Multilineage Differentiating Stress Enduring (Muse) Cells: A New Era of Stem Cell-Based Therapy
Abstract
:1. Introduction
2. Multilineage Differentiating Stress-Enduring (Muse) Cells
3. Characteristics of Muse Stem Cells
3.1. Pluripotency
3.2. Stress Tolerance
3.3. Non-Tumorigenicity
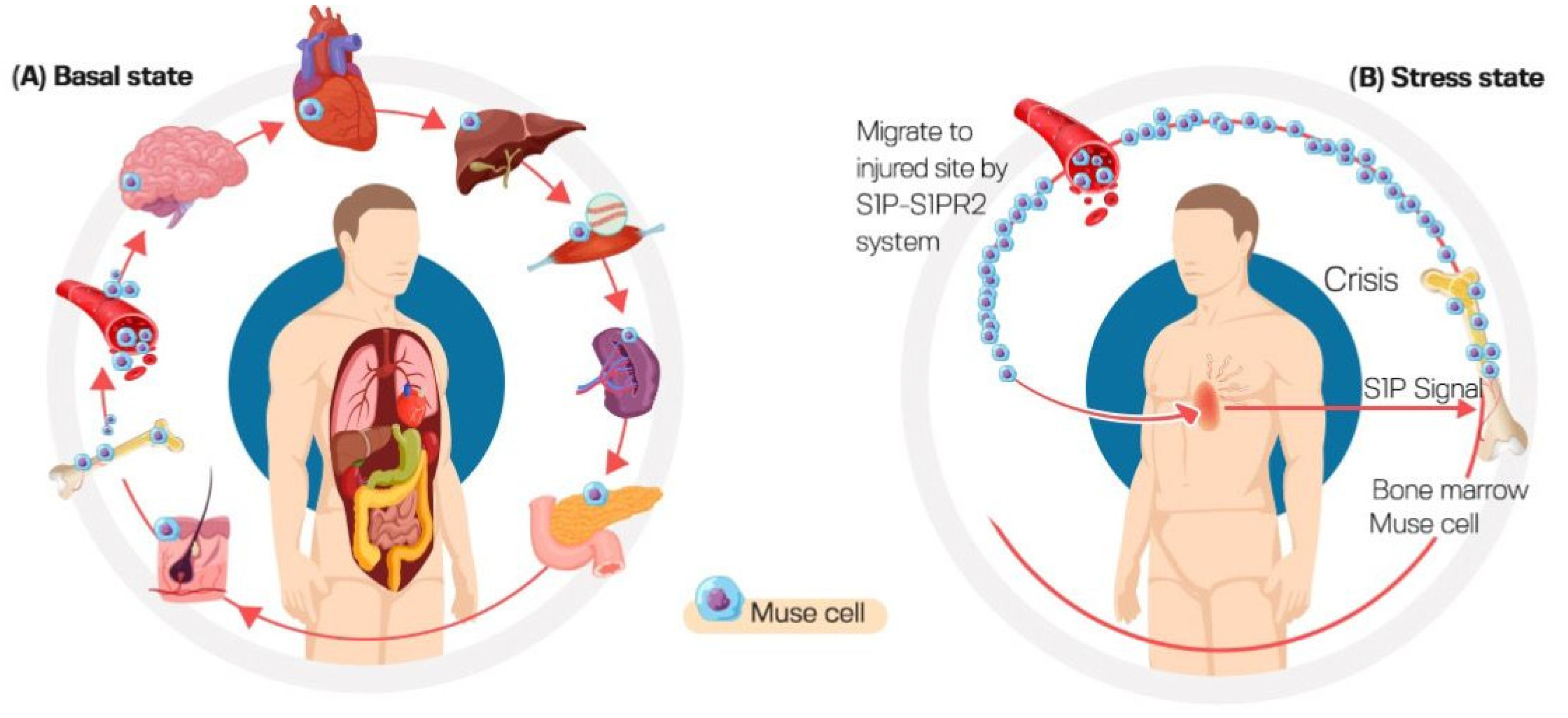
3.4. High Homing Capacity of Muse Cells (Selective Homing to Injured Tissues)
3.5. Adherent–Suspension Switch
3.6. Anti-Immunity
4. Isolation of Muse Cells
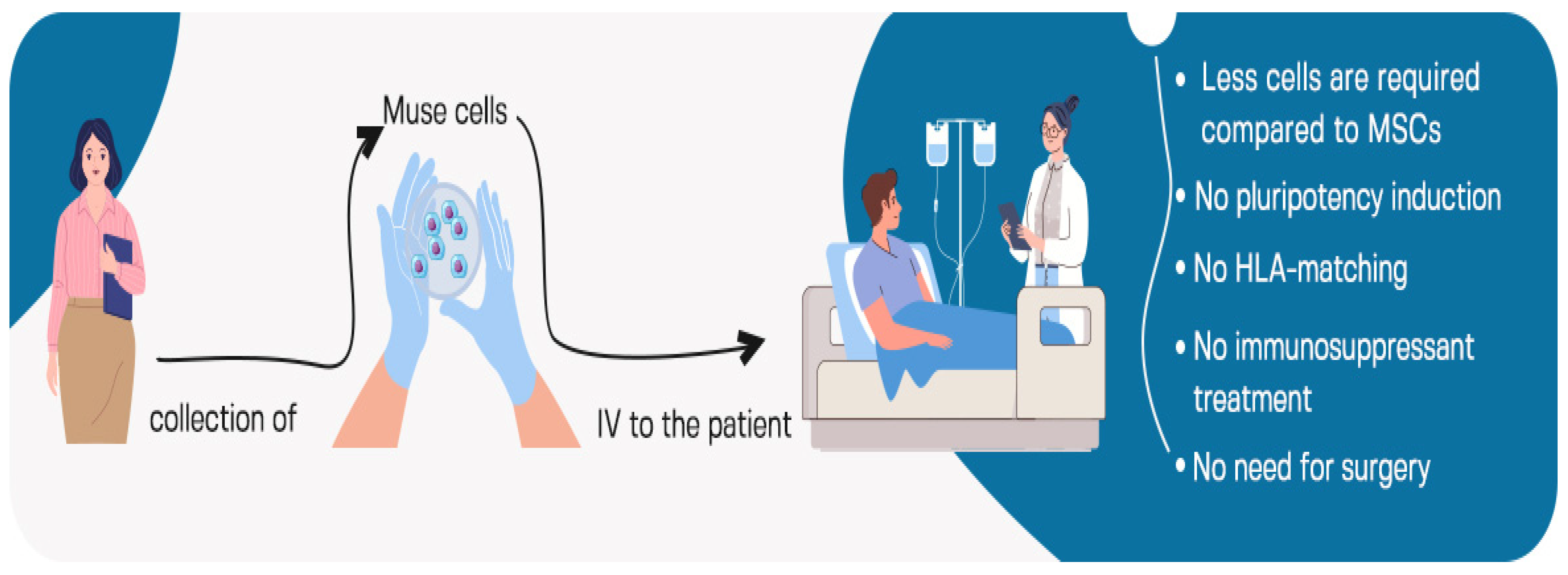
5. Intravenous Administration of Muse Cells
6. Therapeutic Applications of Muse Cells
6.1. Stroke
6.2. Myocardial Infarction
6.3. Neuronal Diseases
6.3.1. Amyotrophic Lateral Sclerosis (ALS)
6.3.2. Perinatal Hypoxic Ischemic Encephalopathy
6.4. Diabetes Mellitus
6.5. Spinal Cord Injury
6.6. Damaged Intestinal Epithelial Cells of Rat
6.7. Acute Lung Ischemia–Reperfusion Injury in a Rat Model
6.8. Bladder Inflammation
6.9. Pancreatitis
6.10. Aortic Aneurism
6.11. Hepatectomy
6.12. Chronic Kidney Disease
7. Clinical Trials Using Muse Cells
8. Challenges in Therapeutic Application of Muse Cells
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Rev. Rep. 2020, 16, 3–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aly, R.M. Current State of Stem Cell-Based Therapies: An Overview. Stem Cell Investig. 2020, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem Cells: Past, Present, and Future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Dezawa, M. Muse Cells: Endogenous Reparative Pluripotent Stem Cells; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2018; ISBN 978-4-431-56845-2. [Google Scholar]
- Kuroda, Y.; Kitada, M.; Wakao, S.; Nishikawa, K.; Tanimura, Y.; Makinoshima, H.; Goda, M.; Akashi, H.; Inutsuka, A.; Niwa, A.; et al. Unique Multipotent Cells in Adult Human Mesenchymal Cell Populations. Proc. Natl. Acad. Sci. USA 2010, 107, 8639–8643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alessio, N.; Squillaro, T.; Özcan, S.; Di Bernardo, G.; Venditti, M.; Melone, M.; Peluso, G.; Galderisi, U. Stress and Stem Cells: Adult Muse Cells Tolerate Extensive Genotoxic Stimuli Better than Mesenchymal Stromal Cells. Oncotarget 2018, 9, 19328–19341. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, T.; Kushida, Y.; Abe, K.; Dezawa, M. Non-Tumorigenic Pluripotent Reparative Muse Cells Provide a New Therapeutic Approach for Neurologic Diseases. Cells 2021, 10, 961. [Google Scholar] [CrossRef]
