisser, M.; Roth, W.; Luginbühl, R.; Grossner, T.; Richter, W. Global chondrocyte gene expression after a single anabolic loading period: Time evolution and re-inducibility of mechano-responses. J. Cell. Physiol. 2018, 233, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Praxenthaler, H.; Kramer, E.; Weisser, M.; Hecht, N.; Fischer, J.; Grossner, T.; Richter, W. Extracellular matrix content and WNT/beta-catenin levels of cartilage determine the chondrocyte response to compressive load. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 851–859. [Google Scholar] [CrossRef]
- Hecht, N.; Johnstone, B.; Angele, P.; Walker, T.; Richter, W. Mechanosensitive MiRs regulated by anabolic and catabolic loading of human cartilage. Osteoarthr. Cartil. 2019, 27, 1208–1218. [Google Scholar] [CrossRef]
- Lückgen, J.; Raque, E.; Reiner, T.; Diederichs, S.; Richter, W. NFkappaB inhibition to lift the mechano-competence of mesenchymal stromal cell-derived neocartilage toward articular chondrocyte levels. Stem. Cell. Res. Ther. 2022, 13, 168. [Google Scholar] [CrossRef]
- Benz, K.; Breit, S.; Lukoschek, M.; Mau, H.; Richter, W. Molecular analysis of expansion, differentiation, and growth factor treatment of human chondrocytes identifies differentiation markers and growth-related genes. Biochem. Biophys. Res. Commun. 2002, 293, 284–292. [Google Scholar] [CrossRef]
- Winter, A.; Breit, S.; Parsch, D.; Benz, K.; Steck, E.; Hauner, H.; Weber, R.M.; Ewerbeck, V.; Richter, W. Cartilage-like gene expression in differentiated human stem cell spheroids: A comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003, 48, 418–429. [Google Scholar] [CrossRef]
- Urban, J.P.G.; Hall, A. Changes in Cartilage Osmotic Pressure in Response to Loads and their Effects on Chondrocyte Metabolism; Springer: Berlin/Heidelberg, Germany, 1992; pp. 513–526. [Google Scholar]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta 1986, 883, 173–177. [Google Scholar] [CrossRef]
- Weiss, S.; Hennig, T.; Bock, R.; Steck, E.; Richter, W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J. Cell. Physiol. 2010, 223, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Aulmann, A.; Dexheimer, V.; Grossner, T.; Richter, W. Intermittent PTHrP(1-34) exposure augments chondrogenesis and reduces hypertrophy of mesenchymal stromal cells. Stem. Cells Dev. 2014, 23, 2513–2523. [Google Scholar] [CrossRef] [Green Version]
- Mueller, M.B.; Fischer, M.; Zellner, J.; Berner, A.; Dienstknecht, T.; Kujat, R.; Prantl, L.; Nerlich, M.; Tuan, R.S.; Angele, P. Effect of parathyroid hormone-related protein in an in vitro hypertrophy model for mesenchymal stem cell chondrogenesis. Int. Orthop. 2013, 37, 945–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adesida, A.B.; Mulet-Sierra, A.; Jomha, N.M. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem. Cell. Res. Ther. 2012, 3, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattappa, G.; Johnstone, B.; Zellner, J.; Docheva, D.; Angele, P. The Importance of Physioxia in Mesenchymal Stem Cell Chondrogenesis and the Mechanisms Controlling Its Response. Int. J. Mol. Sci. 2019, 20, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naski, M.C.; Colvin, J.S.; Coffin, J.D.; Ornitz, D.M. Repression of hedgehog signaling and BMP4 expression in growth plate cartilage by fibroblast growth factor receptor 3. Development 1998, 125, 4977–4988. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Knoch, N.; Sims, T.; Rosshirt, N.; Richter, W. Time-dependent contribution of BMP, FGF, IGF, and HH signaling to the proliferation of mesenchymal stroma cells during chondrogenesis. J. Cell. Physiol. 2018, 233, 8962–8970. [Google Scholar] [CrossRef]
- Bian, L.; Zhai, D.Y.; Zhang, E.C.; Mauck, R.L.; Burdick, J.A. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng. Part A 2012, 18, 715–724. [Google Scholar] [CrossRef]
- Ikeda, Y.; Sakaue, M.; Chijimatsu, R.; Hart, D.A.; Otsubo, H.; Shimomura, K.; Madry, H.; Suzuki, T.; Yoshikawa, H.; Yamashita, T.; et al. IGF-1 Gene Transfer to Human Synovial MSCs Promotes Their Chondrogenic Differentiation Potential without Induction of the Hypertrophic Phenotype. Stem. Cells Int. 2017, 2017, 5804147. [Google Scholar] [CrossRef] [Green Version]
- Weissenberger, M.; Wagenbrenner, M.; Nickel, J.; Ahlbrecht, R.; Blunk, T.; Steinert, A.F.; Gilbert, F. Comparative in vitro treatment of mesenchymal stromal cells with GDF-5 and R57A induces chondrogenic differentiation while limiting chondrogenic hypertrophy. J. Exp. Orthop. 2023, 10, 29. [Google Scholar] [CrossRef]
- Silver, I.A.; Murrills, R.J.; Etherington, D.J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell Res. 1988, 175, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Kunisch, E.; Knauf, A.K.; Hesse, E.; Freudenberg, U.; Werner, C.; Bothe, F.; Diederichs, S.; Richter, W. StarPEG/heparin-hydrogel based in vivo engineering of stable bizonal cartilage with a calcified bottom layer. Biofabrication 2018, 11, 015001. [Google Scholar] [CrossRef] [PubMed]
