The PI3K-Akt-mTOR and Associated Signaling Pathways as Molecular Drivers of Immune-Mediated Inflammatory Skin Diseases: Update on Therapeutic Strategy Using Natural and Synthetic Compounds
Abstract
:1. Introduction to the Structure and Function of the Skin
2. Risk Factors Related to the Pathogenesis of Inflammatory Skin Disorders
3. Overview of the Structure and Function of the PI3K-Akt-mTOR Signaling Pathway
3.1. Class I PI3Ks and the Key Intracellular Effectors
3.2. Class II and Class III PI3Ks
3.3. PKB/Akt
3.4. The Mammalian Target of Rapamycin (mTOR)
4. Role of the PI3K-Akt-mTOR and Related Networks in Skin Development and Homeostasis
4.1. Diagnostic and Therapeutic Consequences of the PI3K-Akt-mTOR and Related Cascades as Drivers of Inflammatory Dermatoses
4.1.1. Role of the PI3K-Akt-mTOR and Allied Networks, and Their Therapeutic Targeting in Major Inflammatory Skin Diseases
Atopic Dermatitis
Therapeutic Targeting the PI3K/Akt/mTOR for Treating Atopic Dermatitis (AD)
Psoriasis
Targeting the PI3K/Akt/mTOR for Treating Psoriasis
Systemic Sclerosis
Targeting the PI3K/Akt/mTOR and Allied Networks for Treating Systemic Sclerosis
Lichen Planus (LP) and Oral Lichen Planus (OLP)
Targeting the PI3K/Akt/mTOR and Allied Networks for Treating LP and OLP
Acne Vulgaris
Targeting the PI3K/Akt/mTOR and Allied Cascades for Treating AV
Hidradenitis Suppurativa
Treatments Strategies for HS
Alopecia Areata
Buruli Ulcer
Wound Healing, Hypertrophic Scars, and Keloids
Targeting the PI3K/Akt/mTOR and Allied Cascades for Treating Wound Healing, Hypertrophic Scars, and Keloids
Mycosis Fungoides
Targeting the PI3K/Akt/mTOR and Allied Cascades for Treating MF
Vitiligo
Targeting the PI3K/Akt/mTOR and Allied Cascades for Treating Vitiligo
5. Therapeutic Strategies for Chronic Immune-Mediated Inflammatory Skin Disorders
5.1. Natural Products Targeting the PI3K/Akt/mTOR Pathway in Inflammatory Dermatoses
5.1.1. Flavonoids
Apigenin
Baicalin and Baicalein
Delphinidin
EGCG
Fisetin
Hesperidin
Kaempferol
Luteolin
Naringenin
Quercetin
Other Flavonoids
5.1.2. Resveratrol and Analogues, Geniposide
5.1.3. Red Ginseng Extract
5.2. Small Molecule Drugs
5.2.1. Corticosteroids
5.2.2. Immunomodulators
5.2.3. Keratolytics
5.2.4. Antimicrobial Drugs
5.3. Biologics
5.3.1. Tumor Necrosis Factor (TNF) Inhibitors
5.3.2. Interleukin (IL) Inhibitors
5.3.3. B-Cells Inhibitors
5.3.4. T-Cells Inhibitors
5.4. Antioxidant Natruceuticals
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chamcheu, J.C.; Adhami, V.M.; Siddiqui, I.A.; Mukhtar, H. Cutaneous Cell- and Gene-Based Therapies for Inherited and Acquired Skin Disorders. In Gene and Cell Therapy: Therapeutic Mechanisms and Strategies; Routledge: Abingdon, UK, 2015; pp. 1091–1122. [Google Scholar]
- Chamcheu, J.C.; Siddiqui, I.A.; Syed, D.N.; Adhami, V.M.; Liovic, M.; Mukhtar, H. Keratin gene mutations in disorders of human skin and its appendages. Arch. Biochem. Biophys. 2011, 508, 123–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckhart, L.; Lippens, S.; Tschachler, E.; Declercq, W. Cell death by cornification. Biochim. Biophys. Acta 2013, 1833, 3471–3480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Yang, X.; Wei, J.; Zhou, S.; Zhao, Z.; Cheng, J.; Duan, H.; Jia, T.; Lei, Q.; et al. Characterization of Th17 and FoxP3+ Treg Cells in Paediatric Psoriasis Patients. Scand. J. Immunol. 2016, 83, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Chamcheu, J.C.; Roy, T.; Uddin, M.B.; Banang-Mbeumi, S.; Chamcheu, R.-C.N.; Walker, A.L.; Liu, Y.-Y.; Huang, S. Role and Therapeutic Targeting of the PI3K/Akt/mTOR Signaling Pathway in Skin Cancer: A Review of Current Status and Future Trends on Natural and Synthetic Agents Therapy. Cells 2019, 8, 803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, W.L.; Qi, J.T.Z.; Yeong, W.Y.; Naing, M.W. Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication 2018, 10, 025005. [Google Scholar] [CrossRef]
- Tharmarajah, G.; Eckhard, U.; Jain, F.; Marino, G.; Prudova, A.; Urtatiz, O.; Fuchs, H.; de Angelis, M.H.; Overall, C.M.; Van Raamsdonk, C.D. Melanocyte development in the mouse tail epidermis requires the Adamts9 metalloproteinase. Pigment. Cell Melanoma Res. 2018, 31, 693–707. [Google Scholar] [CrossRef] [Green Version]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef] [Green Version]
- Bertolesi, G.E.; McFarlane, S. Seeing the light to change colour: An evolutionary perspective on the role of melanopsin in neuroendocrine circuits regulating light-mediated skin pigmentation. Pigment. Cell Melanoma Res. 2018, 31, 354–373. [Google Scholar] [CrossRef] [Green Version]
- De Assis, L.V.M.; Moraes, M.N.; Magalhães-Marques, K.K.; de Lauro Castrucci, A.M. Melanopsin and rhodopsin mediate UVA-induced immediate pigment darkening: Unravelling the photosensitive system of the skin. Eur. J. Cell. Biol. 2018, 97, 150–162. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Hearing, V.J. Physiological factors that regulate skin pigmentation. Biofactors 2009, 35, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, Y.; Morita, A.; Maeda, A.; Hearing, V.J. Regulation of skin pigmentation and thickness by Dickkopf 1 (DKK1). J. Investig. Dermatol. Symp. Proc. 2009, 14, 73–75. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for Skin Care: A Comprehensive Review of Human Clinical Studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef] [Green Version]
- Sagi, Z.; Hieronymus, T. The Impact of the Epithelial-Mesenchymal Transition Regulator Hepatocyte Growth Factor Receptor/Met on Skin Immunity by Modulating Langerhans Cell Migration. Front. Immunol. 2018, 9, 517. [Google Scholar] [CrossRef] [Green Version]
- Deckers, J.; Hammad, H.; Hoste, E. Langerhans Cells: Sensing the Environment in Health and Disease. Front. Immunol. 2018, 9, 93. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, K.; Nümm, T.J.; Koch, S.; Herrmann, N.; Leib, N.; Bieber, T. Langerhans and inflammatory dendritic epidermal cells in atopic dermatitis are tolerized toward TLR2 activation. Allergy 2018, 73, 2205–2213. [Google Scholar] [CrossRef]
- Petersson, M.; Niemann, C. Stem cell dynamics and heterogeneity: Implications for epidermal regeneration and skin cancer. Curr. Med. Chem. 2012, 19, 5984–5992. [Google Scholar] [CrossRef]
- Wang, L.-J.; Wang, Y.-L.; Yang, X. Progress in epidermal stem cells. Yi Chuan 2010, 32, 198–204. [Google Scholar] [CrossRef]
- Flores, E.R.; Halder, G. Stem cell proliferation in the skin: Alpha-catenin takes over the hippo pathway. Sci. Signal. 2011, 4, pe34. [Google Scholar] [CrossRef]
- Uzarska, M.; Porowińska, D.; Bajek, A.; Drewa, T. Epidermal stem cells—Biology and potential applications in regenerative medicine. Postep. Biochem. 2013, 59, 219–227. [Google Scholar]
- Segre, J.A. Epidermal barrier formation and recovery in skin disorders. J. Clin. Investig. 2006, 116, 1150–1158. [Google Scholar] [CrossRef]
- Watt, F.M. Mammalian skin cell biology: At the interface between laboratory and clinic. Science 2014, 346, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, K.A.U.; Fuchs, E. Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev. Cell 2017, 43, 387–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magin, T.M.; Vijayaraj, P.; Leube, R.E. Structural and regulatory functions of keratins. Exp. Cell Res. 2007, 313, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katiyar, S.; Elmets, C.A.; Katiyar, S.K. Green tea and skin cancer: Photoimmunology, angiogenesis and DNA repair. J. Nutr. Biochem. 2007, 18, 287–296. [Google Scholar] [CrossRef]
- Watson, M.; Holman, D.M.; Maguire-Eisen, M. Ultraviolet Radiation Exposure and Its Impact on Skin Cancer Risk. Semin. Oncol. Nurs. 2016, 32, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Gilchrest, B.A.; Eller, M.S.; Geller, A.C.; Yaar, M. The pathogenesis of melanoma induced by ultraviolet radiation. N. Engl. J. Med. 1999, 340, 1341–1348. [Google Scholar] [CrossRef]
- Miyamura, Y.; Coelho, S.G.; Schlenz, K.; Batzer, J.; Smuda, C.; Choi, W.; Brenner, M.; Passeron, T.; Zhang, G.; Kolbe, L.; et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment. Cell Melanoma Res. 2011, 24, 136–147. [Google Scholar] [CrossRef] [Green Version]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [Green Version]
- Bais, A.F.; McKenzie, R.L.; Bernhard, G.; Aucamp, P.J.; Ilyas, M.; Madronich, S.; Tourpali, K. Ozone depletion and climate change: Impacts on UV radiation. Photochem. Photobiol. Sci. 2015, 14, 19–52. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef]
- Diffey, B.L. Solar ultraviolet radiation effects on biological systems. Phys. Med. Biol. 1991, 36, 299–328. [Google Scholar] [CrossRef]
- Abeyama, K.; Eng, W.; Jester, J.V.; Vink, A.A.; Edelbaum, D.; Cockerell, C.J.; Bergstresser, P.R.; Takashima, A. A role for NF-kappaB-dependent gene transactivation in sunburn. J. Clin. Investig. 2000, 105, 1751–1759. [Google Scholar] [CrossRef]
- Murdaca, G.; Greco, M.; Negrini, S.; Casciaro, M.; Gangemi, S. The Role of Skin and Gut Microbiome and Epigenetic Modifications in Skin-Autoimmune Disorders. Curr. Mol. Med. 2021, 21, 283–290. [Google Scholar] [CrossRef]
- Holick, M.F. Sunlight, UV-radiation, vitamin D and skin cancer: How much sunlight do we need? Adv. Exp. Med. Biol. 2008, 624, 1–15. [Google Scholar]
- Juzeniene, A.; Moan, J. Beneficial effects of UV radiation other than via vitamin D production. Dermatoendocrinology 2012, 4, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Avci, P.; Dai, T.; Huang, Y.-Y.; Hamblin, M.R.; Yan, J.-X.; Liao, X.; Li, S.-H.; Liu, H.-W.; Chang, H.-Y.; et al. Ultraviolet Radiation in Wound Care: Sterilization and Stimulation. Adv. Wound Care 2013, 2, 422–437. [Google Scholar] [CrossRef] [Green Version]
- Elias, P.M.; Eichenfield, L.F.; Fowler, J.F., Jr.; Horowitz, P.; McLeod, R.P. Update on the structure and function of the skin barrier: Atopic dermatitis as an exemplar of clinical implications. Semin. Cutan. Med. Surg. 2013, 32 (Suppl. S2), S21–S24. [Google Scholar] [CrossRef]
- Alshobaili, H.A.; Shahzad, M.; Al-Marshood, A.; Khalil, A.; Settin, A.; Barrimah, I. Genetic background of psoriasis. Int. J. Health Sci. 2010, 4, 23–29. [Google Scholar]
- Rahman, P.; Gladman, D.D.; Schentag, C.T.; Petronis, A. Excessive paternal transmission in psoriatic arthritis. Arthritis Rheum. 1999, 42, 1228–1231. [Google Scholar] [CrossRef]
- Trembath, R.C.; Clough, R.L.; Rosbotham, J.L.; Jones, A.B.; Camp, R.D.R.; Frodsham, A.; Browne, J.; Barber, R.; Terwilliger, J.; Lathrop, G.M.; et al. Identification of a major susceptibility locus on chromosome 6p and evidence for further disease loci revealed by a two stage genome-wide search in psoriasis. Hum. Mol. Genet. 1997, 6, 813–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidinger, S.; Rodríguez, E.; Stahl, C.; Wagenpfeil, S.; Klopp, N.; Illig, T.; Novak, N. Filaggrin mutations strongly predispose to early-onset and extrinsic atopic dermatitis. J. Investig. Dermatol. 2007, 127, 724–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roekevisch, E.; Leeflang, M.; Schram, M.; Campbell, L.; McLean, W.I.; Kezic, S.; Bos, J.; Spuls, P.; Middelkamp-Hup, M. Patients with atopic dermatitis with filaggrin loss-of-function mutations show good but lower responses to immunosuppressive treatment. Br. J. Dermatol. 2017, 177, 1745–1746. [Google Scholar] [CrossRef] [Green Version]
- Harkins, C.P.; Leeflang, M.; Schram, M.; Campbell, L.; McLean, W.I.; Kezic, S.; Bos, J.; Spuls, P.; Middelkamp-Hup, M. Loss-of-Function Mutations in the Gene Encoding Filaggrin Are Not Strongly Associated with Chronic Actinic Dermatitis. J. Investig. Dermatol. 2015, 135, 1919–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, M. FLG mutations in ichthyosis vulgaris and atopic eczema: Spectrum of mutations and population genetics. Br. J. Dermatol. 2010, 162, 472–477. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Peng, H.; Qu, L.; Sommar, P.; Wang, A.; Chu, T.; Li, X.; Bi, X.; Liu, Q.; Sérézal, I.G.; et al. miR-19a/b and miR-20a Promote Wound Healing by Regulating the Inflammatory Response of Keratinocytes. J. Investig. Dermatol. 2021, 141, 659–671. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Liu, J.; Cai, T.; Guo, L.; Wang, S.; Wang, J.; Cao, Y.; Ge, J.; Jiang, Y. Hair Follicle and Sebaceous Gland De Novo Regeneration With Cultured Epidermal Stem Cells and Skin-Derived Precursors. Stem Cells Transl. Med. 2016, 5, 1695–1706. [Google Scholar] [CrossRef]
- Fuchs, E. Scratching the surface of skin development. Nature 2007, 445, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Barrett, D.; Brown, V.I.; Grupp, S.A.; Teachey, D.T. Targeting the PI3K/AKT/mTOR signaling axis in children with hematologic malignancies. Paediatr. Drugs 2012, 14, 299–316. [Google Scholar]
- Kawase, Y.; Yanagi, Y.; Takato, T.; Fujimoto, M.; Okochi, H. Characterization of multipotent adult stem cells from the skin: Transforming growth factor-beta (TGF-beta) facilitates cell growth. Exp. Cell Res. 2004, 295, 194–203. [Google Scholar] [CrossRef]
- Fattahi, S.; Langroudi, M.P.; Akhavan-Niaki, H. Hedgehog signaling pathway: Epigenetic regulation and role in disease and cancer development. J. Cell. Physiol. 2018, 233, 5726–5735. [Google Scholar] [CrossRef]
- Schmidt-Ullrich, R.; Paus, R. Molecular principles of hair follicle induction and morphogenesis. Bioessays 2005, 27, 247–261. [Google Scholar] [CrossRef]
- Liang, J.; Slingerland, J.M. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2003, 2, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Dai, Q.; Su, X.; Fu, J.; Feng, X.; Peng, J. Role of PI3K/AKT pathway in cancer: The framework of malignant behavior. Mol. Biol. Rep. 2020, 47, 4587–4629. [Google Scholar] [CrossRef] [Green Version]
- Cai, B.; Zheng, Y.; Ma, S.; Xing, Q.; Wang, X.; Yang, B.; Yin, G.; Guan, F. Long non-coding RNA regulates hair follicle stem cell proliferation and differentiation through PI3K/AKT signal pathway. Mol. Med. Rep. 2018, 17, 5477–5483. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Zhou, F. Inflammasomes in Common Immune-Related Skin Diseases. Front. Immunol. 2020, 11, 882. [Google Scholar] [CrossRef]
- Mercurio, L.; Albanesi, C.; Madonna, S. Recent Updates on the Involvement of PI3K/AKT/mTOR Molecular Cascade in the Pathogenesis of Hyperproliferative Skin Disorders. Front. Med. 2021, 8, 665647. [Google Scholar] [CrossRef]