- Dezawa, M. Muse Cells Provide the Pluripotency of Mesenchymal Stem Cells: Direct Contribution of Muse Cells to Tissue Regeneration. Cell Transplant. 2016, 25, 849–861. [Google Scholar] [CrossRef] [Green Version]
- Suszynska, M.; Zuba-Surma, E.K.; Maj, M.; Mierzejewska, K.; Ratajczak, J.; Kucia, M.; Ratajczak, M.Z. The Proper Criteria for Identification and Sorting of Very Small Embryonic-Like Stem Cells, and Some Nomenclature Issues. Stem Cells Dev. 2014, 23, 702–713. [Google Scholar] [CrossRef] [Green Version]
- Wakao, S.; Kushida, Y.; Dezawa, M. Basic Characteristics of Muse Cells. In Muse Cells; Dezawa, M., Ed.; Advances in Experimental Medicine and Biology; Springer: Tokyo, Japan, 2018; Volume 1103, pp. 13–41. ISBN 978-4-431-56845-2. Available online: http://link.springer.com/10.1007/978-4-431-56847-6_2 (accessed on 1 June 2023).
- Katagiri, H.; Kushida, Y.; Nojima, M.; Kuroda, Y.; Wakao, S.; Ishida, K.; Endo, F.; Kume, K.; Takahara, T.; Nitta, H.; et al. A Distinct Subpopulation of Bone Marrow Mesenchymal Stem Cells, Muse Cells, Directly Commit to the Replacement of Liver Components. Am. J. Transplant. 2016, 16, 468–483. [Google Scholar] [CrossRef]
- Wakao, S.; Kitada, M.; Kuroda, Y.; Shigemoto, T.; Matsuse, D.; Akashi, H.; Tanimura, Y.; Tsuchiyama, K.; Kikuchi, T.; Goda, M.; et al. Multilineage-Differentiating Stress-Enduring (Muse) Cells Are a Primary Source of Induced Pluripotent Stem Cells in Human Fibroblasts. Proc. Natl. Acad. Sci. USA 2011, 108, 9875–9880. [Google Scholar] [CrossRef] [Green Version]
- Hori, E.; Hayakawa, Y.; Hayashi, T.; Hori, S.; Okamoto, S.; Shibata, T.; Kubo, M.; Horie, Y.; Sasahara, M.; Kuroda, S. Mobilization of Pluripotent Multilineage-Differentiating Stress-Enduring Cells in Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 1473–1481. