The Importance of Stem Cells Isolated from Human Dental Pulp and Exfoliated Deciduous Teeth as Therapeutic Approach in Nervous System Pathologies
Abstract
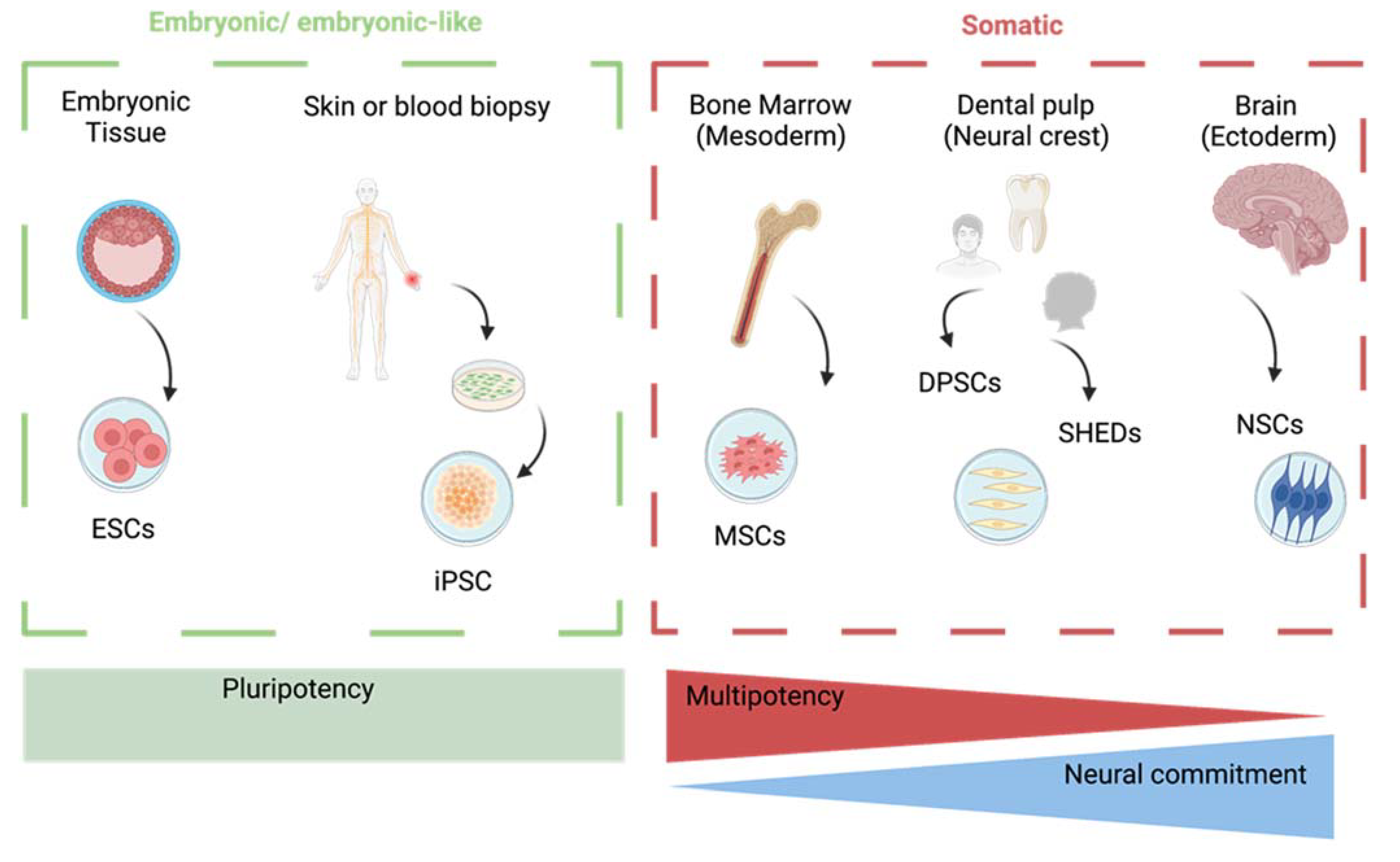
:1. Introduction
2. Types of Stem Cells for Therapy of Neurodegenerative Diseases
3. Induction of Neuronal Lineages in DPSCs and SHEDs
4. Relevance of DPSCs and SHEDs in Neurodegenerative Diseases
4.1. Potential Therapeutic Application of DPSCs and SHEDs for Sensory System Disorders
4.2. DPSCs in Alzhemier’s Disease Models
4.3. DPSCs and SHEDs in Parkinso’s Disease Models
4.4. Application of DPSCs in Other Neurodegenerative Disorders
| Pathology | Pre-Clinical Model | DPSC Administration | Outcome | References |
|---|---|---|---|---|
| Alzheimer’s Disease | Primary rat hippocampal cultures treated with Amyloid-β 1–42 (5–10 µM) or 6-OHDA (5–40 µM) for 24 h | Co-culture with primary neurons | Rescue of cell viability; increase in expression of neuronal markers: release of neurotrophins | [69] |
| Human neuroblastoma SH-SY5Y cells treated with 20 nmol/L Okadaic Acid for 24 h | Transwell insert with porous membrane | Restoration of morphology and cell viability; reduction in apoptosis; reduction in phospho-Tau | [70] | |
| Human neuroblastoma SH-SY5Y cells treated with 5 µM Amyloid-β 1–42 | Transplantation of DPSCs secretome | Increased cell viability; up-regulation of anti-apoptotic Bcl-2; down-regulation of pro-apoptotic Bax | [49] | |
| Mice treated with Kainic Acid | Intrahippocampal transplantation of DPSCs or their secretome | Reduction in cognitive impairment; improved memory acquisition; reduction in neuroinflammation; increase in neurogenesis | [74] | |
| Rats treated with 1 mg/mL Amyloid-β 1–42 | Intrahippocampal transplantation of DPSCs | Increased secretion of neurotrophins; improved cognitive behavior | [75] | |
| Parkinson’s Disease | Rats intraperitoneally injected with 20 mg/kg MPTP | Intranasal | Increased senosory-motor coordination; rescue of olfactory functions; increase in TH-positive neurons | [85] |
| Rats injected unilaterally in the striatum with 10 µg/µL 6-OHDA | Injection of SHEDs in the striatum | Recovery of neurological behavior; increased survival; increase in TH-positive neurons | [87] | |
| Cerebellar Ataxia | Rats injected intraperitoneally with 75 mg/kg 3-Acetylpyridine | Intracerebellar injection | Enhanced motor skills; enhanced muscle activity; rescue of cerebelar volume; reduction in inflammatory cytokines | [88] |
| Vascular Dementia | Two-bessel occlusion in rats | Injection of marked murine DPSCs into tail veins | Successful migration of DPSCs into the lesioned areas observed by PKH compounds; increased neuronal markers; improved behavioral performances | [90] |
| Huntington’s Disease | Rats injected intraperitoneally with 30 mg/kg of 3-nitropropionic acid | Bilateral transplantation of marked DPSCs | Improved motor skills and muscle activity; increased neurite length; reduced astrogliosis and microgliosis; downregulation of Caspase-3 activity; decreased expression of inflammatory cytokines. | [93] |
| Rats injected intraperitoneally with 20 mg/kg of 3-nitropropionic acid | Intravenous injection of SHEDs | SHEDs can cross the BBB; increased expression of neurotrophins; | [95] | |
| Amyotrophic Lateral Sclerosis | Tg-SOD1G93A mouse model | Transplantation of DPSCs secretome | Reduced neuromuscular junction denervation; reduced muscle atrophy; reduced neuronal loss; extended lifespan. | [96] |
5. DPSCs- and SHEDs-Based Clinical Trials for Neuropathological Disorders
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Candelise, N.; Scaricamazza, S.; Salvatori, I.; Ferri, A.; Valle, C.; Manganell, V.; Garofalo, T.; Sorice, M.; Misasi, R. Protein Aggregation Landscape in Neurodegenerative Diseases: Clinical Relevance and Future Applications. Int. J. Mol. Sci. 2021, 22, 6016. [Google Scholar] [CrossRef]