- Mohseni, A.H.; Casolaro, V.; Bermúdez-Humarán, L.G.; Keyvani, H.; Taghinezhad, S. Modulation of the PI3K/Akt/mTOR signaling pathway by probiotics as a fruitful target for orchestrating the immune response. Gut Microbes 2021, 13, 1–17. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X. The role of PI3K/AKT/FOXO signaling in psoriasis. Arch. Dermatol. Res. 2019, 311, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Patni, P.; Bishayee, A.; Sah, A.N.; Bishayee, A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin Cancer Biol. 2019, 80, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fry, M.J. Phosphoinositide 3-kinase signalling in breast cancer: How big a role might it play? Breast Cancer Res. 2001, 3, 304–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug. Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, D.; Yeomanson, D.; Bryant, H.E. PI3King the lock: Targeting the PI3K/Akt/mTOR pathway as a novel therapeutic strategy in neuroblastoma. J. Pediatr. Hematol. Oncol. 2015, 37, 245–251. [Google Scholar] [CrossRef]
- Foster, F.M.; Traer, C.J.; Abraham, S.M.; Fry, M. The phosphoinositide (PI) 3-kinase family. J. Cell Sci. 2003, 116 Pt 15, 3037–3040. [Google Scholar] [CrossRef] [Green Version]
- Rameh, L.E.; Cantley, L.C. The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem. 1999, 274, 8347–8350. [Google Scholar] [CrossRef] [Green Version]
- Katso, R.; Okkenhaug, K.; Ahmadi, K.; White, S.; Timms, J.; Waterfield, M.D. Cellular function of phosphoinositide 3-kinases: Implications for development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2001, 17, 615–675. [Google Scholar] [CrossRef]
- Fruman, D.A.; Meyers, R.E.; Cantley, L.C. Phosphoinositide kinases. Annu. Rev. Biochem. 1998, 67, 481–507. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef]
- Kriplani, N.; Hermida, M.A.; Brown, E.R.; Leslie, N.R. Class I PI 3-kinases: Function and evolution. Adv. Biol. Regul. 2015, 59, 53–64. [Google Scholar] [CrossRef]
- Hassan, B.; Akcakanat, A.; Holder, A.M.; Meric-Bernstam, F. Targeting the PI3-kinase/Akt/mTOR signaling pathway. Surg. Oncol. Clin. N. Am. 2013, 22, 641–664. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.-H.; Yao, H.; Huang, L.J.-S. Cytokine Receptor Endocytosis: New Kinase Activity-Dependent and -Independent Roles of PI3K. Front. Endocrinol. 2017, 8, 78. [Google Scholar] [CrossRef] [Green Version]
- Burris, H.A., III. Overcoming acquired resistance to anticancer therapy: Focus on the PI3K/AKT/mTOR pathway. Cancer Chemother. Pharm. 2013, 71, 829–842. [Google Scholar] [CrossRef]
- Graupera, M.; Guillermet-Guibert, J.; Foukas, L.C.; Phng, L.-K.; Cain, R.J.; Salpekar, A.; Pearce, W.; Meek, S.; Millán, J.; Cutillas, P.R.; et al. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature 2008, 453, 662–666. [Google Scholar] [CrossRef]
- Bénistant, C.; Chapuis, H.; Roche, S. A specific function for phosphatidylinositol 3-kinase alpha (p85alpha-p110alpha) in cell survival and for phosphatidylinositol 3-kinase beta (p85alpha-p110beta) in de novo DNA synthesis of human colon carcinoma cells. Oncogene 2000, 19, 5083–5090. [Google Scholar] [CrossRef] [Green Version]
- Utermark, T.; Rao, T.; Cheng, H.; Wang, Q.; Lee, S.H.; Wang, Z.C.; Iglehart, J.D.; Roberts, T.M.; Muller, W.J.; Zhao, J.J. The p110α and p110β isoforms of PI3K play divergent roles in mammary gland development and tumorigenesis. Genes Dev. 2012, 26, 1573–1586. [Google Scholar] [CrossRef] [Green Version]
- Okkenhaug, K.; Bilancio, A.; Farjot, G.; Priddle, H.; Sancho, S.; Peskett, E.; Pearce, W.; Meek, S.E.; Salpekar, A.; Waterfield, M.D. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science 2002, 297, 1031–1034. [Google Scholar] [CrossRef]
- Ueyama, A.; Yamamoto, M.; Tsujii, K.; Furue, Y.; Imura , C.; Shichijo, M.; Yasui, K. Mechanism of pathogenesis of imiquimod-induced skin inflammation in the mouse: A role for interferon-alpha in dendritic cell activation by imiquimod. J. Dermatol. 2014, 41, 135–143. [Google Scholar] [CrossRef]
- Roller, A.; Perino, A.; Dapavo, P.; Soro, E.; Okkenhaug, K.; Hirsch, E.; Ji, H. Blockade of phosphatidylinositol 3-kinase PI3Kδ or PI3Kγ reduces IL-17 and ameliorates imiquimod-induced psoriasis-like dermatitis. J. Immunol. 2012, 189, 4612–4620. [Google Scholar] [CrossRef] [Green Version]
- Conte, E.; Fruciano, M.; Fagone, E.; Gili, E.; Caraci, F.; Iemmolo, M.; Crimi, N.; Vancheri, C. Inhibition of PI3K prevents the proliferation and differentiation of human lung fibroblasts into myofibroblasts: The role of class I P110 isoforms. PLoS ONE 2011, 6, e24663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehead, M.A.; Bombardieri, M.; Pitzalis, C.; Vanhaesebroeck, B. Isoform-selective induction of human p110δ PI3K expression by TNFα: Identification of a new and inducible PIK3CD promoter. Biochem. J. 2012, 443, 857–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buerger, C. Epidermal mTORC1 Signaling Contributes to the Pathogenesis of Psoriasis and Could Serve as a Therapeutic Target. Front. Immunol. 2018, 9, 2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stark, A.K.; Davenport, E.C.M.; Patton, D.T.; Scudamore, C.L.; Vanhaesebroeck, B.; Veldhoen, M.; Garden, O.A.; Okkenhaug, K. Loss of Phosphatidylinositol 3-Kinase Activity in Regulatory T Cells Leads to Neuronal Inflammation. J. Immunol. 2020, 205, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.K.; Sriskantharajah, S.; Hessel, E.M.; Okkenhaug, K. PI3K inhibitors in inflammation, autoimmunity and cancer. Curr. Opin. Pharm. 2015, 23, 82–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Bloch, W.; Iden, S.; Rüegg, M.A.; Hall, M.N.; Leptin, M.; Partridge, L.; Eming, S.A. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat. Commun. 2016, 7, 13226. [Google Scholar] [CrossRef] [Green Version]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Houghton, P.J. Targeting mTOR signaling for cancer therapy. Curr. Opin. Pharm. 2003, 3, 371–377. [Google Scholar] [CrossRef]
- Guertin, D.A.; Sabatini, D.M. Defining the role of mTOR in cancer. Cancer Cell 2007, 12, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef] [Green Version]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [Green Version]
- Vanhaesebroeck, B.; Stephens, L.; Hawkins, P. PI3K signalling: The path to discovery and understanding. Nat. Rev. Mol. Cell Biol. 2012, 13, 195–203. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Mossmann, D.; Park, S.; Hall, M.N. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat. Rev. Cancer 2018, 18, 744–757. [Google Scholar] [CrossRef]
- Leo, M.S.; Sivamani, R.K. Phytochemical modulation of the Akt/mTOR pathway and its potential use in cutaneous disease. Arch. Dermatol. Res. 2014, 306, 861–871. [Google Scholar] [CrossRef]
- Pópulo, H.; Lopes, J.M.; Soares, P. The mTOR signalling pathway in human cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef] [Green Version]
- Vahidnezhad, H.; Youssefian, L.; Uitto, J. Molecular Genetics of the PI3K-AKT-mTOR Pathway in Genodermatoses: Diagnostic Implications and Treatment Opportunities. J. Investig. Dermatol. 2016, 136, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Lin, X.; Meng, X.; Lin, M. Phosphoinositide-3 kinase/protein kinase-B/mammalian target of rapamycin pathway in psoriasis pathogenesis. A potential therapeutic target? Acta Derm. Venereol. 2014, 94, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Buerger, C.; Shirsath, N.; Lang, V.; Berard, A.; Diehl, S.; Kaufmann, R.; Boehncke, W.-H.; Wolf, P. Inflammation dependent mTORC1 signaling interferes with the switch from keratinocyte proliferation to differentiation. PLoS ONE 2017, 12, e0180853. [Google Scholar] [CrossRef] [Green Version]
- Chamcheu, J.C.; Chaves-Rodriquez, M.-I.; Adhami, V.M.; Siddiqui, I.A.; Wood, G.S.; Longley, B.J.; Mukhtar, H. Upregulation of PI3K/AKT/mTOR, FABP5 and PPARβ/δ in Human Psoriasis and Imiquimod-induced Murine Psoriasiform Dermatitis Model. Acta Derm. Venereol. 2016, 96, 854–856. [Google Scholar] [CrossRef] [Green Version]
- Osada-Oka, M.; Hirai, S.; Izumi, Y.; Misumi, K.; Samukawa, K.; Tomita, S.; Miura, K.; Minamiyama, Y.; Iwao, H. Red ginseng extracts attenuate skin inflammation in atopic dermatitis through p70 ribosomal protein S6 kinase activation. J. Pharm. Sci. 2018, 136, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Novak, N. Atopic dermatitis and filaggrin. Curr. Opin. Immunol. 2016, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef]
- Aoki, V.; Lorenzini, D.; Orfali, R.L.; Zaniboni, M.C.; De Oliveira, Z.N.P.; Rivitti-Machado, M.C.; Takaoka, R.; Weber, M.B.; Cestari, T.; Gontijo, B.; et al. Consensus on the therapeutic management of atopic dermatitis—Brazilian Society of Dermatology. An. Bras. Dermatol. 2019, 94 (Suppl. S1), 67–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orfali, R.L.; Oliveira, L.M.D.S.; de Lima, J.F.; de Carvalho, G.C.; Ramos, Y.A.L.; Pereira, N.Z.; Pereira, N.V.; Zaniboni, M.C.; Sotto, M.N.; Duarte, A.J.D.S.; et al. Staphylococcus aureus enterotoxins modulate IL-22-secreting cells in adults with atopic dermatitis. Sci. Rep. 2018, 8, 6665. [Google Scholar] [CrossRef] [Green Version]
- Leung, D.Y.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef]
- Bürger, C.; Shirsath, N.; Lang, V.; Diehl, S.; Kaufmann, R.; Weigert, A.; Han, Y.; Ringel, C.; Wolf, P. Blocking mTOR Signalling with Rapamycin Ameliorates Imiquimod-induced Psoriasis in Mice. Acta Derm. Venereol. 2017, 97, 1087–1094. [Google Scholar] [CrossRef] [Green Version]
- Ok, S.; Oh, S.-R.; Jung, T.-S.; Jeon, S.-O.; Jung, J.-W.; Ryu, D.-S. Effects of Angelica gigas Nakai as an Anti-Inflammatory Agent in In Vitro and In Vivo Atopic Dermatitis Models. Evid.-Based Complement. Altern. Med. 2018, 2018, 2450712. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Xue, H.-B.; Guan, X.-H.; Shu, C.-M.; Wang, F.; Zhang, J.-H.; An, R.-Z. The Imbalance of Th17 cells and CD4+ CD25highFoxp3+ Treg cells in patients with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1079–1086. [Google Scholar] [CrossRef]
- Fyhrquist, N.; Lehtimäki, S.; Lahl, K.; Savinko, T.; Lappeteläinen, A.-M.; Sparwasser, T.; Wolff, H.; Lauerma, A.; Alenius, H.; Lehtim, S.; et al. Foxp3+ cells control Th2 responses in a murine model of atopic dermatitis. J. Investig. Dermatol. 2012, 132, 1672–1680. [Google Scholar] [CrossRef] [Green Version]
- Siraganian, R.P. Mast cell signal transduction from the high-affinity IgE receptor. Curr. Opin. Immunol. 2003, 15, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Kinet, J.P. The high-affinity IgE receptor (Fc epsilon RI): From physiology to pathology. Annu. Rev. Immunol. 1999, 17, 931–972. [Google Scholar] [CrossRef]
- Buerger, C.; Malisiewicz, B.; Eiser, A.; Hardt, K.; Boehncke, W. Mammalian target of rapamycin and its downstream signalling components are activated in psoriatic skin. Br. J. Dermatol. 2013, 169, 156–159. [Google Scholar] [CrossRef]
- Bae, M.J.; Lim, S.; Lee, D.S.; Ko, K.R.; Lee, W.; Kim, S. Water soluble extracts from Actinidia arguta, PG102, attenuates house dust mite-induced murine atopic dermatitis by inhibiting the mTOR pathway with Treg generation. J. Ethnopharmacol. 2016, 193, 96–106. [Google Scholar] [CrossRef]
- Harir, N.; Boudot, C.; Friedbichler, K.; Sonneck, K.; Kondo, R.; Martin-Lannerée, S.; Kenner, L.; Kerenyi, M.; Yahiaoui, S.; Gouilleux-Gruart, V. Oncogenic Kit controls neoplastic mast cell growth through a Stat5/PI3-kinase signaling cascade. Blood 2008, 112, 2463–2473. [Google Scholar] [CrossRef] [Green Version]
- Sully, K.; Akinduro, O.; Philpott, M.P.; Naeem, A.S.; Harwood, C.A.; Reeve, V.E.; O’Shaughnessy, R.F.; Byrne, C. The mTOR inhibitor rapamycin opposes carcinogenic changes to epidermal Akt1/PKBα isoform signaling. Oncogene 2013, 32, 3254–3262. [Google Scholar] [CrossRef]
- Naeem, A.S.; Tommasi, C.; Cole, C.; Brown, S.J.; Zhu, Y.; Way, B.; Owen, S.A.G.W.; Moffatt, M.; Cookson, W.O.; Harper, J.I.; et al. A mechanistic target of rapamycin complex 1/2 (mTORC1)/V-Akt murine thymoma viral oncogene homolog 1 (AKT1)/cathepsin H axis controls filaggrin expression and processing in skin, a novel mechanism for skin barrier disruption in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2017, 139, 1228–1241. [Google Scholar]
- Otsuka, A.; Doi, H.; Egawa, G.; Maekawa, A.; Fujita, T.; Nakamizo, S.; Nakashima, C.; Nakajima, S.; Watanabe, T.; Miyachi, Y.; et al. Possible new therapeutic strategy to regulate atopic dermatitis through upregulating filaggrin expression. J. Allergy Clin. Immunol. 2014, 133, 139–146.e10. [Google Scholar] [CrossRef]
- Yang, F.; Tanaka, M.; Wataya-Kaneda, M.; Yang, L.; Nakamura, A.; Matsumoto, S.; Attia, M.; Murota, H.; Katayama, I. Topical application of rapamycin ointment ameliorates Dermatophagoides farina body extract-induced atopic dermatitis in NC/Nga mice. Exp. Dermatol. 2014, 23, 568–572. [Google Scholar] [CrossRef]
- Yun, M.Y.; Yang, J.-H.; Kim, D.-K.; Cheong, K.-J.; Song, H.-H.; Kim, D.-H.; Cheong, K.-J.; Kim, Y.-I.; Shin, S.-C. Therapeutic effects of Baicalein on atopic dermatitis-like skin lesions of NC/Nga mice induced by dermatophagoides pteronyssinus. Int. Immunopharmacol. 2010, 10, 1142–1148. [Google Scholar] [CrossRef]
- Obata, K.; Mukai, K.; Tsujimura, Y.; Ishiwata, K.; Kawano, Y.; Minegishi, Y.; Watanabe, N.; Karasuyama, H. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood 2007, 110, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Lv, M.; Deng, J.; Tang, N.; Zeng, Y.; Lu, C. Efficacy and Safety of Tripterygium Wilfordii Hook F on Psoriasis Vulgaris: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid.-Based Complement. Altern. Med. 2018, 2018, 2623085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Duan, X.; Chen, S.; Chen, X.; Yu, R.; Yu, X. Association Between Psoriasis and Erectile Dysfunction: A Meta-Analysis. J. Sex. Med. 2018, 15, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yi, X.; An, Y.; Guo, S.; Li, S.; Song, P.; Chang, Y.; Zhang, S.; Gao, T.; Wang, G.; et al. MicroRNA-17-92 cluster promotes the proliferation and the chemokine production of keratinocytes: Implication for the pathogenesis of psoriasis. Cell Death Dis. 2018, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Liu, X.; Mei, L.; Wang, H.; Fang, F. Epigallocatechin-3-gallate (EGCG) inhibits imiquimod-induced psoriasis-like inflammation of BALB/c mice. BMC Complement. Altern. Med. 2016, 16, 334. [Google Scholar] [CrossRef] [Green Version]