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishigaki, K.; Minatoguchi, S.; Nawa, T.; Yamada, Y.; Kanamori, H.; Mikami, A.; Ushikoshi, H.; Kawasaki, M.; Dezawa, M.; et al. Mobilized Muse Cells After Acute Myocardial Infarction Predict Cardiac Function and Remodeling in the Chronic Phase. Circ. J. 2018, 82, 561–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogura, F.; Wakao, S.; Kuroda, Y.; Tsuchiyama, K.; Bagheri, M.; Heneidi, S.; Chazenbalk, G.; Aiba, S.; Dezawa, M. Human Adipose Tissue Possesses a Unique Population of Pluripotent Stem Cells with Nontumorigenic and Low Telomerase Activities: Potential Implications in Regenerative Medicine. Stem Cells Dev. 2014, 23, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Dezawa, M. The Muse Cell Discovery, Thanks to Wine and Science. Adv. Exp. Med. Biol. 2018, 1103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Simerman, A.A.; Phan, J.D.; Dumesic, D.A.; Chazenbalk, G.D. Muse Cells: Nontumorigenic Pluripotent Stem Cells Present in Adult Tissues-A Paradigm Shift in Tissue Regeneration and Evolution. Stem Cells Int. 2016, 2016, 1463258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wei, J.; Liu, X.; Zhang, P.; Lin, J. Muse Cells: Ushering in a New Era of Stem Cell-Based Therapy for Stroke. Stem Cell Res. Ther. 2022, 13, 421. [Google Scholar] [CrossRef]
- Fisch, S.C.; Gimeno, M.L.; Phan, J.D.; Simerman, A.A.; Dumesic, D.A.; Perone, M.J.; Chazenbalk, G.D. Pluripotent Nontumorigenic Multilineage Differentiating Stress Enduring Cells (Muse Cells): A Seven-Year Retrospective. Stem Cell Res. Ther. 2017, 8, 227. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhang, R.; Li, D.; Cheng, S.; Yang, Y.; Tian, T.; Pan, X. Muse Cells, a New Type of Pluripotent Stem Cell Derived from Human Fibroblasts. Cell. Reprogram. 2016, 18, 67–77. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Z.; Xiao, R.; Pan, B. Regenerative Potential of Pluripotent Nontumorgenetic Stem Cells: Multilineage Differentiating Stress Enduring Cells (Muse Cells). Regen. Ther. 2020, 15, 92–96. [Google Scholar] [CrossRef]
- Kushida, Y.; Wakao, S.; Dezawa, M. Muse Cells Are Endogenous Reparative Stem Cells. Adv. Exp. Med. Biol. 2018, 1103, 43–68. [Google Scholar] [CrossRef]
- Sato, T.; Wakao, S.; Kushida, Y.; Tatsumi, K.; Kitada, M.; Abe, T.; Niizuma, K.; Tominaga, T.; Kushimoto, S.; Dezawa, M. A Novel Type of Stem Cells Double-Positive for SSEA-3 and CD45 in Human Peripheral Blood. Cell Transplant. 2020, 29, 963689720923574. [Google Scholar] [CrossRef]