- Allan, K.S.; Pilliar, R.M.; Wang, J.; Grynpas, M.D.; Kandel, R.A. Formation of biphasic constructs containing cartilage with a calcified zone interface. Tissue Eng. 2007, 13, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Kandel, R.A.; Grynpas, M.; Pilliar, R.; Lee, J.; Wang, J.; Waldman, S.; Zalzal, P.; Hurtig, M. Repair of osteochondral defects with biphasic cartilage-calcium polyphosphate constructs in a sheep model. Biomaterials 2006, 27, 4120–4131. [Google Scholar] [CrossRef]
- St-Pierre, J.P.; Gan, L.; Wang, J.; Pilliar, R.M.; Grynpas, M.D.; Kandel, R.A. The incorporation of a zone of calcified cartilage improves the interfacial shear strength between in vitro-formed cartilage and the underlying substrate. Acta Biomater. 2012, 8, 1603–1615. [Google Scholar] [CrossRef]
- Dexheimer, V.; Gabler, J.; Bomans, K.; Sims, T.; Omlor, G.; Richter, W. Differential expression of TGF-beta superfamily members and role of Smad1/5/9-signalling in chondral versus endochondral chondrocyte differentiation. Sci. Rep. 2016, 6, 36655. [Google Scholar] [CrossRef]
- Diederichs, S.; Klampfleuthner, F.A.M.; Moradi, B.; Richter, W. Chondral differentiation of induced pluripotent stem cells without progression into the endochondral pathway. Front. Cell. Dev. Biol. 2019, 7, 270. [Google Scholar] [CrossRef] [Green Version]
- Diederichs, S.; Tonnier, V.; Marz, M.; Dreher, S.I.; Geisbusch, A.; Richter, W. Regulation of WNT5A and WNT11 during MSC in vitro chondrogenesis: WNT inhibition lowers BMP and hedgehog activity, and reduces hypertrophy. Cell. Mol. Life Sci. 2019, 76, 3875–3889. [Google Scholar] [CrossRef]
- Frerker, N.; Karlsen, T.A.; Stensland, M.; Nyman, T.A.; Rayner, S.; Brinchmann, J.E. Comparison between articular chondrocytes and mesenchymal stromal cells for the production of articular cartilage implants. Front. Bioeng. Biotechnol. 2023, 11, 1116513. [Google Scholar] [CrossRef]
- Mohanraj, B.; Huang, A.H.; Yeger-McKeever, M.J.; Schmidt, M.J.; Dodge, G.R.; Mauck, R.L. Chondrocyte and mesenchymal stem cell derived engineered cartilage exhibits differential sensitivity to pro-inflammatory cytokines. J. Orthop. Res. 2018, 36, 2901–2910. [Google Scholar] [CrossRef] [Green Version]
- Burton, D.W.; Foster, M.; Johnson, K.A.; Hiramoto, M.; Deftos, L.J.; Terkeltaub, R. Chondrocyte calcium-sensing receptor expression is up-regulated in early guinea pig knee osteoarthritis and modulates PTHrP, MMP-13, and TIMP-3 expression. Osteoarthr. Cartil. 2005, 13, 395–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, T.J.; Sims, N.A.; Seeman, E. Physiological and pharmacological roles of PTH and PTHrP in bone using their shared receptor, PTH1R. Endocr. Rev. 2021, 42, 383–406. [Google Scholar] [CrossRef] [PubMed]
- White, A.D.; Fang, F.; Jean-Alphonse, F.G.; Clark, L.J.; An, H.J.; Liu, H.; Zhao, Y.; Reynolds, S.L.; Lee, S.; Xiao, K.; et al. Ca2+ allostery in PTH-receptor signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 3294–3299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Mannstadt, M.; Guo, J.; Kim, S.M.; Yi, H.S.; Khatri, A.; Dean, T.; Okazaki, M.; Gardella, T.J.; Juppner, H. A Homozygous [Cys25]PTH(1-84) Mutation That Impairs PTH/PTHrP Receptor Activation Defines a Novel Form of Hypoparathyroidism. J. Bone Miner. Res. 2015, 30, 1803–1813. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammersen, T.; Buchert, J.; Zietzschmann, S.; Diederichs, S.; Richter, W. Inverse Regulation of Cartilage Neogenesis at Physiologically Relevant Calcium Conditions by Human Articular Chondrocytes and Mesenchymal Stromal Cells. Cells 2023, 12, 1659. https://doi.org/10.3390/cells12121659
Hammersen T, Buchert J, Zietzschmann S, Diederichs S, Richter W. Inverse Regulation of Cartilage Neogenesis at Physiologically Relevant Calcium Conditions by Human Articular Chondrocytes and Mesenchymal Stromal Cells. Cells. 2023; 12(12):1659. https://doi.org/10.3390/cells12121659
Chicago/Turabian StyleHammersen, Tim, Justyna Buchert, Severin Zietzschmann, Solvig Diederichs, and Wiltrud Richter. 2023. "Inverse Regulation of Cartilage Neogenesis at Physiologically Relevant Calcium Conditions by Human Articular Chondrocytes and Mesenchymal Stromal Cells" Cells 12, no. 12: 1659. https://doi.org/10.3390/cells12121659
APA StyleHammersen, T., Buchert, J., Zietzschmann, S., Diederichs, S., & Richter, W. (2023). Inverse Regulation of Cartilage Neogenesis at Physiologically Relevant Calcium Conditions by Human Articular Chondrocytes and Mesenchymal Stromal Cells. Cells, 12(12), 1659. https://doi.org/10.3390/cells12121659