- Egeberg, A.; Skov, L.; Andersen, Y.M.F.; Mallbris, L.; Gislason, G.H.; Silverberg, J.I.; Wu, J.J.; Thyssen, J.P. Ten-year mortality is increased after hospitalization for atopic dermatitis compared with the general population, but reduced compared with psoriasis. J. Am. Acad. Dermatol. 2017, 76, 98–105. [Google Scholar] [CrossRef]
- Shirsath, N.; Mayer, G.; Singh, T.P.; Wolf, P. 8-methoxypsoralen plus UVA (PUVA) therapy normalizes signalling of phosphorylated component of mTOR pathway in psoriatic skin of K5.hTGFβ1 transgenic mice. Exp. Dermatol. 2015, 24, 889–891. [Google Scholar] [CrossRef]
- Yan, K.; Xu, W.; Huang, Y.; Zhang, Z.; Huang, Q.; Xin, K.Z.; Ma, Y.; Han, L. Methotrexate restores the function of peripheral blood Treg cells in psoriasis vulgaris via CD 73/AMPK/mTOR pathway. Br. J. Dermatol. 2018, 179, 896–905. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, F.; Ma, W.; Sun, Q. Metformin inhibits proliferation and proinflammatory cytokines of human keratinocytes in vitro via mTOR-signaling pathway. Pharm. Biol. 2016, 54, 1173–1178. [Google Scholar] [CrossRef] [Green Version]
- Cibrian, D.; de la Fuente, H.; Sánchez-Madrid, F. Metabolic Pathways That Control Skin Homeostasis and Inflammation. Trends Mol. Med. 2020, 26, 975–986. [Google Scholar] [CrossRef]
- Borowczyk, J.; Buerger, C.; Tadjrischi, N.; Drukala, J.; Wolnicki, M.; Wnuk, D.; Modarressi, A.; Boehncke, W.-H.; Brembilla, N.C. IL-17E (IL-25) and IL-17A Differentially Affect the Functions of Human Keratinocytes. J. Investig. Dermatol. 2020, 140, 1379–1389.e2. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Krueger, J.G. Atopic dermatitis and psoriasis: Two different immune diseases or one spectrum? Curr. Opin. Immunol. 2017, 48, 68–73. [Google Scholar] [CrossRef]
- Nguyen, U.T.; Nguyen, L.T.H.; Kim, B.-A.; Choi, M.-J.; Yang, I.-J.; Shin, H.-M. Natural Compound Mixture, Containing Emodin, Genipin, Chlorogenic Acid, Cimigenoside, and Ginsenoside Rb1, Ameliorates Psoriasis-Like Skin Lesions by Suppressing Inflammation and Proliferation in Keratinocytes. Evid.-Based Complement. Altern. Med. 2020, 2020, 9416962. [Google Scholar] [CrossRef]
- Winge, M.C.; Ohyama, B.; Dey, C.N.; Boxer, L.M.; Li, W.; Ehsani-Chimeh, N.; Truong, A.K.; Wu, D.; Armstrong, A.W.; Makino, T.; et al. RAC1 activation drives pathologic interactions between the epidermis and immune cells. J. Clin. Investig. 2016, 126, 2661–2677. [Google Scholar] [CrossRef]
- Gao, M.; Si, X. Rapamycin ameliorates psoriasis by regulating the expression and methylation levels of tropomyosin via ERK 1/2 and mTOR pathways in vitro and in vivo. Exp. Dermatol. 2018, 27, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, E.; Colombo, M.; Franchi, C.; Altomare, A.; Garutti, C.; Altomare, G. Severe psoriasis treated with a new macrolide: Everolimus. Br. J. Dermatol. 2007, 156, 372–374. [Google Scholar] [CrossRef]
- Wei, K.; Lai, P. Combination of everolimus and tacrolimus: A potentially effective regimen for recalcitrant psoriasis. Dermatol. Ther. 2015, 28, 25–27. [Google Scholar] [CrossRef] [Green Version]
- Chamcheu, J.C.; Adhami, V.M.; Esnault, S.; Sechi, M.; Siddiqui, I.A.; Satyshur, K.A.; Syed, D.N.; Dodwad, S.-J.M.; Chaves-Rodriquez, M.-I.; Longley, B.J.; et al. Dual Inhibition of PI3K/Akt and mTOR by the Dietary Antioxidant, Delphinidin, Ameliorates Psoriatic Features In Vitro and in an Imiquimod-Induced Psoriasis-Like Disease in Mice. Antioxid. Redox Signal. 2017, 26, 49–69. [Google Scholar] [CrossRef] [Green Version]
- Umar, M.; Sastry, K.S.; Al Ali, F.; Al-Khulaifi, M.; Wang, E.; Chouchane, A.I. Vitamin D and the Pathophysiology of Inflammatory Skin Diseases. Ski. Pharm. Physiol. 2018, 31, 74–86. [Google Scholar] [CrossRef]
- Shen, H.; Sha, Y.; Huang, J.; Mao, A.-Q.; Zhang, T.; Wu, M.-Y.; Sun, F.; Yu, Y.-Y.; Cheng, Z.-Q.; Gong, Y.-T. The roles of AMPK-mediated autophagy and mitochondrial autophagy in a mouse model of imiquimod-induced psoriasis. Am. J. Transl. Res. 2021, 13, 12626–12637. [Google Scholar] [PubMed]
- Patel, A.B.; Tsilioni, I.; Weng, Z.; Theoharides, T.C. TNF stimulates IL-6, CXCL8 and VEGF secretion from human keratinocytes via activation of mTOR, inhibited by tetramethoxyluteolin. Exp. Dermatol. 2018, 27, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Chamcheu, J.C.; Afaq, F.; Syed, D.N.; Siddiqui, I.A.; Adhami, V.M.; Khan, N.; Singh, S.; Boylan, B.T.; Wood, G.S.; Mukhtar, H. Delphinidin, a dietary antioxidant, induces human epidermal keratinocyte differentiation but not apoptosis: Studies in submerged and three-dimensional epidermal equivalent models. Exp. Dermatol. 2013, 22, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Chamcheu, J.C.; Pal, H.C.; Siddiqui, I.A.; Adhami, V.M.; Ayehunie, S.; Boylan, B.T.; Noubissi, F.K.; Khan, N.; Syed, D.N.; Elmets, C.A.; et al. Prodifferentiation, anti-inflammatory and antiproliferative effects of delphinidin, a dietary anthocyanidin, in a full-thickness three-dimensional reconstituted human skin model of psoriasis. Ski. Pharm. Physiol. 2015, 28, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Pal, H.C.; Chamcheu, J.; Adhami, V.; Wood, G.; Elmets, C.; Mukhtar, H.; Afaq, F. Topical application of delphinidin reduces psoriasiform lesions in the flaky skin mouse model by inducing epidermal differentiation and inhibiting inflammation. Br. J. Dermatol. 2015, 172, 354–364. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.Y.; Lee, K.W.; Kim, J.-E.; Jung, S.K.; Kang, N.J.; Hwang, M.K.; Heo, Y.-S.; Bode, A.M.; Dong, Z.; Lee, H.J. Delphinidin suppresses ultraviolet B-induced cyclooxygenases-2 expression through inhibition of MAPKK4 and PI-3 kinase. Carcinogenesis 2009, 30, 1932–1940. [Google Scholar] [CrossRef] [Green Version]
- Mitra, A.; Raychaudhuri, S.K.; Raychaudhuri, S.P. IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine 2012, 60, 38–42. [Google Scholar] [CrossRef]
- Wu, P.Y.; Lyu, J.-L.; Liu, Y.-J.; Chien, T.-Y.; Hsu, H.-C.; Wen, K.-C.; Chiang, H.-M. Fisetin Regulates Nrf2 Expression and the Inflammation-Related Signaling Pathway to Prevent UVB-Induced Skin Damage in Hairless Mice. Int. J. Mol. Sci. 2017, 18, 2118. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.H.; Wang, C.-N.; Cheng, H.-H.; Liao, J.-W.; Chen, Y.-T.; Chao, Y.-W.; Jiang, J.L.; Lee, C.-C. Baicalin Ameliorates Imiquimod-Induced Psoriasis-Like Inflammation in Mice. Planta Med. 2018, 84, 1110–1117. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.; Tang, B.; Chen, Y.; Han, L.; Yu, J.; Yan, Y.; Lu, C. The Protective Effects of 18β-Glycyrrhetinic Acid on Imiquimod-Induced Psoriasis in Mice via Suppression of mTOR/STAT3 Signaling. J. Immunol. Res. 2020, 2020, 1980456. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharm. Ther. 2020, 207, 107452. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.W.; Wang, Y.-M.; Wang, X.-Y.; Zhang, Q.; Zhu, S.-M.; Zhang, C.-L. Role and mechanism of matrine alone and combined with acitretin for HaCaT cells and psoriasis-like murine models. Chin. Med. J. 2019, 132, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Shetti, D.; Wei, K. Erianin Inhibits Proliferation and Induces Apoptosis of HaCaT Cells via ROS-Mediated JNK/c-Jun and AKT/mTOR Signaling Pathways. Molecules 2019, 24, 2727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, T.P.; Huettner, B.; Koefeler, H.; Mayer, G.; Bambach, I.; Wallbrecht, K.; Schön, M.P.; Wolf, P. Platelet-activating factor blockade inhibits the T-helper type 17 cell pathway and suppresses psoriasis-like skin disease in K5.hTGF-β1 transgenic mice. Am. J. Pathol. 2011, 178, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Keijsers, R.R.; Joosten, I.; van Erp, P.E.J.; Koenen, H.J.P.M.; van de Kerkhof, P.C.M. Cellular sources of IL-17 in psoriasis: A paradigm shift? Exp. Dermatol. 2014, 23, 799–803. [Google Scholar] [CrossRef]
- Beer, L.; Kalinina, P.; Köcher, M.; Laggner, M.; Jeitler, M.; Zadeh, S.A.; Copic, D.; Tschachler, E.; Mildner, M. miR-155 Contributes to Normal Keratinocyte Differentiation and Is Upregulated in the Epidermis of Psoriatic Skin Lesions. Int. J. Mol. Sci. 2020, 21, 9288. [Google Scholar] [CrossRef]
- Nakashima, T.; Jinnin, M.; Yamane, K.; Honda, N.; Kajihara, I.; Makino, T.; Masuguchi, S.; Fukushima, S.; Okamoto, Y.; Hasegawa, M.; et al. Impaired IL-17 signaling pathway contributes to the increased collagen expression in scleroderma fibroblasts. J. Immunol. 2012, 188, 3573–3583. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Chu, H.; Jiang, S.; Liu, Q.; Liu, L.; Xue, Y.; Zheng, S.; Wan, W.; Qiu, J.; Wang, J.; et al. Sirt1 ameliorates systemic sclerosis by targeting the mTOR pathway. J. Dermatol. Sci. 2017, 87, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Ihn, H. Autocrine TGF-β signaling in the pathogenesis of systemic sclerosis. J. Dermatol. Sci. 2008, 49, 103–113. [Google Scholar] [CrossRef]
- Jinnin, M. Mechanisms of skin fibrosis in systemic sclerosis. J. Dermatol. 2010, 37, 11–25. [Google Scholar] [CrossRef]
- Ponticos, M.; Papaioannou, I.; Xu, S.; Holmes, A.M.; Khan, K.; Denton, C.P.; Bou-Gharios, G.; Abraham, D.J. Failed degradation of JunB contributes to overproduction of type I collagen and development of dermal fibrosis in patients with systemic sclerosis. Arthritis Rheumatol. 2015, 67, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Pannu, J.; Trojanowska, M. Recent advances in fibroblast signaling and biology in scleroderma. Curr. Opin. Rheumatol. 2004, 16, 739–745. [Google Scholar] [CrossRef]
- Yoshizaki, A.; Yanaba, K.; Yoshizaki, A.; Iwata, Y.; Komura, K.; Ogawa, F.; Takenaka, M.; Shimizu, K.; Asano, Y.; Hasegawa, M.; et al. Treatment with rapamycin prevents fibrosis in tight-skin and bleomycin-induced mouse models of systemic sclerosis. Arthritis Rheum. 2010, 62, 2476–2487. [Google Scholar] [CrossRef]
- Yasuoka, H.; Yamaguchi, Y.; Feghali-Bostwick, C.A. The membrane-associated adaptor protein DOK5 is upregulated in systemic sclerosis and associated with IGFBP-5-induced fibrosis. PLoS ONE 2014, 9, e87754. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Ghosh, A.K.; Chu, H.; Fang, F.; Hinchcliff, M.E.; Wang, J.; Marangoni, R.G.; Varga, J. The histone deacetylase sirtuin 1 is reduced in systemic sclerosis and abrogates fibrotic responses by targeting transforming growth factor β signaling. Arthritis Rheumatol. 2015, 67, 1323–1334. [Google Scholar] [CrossRef] [Green Version]
- Zerr, P.; Palumbo-Zerr, K.; Huang, J.; Tomcik, M.; Šumová, B.; Distler, O.; Schett, G.; Distler, J.H.W. Sirt1 regulates canonical TGF-β signalling to control fibroblast activation and tissue fibrosis. Ann. Rheum. Dis. 2014, 75, 226–233. [Google Scholar] [CrossRef]
- Jun, J.-B.; Kuechle, M.; Min, J.; Shim, S.C.; Kim, G.; Montenegro, V.; Korn, J.H.; Elkon, K.B. Scleroderma fibroblasts demonstrate enhanced activation of Akt (protein kinase B) in situ. J. Investig. Dermatol. 2005, 124, 298–303. [Google Scholar] [CrossRef] [Green Version]
- Forestier, A.; Guerrier, T.; Jouvray, M.; Giovannelli, J.; Lefèvre, G.; Sobanski, V.; Hauspie, C.; Hachulla, E.; Hatron, P.-Y.; Zéphir, H.; et al. Altered B lymphocyte homeostasis and functions in systemic sclerosis. Autoimmun. Rev. 2018, 17, 244–255. [Google Scholar] [CrossRef]
- Liang, M.; Lv, J.; Chu, H.; Wang, J.; Chen, X.; Zhu, X.; Xue, Y.; Guan, M.; Zou, H. Vertical inhibition of PI3K/Akt/mTOR signaling demonstrates in vitro and in vivo anti-fibrotic activity. J. Dermatol. Sci. 2014, 76, 104–111. [Google Scholar] [CrossRef]
- Mitra, A.; Luna, J.I.; Marusina, A.I.; Merleev, A.; Kundu-Raychaudhuri, S.; Fiorentino, D.; Raychaudhuri, S.P.; Maverakis, E. Dual mTOR inhibition is required to prevent TGF-β-mediated fibrosis: Implications for scleroderma. J. Investig. Dermatol. 2015, 135, 2873. [Google Scholar] [CrossRef] [Green Version]
- Cong, L.; Xia, Z.-K.; Yang, R.-Y. Targeting the TGF-β Receptor with Kinase Inhibitors for Scleroderma Therapy. Arch. Pharm. 2014, 347, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Z.; Wen, D.; Zhang, M.; Xie, Q.; Ma, L.; Guan, Y.; Ren, Y.; Chen, J.; Hao, C.-M. Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF-β/Smad3 pathway. J. Cell. Biochem. 2014, 115, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Mao, Y.; Tian, Y.; Zhu, K.; Cha, X.; Wu, M.; Zhou, X. Geniposide inhibited endothelial-mesenchymal transition via the mTOR signaling pathway in a bleomycin-induced scleroderma mouse model. Am. J. Transl. Res. 2017, 9, 1025–1036. [Google Scholar]
- Zhou, X.; Liu, C.; Lu, J.; Zhu, L.; Li, M. 2-Methoxyestradiol inhibits hypoxia-induced scleroderma fibroblast collagen synthesis by phosphatidylinositol 3-kinase/Akt/mTOR signalling. Rheumatology 2018, 57, 1675–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vescovi, P.; Manfredi, M.; Savi, A.; Bonanini, M. Neoplastic transformation of oral lichen planus. I: Review of the literature. Minerva Stomatol. 2000, 49, 249–255. [Google Scholar] [PubMed]
- Loré, B.; Saraceno, R.; Poladas, G.; Fida, M.; Khoury, C.; Arcuri, C.; Magnato, R. Oral lichen planus: Therapy and phenotype. G. Ital. Dermatol. Venereol. 2018, 153, 459–463. [Google Scholar] [CrossRef]
- Lavanya, N.; Jayanthi, P.; Rao, U.K.; Ranganathan, K. Oral lichen planus: An update on pathogenesis and treatment. J. Oral Maxillofac. Pathol. 2011, 15, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Baek, K.; Choi, Y. The microbiology of oral lichen planus: Is microbial infection the cause of oral lichen planus? Mol. Oral Microbiol. 2018, 33, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Shiva, A.; Zamanian, A.; Arab, S.; Boloki, M. Immunohistochemical Study of p53 Expression in Patients with Erosive and Non-Erosive Oral Lichen Planus. J. Dent. 2018, 19, 118. [Google Scholar]
- Zhang, N.; Zhang, J.; Tan, Y.-Q.; Du, G.-F.; Lu, R.; Zhou, G. Activated Akt/mTOR-autophagy in local T cells of oral lichen planus. Int. Immunopharmacol. 2017, 48, 84–90. [Google Scholar] [CrossRef]