- Soto, C.; Pritzkow, S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1332–1340. [Google Scholar] [CrossRef]
- Bjerke, M.; Engelborghs, S. Cerebrospinal Fluid Biomarkers for Early and Differential Alzheimer’s Disease Diagnosis. J. Alzheimers Dis. 2018, 62, 1199–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candelise, N.; Baiardi, S.; Franceschini, A.; Rossi, M.; Parchi, P. Towards an improved early diagnosis of neurodegenerative diseases: The emerging role of in vitro conversion assays for protein amyloids. Acta Neuropathol. Commun. 2020, 8, 117. [Google Scholar] [CrossRef]
- Candelise, N.; Schmitz, M.; Da Silva Correia, S.M.; Arora, A.S.; Villar-Piqué, A.; Zafar, S.; Llorens, F.; Cramm, M.; Zerr, I. Applications of the real-time quaking-induced conversion assay in diagnosis, prion strain-typing, drug pre-screening and other amyloidopathies. Expert Rev. Mol. Diagn. 2017, 17, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Kishi, T.; Matsunaga, S.; Oya, K.; Nomura, I.; Ikuta, T.; Iwata, N. Memantine for Alzheimer’s Disease: An Updated Systematic Review and Meta-analysis. J. Alahimers Dis. 2017, 60, 401–425. [Google Scholar] [CrossRef]
- Song, C.; Shi, J.; Zhang, P.; Zhang, Y.; Xu, J.; Zhao, L.; Zhang, R.; Wang, H.; Chen, H. Immunotherapy for Alzheimer’s disease: Targeting β-amyloid and beyond. Transl. Neurodegener. 2022, 11, 18. [Google Scholar] [CrossRef]
- Carrasco, A.J.P.; Waldthaler, J.; Mügge, F.; Timmermann, L.; Pedrosa, D.J. Non-lesional treatments for tremor in Parkinson’s disease: A systematic review and meta-analysis. Eur. J. Neurol. 2023. [Google Scholar] [CrossRef]
- Mazumder, S.; McCann, H.; D’Silva, S.; Furlong, S.; Shepherd, C.E.; Kril, J.J.; Halliday, G.M.; Rowe, D.B.; Kiernan, M.C.; Tan, R.H. Riluzole is associated with decreasing neuritic plaque severity in amyotrophic lateral sclerosis. Brain 2023, 146, e17–e19. [Google Scholar] [CrossRef] [PubMed]
- Delle Monache, S.; Pulcini, F.; Frosini, R.; Mattei, V.; Talesa, V.N.; Antognelli, C. Methylglyoxal-Dependent Glycative Stress Is Prevented by the Natural Antioxidant Oleuropein in Human Dental Pulp Stem Cells through Nrf2/Glo1 Pathway. Antioxidants 2021, 10, 716. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, S.; Manganelli, V.; Santacroce, C.; Santilli, F.; Piccoli, L.; Sorice, M.; Mattei, V. Role of Prion protein-EGFR multimolecular complex during neuronal differentiation of human dental pulp-derived stem cells. Prion 2018, 12, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Poetsch, M.S.; Strano, A.; Guan, K. Human Induced Pluripotent Stem Cells: From Cell Origin, Genomic Stability, and Epigenetic Memory to Translational Medicine. Stem Cells 2022, 40, 546–555. [Google Scholar] [CrossRef]
- Purwaningrum, M.; Jamilah, N.S.; Purbantoro, S.D.; Sawangmake, C.; Nantavisai, S. Comparative characteristic study from bone marrow-derived mesenchymal stem cells. J. Vet. Sci. 2021, 22, e74. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [Green Version]
- Santilli, F.; Fabrizi, J.; Pulcini, F.; Santacroce, C.; Sorice, M.; Delle Monache, S.; Mattei, V. Gangliosides and Their Role in Multilineage Differentiation of Mesenchymal Stem Cells. Biomedicines 2022, 10, 3112. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, L. Neural stem cell therapies and hypoxic-ischemic brain injury. Prog. Neurobiol. 2019, 173, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, W. Neural Stem Cell Niche and Adult Neurogenesis. Neuroscientist 2021, 27, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.G.; Daley, G.Q. Induced pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Genet. 2019, 20, 377–388. [Google Scholar] [CrossRef] [PubMed]