- Yamada, Y.; Wakao, S.; Kushida, Y.; Minatoguchi, S.; Mikami, A.; Higashi, K.; Baba, S.; Shigemoto, T.; Kuroda, Y.; Kanamori, H.; et al. S1P-S1PR2 Axis Mediates Homing of Muse Cells into Damaged Heart for Long-Lasting Tissue Repair and Functional Recovery After Acute Myocardial Infarction. Circ. Res. 2018, 122, 1069–1083. [Google Scholar] [CrossRef]
- Iseki, M.; Kushida, Y.; Wakao, S.; Akimoto, T.; Mizuma, M.; Motoi, F.; Asada, R.; Shimizu, S.; Unno, M.; Chazenbalk, G.; et al. Muse Cells, Nontumorigenic Pluripotent-Like Stem Cells, Have Liver Regeneration Capacity Through Specific Homing and Cell Replacement in a Mouse Model of Liver Fibrosis. Cell Transplant. 2017, 26, 821–840. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Kushida, Y.; Wakao, S.; Kitada, M.; Tatsumi, K.; Dezawa, M. Cardiotrophic Growth Factor-Driven Induction of Human Muse Cells into Cardiomyocyte-Like Phenotype. Cell Transplant. 2018, 27, 285–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, W. Future of Muse Cells. In Muse Cells; Dezawa, M., Ed.; Advances in Experimental Medicine and Biology; Springer: Tokyo, Japan, 2018; Volume 1103, pp. 309–315. ISBN 978-4-431-56845-2. Available online: http://link.springer.com/10.1007/978-4-431-56847-6_18 (accessed on 9 December 2022).
- Tatsumi, K.; Kushida, Y.; Wakao, S.; Kuroda, Y.; Dezawa, M. Protocols for Isolation and Evaluation of Muse Cells. In Muse Cells; Dezawa, M., Ed.; Advances in Experimental Medicine and Biology; Springer: Tokyo, Japan, 2018; Volume 1103, pp. 69–101. ISBN 978-4-431-56845-2. Available online: http://link.springer.com/10.1007/978-4-431-56847-6_4 (accessed on 9 December 2022).
- Noda, T.; Nishigaki, K.; Minatoguchi, S. Safety and Efficacy of Human Muse Cell-Based Product for Acute Myocardial Infarction in a First-in-Human Trial. Circ. J. 2020, 84, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Minatoguchi, S.; Mikami, A.; Tanaka, T.; Minatoguchi, S.; Yamada, Y. Acute Myocardial Infarction, Cardioprotection, and Muse Cells. In Muse Cells; Dezawa, M., Ed.; Advances in Experimental Medicine and Biology; Springer: Tokyo, Japan, 2018; Volume 1103, pp. 153–166. ISBN 978-4-431-56845-2. Available online: http://link.springer.com/10.1007/978-4-431-56847-6_8 (accessed on 9 December 2022).