- Wang, J.; Luo, H.; Xiao, Y.; Wang, L. miR-125b inhibits keratinocyte proliferation and promotes keratinocyte apoptosis in oral lichen planus by targeting MMP-2 expression through PI3K/Akt/mTOR pathway. Biomed. Pharmacother. 2016, 80, 373–380. [Google Scholar] [CrossRef]
- Eisen, D.; Carrozzo, M.; Sebastian, J.-V.B.; Thongprasom, K. Number V Oral lichen planus: Clinical features and management. Oral Dis. 2005, 11, 338–349. [Google Scholar] [CrossRef]
- Mostafa, B.; Zakaria, M. Evaluation of Combined Topical Ozone and Steroid Therapy in Management of Oral Lichen Planus. Open Access Maced. J. Med. Sci. 2018, 6, 879. [Google Scholar] [CrossRef] [Green Version]
- Larsen, K.R.; Johansen, J.D.; Reibel, J.; Zachariae, C.; Pedersen, A.M.L. Serum cytokine profile and clinicopathological findings in oral lichen planus, oral lichenoid lesions and stomatitis. Clin. Exp. Dent. Res. 2017, 3, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Nosratzehi, T. Oral Lichen Planus: An Overview of Potential Risk Factors, Biomarkers and Treatments. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 1161–1167. [Google Scholar]
- Zhao, Z.; Han, Y.; Zhang, Z.; Li, W.; Ji, X.; Liu, X.; Jin, J.; Xu, S.; Cui, H.; Cheng, Z.; et al. Total glucosides of paeony improves the immunomodulatory capacity of MSCs partially via the miR-124/STAT3 pathway in oral lichen planus. Biomed. Pharmacother. 2018, 105, 151–158. [Google Scholar] [CrossRef]
- Han, X.; Zhang, J.; Tan, Y.; Zhou, G. Probiotics: A non-conventional therapy for oral lichen planus. Arch. Oral Biol. 2017, 81, 90–96. [Google Scholar] [CrossRef]
- Prodromidis, G.; Nikitakis, N.G.; Sklavounou, A. Immunohistochemical Analysis of the Activation Status of the Akt/mTOR/pS6 Signaling Pathway in Oral Lichen Planus. Int. J. Dent. 2013, 2013, 743456. [Google Scholar] [CrossRef]
- Mahévas, T.; Bertinchamp, R.; Battistella, M.; Reygagne, P.; Oksenhendler, E.; Fieschi, C.; Bachelez, H. Efficacy of oral sirolimus as salvage therapy in refractory lichen planus associated with immune deficiency. Br. J. Dermatol. 2018, 179, 771–773. [Google Scholar] [CrossRef]
- Li, X.; Ponandai-Srinivasan, S.; Nandakumar, K.S.; Fabre, S.; Landén, N.X.; Mavon, A.; Khmaladze, I. Targeting microRNA for improved skin health. Health Sci. Rep. 2021, 4, e374. [Google Scholar] [CrossRef]
- Song, Y.; Xu, S.; Shao, Y.; Ge, S.; Zhou, H. Expression profile of circular RNAs in oral lichen planus. Ann. Palliat. Med. 2021, 10, 5205–5217. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Xie, H.; Wang, L.; Li, R.; Zhang, F.; Xu, J.; Zhao, B.; Du, J. MicroRNA-122 promotes apoptosis of keratinocytes in oral lichen planus through suppressing VDR expression. J. Cell. Mol. Med. 2021, 25, 3400–3407. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhong, W.; Ren, X.; Huang, X.; Li, Z.; Chen, C.; Jiang, B.; Chen, Z.; Jian, X.; Yang, L.; et al. MiR-193b-3p-ERBB4 axis regulates psoriasis pathogenesis via modulating cellular proliferation and inflammatory-mediator production of keratinocytes. Cell Death Dis. 2021, 12, 963. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; E, C.; Wu, S.; Meng, Z.; Qin, G.; Wang, R. Circ-IGF1R plays a significant role in psoriasis via regulation of a miR-194-5p/CDK1 axis. Cytotechnology 2021, 73, 775–785. [Google Scholar] [CrossRef]
- Solvin, Å.; Chawla, K.; Olsen, L.C.; Hegre, S.A.; Danielsen, K.; Jenssen, M.; Furberg, A.; Saunes, M.; Hveem, K.; Sætrom, P.; et al. MicroRNA profiling of psoriatic skin identifies 11 miRNAs associated with disease severity. Exp. Dermatol. 2021, 31, 535–547. [Google Scholar] [CrossRef]
- Bao, L.; Chau, C.S.; Lei, Z.; Hu, H.; Chan, A.G.; Amber, K.T.; Maienschein-Cline, M.; Tsoukas, M.M. Dysregulated microRNA expression in IL-4 transgenic mice, an animal model of atopic dermatitis. Arch. Dermatol. Res. 2021, 313, 837–846. [Google Scholar] [CrossRef]
- Zhong, Y.; Qin, K.; Li, L.; Liu, H.; Xie, Z.; Zeng, K. Identification of Immunological Biomarkers of Atopic Dermatitis by Integrated Analysis to Determine Molecular Targets for Diagnosis and Therapy. Int. J. Gen. Med. 2021, 14, 8193–8209. [Google Scholar] [CrossRef]
- Huang, J.; Xu, X.; Wang, X.; Yang, J.; Xue, M.; Yang, Y.; Zhang, R.; Yang, X. MicroRNA-590-3p inhibits T helper 17 cells and ameliorates inflammation in lupus mice. Immunology 2021, 165, 260–273. [Google Scholar] [CrossRef]
- Yang, X.; Dang, X.; Zhang, X.; Zhao, S. Liquiritin reduces lipopolysaccharide-aroused HaCaT cell inflammation damage via regulation of microRNA-31/MyD88. Int. Immunopharmacol. 2021, 101 Pt B, 108283. [Google Scholar] [CrossRef]
- Danielsson, K.; Wahlin, Y.B.; Gu, X.; Boldrup, L.; Nylander, K. Altered expression of miR-21, miR-125b, and miR-203 indicates a role for these microRNAs in oral lichen planus. J. Oral Pathol. Med. 2012, 41, 90–95. [Google Scholar] [CrossRef]
- Mochtar, M.; Murasmita, A.; Irawanto, M.E.; Julianto, I.; Kariosentono, H.; Waskito, F. The Difference in Interleukin-19 Serum on Degrees of Acne Vulgaris Severity. Int. J. Inflamm. 2018, 2018, 4141579. [Google Scholar] [CrossRef] [Green Version]
- Mansu, S.S.; Liang, H.; Parker, S.; Coyle, M.E.; Wang, K.; Zhang, A.L.; Guo, X.; Lu, C.; Xue, C.C.L. Acupuncture for Acne Vulgaris: A Systematic Review and Meta-Analysis. Evid.-Based Complement. Altern. Med. 2018, 2018, 4806734. [Google Scholar] [CrossRef] [Green Version]
- Melnik, B.C. Acne vulgaris: The metabolic syndrome of the pilosebaceous follicle. Clin. Dermatol. 2018, 36, 29–40. [Google Scholar] [CrossRef]
- Smith, T.M.; Gilliland, K.; Clawson, G.A.; Thiboutot, D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J. Investig. Dermatol. 2008, 128, 1286–1293. [Google Scholar] [CrossRef] [Green Version]
- Oge, L.K.; Broussard, A.; Marshall, M.D. Acne Vulgaris: Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 475–484. [Google Scholar]
- Xu, H.; Li, H. Acne, the Skin Microbiome, and Antibiotic Treatment. Am. J. Clin. Dermatol. 2019, 20, 335–344. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, H.; Zhou, J.; Mou, Y.; Wang, G.; Xiong, X. Patients with Acne Vulgaris Have a Distinct Gut Microbiota in Comparison with Healthy Controls. Acta Derm.-Venereol. 2018, 98, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Bakus, A.D.; Yaghmai, D.; Massa, M.C.; Garden, B.C.; Garden, J.M. Sustained Benefit After Treatment of Acne Vulgaris Using Only a Novel Combination of Long-Pulsed and Q-Switched 1064-nm Nd: YAG Lasers. Dermatol. Surg. 2018, 44, 1402–1410. [Google Scholar] [CrossRef]
- Connolly, D.; Vu, H.L.; Mariwalla, K.; Saedi, N. Acne scarring—Pathogenesis, evaluation, and treatment options. J. Clin. Aesthetic Dermatol. 2017, 10, 12. [Google Scholar]
- Suh, Y.; Yang, J.H.; Yoon, J.Y.; Choi, Y.S. Platycodin D May Improve Acne and Prevent Scarring by Downregulating SREBP-1 Expression Via Inhibition of IGF-1R/PI3K/Akt Pathway and Modulating Inflammation with an Increase in Collagen. Ann. Dermatol. 2018, 30, 581–587. [Google Scholar] [CrossRef]
- Kwon, H.H.; Yoon, J.Y.; Park, S.Y.; Min, S.; Kim, Y.-I.; Park, J.Y.; Lee, Y.-S.; Thiboutot, D.M.; Suh, D.H. Activity-guided purification identifies lupeol, a pentacyclic triterpene, as a therapeutic agent multiple pathogenic factors of acne. J. Investig. Dermatol. 2015, 135, 1491–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanwar, I.L.; Haider, T.; Kumari, A.; Dubey, S.; Jain, P.; Soni, V. Models for acne: A comprehensive study. Drug Discov. Ther. 2018, 12, 329–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melnik, B.C. FoxO1—The key for the pathogenesis and therapy of acne? J. Dtsch. Dermatol. Ges. 2010, 8, 105–114. [Google Scholar] [CrossRef]
- Monfrecola, G.; Lembo, S.; Caiazzo, G.; De Vita, V.; Di Caprio, R.; Balato, A.; Fabbrocini, G. Mechanistic target of rapamycin (mTOR) expression is increased in acne patients’ skin. Exp. Dermatol. 2016, 25, 153–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agamia, N.F.; Abdallah, D.M.; Sorour, O.; Mourad, B.; Younan, D.N. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br. J. Dermatol. 2016, 174, 1299–1307. [Google Scholar] [CrossRef]
- Melnik, B.C.; Zouboulis, C.C. Potential role of FoxO1 and mTORC1 in the pathogenesis of Western diet-induced acne. Exp. Dermatol. 2013, 22, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Simonart, T. Newer approaches to the treatment of acne vulgaris. Am. J. Clin. Dermatol. 2012, 13, 357–364. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Shamban, A.; Webster, G.; Del Rosso, J.; Dover, J.S.; Swinyer, L.; Stein, L.; Lin, X.; Draelos, Z.; Gold, M.; et al. A phase IV, open-label study evaluating the use of triple-combination therapy with minocycline HCl extended-release tablets, a topical antibiotic/retinoid preparation and benzoyl peroxide in patients with moderate to severe acne vulgaris. J. Drugs Dermatol. 2013, 12, 619–625. [Google Scholar]
- Sardana, K.; Gupta, T.; Garg, V.K.; Ghunawat, S. Antibiotic resistance to Propionobacterium acnes: Worldwide scenario, diagnosis and management. Expert Rev. Anti-Infect. Ther. 2015, 13, 883–896. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. Are therapeutic effects of antiacne agents mediated by activation of FoxO1 and inhibition of mTORC1? Exp. Dermatol. 2013, 22, 502–504. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, P.; Azizian, Z.; Hejazi, S.; Chalangari, R.; Chalangari, K.M. Evaluation the efficacy of trichloroacetic acid (TCA) 33% in treatment of oral retinoid-induced cheilitis compared with placebo (Vaseline): A randomized pilot study. J. Dermatol. Treat. 2018, 29, 694–697. [Google Scholar] [CrossRef]
- De Vita, V.; Melnik, B.C. The Magnitude of mTORC1 Signalling May Predict the Response to Isotretinoin Treatment in Patients with Hidradenitis Suppurativa. Dermatology 2017, 233, 399–400. [Google Scholar] [CrossRef] [Green Version]
- Fabbrocini, G.; Staibano, S.; De Rosa, G.; Battimiello, V.; Fardella, N.; Ilardi, G.; La Rotonda, M.I.; Longobardi, A.; Mazzella, M.; Siano, M. Resveratrol-containing gel for the treatment of acne vulgaris: A single-blind, vehicle-controlled, pilot study. Am. J. Clin. Dermatol. 2011, 12, 133–141. [Google Scholar] [CrossRef]
- Kim, S.Y.; Hyun, M.Y.; Go, K.C.; Zouboulis, C.C.; Kim, B.J. Resveratrol exerts growth inhibitory effects on human SZ95 sebocytes through the inactivation of the PI3-K/Akt pathway. Int. J. Mol. Med. 2015, 35, 1042–1050. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.-K.; Ha, J.-M.; Lee, Y.H.; Seo, Y.-J.; Kim, C.D.; Lee, J.-H.; Im, M. Comparison of vitamin D levels in patients with and without acne: A case-control study combined with a randomized controlled trial. PLoS ONE 2016, 11, e0161162. [Google Scholar] [CrossRef] [Green Version]
- Melnik, B. Dietary intervention in acne: Attenuation of increased mTORC1 signaling promoted by Western diet. Dermato-endocrinology 2012, 4, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Zou, N.; Wei, Y.; Li, F.; Yang, Y.; Cheng, X.; Wang, C. The inhibitory effects of compound Muniziqi granule against B16 cells and harmine induced autophagy and apoptosis by inhibiting Akt/mTOR pathway. BMC Complement. Altern. Med. 2017, 17, 517. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wang, Q.; Tuo, J.; Chang, Y.; Ying, J.; Jiang, M.; Zouboulis, C.C.; Xiang, L. ALA-PDT suppressed the cell growth by Akt-/Erk-mTOR-p70 s6k pathway in human SZ95 sebocytes in vitro. Photodiagn. Photodyn. Ther. 2018, 24, 1–6. [Google Scholar] [CrossRef]
- Rosi, E.; Fastame, M.T.; Scandagli, I.; Di Cesare, A.; Ricceri, F.; Pimpinelli, N.; Prignano, F. Insights into the Pathogenesis of HS and Therapeutical Approaches. Biomedicines 2021, 9, 1168. [Google Scholar] [CrossRef]
- Scala, E.; Cacciapuoti, S.; Garzorz-Stark, N.; Megna, M.; Marasca, C.; Seiringer, P.; Volz, T.; Eyerich, K.; Fabbrocini, G. Hidradenitis Suppurativa: Where We Are and Where We Are Going. Cells 2021, 10, 2094. [Google Scholar] [CrossRef]
- Vossen, A.R.J.V.; Van Der Zee, H.H.; Prens, E.P. Hidradenitis Suppurativa: A Systematic Review Integrating Inflammatory Pathways into a Cohesive Pathogenic Model. Front. Immunol. 2018, 9, 2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napolitano, M.; Megna, M.; A Timoshchuk, E.; Patruno, C.; Balato, N.; Fabbrocini, G.; Monfrecola, G. Hidradenitis suppurativa: From pathogenesis to diagnosis and treatment. Clin. Cosmet. Investig. Dermatol. 2017, 10, 105–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pink, A.; Anzengruber, F.; Navarini, A. Acne and hidradenitis suppurativa. Br. J. Dermatol. 2018, 178, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, A.; Sayed, C.; Alavi, A.; Alhusayen, R.; Brassard, A.; Burkhart, C.; Crowell, K.; Eisen, D.B.; Gottlieb, A.B.; Hamzavi, I.; et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part I: Diagnosis, evaluation, and the use of complementary and procedural management. J. Am. Acad. Dermatol. 2019, 81, 76–90. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, J.M.; Hendricks, A.J.; Thompson, A.M.; Mata, E.M.; Collier, E.K.; Grogan, T.R.; Shi, V.Y.; Hsiao, J.L. Menses, pregnancy, delivery, and menopause in hidradenitis suppurativa: A patient survey. Int. J. Womens Dermatol. 2020, 6, 368–371. [Google Scholar] [CrossRef]
- Melnik, B.C.; Plewig, G. Impaired Notch-MKP-1 signalling in hidradenitis suppurativa: An approach to pathogenesis by evidence from translational biology. Exp. Dermatol. 2013, 22, 172–177. [Google Scholar] [CrossRef]
- Dajnoki, Z.; Somogyi, O.; Medgyesi, B.; Jenei, A.; Szabó, L.; Gáspár, K.; Hendrik, Z.; Gergely, P.; Imre, D.; Póliska, S.; et al. Primary alterations during the development of hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2021, 36, 462–471. [Google Scholar] [CrossRef]
- Dmitriev, A.; König, A.; Lang, V.; Diehl, S.; Kaufmann, R.; Pinter, A.; Buerger, C. mTORC1—A potential player in the pathogenesis of hidradenitis suppurativa? J. Eur. Acad. Dermatol. Venereol. 2021, 35, e444–e447. [Google Scholar] [CrossRef]
- De Vita, V. Altered mTORC1 signalling may contribute to macrophage dysregulation in hidradenitis suppurativa. Inflamm. Res. 2018, 67, 207–208. [Google Scholar] [CrossRef]
- De Vita, V.; Melnik, B.C. Activated mTORC1 signaling: The common driving force of type 2 diabetes and hidradenitis suppurativa. J. Am. Acad. Dermatol. 2018, 78, e121. [Google Scholar] [CrossRef] [Green Version]