- King, N.M.; Perrin, J. Ethical issues in stem cell research and therapy. Stem Cell Res. Ther. 2014, 5, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodgerson, D.O.; Harris, A.G. A comparison of stem cells for therapeutic use. Stem Cell Rev. Rep. 2011, 7, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Song, S.; Zhang, H.; Cuevas, J.; Sanchez-Ramos, J. Comparison of neuron-like cells derived from bone marrow stem cells to those differentiated from adult brain neural stem cells. Stem Cells Dev. 2007, 16, 747–756. [Google Scholar] [CrossRef]
- Bai, W.F.; Zhang, Y.; Xu, W.; Li, W.; Li, M.; Yuan, F.; Luo, X.; Zhang, M. Isolation and Characterization of Neural Progenitor Cells from Bone Marrow in Cell Replacement Therapy of Brain Injury. Front. Cell. Neurosci. 2020, 14, 49. [Google Scholar] [CrossRef]
- Karakaş, N.; Bay, S.; Türkel, N.; Öztunç, N.; Öncül, M.; Bilgen, H.; Shah, K.; Şahin, F.; Öztürk, G. Neurons from human mesenchymal stem cells display both spontaneous and stimuli responsive activity. PLoS ONE 2020, 15, e0228510. [Google Scholar] [CrossRef]
- Goorha, S.; Victor, A.K.; Reiter, L.T. Culturing and Neuronal Differentiation of Human Dental Pulp Stem Cells. Curr. Protoc. 2022, 2, e600. [Google Scholar] [CrossRef]
- Rodas-Junco, B.A.; Villicaña, C. Dental Pulp Stem Cells: Current Advances in Isolation, Expansion and Preservation. Tissue Eng. Regen. Med. 2017, 14, 333–347. [Google Scholar] [CrossRef]
- Goorha, S.; Reiter, L.T. Culturing and Neuronal Differentiation of Human Dental Pulp Stem Cells. Curr. Protoc. Hum. Genet. 2017, 92, 21.6.1–21.6.10. [Google Scholar] [CrossRef] [Green Version]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, S.; Santacroce, C.; Santilli, F.; Manganelli, V.; Sorice, M.; Mattei, V. Prion Protein in Stem Cells: A Lipid Raft Component Involved in the Cellular Differentiation Process. Int. J. Mol. Sci. 2020, 21, 4168. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, S.; Santacroce, C.; Manganelli, V.; Santilli, F.; Piccoli, L.; Cassetta, M.; Misasi, R.; Sorice, M.; Mattei, V. Isolation, Propagation, and Prion Protein Expression During Neuronal Differentiation of Human Dental Pulp Stem Cells. J. Vis. Exp. 2019, 2019. 145, e59282. [Google Scholar] [CrossRef] [Green Version]
- Rafiee, F.; Pourteymourfard-Tabrizi, Z.; Mahmoudian-Sani, M.R.; Mehri-Ghahfarrokhi, A.; Soltani, A.; Hashemzadeh-Chaleshtori, M.; Jami, M.S. Differentiation of dental pulp stem cells into neuron-like cells. Int. J. Neurosci. 2020, 130, 107–116. [Google Scholar] [CrossRef]
- Bonnamain, V.; Thinard, R.; Sergent-Tanguy, S.; Huet, P.; Bienvenu, G.; Naveilhan, P.; Farges, J.C.; Alliot-Licht, B. Human dental pulp stem cells cultured in serum-free supplemented medium. Front. Physiol. 2013, 4, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delle Monache, S.; Pulcini, F.; Santilli, F.; Martellucci, S.; Santacroce, C.; Fabrizi, J.; Angelucci, A.; Sorice, M.; Mattei, V. Hypoxia Induces DPSC Differentiation versus a Neurogenic Phenotype by the Paracrine Mechanism. Biomedicines 2022, 10, 1056. [Google Scholar] [CrossRef]
- Kawase-Koga, Y.; Fujii, Y.; Yamakawa, D.; Sato, M.; Chikazu, D. Identification of neurospheres generated from human dental pulp stem cells in xeno-/serum-free conditions. Regen. Ther. 2020, 14, 128–135. [Google Scholar] [CrossRef]
- Putra, P.; Thompson, T.B.; Chaggar, P.; Goriely, A. Braiding Braak and Braak: Staging patterns and model selection in network neurodegeneration. Netw. Neurosci. 2021, 5, 929–956. [Google Scholar] [CrossRef]
- Del Tredici, K.; Braak, H. To stage, or not to stage. Curr. Opin. Neurobiol. 2020, 61, 10–22. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Niculescu, A.G.; Roza, E.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters-Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef]
- Liu, A.K.; Chang, R.C.; Pearce, R.K.; Gentleman, S.M. Nucleus basalis of Meynert revisited: Anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. 2015, 129, 527–540. [Google Scholar] [CrossRef]