- Park, Y.J.; Niizuma, K.; Mokin, M.; Dezawa, M.; Borlongan, C.V. Cell-Based Therapy for Stroke: Musing with Muse Cells. Stroke 2020, 51, 2854–2862. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic Stroke. Nat. Rev. Dis. Primer 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Boese, A.C.; Le, Q.-S.E.; Pham, D.; Hamblin, M.H.; Lee, J.-P. Neural Stem Cell Therapy for Subacute and Chronic Ischemic Stroke. Stem Cell Res. Ther. 2018, 9, 154. [Google Scholar] [CrossRef]
- Uchida, H.; Morita, T.; Niizuma, K.; Kushida, Y.; Kuroda, Y.; Wakao, S.; Sakata, H.; Matsuzaka, Y.; Mushiake, H.; Tominaga, T.; et al. Transplantation of Unique Subpopulation of Fibroblasts, Muse Cells, Ameliorates Experimental Stroke Possibly via Robust Neuronal Differentiation. Stem Cells 2016, 34, 160–173. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kuroda, Y.; Morita, T.; Shichinohe, H.; Houkin, K.; Dezawa, M.; Kuroda, S. Therapeutic Effects of Human Multilineage-Differentiating Stress Enduring (MUSE) Cell Transplantation into Infarct Brain of Mice. PLoS ONE 2015, 10, e0116009. [Google Scholar] [CrossRef] [Green Version]
- Uchida, H.; Niizuma, K.; Kushida, Y.; Wakao, S.; Tominaga, T.; Borlongan, C.V.; Dezawa, M. Human Muse Cells Reconstruct Neuronal Circuitry in Subacute Lacunar Stroke Model. Stroke 2017, 48, 428–435. [Google Scholar] [CrossRef]
- Abe, T.; Aburakawa, D.; Niizuma, K.; Iwabuchi, N.; Kajitani, T.; Wakao, S.; Kushida, Y.; Dezawa, M.; Borlongan, C.V.; Tominaga, T. Intravenously Transplanted Human Multilineage-Differentiating Stress-Enduring Cells Afford Brain Repair in a Mouse Lacunar Stroke Model. Stroke 2020, 51, 601–611. [Google Scholar] [CrossRef]
- Shimamura, N.; Kakuta, K.; Wang, L.; Naraoka, M.; Uchida, H.; Wakao, S.; Dezawa, M.; Ohkuma, H. Neuro-Regeneration Therapy Using Human Muse Cells Is Highly Effective in a Mouse Intracerebral Hemorrhage Model. Exp. Brain Res. 2017, 235, 565–572. [Google Scholar] [CrossRef]
- Cannon, C.P.; Gibson, C.M.; Lambrew, C.T.; Shoultz, D.A.; Levy, D.; French, W.J.; Gore, J.M.; Weaver, W.D.; Rogers, W.J.; Tiefenbrunn, A.J. Relationship of Symptom-Onset-to-Balloon Time and Door-to-Balloon Time with Mortality in Patients Undergoing Angioplasty for Acute Myocardial Infarction. JAMA 2000, 283, 2941–2947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, Y.; Minatoguchi, S.; Kanamori, H.; Mikami, A.; Okura, H.; Dezawa, M.; Minatoguchi, S. Stem Cell Therapy for Acute Myocardial Infarction—Focusing on the Comparison between Muse Cells and Mesenchymal Stem Cells. J. Cardiol. 2022, 80, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, Y.; Yahata, T.; Miyatake, H.; Sato, T.; Uno, T.; Itoh, J.; Kato, S.; Ito, M.; Hotta, T.; Ando, K. Reconstitution of the Functional Human Hematopoietic Microenvironment Derived from Human Mesenchymal Stem Cells in the Murine Bone Marrow Compartment. Blood 2006, 107, 1878–1887. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.R.; Abadeh, A.; Connelly, K.A. Concise Review: Rational Use of Mesenchymal Stem Cells in the Treatment of Ischemic Heart Disease. Stem Cells Transl. Med. 2018, 7, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Minatoguchi, S.; Baba, S.; Shibata, S.; Takashima, S.; Wakao, S.; Okura, H.; Dezawa, M.; Minatoguchi, S. Human Muse Cells Reduce Myocardial Infarct Size and Improve Cardiac Function without Causing Arrythmias in a Swine Model of Acute Myocardial Infarction. PLoS ONE 2022, 17, e0265347. [Google Scholar] [CrossRef]