- Amat-Samaranch, V.; Agut-Busquet, E.; Vilarrasa, E.; Puig, L. New perspectives on the treatment of hidradenitis suppurativa. Ther. Adv. Chronic Dis. 2021, 12, 20406223211055920. [Google Scholar] [CrossRef]
- Haferland, I.; Wallenwein, C.M.; Ickelsheimer, T.; Diehl, S.; Wacker, M.G.; Schiffmann, S.; Buerger, C.; Kaufmann, R.; Koenig, A.; Pinter, A. Mechanism of anti-inflammatory effects of Rifampicin in an ex vivo culture system of Hidradenitis Suppurativa. Exp. Dermatol. 2022, 31, 1005–1013. [Google Scholar] [CrossRef]
- Lipner, S.R.; Scher, R.K. Alopecia areata. J. Am. Acad. Dermatol. 2018, 79, e9–e10. [Google Scholar] [CrossRef]
- Żeberkiewicz, M.; Rudnicka, L.; Malejczyk, J. Immunology of alopecia areata. Cent. Eur. J. Immunol. 2020, 45, 325–333. [Google Scholar] [CrossRef]
- Pratt, C.H. Alopecia areata. Nat. Rev. Dis. Prim. 2017, 3, 17011. [Google Scholar] [CrossRef] [Green Version]
- Juárez-Rendón, K.J.; Sánchez, G.R.; Reyes-López, M.Á.; García-Ortiz, J.E.; Bocanegra-García, V.; Guardiola-Avila, I.; Altamirano-García, M.L. Alopecia Areata. Current situation and perspectives. Arch. Argent. Pediatr. 2017, 115, e404–e411. [Google Scholar]
- Villasante Fricke, A.C.; Miteva, M. Epidemiology and burden of alopecia areata: A systematic review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 397–403. [Google Scholar]
- Mace, E.M. Phosphoinositide-3-Kinase Signaling in Human Natural Killer Cells: New Insights from Primary Immunodeficiency. Front. Immunol. 2018, 9, 445. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Mueller, U.; Freyschmidt-Paul, P.; Zöller, M. Delayed type hypersensitivity-induced myeloid-derived suppressor cells regulate autoreactive T cells. Eur. J. Immunol. 2011, 41, 2871–2882. [Google Scholar] [CrossRef]
- Cheon, H.I.; Bae, S.; Ahn, K.J. Flavonoid Silibinin Increases Hair-Inductive Property Via Akt and Wnt/β-Catenin Signaling Activation in 3-Dimensional-Spheroid Cultured Human Dermal Papilla Cells. J. Microbiol. Biotechnol. 2019, 29, 321–329. [Google Scholar] [CrossRef]
- Kang, J.I.; Choi, Y.K.; Koh, Y.-S.; Hyun, J.-W.; Lee, K.S.; Lee, C.M.; Yoo, E.-S.; Kang, H.-K. Vanillic Acid Stimulates Anagen Signaling via the PI3K/Akt/β-Catenin Pathway in Dermal Papilla Cells. Biomol. Ther. 2020, 28, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Hosking, A.-M.; Juhasz, M.; Mesinkovska, N.A. Complementary and Alternative Treatments for Alopecia: A Comprehensive Review. Skin Appendage Disord. 2019, 5, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, X.; Wang, C.; Zhang, J. Alopecia Areata: An Update on Etiopathogenesis, Diagnosis, and Management. Clin. Rev. Allergy Immunol. 2021, 61, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Bibert, S.; Bratschi, M.W.; Aboagye, S.Y.; Collinet, E.; Scherr, N.; Yeboah-Manu, D.; Beuret, C.; Pluschke, G.; Bochud, P.-Y. Susceptibility to Mycobacterium ulcerans Disease (Buruli ulcer) Is Associated with IFNG and iNOS Gene Polymorphisms. Front. Microbiol. 2017, 8, 1903. [Google Scholar] [CrossRef] [Green Version]
- Vincent, Q.B.; Ardant, M.-F.; Adeye, A.; Goundote, A.; Saint-André, J.-P.; Cottin, J.; Kempf, M.; Agossadou, D.; Johnson, C.; Abel, L.; et al. Clinical epidemiology of laboratory-confirmed Buruli ulcer in Benin: A cohort study. Lancet Glob. Health 2014, 2, e422–e430. [Google Scholar] [CrossRef] [Green Version]
- Röltgen, K.; Pluschke, G.; Johnson, P.D.R.; Fyfe, J. Mycobacterium ulcerans DNA in Bandicoot Excreta in Buruli Ulcer–Endemic Area, Northern Queensland, Australia. Emerg. Infect. Dis. 2017, 23, 2042. [Google Scholar] [CrossRef] [Green Version]
- Demangel, C.; Stinear, T.P.; Cole, S.T. Buruli ulcer: Reductive evolution enhances pathogenicity of Mycobacterium ulcerans. Nat. Rev. Microbiol. 2009, 7, 50–60. [Google Scholar] [CrossRef]
- Guarner, J.; Bartlett, J.; Whitney, E.A.S.; Raghunathan, P.L.; Stienstra, Y.; Asamoa, K.; Etuaful, S.; Klutse, E.; Quarshie, E.; van der Werf, T.; et al. Histopathologic features of Mycobacterium ulcerans infection. Emerg. Infect. Dis. 2003, 9, 651–656. [Google Scholar] [CrossRef]
- Zingue, D.; Bouam, A.; Tian, R.B.D.; Drancourt, M. Buruli Ulcer, a Prototype for Ecosystem-Related Infection, Caused by Mycobacterium ulcerans. Clin. Microbiol. Rev. 2018, 31, e00045-17. [Google Scholar] [CrossRef] [Green Version]
- Guarner, J. Buruli ulcer. Review of a neglected skin mycobacterial disease. J. Clin. Microbiol. 2018, 56, e01507–e01517. [Google Scholar] [CrossRef] [Green Version]
- Buultjens, A.H.; Vandelannoote, K.; Meehan, C.J.; Eddyani, M.; de Jong, B.C.; Fyfe, J.A.M.; Globan, M.; Tobias, N.J.; Porter, J.L.; Tomita, T.; et al. Comparative Genomics Shows That Mycobacterium ulcerans Migration and Expansion Preceded the Rise of Buruli Ulcer in Southeastern Australia. Appl. Environ. Microbiol. 2018, 84, e02612–e02617. [Google Scholar] [CrossRef] [Green Version]
- Bieri, R.; Scherr, N.; Ruf, M.-T.; Dangy, J.-P.; Gersbach, P.; Gehringer, M.; Altmann, K.-H.; Pluschke, G. The macrolide toxin mycolactone promotes bim-dependent apoptosis in Buruli Ulcer through inhibition of mTOR. ACS Chem. Biol. 2017, 12, 1297–1307. [Google Scholar] [CrossRef]
- Guertin, D.A.; Stevens, D.M.; Thoreen, C.C.; Burds, A.A.; Kalaany, N.Y.; Moffat, J.; Brown, M.; Fitzgerald, K.J.; Sabatini, D.M. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell 2006, 11, 859–871. [Google Scholar] [CrossRef] [Green Version]
- Janes, M.R.; Limon, J.J.; So, L.; Chen, J.; Lim, R.J.; Chavez, M.A.; Vu, C.; Lilly, M.B.; Mallya, S.; Ong, S.T.; et al. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat. Med. 2010, 16, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Hermans, M.H. Wounds and ulcers: Back to the old nomenclature. Wounds 2010, 22, 289–293. [Google Scholar]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Squarize, C.H.; Castilho, R.M.; Bugge, T.H.; Gutkind, J.S. Accelerated wound healing by mTOR activation in genetically defined mouse models. PLoS ONE 2010, 5, e10643. [Google Scholar] [CrossRef]
- Wang, P.H. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Forsberg, S.; Rollman, O. Re-epithelialization from human skin explant cultures is promoted by ligand-activated HER3 receptor. J. Dermatol. Sci. 2010, 59, 7–15. [Google Scholar] [CrossRef]
- Forsberg, S.; Ostman, A.; Rollman, O. Regeneration of human epidermis on acellular dermis is impeded by small-molecule inhibitors of EGF receptor tyrosine kinase. Arch. Dermatol. Res. 2008, 300, 505–516. [Google Scholar] [CrossRef]
- Xiao, W.; Tang, H.; Wu, M.; Liao, Y.; Li, K.; Li, L.; Xu, X. Ozone oil promotes wound healing by increasing the migration of fibroblasts via PI3K/Akt/mTOR signaling pathway. Biosci. Rep. 2017, 37, BSR20170658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinke, J.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Chung, S.; Takahashi, A.; Kamatani, N.; Kawaguchi, T.; Tsunoda, T.; Hosono, N.; Kubo, M.; Nakamura, Y.; Zembutsu, H. A genome-wide association study identifies four susceptibility loci for keloid in the Japanese population. Nat. Genet. 2010, 42, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Mall, J.W.; Pollmann, C.; Müller, J.M.; Büttemeyer, R. Keloid of the earlobe after ear piercing. Not only a surgical problem. Chirurg 2002, 73, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.; Sherris, D.; Paus, R.; Varmeh, S.; Singh, S.; Pandolfi, P.P.; Bayat, A. Keloid disease can be inhibited by antagonizing excessive mTOR signaling with a novel dual TORC1/2 inhibitor. Am. J. Pathol. 2012, 181, 1642–1658. [Google Scholar] [CrossRef]
- Vincent, A.S.; Phan, T.T.; Mukhopadhyay, A.; Lim, H.Y.; Halliwell, B.; Wong, K.P. Human skin keloid fibroblasts display bioenergetics of cancer cells. J. Investig. Dermatol. 2008, 128, 702–709. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Ishihara, H.; Ohtsuru, A.; Akino, K.; Murakami, R.; Kuroda, H.; Namba, H.; Ito, M.; Fujii, T.; Yamashita, S. Overexpression of insulin-like growth factor-1 (IGF-I) receptor and the invasiveness of cultured keloid fibroblasts. Am. J. Pathol. 1999, 154, 883–889. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.Y.; Fu, X.-B.; Chen, W.; Sun, T.-Z. Relationship of overexpression of angiogenesis factors and their receptors with invasive growth of keloid. Zhonghua Zheng Xing Wai Ke Za Zhi 2004, 20, 128–131. [Google Scholar]
- Ong, C.T.; Khoo, Y.T.; Mukhopadhyay, A.; Do, D.V.; Lim, I.J.; Aalami, O.; Phan, T.T. mTOR as a potential therapeutic target for treatment of keloids and excessive scars. Exp. Dermatol. 2007, 16, 394–404. [Google Scholar] [CrossRef]
- Zhang, Q.; Kelly, A.P.; Wang, L.; French, S.W.; Tang, X.; Duong, H.S.; Messadi, D.V.; Le, A.D. Green tea extract and (−)-epigallocatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in keloid fibroblasts via blocking PI-3K/AkT signaling pathways. J. Investig. Dermatol. 2006, 126, 2607–2613. [Google Scholar] [CrossRef] [Green Version]
- Bujor, A.M.; Pannu, J.; Bu, S.; Smith, E.A.; Muise-Helmericks, R.C.; Trojanowska, M. Akt blockade downregulates collagen and upregulates MMP1 in human dermal fibroblasts. J. Investig. Dermatol. 2008, 128, 1906–1914. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.W.; Cheng, B.; Yu, W.-L.; Sun, R.-X.; Zeng, D.; Wang, J.; Liao, Y.-X.; Fu, X.-B. Angiotensin II regulates phosphoinositide 3 kinase/Akt cascade via a negative crosstalk between AT1 and AT2 receptors in skin fibroblasts of human hypertrophic scars. Life Sci. 2006, 79, 475–483. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Favara, G.; Lio, R.M.S.; Evola, G.; Agodi, A.; Basile, G. Nutrition and Wound Healing: An Overview Focusing on the Beneficial Effects of Curcumin. Int. J. Mol. Sci. 2019, 20, 1119. [Google Scholar] [CrossRef] [Green Version]
- Syed, F.; Sanganee, H.J.; Bahl, A.; Bayat, A. Potent dual inhibitors of TORC1 and TORC2 complexes (KU-0063794 and KU-0068650) demonstrate in vitro and ex vivo anti-keloid scar activity. J. Investig. Dermatol. 2013, 133, 1340–1350. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Liu, Z.; Liu, J.; Liu, Y.; Zhang, W.; Zhang, Y. Mycosis Fungoides and Variants of Mycosis Fungoides: A Retrospective Study of 93 Patients in a Chinese Population at a Single Center. Ann. Dermatol. 2020, 32, 14–20. [Google Scholar] [CrossRef]
- Akinbami, A.A.; Osikomaiya, B.I.; John-Olabode, S.O.; Adediran, A.A.; Osinaike, O.; Uche, E.I.; Ismail, A.K.; Dosunmu, A. Mycosis fungoides: Case report and literature review. Clin. Med. Insights Case Rep. 2014, 7, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Oka, T.; Miyagaki, T. Novel and Future Therapeutic Drugs for Advanced Mycosis Fungoides and Sézary Syndrome. Front. Med. 2019, 6, 116. [Google Scholar] [CrossRef]
- Virmani, P.; Myskowski, P.L.; Pulitzer, M. Unusual variants of mycosis fungoides. Diagn. Histopathol. 2016, 22, 142–151. [Google Scholar] [CrossRef] [Green Version]
- McGirt, L.Y.; Jia, P.; Baerenwald, D.A.; Duszynski, R.J.; Dahlman, K.B.; Zic, J.A.; Zwerner, J.P.; Hucks, D.; Dave, U.; Zhao, Z.; et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood 2015, 126, 508–519. [Google Scholar] [CrossRef] [Green Version]
- Makdisi, J.; Friedman, A. Myocosis fungoides—An update on a non-mycotic disease. J. Drugs Derm. 2013, 12, 825–831. [Google Scholar]
- Bresin, A.; Cristofoletti, C.; Caprini, E.; Cantonetti, M.; Monopoli, A.; Russo, G.; Narducci, M.G. Preclinical Evidence for Targeting PI3K/mTOR Signaling with Dual-Inhibitors as a Therapeutic Strategy against Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2020, 140, 1045–1053.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.C.; Lin, C.-L.; Hong, C.-H.; Yu, H.-S.; Chen, G.-S.; Lee, C.-H. CCR7 expression correlates with subcutaneous involvement in mycosis fungoides skin lesions and promotes migration of mycosis fungoides cells (MyLa) through mTOR activation. J. Dermatol. Sci. 2014, 74, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Witzig, T.E.; Reeder, C.; Han, J.J.; LaPlant, B.; Stenson, M.; Tun, H.W.; Macon, W.; Ansell, S.M.; Habermann, T.M.; Inwards, D.J.; et al. The mTORC1 inhibitor everolimus has antitumor activity in vitro and produces tumor responses in patients with relapsed T-cell lymphoma. Blood 2015, 126, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarabadkar, E.S.; Shinohara, M.M. Skin Directed Therapy in Cutaneous T-Cell Lymphoma. Front. Oncol. 2019, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Chen, G.-S.; Lin, P.-Y.; Pan, I.-H.; Wang, S.-T.; Lin, S.H.; Yu, H.-S.; Lin, C.-C. Tazarotene induces apoptosis in human basal cell carcinoma via activation of caspase-8/t-Bid and the reactive oxygen species-dependent mitochondrial pathway. DNA Cell Biol. 2014, 33, 652–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, K.; Ramachandran, V.; Fassihi, H.; Sebaratnam, D.F. Comparison of Narrowband UV-B With Psoralen-UV-A Phototherapy for Patients with Early-Stage Mycosis Fungoides: A Systematic Review and Meta-analysis. JAMA Dermatol. 2019, 155, 335–341. [Google Scholar] [CrossRef]
- Wan, J.; Lin, F.; Zhang, W.; Xu, A.; DeGiorgis, J.; Lu, H.; Wan, Y. Novel approaches to vitiligo treatment via modulation of mTOR and NF-κB pathways in human skin melanocytes. Int. J. Biol. Sci. 2017, 13, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Sureda, A.; Belwal, T.; Çetinkaya, S.; Süntar, I.; Tejada, S.; Devkota, H.P.; Ullah, H.; Aschner, M. Polyphenols in the treatment of autoimmune diseases. Autoimmun. Rev. 2019, 18, 647–657. [Google Scholar] [CrossRef]
- Shi, H.; Lin, B.; Huang, Y.; Wu, J.; Zhang, H.; Lin, C.; Wang, Z.; Zhu, J.; Zhao, Y.; Fu, X.; et al. Basic fibroblast growth factor promotes melanocyte migration via activating PI3K/Akt-Rac1-FAK-JNK and ERK signaling pathways. IUBMB Life 2016, 68, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Niu, C.; Liao, L.-X.; Dou, J.; Habasi, M.; Aisa, H.A. An Isoxazole Chalcone Derivative Enhances Melanogenesis in B16 Melanoma Cells via the Akt/GSK3β/β-Catenin Signaling Pathways. Molecules 2017, 22, 2077. [Google Scholar] [CrossRef] [Green Version]
- Wahid, M.; Jawed, A.; Mandal, R.K.; Dar, S.A.; Akhter, N.; Somvanshi, P.; Khan, F.; Lohani, M.; Areeshi, M.Y.; Haque, S. Recent developments and obstacles in the treatment of melanoma with BRAF and MEK inhibitors. Crit. Rev. Oncol. Hematol. 2018, 125, 84–88. [Google Scholar] [CrossRef]