- Sramkó, B.; Földes, A.; Kádár, K.; Varga, G.; Zsembery, Á.; Pircs, K. The Wisdom in Teeth: Neuronal Differentiation of Dental Pulp Cells. Cell. Reprogram. 2023, 25, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Osathanon, T.; Nowwarote, N.; Pavasant, P. Basic fibroblast growth factor inhibits mineralization but induces neuronal differentiation by human dental pulp stem cells through a FGFR and PLCγ signaling pathway. J. Cell. Biochem. 2011, 112, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Király, M.; Porcsalmy, B.; Pataki, A.; Kádár, K.; Jelitai, M.; Molnár, B.; Hermann, P.; Gera, I.; Grimm, W.D.; Ganss, B.; et al. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem. Int. 2009, 55, 323–332. [Google Scholar] [CrossRef]
- Cho, Y.A.; Kim, D.S.; Song, M.; Bae, W.J.; Lee, S.; Kim, E.C. Protein Interacting with Never in Mitosis A-1 Induces Glutamatergic and GABAergic Neuronal Differentiation in Human Dental Pulp Stem Cells. J. Endod. 2016, 42, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, D.; Kanafi, M.; Bhonde, R.; Gupta, P.; Datta, I. Differential Neuronal Plasticity of Dental Pulp Stem Cells from Exfoliated Deciduous and Permanent Teeth Towards Dopaminergic Neurons. J. Cell. Physiol. 2016, 231, 2048–2063. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chang, K.C.; Tsai, S.J.; Chang, H.H.; Lin, C.P. Neurogenic differentiation of dental pulp stem cells to neuron-like cells in dopaminergic and motor neuronal inductive media. J. Formos. Med. Assoc. 2014, 113, 956–965. [Google Scholar] [CrossRef] [Green Version]
- Arimura, Y.; Shindo, Y.; Yamanaka, R.; Mochizuki, M.; Hotta, K.; Nakahara, T.; Ito, E.; Yoshioka, T.; Oka, K. Peripheral-neuron-like properties of differentiated human dental pulp stem cells (hDPSCs). PLoS ONE 2021, 16, e0251356. [Google Scholar] [CrossRef]
- Ahmed, N.-M.; Murakami, M.; Hirose, Y.; Nakashima, M. Therapeutic Potential of Dental Pulp Stem Cell Secretome for Alzheimer’s Disease Treatment: An In Vitro Study. Stem Cells Int. 2016, 2016, 8102478. [Google Scholar] [CrossRef]
- Kerkis, I.; Kerkis, A.; Dozortsev, D.; Stukart-Parsons, G.C.; Gomes Massironi, S.M.; Pereira, L.V.; Caplan, A.I.; Cerruti, H.F. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs 2006, 184, 105–116. [Google Scholar] [CrossRef]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: Comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS ONE 2014, 9, e109305. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, S.; Venugopal, C.; Parveen, S.K.S.; Rai, K.S.; Kutty, B.M.; Dhanushkodi, A. Remarkable migration propensity of dental pulp stem cells towards neurodegenerative milieu: An in vitro analysis. Neurotoxicology 2020, 81, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Darabi, S.; Tiraihi, T.; Nazm Bojnordi, M.; Ghasemi Hamidabadi, H.; Rezaei, N.; Zahiri, M.; Alizadeh, R. Trans-Differentiation of Human Dental Pulp Stem Cells into Cholinergic-Like Neurons Via Nerve Growth Factor. Basic Clin. Neurosci. 2019, 10, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, calcium and mitochondria: A triad in synaptic neurodegeneration. Transl. Neurodegener. 2022, 11, 3. [Google Scholar] [CrossRef]
- Pisciotta, A.; Bertoni, L.; Vallarola, A.; Bertani, G.; Mecugni, D.; Carnevale, G. Neural crest derived stem cells from dental pulp and tooth-associated stem cells for peripheral nerve regeneration. Neural Regen. Res. 2020, 15, 373–381. [Google Scholar] [CrossRef]
- Ullah, I.; Park, J.M.; Kang, Y.H.; Byun, J.H.; Kim, D.G.; Kim, J.H.; Kang, D.H.; Rho, G.J.; Park, B.W. Transplantation of Human Dental Pulp-Derived Stem Cells or Differentiated Neuronal Cells from Human Dental Pulp-Derived Stem Cells Identically Enhances Regeneration of the Injured Peripheral Nerve. Stem Cells Dev. 2017, 26, 1247–1257. [Google Scholar] [CrossRef]
- Barthels, D.; Das, H. Current advances in ischemic stroke research and therapies. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165260. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Tian, Q.; He, Y.; He, P.; Wang, J.; Guo, J.; Ye, Q.; Li, M. Dental pulp stem cell transplantation facilitates neuronal neuroprotection following cerebral ischemic stroke. Biomed. Pharmacother. 2022, 152, 113234. [Google Scholar] [CrossRef]