- Rojas, P.; Ramírez, A.I.; Fernández-Albarral, J.A.; López-Cuenca, I.; Salobrar-García, E.; Cadena, M.; Elvira-Hurtado, L.; Salazar, J.J.; de Hoz, R.; Ramírez, J.M. Amyotrophic Lateral Sclerosis: A Neurodegenerative Motor Neuron Disease with Ocular Involvement. Front. Neurosci. 2020, 14, 566858. [Google Scholar] [CrossRef]
- Yamashita, T.; Kushida, Y.; Wakao, S.; Tadokoro, K.; Nomura, E.; Omote, Y.; Takemoto, M.; Hishikawa, N.; Ohta, Y.; Dezawa, M.; et al. Therapeutic Benefit of Muse Cells in a Mouse Model of Amyotrophic Lateral Sclerosis. Sci. Rep. 2020, 10, 17102. [Google Scholar] [CrossRef]
- Suzuki, T.; Sato, Y.; Kushida, Y.; Tsuji, M.; Wakao, S.; Ueda, K.; Imai, K.; Iitani, Y.; Shimizu, S.; Hida, H.; et al. Intravenously Delivered Multilineage-Differentiating Stress Enduring Cells Dampen Excessive Glutamate Metabolism and Microglial Activation in Experimental Perinatal Hypoxic Ischemic Encephalopathy. J. Cereb. Blood Flow Metab. 2021, 41, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Burrack, A.L.; Martinov, T.; Fife, B.T. T Cell-Mediated Beta Cell Destruction: Autoimmunity and Alloimmunity in the Context of Type 1 Diabetes. Front. Endocrinol. 2017, 8, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perone, M.J.; Gimeno, M.L.; Fuertes, F. Immunomodulatory Properties and Potential Therapeutic Benefits of Muse Cells Administration in Diabetes. Adv. Exp. Med. Biol. 2018, 1103, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Kuno, S.; Ishimine, H.; Aoi, N.; Mineda, K.; Kato, H.; Doi, K.; Kanayama, K.; Feng, J.; Mashiko, T.; et al. Therapeutic Potential of Adipose-Derived SSEA-3-Positive Muse Cells for Treating Diabetic Skin Ulcers. Stem Cells Transl. Med. 2015, 4, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Al Shehabi, T.S.; Eid, A.H. Inflammogenesis of Secondary Spinal Cord Injury. Front. Cell. Neurosci. 2016, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yin, X.-Y.; Zhao, Y.-Y.; Wang, C.-C.; Du, P.; Lu, Y.-C.; Jin, H.-B.; Yang, C.-C.; Ying, J.-L. Human Muse Cells-Derived Neural Precursor Cells as the Novel Seed Cells for the Repair of Spinal Cord Injury. Biochem. Biophys. Res. Commun. 2021, 568, 103–109. [Google Scholar] [CrossRef]
- Kajitani, T.; Endo, T.; Iwabuchi, N.; Inoue, T.; Takahashi, Y.; Abe, T.; Niizuma, K.; Tominaga, T. Association of Intravenous Administration of Human Muse Cells with Deficit Amelioration in a Rat Model of Spinal Cord Injury. J. Neurosurg. Spine 2021, 34, 648–655. [Google Scholar] [CrossRef]
- Sun, D.; Yang, L.; Cao, H.; Shen, Z.-Y.; Song, H.-L. Study of the Protective Effect on Damaged Intestinal Epithelial Cells of Rat Multilineage-Differentiating Stress-Enduring (Muse) Cells. Cell Biol. Int. 2020, 44, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Yabuki, H.; Wakao, S.; Kushida, Y.; Dezawa, M.; Okada, Y. Human Multilineage-Differentiating Stress-Enduring Cells Exert Pleiotropic Effects to Ameliorate Acute Lung Ischemia-Reperfusion Injury in a Rat Model. Cell Transplant. 2018, 27, 979–993. [Google Scholar] [CrossRef]
- Marcu, I.; Campian, E.; Tu, F. Interstitial Cystitis/Bladder Pain Syndrome. Semin. Reprod. Med. 2018, 36, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Furuta, A.; Yamamoto, T.; Igarashi, T.; Suzuki, Y.; Egawa, S.; Yoshimura, N. Bladder Wall Injection of Mesenchymal Stem Cells Ameliorates Bladder Inflammation, Overactivity, and Nociception in a Chemically Induced Interstitial Cystitis-like Rat Model. Int. Urogynecol. J. 2018, 29, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Furuta, A.; Kuroda, Y.; Yamamoto, T.; Egawa, S.; Dezawa, M.; Yoshimura, N. Effects of Human Muse Cells on Bladder Inflammation, Overactivity, and Nociception in a Chemically Induced Hunner-Type Interstitial Cystitis-like Rat Model. Int. Urogynecol. J. 2022, 33, 1293–1301. [Google Scholar] [CrossRef]