- Luan, W.; Li, R.; Liu, L.; Ni, X.; Shi, Y.; Xia, Y.; Wang, J.; Lu, F.; Xu, B. Long non-coding RNA HOTAIR acts as a competing endogenous RNA to promote malignant melanoma progression by sponging miR-152-3p. Oncotarget 2017, 8, 85401–85414. [Google Scholar] [CrossRef] [Green Version]
- Zang, D.; Niu, C.; Aisa, H.A. Amine derivatives of furocoumarin induce melanogenesis by activating Akt/GSK-3β/β-catenin signal pathway. Drug. Des. Devel Ther. 2019, 13, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Yang, Q.; Yang, X.; Yan, H.-B.; Lu, Q.-P. Hyperoside protects human primary melanocytes against H2O2-induced oxidative damage. Mol. Med. Rep. 2016, 13, 4613–4619. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S. Ginkgo biloba extract protects human melanocytes from H2O2-induced oxidative stress by activating Nrf2. J. Cell. Mol. Med. 2019, 23, 5193–5199. [Google Scholar] [CrossRef] [Green Version]
- Bernardini, S.; Tiezzi, A.; Masci, V.L.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef]
- Hussein, R.A.; El-Anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. Herb. Med. 2019, 1. [Google Scholar]
- Aharoni, A.; Galili, G. Metabolic engineering of the plant primary-secondary metabolism interface. Curr. Opin. Biotechnol. 2011, 22, 239–244. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [Green Version]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived from the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant. Sci. 2019, 10, 835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids—Food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar] [PubMed]
- Clark, J.; Zahradka, P.; Taylor, C.G. Efficacy of flavonoids in the management of high blood pressure. Nutr. Rev. 2015, 73, 799–822. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. Biomed. Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Man, M.Q.; Hupe, M.; Sun, R.; Man, G.; Mauro, T.M.; Elias, P.M. Topical apigenin alleviates cutaneous inflammation in murine models. Evid.-Based Complement. Altern. Med. 2012, 2012, 912028. [Google Scholar] [CrossRef] [Green Version]
- Hou, M.; Sun, R.; Hupe, M.; Kim, P.L.; Park, K.; Crumrine, D.; Lin, T.-K.; Santiago, J.L.; Mauro, T.M.; Elias, P.M.; et al. Topical apigenin improves epidermal permeability barrier homoeostasis in normal murine skin by divergent mechanisms. Exp. Dermatol. 2013, 22, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Arsić, I.; Tadić, V.; Vlaović, D.; Homšek, I.; Vesić, S.; Isailović, G.; Vuleta, G. Preparation of novel apigenin-enriched, liposomal and non-liposomal, antiinflammatory topical formulations as substitutes for corticosteroid therapy. Phytother. Res. 2011, 25, 228–233. [Google Scholar] [CrossRef]
- Jelić, D.; Lower-Nedza, A.D.; Brantner, A.H.; Blažeković, B.; Bian, B.; Yang, J.; Brajša, K.; Vladimir-Knežević, S. Baicalin and Baicalein Inhibit Src Tyrosine Kinase and Production of IL-6. J. Chem. 2016, 2016, 2510621. [Google Scholar] [CrossRef] [Green Version]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. Neuroprotective and Cognitive Enhancement Potentials of Baicalin: A Review. Brain Sci. 2018, 8, 104. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Li, Z.; Zhu, G. Immunological regulatory effect of flavonoid baicalin on innate immune toll-like receptors. Pharm. Res. 2020, 158, 104890. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, R.; Shi, W.; Li, L.; Liu, H.; Liu, Z.; Wu, L. The Multifunctional Benefits of Naturally Occurring Delphinidin and Its Glycosides. J. Agric. Food Chem. 2019, 67, 11288–11306. [Google Scholar] [CrossRef]
- Wolfram, S. Effects of green tea and EGCG on cardiovascular and metabolic health. J. Am. Coll. Nutr. 2007, 26, 373s–388s. [Google Scholar] [CrossRef]
- Sanna, V.; Singh, C.K.; Jashari, R.; Adhami, V.M.; Chamcheu, J.C.; Rady, I.; Sechi, M.; Mukhtar, H.; Siddiqui, I.A. Targeted nanoparticles encapsulating (−)-epigallocatechin-3-gallate for prostate cancer prevention and therapy. Sci. Rep. 2017, 7, 41573. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, I.A.; Bharali, D.J.; Nihal, M.; Adhami, V.M.; Khan, N.; Chamcheu, J.C.; Khan, M.I.; Shabana, S.; Mousa, S.A.; Mukhtar, H. Excellent anti-proliferative and pro-apoptotic effects of (−)-epigallocatechin-3-gallate encapsulated in chitosan nanoparticles on human melanoma cell growth both in vitro and in vivo. Nanomedicine 2014, 10, 1619–1626. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Siddiqui, I.A.; Adhami, V.M.; Esnault, S.; Bharali, D.J.; Babatunde, A.S.; Adame, S.; Massey, R.J.; Wood, G.S.; Longley, B.J. Chitosan-based nanoformulated (−)-epigallocatechin-3-gallate (EGCG) modulates human keratinocyte-induced responses and alleviates imiquimod-induced murine psoriasiform dermatitis. Int. J. Nanomed. 2018, 13, 4189–4206. [Google Scholar] [CrossRef] [Green Version]
- Frasheri, L.; Schielein, M.C.; Tizek, L.; Mikschl, P.; Biedermann, T.; Zink, A. Great green tea ingredient? A narrative literature review on epigallocatechin gallate and its biophysical properties for topical use in dermatology. Phytother. Res. 2020, 34, 2170–2179. [Google Scholar] [CrossRef] [PubMed]
- Dondalska, A.; Rönnberg, E.; Ma, H.; Pålsson, S.A.; Magnusdottir, E.; Gao, T.; Adam, L.; Lerner, E.A.; Nilsson, G.; Lagerström, M.; et al. Amelioration of Compound 48/80-Mediated Itch and LL-37-Induced Inflammation by a Single-Stranded Oligonucleotide. Front. Immunol. 2020, 11, 559589. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, H.; Zhao, Z.; Huang, X.; Xiong, H.; Mei, Z. Thymol activates TRPM8-mediated Ca(2+) influx for its antipruritic effects and alleviates inflammatory response in Imiquimod-induced mice. Toxicol. Appl. Pharm. 2020, 407, 115247. [Google Scholar] [CrossRef] [PubMed]
- Follansbee, T.; Zhou, Y.; Wu, X.; Delahanty, J.; Nguyen, A.; Domocos, D.; Carstens, M.I.; Hwang, S.T.; Carstens, E. Signs of chronic itch in the mouse imiquimod model of psoriasiform dermatitis: Sex differences and roles of TRPV1 and TRPA1. Itch 2019, 4, e25. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zhou, F.-M.; Su, C.-J.; Liu, T.-T.; Zhou, Y.; Fan, L.; Wang, Z.-H.; Liu, X.; Huang, Y.; Liu, T.; et al. Epigallocatechin-3-gallate attenuates acute and chronic psoriatic itch in mice: Involvement of antioxidant, anti-inflammatory effects and suppression of ERK and Akt signaling pathways. Biochem. Biophys. Res. Commun. 2018, 496, 1062–1068. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Kwon, H.H.; Min, S.U.; Thiboutot, D.M.; Suh, D.H. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J. Investig. Dermatol. 2013, 133, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.H.; Hsu, C.H. Does supplementation with green tea extract improve acne in post-adolescent women? A randomized, double-blind, and placebo-controlled clinical trial. Complement. Ther. Med. 2016, 25, 159–163. [Google Scholar] [CrossRef]
- Jones, V.A.; Patel, P.M.; Wilson, C.; Wang, H.; Ashack, K.A. Complementary and alternative medicine treatments for common skin diseases: A systematic review and meta-analysis. JAAD Int. 2021, 2, 76–93. [Google Scholar] [CrossRef]
- Maher, P. How fisetin reduces the impact of age and disease on CNS function. Front. Biosci. Sch. Ed. 2015, 7, 58–82. [Google Scholar] [CrossRef]
- Chiruta, C.; Schubert, D.; Dargusch, R.; Maher, P. Chemical modification of the multitarget neuroprotective compound fisetin. J. Med. Chem. 2012, 55, 378–389. [Google Scholar] [CrossRef]
- Roy, T.; Boateng, S.T.; Banang-Mbeumi, S.; Singh, P.K.; Basnet, P.; Chamcheu, R.-C.N.; Ladu, F.; Chauvin, I.; Spiegelman, V.S.; Hill, R.A.; et al. Synthesis, inverse docking-assisted identification and in vitro biological characterization of Flavonol-based analogs of fisetin as c-Kit, CDK2 and mTOR inhibitors against melanoma and non-melanoma skin cancers. Bioorg. Chem. 2021, 107, 104595. [Google Scholar] [CrossRef]
- Lall, R.K.; Adhami, V.M.; Mukhtar, H. Dietary flavonoid fisetin for cancer prevention and treatment. Mol. Nutr. Food Res. 2016, 60, 1396–1405. [Google Scholar] [CrossRef]
- Maher, P.; Akaishi, T.; Abe, K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc. Natl. Acad. Sci. USA 2006, 103, 16568–16573. [Google Scholar] [CrossRef] [Green Version]
- Sahu, B.D.; Kalvala, A.K.; Koneru, M.; Kumar, J.M.; Kuncha, M.; Rachamalla, S.S.; Sistla, R. Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-κB activation and antioxidant defence. PLoS ONE 2014, 9, e105070. [Google Scholar] [CrossRef]
- Suh, Y.; Afaq, F.; Khan, N.; Johnson, J.J.; Khusro, F.H.; Mukhtar, H. Fisetin induces autophagic cell death through suppression of mTOR signaling pathway in prostate cancer cells. Carcinogenesis 2010, 31, 1424–1433. [Google Scholar] [CrossRef] [Green Version]
- Chamcheu, J.C.; Esnault, S.; Adhami, V.M.; Noll, A.L.; Banang-Mbeumi, S.; Roy, T.; Singh, S.S.; Huang, S.; Kousoulas, K.G.; Mukhtar, H. Fisetin, a 3,7,3′,4′-Tetrahydroxyflavone Inhibits the PI3K/Akt/mTOR and MAPK Pathways and Ameliorates Psoriasis Pathology in 2D and 3D Organotypic Human Inflammatory Skin Models. Cells 2019, 8, 1089. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.D.; Lee, S.E.; Park, Y.S.; Shin, D.-H.; Park, G.G.; Park, C.-S. Immunosuppressive effects of fisetin against dinitrofluorobenzene-induced atopic dermatitis-like symptoms in NC/Nga mice. Food Chem. Toxicol. 2014, 66, 341–349. [Google Scholar] [CrossRef]
- Che, D.N.; Cho, B.O.; Shin, J.Y.; Kang, H.J.; Kim, Y.-S.; Jang, S.I. Fisetin inhibits IL-31 production in stimulated human mast cells: Possibilities of fisetin being exploited to treat histamine-independent pruritus. Life Sci. 2018, 201, 121–129. [Google Scholar] [CrossRef]
- Kawai, M.; Hirano, T.; Higa, S.; Arimitsu, J.; Maruta, M.; Kuwahara, Y.; Ohkawara, T.; Hagihara, K.; Yamadori, T.; Shima, Y.; et al. Flavonoids and related compounds as anti-allergic substances. Allergol. Int. 2007, 56, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.M.; Bautista, R.J.H.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef] [Green Version]
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Chapter 76—Cardiovascular Effects of Hesperidin: A Flavanone Glycoside. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 989–992. [Google Scholar]
- Hou, M.; Man, M.; Man, W.; Zhu, W.; Hupe, M.; Park, K.; Crumrine, D.; Elias, P.M.; Man, M.-Q. Topical hesperidin improves epidermal permeability barrier function and epidermal differentiation in normal murine skin. Exp. Dermatol. 2012, 21, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Nagashio, Y.; Matsuura, Y.; Miyamoto, J.; Kometani, T.; Suzuki, T.; Tanabe, S. Hesperidin inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice by suppressing Th17 activity. J. Funct. Foods 2013, 5, 1633–1641. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Özay, Y.; Güzel, S.; Yumrutaş, Ö.; Pehlivanoğlu, B.; Erdoğdu, İ.H.; Yildirim, Z.; Türk, B.A.; Darcan, S. Wound Healing Effect of Kaempferol in Diabetic and Nondiabetic Rats. J. Surg. Res. 2019, 233, 284–296. [Google Scholar] [CrossRef]
- Kritas, S.K.; Saggini, A.; Varvara, G.; Murmura, G.; Caraffa, A.; Antinolfi, P.; Toniato, E.; Pantalone, A.; Neri, G.; Frydas, S.; et al. Luteolin inhibits mast cell-mediated allergic inflammation. J. Biol. Regul. Homeost. Agents 2013, 27, 955–959. [Google Scholar]
- Zhou, W.; Hu, M.; Zang, X.; Liu, Q.; Du, J.; Hu, J.; Zhang, L.; Du, Z.; Xiang, Z. Luteolin attenuates imiquimod-induced psoriasis-like skin lesions in BALB/c mice via suppression of inflammation response. Biomed. Pharm. 2020, 131, 110696. [Google Scholar] [CrossRef]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. Biofactors 2021, 47, 170–180. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119 Pt A, 1–11. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef]
- Assini, J.M.; Mulvihill, E.E.; Huff, M.W. Citrus flavonoids and lipid metabolism. Curr. Opin. Lipidol. 2013, 24, 34–40. [Google Scholar] [CrossRef]
- Joshi, R.; Kulkarni, Y.A.; Wairkar, S. Pharmacokinetic, pharmacodynamic and formulations aspects of Naringenin: An update. Life Sci. 2018, 215, 43–56. [Google Scholar] [CrossRef]
- Escribano-Ferrer, E.; Regué, J.Q.; Garcia-Sala, X.; Montañés, A.B.; Lamuela-Raventos, R.M. In Vivo Anti-inflammatory and Antiallergic Activity of Pure Naringenin, Naringenin Chalcone, and Quercetin in Mice. J. Nat. Prod. 2019, 82, 177–182. [Google Scholar] [CrossRef]
- Akrawi, S.H.; Gorain, B.; Nair, A.B.; Choudhury, H.; Pandey, M.; Shah, J.N.; Venugopala, K.N. Development and Optimization of Naringenin-Loaded Chitosan-Coated Nanoemulsion for Topical Therapy in Wound Healing. Pharmaceutics 2020, 12, 893. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Vargas, A.J.; Burd, R. Hormesis and synergy: Pathways and mechanisms of quercetin in cancer prevention and management. Nutr. Rev. 2010, 68, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Shin, S.A.; Choo, G.S.; Kim, H.J.; Park, Y.S.; Kim, B.S.; Kim, S.K.; Cho, S.D.; Nam, J.S.; Choi, C.S.; et al. Anti-inflammatory effect of quercetin and galangin in LPS-stimulated RAW264.7 macrophages and DNCB-induced atopic dermatitis animal models. Int. J. Mol. Med. 2018, 41, 888–898. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Lu, C.; Liu, H.; Wang, M.; Zhao, H.; Yan, Y.; Han, L. Quercetin ameliorates imiquimod-induced psoriasis-like skin inflammation in mice via the NF-κB pathway. Int. Immunopharmacol. 2017, 48, 110–117. [Google Scholar] [CrossRef]
- Doersch, K.M.; Newell-Rogers, M.K. The impact of quercetin on wound healing relates to changes in αV and β1 integrin expression. Exp. Biol. Med. 2017, 242, 1424–1431. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Sumiyoshi, M. Effects of baicalein and wogonin isolated from Scutellaria baicalensis roots on skin damage in acute UVB-irradiated hairless mice. Eur. J. Pharmacol. 2011, 661, 124–132. [Google Scholar] [CrossRef]
- Noh, D.; Choi, J.G.; Huh, E.; Oh, M.S. Tectorigenin, a Flavonoid-Based Compound of Leopard Lily Rhizome, Attenuates UV-B-Induced Apoptosis and Collagen Degradation by Inhibiting Oxidative Stress in Human Keratinocytes. Nutrients 2018, 10, 1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Li, S.; Yang, Y.; Yuan, L.; Wang, P.; Zhao, H.; Ho, C.-T.; Lin, C.-C. Nobiletin and 5-Hydroxy-6,7,8,3′,4′-pentamethoxyflavone Ameliorate 12- O-Tetradecanoylphorbol-13-acetate-Induced Psoriasis-Like Mouse Skin Lesions by Regulating the Expression of Ki-67 and Proliferating Cell Nuclear Antigen and the Differentiation of CD4(+) T Cells through Mitogen-Activated Protein Kinase Signaling Pathways. J. Agric. Food Chem. 2018, 66, 8299–8306. [Google Scholar] [PubMed]