- Lan, X.; Sun, Z.; Chu, C.; Boltze, J.; Li, S. Dental Pulp Stem Cells: An Attractive Alternative for cell therapy in Ischemic Stroke. Front. Neurol. 2019, 10, 824. [Google Scholar] [CrossRef]
- Nito, C.; Suda, S.; Nitahara-Kasahara, Y.; Okada, T.; Kimura, K. Dental-Pulp Stem Cells as a Therapeutic Strategy for Ischemic Stroke. Biomedicines 2022, 10, 737. [Google Scholar] [CrossRef]
- Alsaeedi, H.A.; Lam, C.; Koh, A.E.; Teh, S.W.; Mok, P.L.; Higuchi, A.; Then, K.Y.; Bastion, M.C.; Alzahrani, B.; Farhana, A.; et al. Looking into dental pulp stem cells in the therapy of photoreceptors and retinal degenerative disorders. J. Photochem. Photobiol. B 2020, 203, 111727. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Alsaeedi, H.A.; Koh, A.E.; Harun, M.H.N.; Hwei, A.N.M.; Mok, P.L.; Luu, C.D.; Yong, T.K.; Subbiah, S.K.; Bastion, M.C. Human Dental Pulp Stem Cells (DPSCs) Therapy in Rescuing Photoreceptors and Establishing a Sodium Iodate-Induced Retinal Degeneration Rat Model. Tissue Eng. Regen. Med. 2021, 18, 143–154. [Google Scholar] [CrossRef]
- Gonmanee, T.; Sritanaudomchai, H.; Vongsavan, K.; Faisaikarm, T.; Songsaad, A.; White, K.L.; Thonabulsombat, C. Neuronal differentiation of dental pulp stem cells from human permanent and deciduous teeth following coculture with rat auditory brainstem slices. Anat. Rec. 2020, 303, 2931–2946. [Google Scholar] [CrossRef] [PubMed]
- Adriztina, I.; Munir, D.; Sandra, F.; Ichwan, M.; Bashiruddin, J.; Putra, I.B.; Farhat; Sembiring, R.J.; Sartika, C.R.; Chouw, A.; et al. Differentiation capacity of dental pulp stem cell into inner ear hair cell using an in vitro assay: A preliminary step toward treating sensorineural hearing loss. Eur. Arch. Otorhinolaryngol. 2022, 279, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Crestini, A.; Santilli, F.; Martellucci, S.; Carbone, E.; Sorice, M.; Piscopo, P.; Mattei, V. Prions and Neurodegenerative Diseases: A Focus on Alzheimer’s Disease. J. Alzheimers Dis. 2022, 85, 503–518. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lee, Y.; Lee, S.; Kim, K.; Song, M.; Lee, J. Mesenchymal Stem CT and Alzheimer’s Disease: Current Status and Future Perspectives. J. Alzheimers Dis. 2020, 77, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Yin, Z.; Chen, F.; Lei, P. Mesenchymal stem cell-derived exosome: A promising alternative in the therapy of Alzheimer’s disease. Alzheimers Res. Ther. 2020, 12, 109. [Google Scholar] [CrossRef]
- Apel, C.; Forlenza, O.V.; de Paula, V.J.; Talib, L.L.; Denecke, B.; Eduardo, C.P.; Gattaz, W.F. The neuroprotective effect of dental pulp cells in models of Alzheimer’s and Parkinson’s disease. J. Neural Transm. 2009, 116, 71–78. [Google Scholar] [CrossRef]
- Wang, F.; Jia, Y.; Liu, J.; Zhai, J.; Cao, N.; Yue, W.; He, H.; Pei, X. Dental pulp stem cells promote regeneration of damaged neuron cells on the cellular model of Alzheimer’s disease. Cell Biol. Int. 2017, 41, 639–650. [Google Scholar] [CrossRef]
- Ueda, T.; Inden, M.; Ito, T.; Kurita, H.; Hozumi, I. Characteristics and Therapeutic Potential of Dental Pulp Stem Cells on Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 407. [Google Scholar] [CrossRef]
- Zheng, L.; Calvo-Garrido, J.; Hallbeck, M.; Hultenby, K.; Marcusson, J.; Cedazo-Minguez, A.; Terman, A. Intracellular localization of amyloid-β peptide in SH-SY5Y neuroblastoma cells. J. Alzheimers Dis. 2013, 37, 713–733. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Studzinski, C.; Beckett, T.; Murphy, M.P.; Klein, R.L.; Hersh, L.B. Circulating neprilysin clears brain amyloid. Mol. Cell. Neurosci. 2010, 45, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Venugopal, C.; Shobha, K.; Rai, K.S.; Dhanushkodi, A. Neurogenic and cognitive enhancing effects of human dental pulp stem cells and its secretome in animal model of hippocampal neurodegeneration. Brain Res. Bull. 2022, 180, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Ouyang, Y.J.; Yu, B.Q.; Li, W.; Yu, M.Y.; Li, J.Y.; Jiao, Z.M.; Yang, D.; Li, N.; Shi, Y.; et al. Therapeutic potential of dental pulp stem cell transplantation in a rat model of Alzheimer’s disease. Neural Regen. Res. 2021, 16, 893–898. [Google Scholar] [CrossRef]