- Habtezion, A.; Gukovskaya, A.S.; Pandol, S.J. Acute Pancreatitis: A Multifaceted Set of Organelle and Cellular Interactions. Gastroenterology 2019, 156, 1941–1950. [Google Scholar] [CrossRef]
- Fukase, M.; Sakata, N.; Kushida, Y.; Wakao, S.; Unno, M.; Dezawa, M. Intravenous Injection of Human Multilineage-Differentiating Stress-Enduring Cells Alleviates Mouse Severe Acute Pancreatitis without Immunosuppressants. Surg. Today 2022, 52, 603–615. [Google Scholar] [CrossRef]
- Motoki, T.; Kurobe, H.; Hirata, Y.; Nakayama, T.; Kinoshita, H.; Rocco, K.A.; Sogabe, H.; Hori, T.; Sata, M.; Kitagawa, T. PPAR-γ Agonist Attenuates Inflammation in Aortic Aneurysm Patients. Gen. Thorac. Cardiovasc. Surg. 2015, 63, 565–571. [Google Scholar] [CrossRef]
- Hosoyama, K.; Saiki, Y. Muse Cells and Aortic Aneurysm. In Muse Cells; Dezawa, M., Ed.; Advances in Experimental Medicine and Biology; Springer: Tokyo, Japan, 2018; Volume 1103, pp. 273–291. ISBN 978-4-431-56845-2. Available online: https://link.springer.com/10.1007/978-4-431-56847-6_15 (accessed on 13 March 2023).
- Hosoyama, K.; Wakao, S.; Kushida, Y.; Ogura, F.; Maeda, K.; Adachi, O.; Kawamoto, S.; Dezawa, M.; Saiki, Y. Intravenously Injected Human Multilineage-Differentiating Stress-Enduring Cells Selectively Engraft into Mouse Aortic Aneurysms and Attenuate Dilatation by Differentiating into Multiple Cell Types. J. Thorac. Cardiovasc. Surg. 2018, 155, 2301–2313.e4. [Google Scholar] [CrossRef]
- Ezzat, T.M.; Dhar, D.K.; Newsome, P.N.; Malagó, M.; Olde Damink, S.W.M. Use of Hepatocyte and Stem Cells for Treatment of Post-Resectional Liver Failure: Are We There Yet? Liver Int. 2011, 31, 773–784. [Google Scholar] [CrossRef]
- Iseki, M.; Mizuma, M.; Wakao, S.; Kushida, Y.; Kudo, K.; Fukase, M.; Ishida, M.; Ono, T.; Shimura, M.; Ise, I.; et al. The Evaluation of the Safety and Efficacy of Intravenously Administered Allogeneic Multilineage-Differentiating Stress-Enduring Cells in a Swine Hepatectomy Model. Surg. Today 2021, 51, 634–650. [Google Scholar] [CrossRef]
- Uchida, N.; Kumagai, N.; Kondo, Y. Application of Muse Cell Therapy for Kidney Diseases. Adv. Exp. Med. Biol. 2018, 1103, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Kushida, Y.; Kitada, M.; Wakao, S.; Kumagai, N.; Kuroda, Y.; Kondo, Y.; Hirohara, Y.; Kure, S.; Chazenbalk, G.; et al. Beneficial Effects of Systemically Administered Human Muse Cells in Adriamycin Nephropathy. J. Am. Soc. Nephrol. JASN 2017, 28, 2946–2960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, Y.; Nohara, T.; Takashima, S.; Natsuga, K.; Adachi, M.; Yoshida, K.; Shinkuma, S.; Takeichi, T.; Nakamura, H.; Wada, O.; et al. Intravenous Allogeneic Multilineage-Differentiating Stress-Enduring Cells in Adults with Dystrophic Epidermolysis Bullosa: A Phase 1/2 Open-Label Study. J. Eur. Acad. Dermatol. Venereol. JEADV 2021, 35, e528–e531. [Google Scholar] [CrossRef] [PubMed]


| Model | Stem Cells Source | Results | Reference |
|---|---|---|---|
| Middle cerebral artery occlusion in immunodeficient mice | Human BM MSCs-derived Muse cells | Muse cells were integrated into the peri-infarct cortex, spontaneously differentiated into neuronal markers (Tuj 1 and NeuN)-positive cells, replaced lost neurons and restored motor function | [35] |