- Jang, S.E.; Ryu, K.-R.; Park, S.-H.; Chung, S.; Teruya, Y.; Han, M.J.; Woo, J.-T.; Kim, D.-H. Nobiletin and tangeretin ameliorate scratching behavior in mice by inhibiting the action of histamine and the activation of NF-κB, AP-1 and p38. Int. Immunopharmacol. 2013, 17, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Cho, J.-G.; Hwang, E.-S.; Yang, J.-E.; Gao, W.; Fang, M.-Z.; Zheng, S.-D.; Yi, T.-H. Enhancement of Protective Effects of Radix Scutellariae on UVB-induced Photo Damage in Human HaCaT Keratinocytes. Appl. Biochem. Biotechnol. 2018, 184, 1073–1093. [Google Scholar] [CrossRef] [PubMed]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an active ingredient for cosmetic and dermatological applications: A review. J. Cosmet. Laser Ther. 2019, 21, 84–90. [Google Scholar] [CrossRef]
- Kang, M.C.; Cho, K.; Lee, J.H.; Subedi, L.; Yumnam, S.; Kim, S.Y. Effect of Resveratrol-Enriched Rice on Skin Inflammation and Pruritus in the NC/Nga Mouse Model of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 1428. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.; Zhang, J.; Yang, B.; Elias, P.M.; Man, M.-Q. Role of Resveratrol in Regulating Cutaneous Functions. Evid.-Based Complement. Altern. Med. 2020, 2020, 2416837. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Atala, A. Small molecules and small molecule drugs in regenerative medicine. Drug Discov. Today 2014, 19, 801–808. [Google Scholar] [CrossRef]
- Uva, L.; Miguel, D.; Pinheiro, C.; Antunes, J.; Cruz, D.; Ferreira, J.; Filipe, P. Mechanisms of action of topical corticosteroids in psoriasis. Int. J. Endocrinol. 2012, 2012, 561018. [Google Scholar] [CrossRef] [Green Version]
- Jovanović, M.; Golušin, Z. Nonsteroidal Topical Immunomodulators in Allergology and Dermatology. Biomed. Res. Int. 2016, 2016, 5185303. [Google Scholar] [CrossRef] [Green Version]
- No Author Listed. Drugs for acne. Med. Lett. Drugs Ther. 2020, 62, 188–191. [Google Scholar]
- Edslev, S.M.; Andersen, P.S.; Agner, T.; Saunte, D.M.L.; Ingham, A.C.; Johannesen, T.B.; Clausen, M.-L. Identification of cutaneous fungi and mites in adult atopic dermatitis: Analysis by targeted 18S rRNA amplicon sequencing. BMC Microbiol. 2021, 21, 72. [Google Scholar] [CrossRef]
- Wollina, U. Microbiome in atopic dermatitis. Clin. Cosmet. Investig. Dermatol. 2017, 10, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Dimitrov, D.S. Therapeutic proteins. Methods Mol. Biol. 2012, 899, 1–26. [Google Scholar]
- Dogra, S.; Mahajan, R. Biologics in pediatric psoriasis—Efficacy and safety. Expert. Opin. Drug. Saf. 2018, 17, 9–16. [Google Scholar] [CrossRef]
- Plachouri, K.-M.; Georgiou, S. Special aspects of biologics treatment in psoriasis: Management in pregnancy, lactation, surgery, renal impairment, hepatitis and tuberculosis. J. Dermatol. Treat. 2019, 30, 668–673. [Google Scholar] [CrossRef]
- Van den Reek, J. Predicting treatment success with biologics in psoriasis. Br. J. Dermatol. 2019, 181, 438. [Google Scholar] [CrossRef] [Green Version]
- Sethu, S.; Govindappa, K.; Alhaidari, M.; Pirmohamed, M.; Park, K.; Sathish, J. Immunogenicity to biologics: Mechanisms, prediction and reduction. Arch. Immunol. Ther. Exp. 2012, 60, 331–344. [Google Scholar] [CrossRef]
- Blauvelt, A.; Papp, K.A.; Lebwohl, M.G.; Green, L.J.; Hsu, S.; Bhatt, V.; Rastogi, S.; Pillai, R.; Israel, R. Rapid onset of action in patients with moderate-to-severe psoriasis treated with brodalumab: A pooled analysis of data from two phase 3 randomized clinical trials (AMAGINE-2 and AMAGINE-3). J. Am. Acad. Dermatol. 2017, 77, 372–374. [Google Scholar] [CrossRef] [Green Version]
- Gordon, K.B.; Blauvelt, A.; Papp, K.A.; Langley, R.G.; Luger, T.; Ohtsuki, M.; Reich, K.; Amato, D.; Ball, S.G.; Braun, D.K.; et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2016, 375, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.G.; Elewski, B.E.; Lebwohl, M.; Reich, K.; Griffiths, C.E.; Papp, K.; Puig, L.; Nakagawa, H.; Spelman, L.; Sigurgeirsson, B.; et al. Secukinumab in plaque psoriasis—Results of two phase 3 trials. N. Engl. J. Med. 2014, 371, 326–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chima, M.; Lebwohl, M. TNF inhibitors for psoriasis. Semin. Cutan. Med. Surg. 2018, 37, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Strober, B.E.; Kaplan, D.H.; Kivelevitch, D.; Prater, E.F.; Stoff, B.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Davis, D.M.; et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J. Am. Acad. Dermatol. 2019, 80, 1029–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerriets, V.; Goyal, A.; Khaddour, K. Tumor Necrosis Factor Inhibitors. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2021. [Google Scholar]
- Li, H.; Xie, S.; Qi, Y.; Li, H.; Zhang, R.; Lian, Y. TNF-α increases the expression of inflammatory factors in synovial fibroblasts by inhibiting the PI3K/AKT pathway in a rat model of monosodium iodoacetate-induced osteoarthritis. Exp. Ther. Med. 2018, 16, 4737–4744. [Google Scholar] [CrossRef] [Green Version]
- Jensen, L.E. Targeting the IL-1 family members in skin inflammation. Curr. Opin. Investig. Drugs 2010, 11, 1211–1220. [Google Scholar]
- Gaspari, A.A.; Tyring, S. New and emerging biologic therapies for moderate-to-severe plaque psoriasis: Mechanistic rationales and recent clinical data for IL-17 and IL-23 inhibitors. Dermatol. Ther. 2015, 28, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Vu, Y.H.; Furue, M.; Tsuji, G. The role of interleukin-24 in atopic dermatitis. Exploration of Immunology. Explor. Immunol. 2021, 1, 4–15. [Google Scholar]
- Nagel, A.; Hertl, M.; Eming, R. B-cell-directed therapy for inflammatory skin diseases. J. Investig. Dermatol. 2009, 129, 289–301. [Google Scholar] [CrossRef] [Green Version]
- Didona, D.; Maglie, R.; Eming, R.; Hertl, M. Pemphigus: Current and Future Therapeutic Strategies. Front. Immunol. 2019, 10, 1418. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, R.; Wisniewski, J.A.; Woodfolk, J.A. The role of regulatory T cells in atopic dermatitis. Curr. Probl. Dermatol. 2011, 41, 112–124. [Google Scholar]
- Gordon, K.B.; McCormick, T.S. Evolution of biologic therapies for the treatment of psoriasis. Skinmed 2003, 2, 286–294. [Google Scholar]
- Chen, Y.; Zhang, Q.; Liu, H.; Lu, C.; Liang, C.-L.; Qiu, F.; Han, L.; Dai, Z. Esculetin Ameliorates Psoriasis-Like Skin Disease in Mice by Inducing CD4(+)Foxp3(+) Regulatory T Cells. Front. Immunol. 2018, 9, 2092. [Google Scholar] [CrossRef]
- Wu, Z.; Uchi, H.; Morino-Koga, S.; Shi, W.; Furue, M. Resveratrol inhibition of human keratinocyte proliferation via SIRT1/ARNT/ERK dependent downregulation of aquaporin 3. J. Dermatol. Sci. 2014, 75, 16–23. [Google Scholar] [CrossRef]
- Sun, Y.; Lenon, G.B.; Yang, A.W.H. Phellodendri Cortex: A Phytochemical, Pharmacological, and Pharmacokinetic Review. Evid.-Based Complement. Alternat Med. 2019, 2019, 7621929. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.Y. Hair-Growth Potential of Ginseng and Its Major Metabolites: A Review on Its Molecular Mechanisms. Int. J. Mol. Sci. 2018, 19, 2703. [Google Scholar] [CrossRef] [Green Version]
- Varshney, P.; Saini, N. PI3K/AKT/mTOR activation and autophagy inhibition plays a key role in increased cholesterol during IL-17A mediated inflammatory response in psoriasis. Biochim. Biophys. Mol. Basis Dis. 2018, 1864, 1795–1803. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Sengupta, S.; Sheen, J.-H.; Hsu, P.P.; Bagley, A.F.; Markhard, A.L.; Sabatini, D.M. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 2006, 22, 159–168. [Google Scholar] [CrossRef]
- Agarwal, S.; Mirzoeva, S.; Readhead, B.; Dudley, J.T.; Budunova, I. PI3K inhibitors protect against glucocorticoid-induced skin atrophy. EBioMedicine 2019, 41, 526–537. [Google Scholar] [CrossRef] [Green Version]
- Im, A.R.; Kim, Y.-M.; Chin, Y.-W.; Chae, S. Protective effects of compounds from Garcinia mangostana L. (mangosteen) against UVB damage in HaCaT cells and hairless mice. Int. J. Mol. Med. 2017, 40, 1941–1949. [Google Scholar] [CrossRef] [Green Version]
- Jeon, Y.J.; Kim, B.-H.; Kim, S.; Oh, I.; Lee, S.; Shin, J.; Kim, T.-Y. Rhododendrin ameliorates skin inflammation through inhibition of NF-κB, MAPK, and PI3K/Akt signaling. Eur. J. Pharmacol. 2013, 714, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, C.D.; Mendes, D.A.G.B.; Nolte, S.; de Brito, P.S.; da Silva Soley, B.; Favero, G.M.; Facundo, V.A.; Santos, A.R.S.; de Almeida Cabrini, D.; Otuki, M.F. Anti-proliferative and anti-inflammatory effects of 3β,6β,16β-Trihydroxylup-20(29)-ene on cutaneous inflammation. J. Ethnopharmacol. 2017, 195, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Pal, H.C.; Athar, M.; Elmets, C.A.; Afaq, F. Fisetin inhibits UVB-induced cutaneous inflammation and activation of PI3K/AKT/NFκB signaling pathways in SKH-1 hairless mice. Photochem. Photobiol. 2015, 91, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Ito, T.; Gilhar, A.; Tokura, Y.; Reich, K.; Paus, R. The hair follicle-psoriasis axis: Shared regulatory mechanisms and therapeutic targets. Exp. Dermatol. 2021, 31, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Pourang, A.; Mesinkovska, N.A. New and Emerging Therapies for Alopecia Areata. Drugs 2020, 80, 635–646. [Google Scholar] [CrossRef]
- Milani, G.B.; Camponogara, C.; Piana, M.; Silva, C.R.; Oliveira, S.M. Cariniana domestica fruit peels present topical anti-inflammatory efficacy in a mouse model of skin inflammation. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 513–528. [Google Scholar] [CrossRef]
- Camponogara, C.; Casoti, R.; Brusco, I.; Piana, M.; Boligon, A.A.; Cabrini, D.A.; Trevisan, G.; Ferreira, J.; Silva, C.R.; Oliveira, S.M. Tabernaemontana catharinensis leaves effectively reduce the irritant contact dermatitis by glucocorticoid receptor-dependent pathway in mice. Biomed. Pharmacother. 2019, 109, 646–657. [Google Scholar] [CrossRef]
- Camponogara, C.; Silva, C.R.; Brusco, I.; Piana, M.; Faccin, H.; de Carvalho, L.M.; Schuch, A.; Trevisan, G.; Oliveira, S.M. Nasturtium officinale R. Br. effectively reduces the skin inflammation induced by croton oil via glucocorticoid receptor-dependent and NF-κB pathways without causing toxicological effects in mice. J. Ethnopharmacol. 2019, 229, 190–204. [Google Scholar] [CrossRef]
- Yue, L.; Ailin, W.; Jinwei, Z.; Leng, L.; Jianan, W.; Li, L.; Haiming, C.; Ling, H.; Chuanjian, L. PSORI-CM02 ameliorates psoriasis in vivo and in vitro by inducing autophagy via inhibition of the PI3K/Akt/mTOR pathway. Phytomedicine 2019, 64, 153054. [Google Scholar] [CrossRef]
- McLoone, P.; Oluwadun, A.; Warnock, M.; Fyfe, L. Honey: A Therapeutic Agent for Disorders of the Skin. Cent. Asian J. Glob. Health 2016, 5, 241. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Tyagi, A.K.; Deshmukh-Taskar, P.; Hinojosa, M.; Prasad, S.; Aggarwal, B.B. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch. Biochem. Biophys. 2014, 559, 91–99. [Google Scholar] [CrossRef]
- Helmy, A.Y.; El Batreek, M.H.; Fadeel, D.A.A.; Tawfik, A.A.; Samy, N.A. Efficacy of the topical cyclosporine cream assisted by fractional carbon dioxide laser vs topical clobetasol cream for the treatment of plaque psoriasis: Randomized comparative study. J. Cosmet. Dermatol. 2021, 21, 3362–3370. [Google Scholar] [CrossRef]
- Lee, S.H.; Yu, J.; Cho, S.H. Effect of a combination of Korean red ginseng extract and probiotics on the prevention of atopic dermatitis in a murine model. J. Ethnopharmacol. 2022, 283, 114687. [Google Scholar] [CrossRef]
- Kumsa, S.M.; Tadesse, T.A.; Woldu, M.A. Management practice, quality of life and associated factors in psoriasis patients attending a dermatological center in Ethiopia. PLoS ONE 2021, 16, e0260243. [Google Scholar] [CrossRef]
- Van den Bogaard, E.H.; Esser, C.; Perdew, G.H. The aryl hydrocarbon receptor at the forefront of host-microbe interactions in the skin: A perspective on current knowledge gaps and directions for future research and therapeutic applications. Exp. Dermatol. 2021, 30, 1477–1483. [Google Scholar] [CrossRef]
- Kansal, N.K.; Kumar, A. Psoriasis Alba (White Psoriasis) of the Perianal Region. Skinmed 2021, 19, 390–391. [Google Scholar]
- Bell, M.A.; Whang, K.A.; Thomas, J.; Aguh, C.; Kwatra, S.G. Racial and Ethnic Disparities in Access to Emerging and Frontline Therapies in Common Dermatological Conditions: A Cross-Sectional Study. J. Natl. Med. Assoc. 2020, 112, 650–653. [Google Scholar] [CrossRef]
- Mahasaksiri, T.; Kositkuljorn, C.; Anuntrangsee, T.; Suchonwanit, P. Application of Topical Immunotherapy in the Treatment of Alopecia Areata: A Review and Update. Drug Des. Dev. Ther. 2021, 15, 1285–1298. [Google Scholar] [CrossRef]
- Datta-Mitra, A.; Mitra, A.; Ray, R.; Raychaudhuri, S.P.; Kundu-Raychaudhuri, S. 1,25-Dihydroxyvitamin D3-3-bromoacetate, a novel vitamin D analog induces immunosuppression through PI3K/Akt/mTOR signaling cascade. Int. Immunopharmacol. 2013, 17, 744–751. [Google Scholar] [CrossRef]
- Datta Mitra, A.; Raychaudhuri, S.P.; Abria, C.J.; Mitra, A.; Wright, R.; Ray, R.; Kundu-Raychaudhuri, S. 1α,25-Dihydroxyvitamin-D3-3-bromoacetate regulates AKT/mTOR signaling cascades: A therapeutic agent for psoriasis. J. Investig. Dermatol. 2013, 133, 1556–1564. [Google Scholar] [CrossRef] [Green Version]
- Voûte, A.B.; Schulten, E.A.; Langendijk, P.N.; Kostense, P.J.; van der Waal, I. Fluocinonide in an adhesive base for treatment of oral lichen planus. A double-blind, placebo-controlled clinical study. Oral Surg. Oral Med. Oral Pathol. 1993, 75, 181–185. [Google Scholar] [CrossRef] [PubMed]
- No author listed. Drugs for atopic dermatitis. Med. Lett. Drugs Ther. 2020, 62, 89–96. [Google Scholar]
- Dasht Bozorg, B.; Bhattaccharjee, S.A.; Somayaji, M.R.; Banga, A.K. Topical and transdermal delivery with diseased human skin: Passive and iontophoretic delivery of hydrocortisone into psoriatic and eczematous skin. Drug Deliv. Transl. Res. 2022, 12, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Hassan, S.K.U. Two Percentage of Ketoconazole Cream for the Treatment of Adult Female Acne: A Placebo-Controlled Trial. Cureus 2020, 12, e11581. [Google Scholar] [CrossRef]