- Ballatore, C.; Lee, V.M.; Trojanowski, J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [Google Scholar] [CrossRef]
- Gazarian, K.; Ramirez-Garcia, L.; Tapía Orozco, L.; Luna-Muñoz, J.; Pacheco-Herrero, M. Human Dental Pulp Stem Cells Display a Potential for Modeling Alzheimer Disease-Related Tau Modifications. Front. Neurol. 2021, 11, 612657. [Google Scholar] [CrossRef] [PubMed]
- Mondragón-Rodríguez, S.; Perry, G.; Luna-Muñoz, J.; Acevedo-Aquino, M.C.; Williams, S. Phosphorylation of tau protein at sites Ser(396-404) is one of the earliest events in Alzheimer’s disease and Down syndrome. Neuropathol. Appl. Neurobiol. 2014, 40, 121–135. [Google Scholar] [CrossRef]
- Hasegawa, M.; Jakes, R.; Crowther, R.A.; Lee, V.M.; Ihara, Y.; Goedert, M. Characterization of mAb AP422, a novel phosphorylation-dependent monoclonal antibody against tau protein. FEBS Lett. 1996, 384, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Green, K.N.; Steffan, J.S.; Martinez-Coria, H.; Sun, X.; Schreiber, S.S.; Thompson, L.M.; LaFerla, F.M. Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J. Neurosci. 2008, 28, 11500–11510. [Google Scholar] [CrossRef] [Green Version]
- Drummond, E.; Pires, G.; MacMurray, C.; Askenazi, M.; Nayak, S.; Bourdon, M.; Safar, J.; Ueberheide, B.; Wisniewski, T. Phosphorylated tau interactome in the human Alzheimer’s disease brain. Brain 2020, 143, 2803–2817. [Google Scholar] [CrossRef]
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Xiao, Z.; Lei, T.; Liu, Y.; Yang, Y.; Bi, W.; Du, H. The potential therapy with dental tissue-derived mesenchymal stem cells in Parkinson’s disease. Stem Cell Res. Ther. 2021, 12, 5. [Google Scholar] [CrossRef]
- Simon, C.; Gan, Q.F.; Kathivaloo, P.; Mohamad, N.A.; Dhamodharan, J.; Krishnan, A.; Sengodan, B.; Palanimuthu, V.R.; Marimuthu, K.; Rajandas, H.; et al. Deciduous DPSCs Ameliorate MPTP-Mediated Neurotoxicity, Sensorimotor Coordination and Olfactory Function in Parkinsonian Mice. Int. J. Mol. Sci. 2019, 20, 568. [Google Scholar] [CrossRef] [Green Version]
- Burns, R.S.; LeWitt, P.A.; Ebert, M.H.; Pakkenberg, H.; Kopin, I.J. The clinical syndrome of striatal dopamine deficiency. Parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). N. Engl. J. Med. 1985, 312, 1418–1421. [Google Scholar] [CrossRef]
- Fujii, H.; Matsubara, K.; Sakai, K.; Ito, M.; Ohno, K.; Ueda, M.; Yamamoto, A. Dopaminergic differentiation of stem cells from human deciduous teeth and their therapeutic benefits for Parkinsonian rats. Brain Res. 2015, 1613, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Aliaghaei, A.; Boroujeni, M.E.; Ahmadi, H.; Bayat, A.H.; Tavirani, M.R.; Abdollahifar, M.A.; Pooyafar, M.H.; Mansouri, V. Dental pulp stem cell transplantation ameliorates motor function and prevents cerebellar atrophy in rat model of cerebellar ataxia. Cell Tissue Res. 2019, 376, 179–187. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.T.; Thomas, A. Vascular Dementia. Lancet. 2015, 386, 1698–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.M.; Sun, Y.; Zhou, Y.L.; Jiao, Z.M.; Yang, D.; Ouyang, Y.J.; Yu, M.Y.; Li, J.Y.; Li, W.; Wang, D.; et al. Therapeutic effects of dental pulp stem cells on vascular dementia in rat models. Neural Regen. Res. 2021, 16, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Tabrizi, S.J. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef]