| Transient middle cerebral artery occlusion in rats | Human fibroblast-derived Muse cells | Muse cells integrated with brain microenvironment demonstrated a high rate of differentiation into neuronal cells with the subsequent reconstruction of the neuronal circuit and alleviation of stroke symptoms | [34] |
| Subacute lacunar stroke model in immunodeficient mice | Human BM MSCs-derived Muse cells | Muse cells differentiated into neural cells, facilitated neural recovery, improved behavioral score, and demonstrated solid safety outcomes over the experimental period | [36] |
| Immunodeficient mouse lacunar stroke model | Clinical-grade multilineage-differentiating stress-enduring cell-based product CL2020 | CL2020 was safe with no tumorigenesis or adverse effects detected. CL2020 migrated to the peri-infarct area, expressed neuronal markers, and showed functional recovery | [37] |
| Mouse intracerebral hemorrhage (ICH) model | Human BM MSCs-derived Muse cells | Muse cells resided in the ICH brain, differentiated into NeuN and MAP-2 positive neurons and improved survival rate and motor function | [38] |
| Model | Stem Cells Source | Results | Reference |
|---|---|---|---|
| Swine model of acute myocardial infarction | Semi-clinical grade human Muse cell product | Muse cells homed into the infarct border area, differentiated into cardiomyocytes (Troponin I positive) and microvessels (CD31-positive) reduced infarct size, improved the left ventricular (LV) function and remodeling | [43] |
| Rabbit acute myocardial infarction model | Human BM-MSCs derived Muse cells | Muse cell xenografts and allografts successfully engrafted, reduced infarct size and restored functions. Allografts resided in the tissue and maintained functional recovery for up to 6 months with no need for immunosuppressive treatment | [24] |
| Model | Stem Cells Source | Results | Reference |
|---|---|---|---|
| Rat model of thoracic spinal cord contusion injury | Clinical product CL2020 containing 300,000 Muse cells | Muse cells in CL2020 differentiated into neuronal cells with improvement of hindlimb motor function, smaller cystic cavity and preservation of 5-hydroxytryptamine (5-HT) fibers | [53] |
| Spinal cord injury induced in rats | BM-MSCs-derived muse cells induced into neural precursor cells | Restoration of motor function | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, R.F.; Alhwity, B.S.; Almahlawi, R.M.; Alatawi, B.D.; Albalawi, S.A.; Albalawi, R.A.; Albalawi, A.A.; Abdel-Maksoud, M.S.; Elsherbiny, N. Multilineage Differentiating Stress Enduring (Muse) Cells: A New Era of Stem Cell-Based Therapy. Cells 2023, 12, 1676. https://doi.org/10.3390/cells12131676
Alanazi RF, Alhwity BS, Almahlawi RM, Alatawi BD, Albalawi SA, Albalawi RA, Albalawi AA, Abdel-Maksoud MS, Elsherbiny N. Multilineage Differentiating Stress Enduring (Muse) Cells: A New Era of Stem Cell-Based Therapy. Cells. 2023; 12(13):1676. https://doi.org/10.3390/cells12131676
Chicago/Turabian StyleAlanazi, Raghad F., Basma S. Alhwity, Raghad M. Almahlawi, Bashayer D. Alatawi, Shatha A. Albalawi, Raneem A. Albalawi, Amaal A. Albalawi, Mohamed S. Abdel-Maksoud, and Nehal Elsherbiny. 2023. "Multilineage Differentiating Stress Enduring (Muse) Cells: A New Era of Stem Cell-Based Therapy" Cells 12, no. 13: 1676. https://doi.org/10.3390/cells12131676