- Ochoa-Gonzalez, F.; Cervantes-Villagrana, A.R.; Fernandez-Ruiz, J.C.; Nava-Ramirez, H.S.; Hernandez-Correa, A.C.; Enciso-Moreno, J.A.; Castañeda-Delgado, J.E. Metformin Induces Cell Cycle Arrest, Reduced Proliferation, Wound Healing Impairment In Vivo and Is Associated to Clinical Outcomes in Diabetic Foot Ulcer Patients. PLoS ONE 2016, 11, e0150900. [Google Scholar]
- Pretel, M.; España, A.; Marquina, M.; Pelacho, B.; López-Picazo, J.M.; López-Zabalza, M.J. An imbalance in Akt/mTOR is involved in the apoptotic and acantholytic processes in a mouse model of pemphigus vulgaris. Exp. Dermatol. 2009, 18, 771–780. [Google Scholar] [CrossRef]
- Eichenfield, D.Z.; Sprague, J.; Eichenfield, L.F. Management of Acne Vulgaris: A Review. JAMA 2021, 326, 2055–2067. [Google Scholar] [CrossRef]
- Jenerowicz, D.; Kaznowska, J.; Bartkiewicz, P.; Sadowska-Przytocka, A.; Szymkowiak, M.; Adamski, Z.; Czarnecka-Operacz, M. Contemporary treatment patterns in plaque psoriasis and psoriatic arthritis. Postepy Dermatol. Alergol. 2021, 38, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Ricardo, J.W.; Lipner, S.R. Nail Psoriasis in Older Adults: Intralesional, Systemic, and Biological Therapy. Dermatol. Clin. 2021, 39, 195–210. [Google Scholar] [CrossRef]
- Gallagher, T.; Taliercio, M.; Nia, J.K.; Hashim, P.W.; Zeichner, J.A. Dermatologist Use of Intralesional Triamcinolone in the Treatment of Acne. J. Clin. Aesthet. Dermatol. 2020, 13, 41–43. [Google Scholar]
- Liu, H.; Yu, H.; Xia, J.; Liu, L.; Liu, G.J.; Sang, H.; Peinemann, F. Topical azelaic acid, salicylic acid, nicotinamide, sulphur, zinc and fruit acid (alpha-hydroxy acid) for acne. Cochrane Database Syst. Rev. 2020, 5, Cd011368. [Google Scholar]
- Sanad, E.M.; El-Fallah, A.A.; Al-Doori, A.R.; Salem, R.M. Serum Zinc and Inflammatory Cytokines in Vitiligo. J. Clin. Aesthetic Dermatol. 2020, 13 (Suppl. S1), S29–S33. [Google Scholar]
- Pan, R.; Wang, X.; Shu, M.; Das, J.; Kalra, M.; Wang, Z. Comparative efficacy of secukinumab against adalimumab and infliximab in patients with moderate-to-severe plaque psoriasis. Chin. Med. J. 2021, 135, 11–19. [Google Scholar] [CrossRef]
- Pagnanelli, G.; Cavani, A.; Canzona, F.; Mazzanti, C. Mild therapeutic response of alopecia areata during treatment of psoriasis with secukinumab. Eur. J. Dermatol. 2020, 30, 602–603. [Google Scholar] [CrossRef]
- Cline, A.; Bartos, G.J.; Strowd, L.C.; Feldman, S.R. Biologic Treatment Options for Pediatric Psoriasis and Atopic Dermatitis. Children 2019, 6, 103. [Google Scholar] [CrossRef] [Green Version]
- Mälkönen, T.; Nuutinen, P.; Hallinen, T.; Soini, E.; Nissinen, R.; Wennerstöm, C.; Rantanen, T.; Hagman, J.H.; Harvima, R.; Höök-Nikanne, J.; et al. Guselkumab Treatment Outcomes and Persistence in a Nationwide Real-world Cohort of Patients with Plaque Psoriasis. Acta Derm.-Venereol. 2022, 102, adv00631. [Google Scholar] [CrossRef]
- Sotiriou, E.; Bakirtzi, K.; Papadimitriou, I.; Tsentemeidou, A.; Eftychidou, P.; Eleftheriadis, V.; Lallas, A.; Ioannides, D.; Vakirlis, E. A head-to-head comparison of risankizumab and ixekizumab for genital psoriasis: A real-life, 24-week, prospective study. J. Eur. Acad. Dermatol. Venereol. 2021, 36, e359–e361. [Google Scholar] [CrossRef]
- Lebwohl, M.G.; Soliman, A.M.; Yang, H.; Wang, J.; Hagan, K.; Padilla, B.; Pinter, A. Impact of Risankizumab on PASI90 and DLQI0/1 Duration in Moderate-to-Severe Psoriasis: A Post Hoc Analysis of Four Phase 3 Clinical Trials. Dermatol. Ther. 2021, 12, 407–418. [Google Scholar] [CrossRef]
- Tewari, D.; Samoilă, O.; Gocan, D.; Mocan, A.; Moldovan, C.; Devkota, H.P.; Atanasov, A.G.; Zengin, G.; Echeverría, J.; Vodnar, D.; et al. Medicinal Plants and Natural Products Used in Cataract Management. Front. Pharmacol. 2019, 10, 466. [Google Scholar] [CrossRef] [Green Version]
- Tewari, D.; Mocan, A.; Parvanov, E.D.; Sah, A.N.; Nabavi, S.M.; Huminiecki, L.; Ma, Z.F.; Lee, Y.Y.; Horbańczuk, J.O.; Atanasov, A.G. Ethnopharmacological Approaches for Therapy of Jaundice: Part II. Highly Used Plant Species from Acanthaceae, Euphorbiaceae, Asteraceae, Combretaceae, and Fabaceae Families. Front. Pharmacol. 2017, 8, 519. [Google Scholar] [CrossRef] [Green Version]
- Guarneri, F.; Bertino, L.; Pioggia, G.; Casciaro, M.; Gangemi, S. Therapies with Antioxidant Potential in Psoriasis, Vitiligo, and Lichen Planus. Antioxidants 2021, 10, 1087. [Google Scholar] [CrossRef] [PubMed]

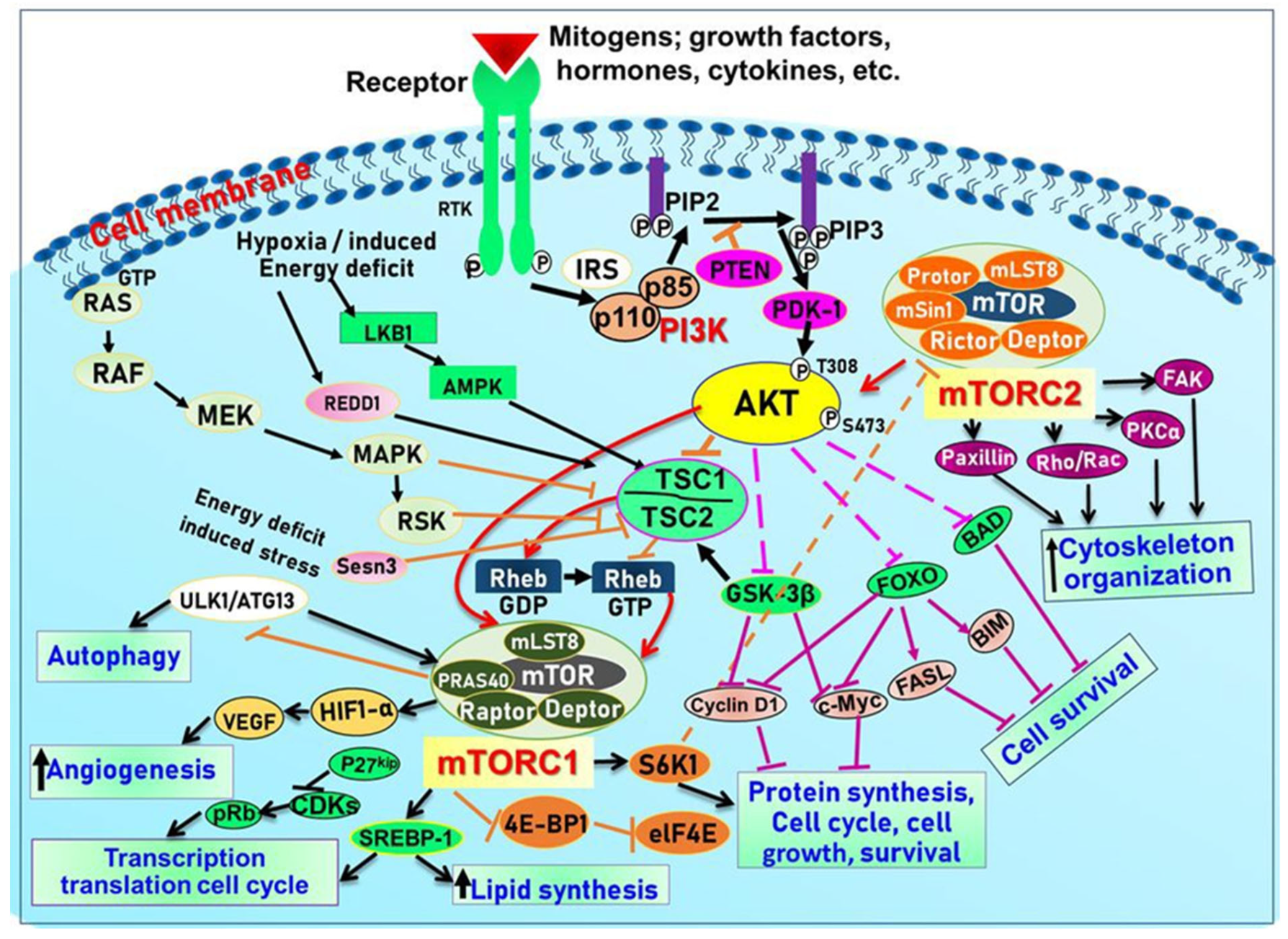
| Phytochemical | Class | Chemical Structures | Protein Target(s) | Chronic Skin Inflammation Type | References |
|---|---|---|---|---|---|
| Esculetin | Coumarin derivatives |  | IKKα and P65 | Psoriasis | [406] |
| ALA-PDT Aminolevulinic acid |  | Akt-/Erk- mTOR -p70S6K pathway | Acne Vulgaris | [229] | |
| Apigenin | Flavone | 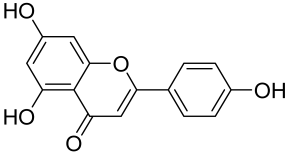 | Inflammatory dermatoses | [318,319,320,321,322,323] | |
| 18-ß glycyrrhetinic acid | Pentacyclic triterpenoid |  | mTOR | Psoriasis | [151] |
| Caffeine | Methylxanthine |  | mTOR | Mycosis fungoides, pemphigus vulgaris, alopecia areata | [96] |
| Curcumin | Diarylheptanoid |  | mTOR/Akt/S6K1 | Mycosis fungoides, pemphigus vulgaris, psoriasis | [96] |
| Epigallocaechin-3- gallate | Catechin | 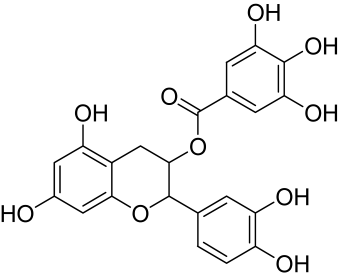 | mTOR/Akt | Mycosis fungoides Pemphigus vulgaris Acne vulgaris | [330,331,332] |
| Erianin | Bibenzyl |  | Akt/mTOR pathway | Psoriasis | [154] |
| Gartanin | Xanthones |  | mTOR/Akt/S6K1 | Mycosis fungoides, pemphigus vulgaris, psoriasis | [96] |
| Hesperidin | Bioflavonoid |  | IL-17 and IFN-α axis | Atopic dermatitis | [352,353,354] |
| Isotretinoin/Retionoic acid | Retinoid |  | FoxO1 mTORC1 | Acne Vulgaris, hidradenitis suppurativa | [244,259] |
| Imperatorin | Furocoumarin | 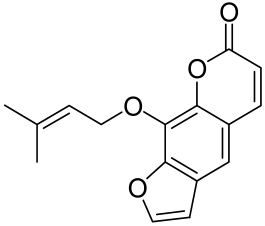 | IGF-1/Akt PPAR-y | Acne Vulgaris | [304] |
| Kaempferol | Flavonol | 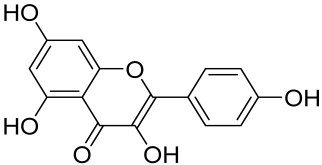 | Reactive oxygen species pathway | Chronic wounds | [355,356] |
| Luteolin | Flavone |  | NF-κB, MAPK, PI3K | Contact dermatitis Psoriasis | [357,358,359,360] |
| Naringenin | Flavanones | 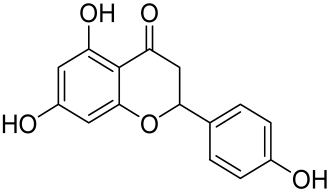 | Reactive oxygen species pathway | Wounds | [361,362,363,364,365,366] |
| Matrine | Alkaloid |  | PI3K/Akt/mTOR pathway | Psoriasis | [153] |
| Paeoniflorin | Monoterpene glycoside | 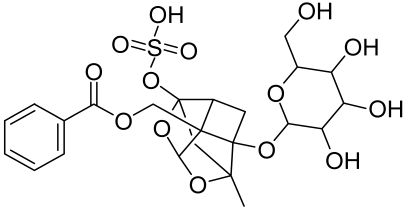 | PI3K/Akt/mTOR pathway | Psoriasis | [152] |
| Psoralen derivatives | Linear furanocoumarins |  | Akt/GSK-3ß/ß-catenin pathway | Vitiligo | [304] |
| Resveratrol | Stilbenol |  | PI3K/mTOR | Mycosis fungoides Pemphigus vulgaris Acne vulgaris | [96,407] |
| Quercetin | Flavonoid | 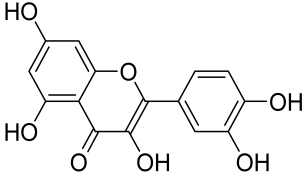 | Akt | Mycosis fungoides, pemphigus vulgaris, acne vulgaris | [96] |
| Obacunone | Tetranortriterpenoids | 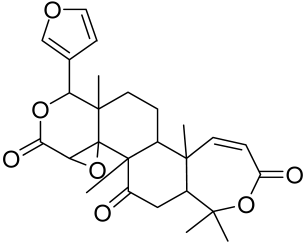 | Akt | Atopic dermatitis | [408] |
| Obaculactone | 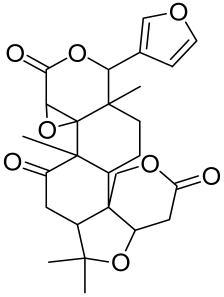 | Akt | Atopic dermatitis | [408] | |
| Ginsenoside Rb1 | Triterpenoid saponins |  | Akt | Alopeciea areata | [409] |
| Ginsenoside Rd | Triterpenoid saponins |  | Akt | Alopeciea areata | [409] |
| Ginsenoside Rh3 | Triterpenoid saponins |  | Akt | Alopeciea areata | [409] |
| Ginsenoside Rg1 | Triterpenoid saponins |  | Akt | Alopeciea areata | [409] |
| Ginsenoside Rg3 | Triterpenoid saponins |  | Akt | Alopeciea areata | [409] |
| Rapamycin | Macrolide | 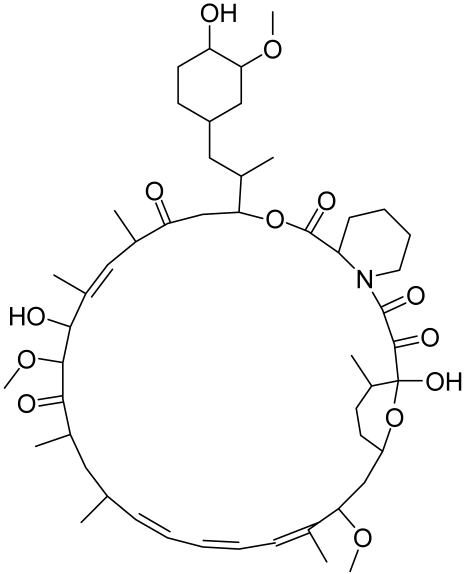 | mTOR | Psoriasis Systemic sclerosis | [408,409,410,411] |
| Wortmannin | Oxa-steroid. |  | PI3K/Akt/ mTOR pathway | Skin atrophy | [412] |
| α-Mangostin | Xanthones |  | MAPK downstream proteins | Antiaging, anti-wrinkle | [413] |
| Rhododendrin | Arylbutanoid glycoside |  | PI3K/Akt/mTOR pathway | Psoriasis | [414] |
| 3β,6β,16β-Trihydroxylup-20(29)-ene | Pentacyclic triterpene |  | Psoriasis | [415] | |
| Geniposide | Iridoid glycoside |  | Akt/mTOR | Systemic sclerosis | [416] |
| Delphinidin | Anthocyanidin |  | PI3K, mTOR/p70S6K | Psoriasis | [140] |
| Fisetin | Flavonol | 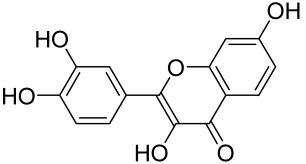 | Akt/mTOR, p70S6K | Psoriasis, UV-induced skin inflammation | [417,418,419] |
| Phytochemical | Class | Protein Target(s) | Chronic Skin Inflammation Type | References |
|---|---|---|---|---|
| Actinidia arguta | Extract | mTOR | Atopic Dermatitis | [110] |
| Crude extract of Carinianadomestica fruit peels (CdE) | Extract | - | Acute and chronic irritant contact dermatitis | [419] |
| Tabernaemontanacatharinensis leaf extract (snakeskin) | Extract | - | Irritant contact dermatitis, skin edema | [420] |
| Nasturtium officinale (watercress) | Extract | - | Irritant contact dermatitis | [421] |
| PSORI-CM02 | Herbs mixture | PI3K/Akt/mTOR | Psoriasis | [422] |
| Honey | Polyphenols and other antioxidants | - | Wounds | [423] |
| Active Agent | Class | Chemical Structures | Protein Target(s) | Chronic Skin Inflammation Type | References |
|---|---|---|---|---|---|
| Everolimus | Immunomodulator/macrolactams |  | mTOR | Psoriasis and tuberous sclerosis | [400,401] |
| Anthralin | Immunomodulator |  | IL, TNF-α and IFN-α axis | Alopecia areata and psoriasis | [159,167,424] |
| Betamethasone dipropionate | Corticosteroid |  | IL and IFN-α axis | Psoriasis and dermatitis | [382] |
| BEZ235 | Kinase inhibitor |  | PI3K/Akt/mTOR pathway | Systemic sclerosis | [170] |
| Cyclosporine | Immunomodulator/macrolactams |  | IL and IFN-α axis | Atopic dermatitis and psoriasis | [425] |
| Clobetasol 17-propionate | Corticosteroid |  | IL and IFN-α axis | Psoriasis and dermatitis | [382,426,427] |
| Coal tar | Keratolytics | Aryl hydrocarbon receptor | Atopic dermatitis and psoriasis | [428] | |
| Desonide | Corticosteroid | 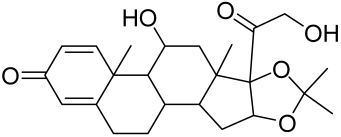 | IL and IFN-α axis | Atopic dermatitis and psoriasis | [382,428,429,430] |
| Dinitrochlorobenzene | Immunomodulator/contact sensitizers |  | IFN-γ, IL-1β, and IL-2, | Alopecia areata | [431] |
| 1α,25-dihydroxy vitamin D3 | Vitamin D analog | 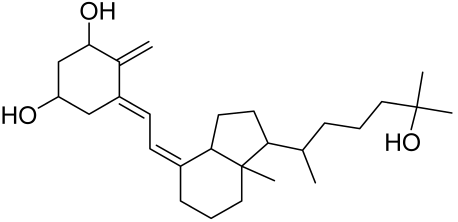 | Akt/mTOR | Psoriasis | [432,433] |
| Diphenylcyclo-propenone | Immunomodulator/contact sensitizers |  | IFN-γ, IL-1β, and IL-2, | Alopecia areata | [431] |
| Sirulumus | Immunomodulator/macrolactams |  | mTOR | Psoriasis and tuberous sclerosis | [400,401] |
| Fluocinonide | Corticosteroid | 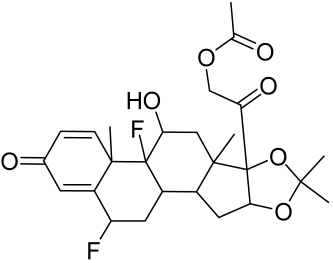 | IL and IFN-α axis | Oral lichen planus and alopecia areata | [96,97,434] |
| Tacrolimus | Immunomodulator/macrolactams | 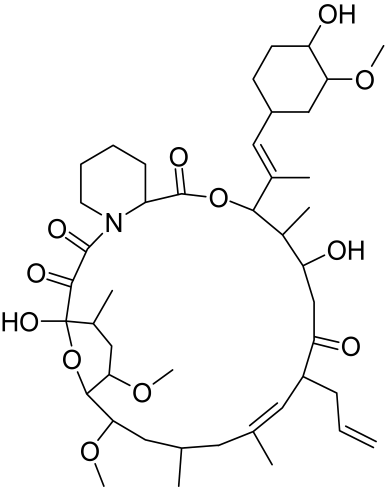 | mTOR | Psoriasis and tuberous sclerosis | [400,401] |
| Erlotinib | Kinase inhibitor |  | PI3K/Akt/ mTOR pathway | Pemphigus vulgaris | |
| Hydrocortisone | Corticosteroid |  | IL and IFN-α axis | Atopic dermatitis and psoriasis | [98,435,436] |
| Ketoconazole | Antimicrobial |  | Acne vulgaris | [99,437] | |
| Metformin | Biguanides |  | mTOR | Diabetic foot ulcer | [403,438] |
| PP1{4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]- pyrimidine} | Kinase inhibitor |  | PI3K/Akt/ mTOR pathway | Pemphigus vulgaris | [439] |
| Rifampicin | Antimicrobial |  | Buruli ulcer | [243] | |
| Salicylic acid | Keratolytics | 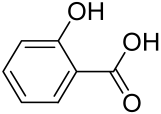 | Acne vulgaris and psoriasis | [439,440] | |
| Triamcinolone acetonide | Corticosteroid | 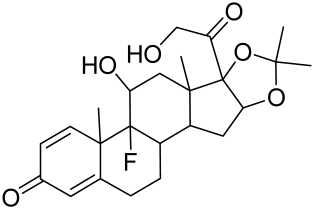 | IL and IFN-α axis | Acne vulgaris, hidradenitis suppurativa and psoriasis | [259,441,442] |
| Zinc | Immunomodulator | IL axis and Reactive oxygen species (ROS) | Acne vulgaris, vitiligo | [443,444,445] |
| Active Agent | Class | Protein Target(s) | Chronic Skin Inflammation Type | References |
|---|---|---|---|---|
| Secukinumab | IL inhibitors | IL axis | Psoriasis | [395,446,447] |
| Adalimumab | TNF and T-cells inhibitor | TNF axis | Psoriasis and hidradenitis suppurativa | [448] |
| Etanercept | TNF and T-cells inhibitor | TNF axis | Psoriasis | [419] |
| Dupilumab | IL inhibitors | IL axis | Atopic Dermatitis | [126] |
| Guselkumab | IL inhibitors | IL axis | Psoriasis | [449] |
| Infiximab | TNF and T-cells inhibitor | TNF axis | Psoriasis and hidradenitis suppurativa/acne inversa | [259] |
| Ixekizumab | IL inhibitors | IL axis | Psoriasis | [394,450] |
| Risankizumab | IL inhibitors | IL axis | Psoriasis | [450,451] |
| Ustekinumab | IL inhibitors | IL axis | Psoriasis | [126,448] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, T.; Boateng, S.T.; Uddin, M.B.; Banang-Mbeumi, S.; Yadav, R.K.; Bock, C.R.; Folahan, J.T.; Siwe-Noundou, X.; Walker, A.L.; King, J.A.; et al. The PI3K-Akt-mTOR and Associated Signaling Pathways as Molecular Drivers of Immune-Mediated Inflammatory Skin Diseases: Update on Therapeutic Strategy Using Natural and Synthetic Compounds. Cells 2023, 12, 1671. https://doi.org/10.3390/cells12121671
Roy T, Boateng ST, Uddin MB, Banang-Mbeumi S, Yadav RK, Bock CR, Folahan JT, Siwe-Noundou X, Walker AL, King JA, et al. The PI3K-Akt-mTOR and Associated Signaling Pathways as Molecular Drivers of Immune-Mediated Inflammatory Skin Diseases: Update on Therapeutic Strategy Using Natural and Synthetic Compounds. Cells. 2023; 12(12):1671. https://doi.org/10.3390/cells12121671
Chicago/Turabian StyleRoy, Tithi, Samuel T. Boateng, Mohammad B. Uddin, Sergette Banang-Mbeumi, Rajesh K. Yadav, Chelsea R. Bock, Joy T. Folahan, Xavier Siwe-Noundou, Anthony L. Walker, Judy A. King, and et al. 2023. "The PI3K-Akt-mTOR and Associated Signaling Pathways as Molecular Drivers of Immune-Mediated Inflammatory Skin Diseases: Update on Therapeutic Strategy Using Natural and Synthetic Compounds" Cells 12, no. 12: 1671. https://doi.org/10.3390/cells12121671
APA StyleRoy, T., Boateng, S. T., Uddin, M. B., Banang-Mbeumi, S., Yadav, R. K., Bock, C. R., Folahan, J. T., Siwe-Noundou, X., Walker, A. L., King, J. A., Buerger, C., Huang, S., & Chamcheu, J. C. (2023). The PI3K-Akt-mTOR and Associated Signaling Pathways as Molecular Drivers of Immune-Mediated Inflammatory Skin Diseases: Update on Therapeutic Strategy Using Natural and Synthetic Compounds. Cells, 12(12), 1671. https://doi.org/10.3390/cells12121671