- Nance, M.A. Genetics of Huntington disease. Handb. Clin. Neurol. 2017, 144, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, N.; Boroujeni, M.E.; Abdollahifar, M.A.; Piryaei, A.; Khodagholi, F.; Mirbehbahani, S.H.; Siroosi, S.; Moghaddam, M.H.; Aliaghaei, A.; Sadeghi, Y. Transplantation of human dental pulp stem cells compensates for striatal atrophy and modulates neuro-inflammation in 3-nitropropionic acid rat model of Huntington’s disease. Neurosci. Res. 2021, 170, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Túnez, I.; Tasset, I.; Pérez-De La Cruz, V.; Santamaría, A. 3-Nitropropionic acid as a tool to study the mechanisms involved in Huntington’s disease: Past, present and future. Molecules 2010, 15, 878–916. [Google Scholar] [CrossRef] [Green Version]
- Wenceslau, C.V.; de Souza, D.M.; Mambelli-Lisboa, N.C.; Ynoue, L.H.; Araldi, R.P.; da Silva, J.M.; Pagani, E.; Haddad, M.S.; Kerkis, I. Restoration of BDNF, DARPP32, and D2R Expression Following Intravenous Infusion of Human Immature Dental Pulp Stem Cells in Huntington’s Disease 3-NP Rat Model. Cells 2022, 11, 1664. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zuzzio, K.; Walker, C.L. Systemic Dental Pulp Stem Cell Secretome Therapy in a Mouse Model of Amyotrophic Lateral Sclerosis. Brain Sci. 2019, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic Lateral Sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef] [Green Version]
- Pfohl, S.R.; Halicek, M.T.; Mitchell, C.S. Characterization of the Contribution of Genetic Background and Gender to Disease Progression in the SOD1 G93A Mouse Model of Amyotrophic Lateral Sclerosis: A Meta-Analysis. J. Neuromuscul. Dis. 2015, 2, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Bethesda (MD): National Library of Medicine (US). Safety and Efficacy Study of Allogeneic Human Dental Pulp Mesenchymal Stem Cells to Treat Severe COVID-19 Patients. 2000. Available online: https://clinicaltrials.gov/ct2/show/NCT04336254?term=dental+pulp+stem+cell&recrs=abdefhm&draw=2&rank=5 (accessed on 7 April 2020).
- Bethesda (MD): National Library of Medicine (US). Novel Coronavirus Induced Severe Pneumonia Treated by Dental Pulp Mesenchymal Stem Cells. 2000. Available online: https://clinicaltrials.gov/ct2/show/NCT04302519?term=dental+pulp+stem+cell&recrs=abdefhm&draw=2&rank=9 (accessed on 10 March 2020).
- Bethesda (MD): National Library of Medicine (US). A Randomized Placebo-Controlled Multicenter Trial to Evaluate the Efficacy and Safety of JTR-161, Allogeneic Human Dental Pulp Stem Cell, in Patients with Acute Ischemic stRoke (J-REPAIR) (J-REPAIR). 2000. Available online: https://clinicaltrials.gov/ct2/show/NCT04608838?term=dental+pulp+stem+cell&recrs=abdefhm&draw=2&rank=7 (accessed on 29 October 2020).
- Bethesda (MD): National Library of Medicine (US). Clinical Study of Pulp Mesenchymal Stem Cells in the Treatment of Primary Mild to Moderate Knee Osteoarthritis. 2000. Available online: https://clinicaltrials.gov/ct2/show/NCT04130100?term=dental+pulp+stem+cell&recrs=abdefhm&draw=2&rank=15 (accessed on 17 October 2019).
- Bethesda (MD): National Library of Medicine (US). Stem Cells from Human Exfoliated Teeth in Treatment of Diabetic Patients with Significantly Reduced Islet Function. 2000. Available online: https://clinicaltrials.gov/ct2/show/NCT03912480?term=dental+pulp+stem+cell&recrs=abdefhm&draw=2&rank=22 (accessed on 11 April 2019).
- Bethesda (MD): National Library of Medicine (US). Pilot Trial of Mesenchymal Stem Cells for Systemic Lupus Erythematosus. 2000. Available online: https://clinicaltrials.gov/ct2/show/NCT03171194?term=dental+pulp+stem+cell&recrs=abdefhm&draw=2&rank=24 (accessed on 31 May 2017).
- Bethesda (MD): National Library of Medicine (US). Safety Evaluation of Cellavita HD Administered Intravenously in Participants with Huntington’s Disease (SAVE-DH). 2000. Available online: https://clinicaltrials.gov/ct2/show/NCT02728115?term=dental+pulp+stem+cell&recrs=abdefhm&draw=2&rank=12 (accessed on 5 April 2016).
- Bethesda (MD): National Library of Medicine (US). Dose-Response Evaluation of the Cellavita HD Product in Patients with Huntington’s Disease (ADORE-DH). 2000. Available online: https://clinicaltrials.gov/ct2/show/NCT03252535?term=dental+pulp+stem+cell&recrs=abdefhm&draw=2&rank=13 (accessed on 17 August 2017).
- Bethesda (MD): National Library of Medicine (US). Clinical Extension Study for Safety and Efficacy Evaluation of Cellavita-HD Administration in Huntington’s Patients. (ADORE-EXT). 2000. Available online: https://clinicaltrials.gov/ct2/show/NCT04219241?term=dental+pulp+stem+cell&recrs=abdefhm&draw=2&rank=10 (accessed on 7 January 2020).
- Song, W.P.; Jin, L.Y.; Zhu, M.D.; Wang, H.; Xia, D.S. Clinical trials using dental stem cells: 2022 update. World J. Stem Cells 2023, 15, 31–51. [Google Scholar] [CrossRef]
- Doğan, A.; Demirci, S.; Apdik, H.; Apdik, E.A.; Şahin, F. Dental pulp stem cells (DPSCs) increase prostate cancer cell proliferation and migration under in vitro conditions. Tissue Cell 2017, 49, 711–718. [Google Scholar] [CrossRef]
- Nikkhah, E.; Kalalinia, F.; Asgharian Rezaee, M.; Tayarani-Najaran, Z. Suppressive effects of dental pulp stem cells and its conditioned medium on development and migration of colorectal cancer cells through MAPKinase pathways. Iran. J. Basic Med. Sci. 2021, 24, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Vandana, K.L.; Desai, R.; Dalvi, P.J. Autologous Stem Cell Application in Periodontal Regeneration Technique (SAI-PRT) Using PDLSCs Directly From an Extracted Tooth—An Insight. Int. J. Stem Cells 2015, 8, 235–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anoop, M.; Datta, I. Stem Cells Derived from Human Exfoliated Deciduous Teeth (SHED) in Neuronal Disorders: A Review. Curr. Stem Cell Res. Ther. 2021, 16, 535–550. [Google Scholar] [CrossRef] [PubMed]
| Type and Source of Stem Cells | Medium for Neuronal Lineage | Neuronal Markers | Neuronal Sub-Population | References |
|---|---|---|---|---|
| Adult DPSCs from third molar | Neurobasal + 2% B27 + 2 mM Gln + 20 ng/mL basic FGF + 20 ng/mL EGF | β3-Tubulin; PLCγ activity | Pan-neuronal | [43] |
| Adult DPSCs from third molar | DMEM/F12 (1:1) + 2.5% FCS + 10 μM 5-azacytidine + 10 ng/mL basic FGF. After 48 h: 250 μM IBMX + 50 μM forskolin + 200 nM TPA + 1 mM dbcAMP + 10 ng/mL bFGF + 10 ng/mL NGF + 30 ng/mL NT-3 + 1% of insulin-transferrin-sodium selenite premix. | N-tub; NeuN; Neurofilament-M. Electrical activity | Pan-neuronal | [44] |
| Adult DPSCs from premolar teeth | Neuronal medium + N2 + 20 ng/mL EGF + 20 ng/mL FGF; PIN1 inhibitor (juglone) or PIN1 overepression (through adenovirus) | NeurN; Nestin; VGluT1; GABA; TH | Pan-neuronal; GABAergic; Glutamatergic | [45] |
| SHEDs from deciduous baby teeth; DPSCs from adult third molar | Neurobasal + 0.5% B27 + 200 ng/mL SHH + 100 ng/mL FGF8 + 50 ng/mL basic FGF + BDNF for 72 h | Nurr1; Engrailed1; Pitx3; Nestin; β3-Tubulin; TH; Ca2+ influx | Dopaminergic Neurons | [46] |
| Adult DPSCs from third molar | For cholinergic neurons: DMEM:F12 (1:1) + 1% N2 + 1% non-essential aminoacids + 0.2% Heparin + 0.1 µM RA. After 96 hours: + 100 ng/mL SHH. After 48 h: + 1 µM cAMP 200 ng/mL ascorbic acid. After 72 h: + 10 ng/mL BDNF + 10 ng/mL GDNF + 10 ng/mL IGF-1. For dopaminergic neurons: DMEM:F12 (1:1) + 1% N2 + 300 ng/mL Noggin. After 96 h: + 50 ng/mL BDNF + 200 mM Ascorbic acid + 50 µg/mL SHH + 50 µg/mL FGF8b. After 120 h:—bFGF After 72 h: + 10 ng/mL GDNF + 2 µg/mL TGF-βIII + 200 mM cAMP. | Nestin; β3-Tubulin; NeuN; TH; Choline AcetylTransferase | Dopaminergic and Cholinergic Neurons | [47] |
| Adult DPSCs from third molar | DMEM:F12 (1:1) + 5% FBS + 10 µM non-essential amino acids + 2 mM Glutamatec+ 10 mM RA + 50 µM Ascorbic Acid + 5 µM Insulin + 10 nM Dexamethasone + 20 nM Progesterone + 20 nM Estradiol + 50 ng/mL NGF + 10 ng/mL Thyroxine | Nestin; β3-Tubulin; Brn-3a; TRPV1; substance-P; Ca2+ imaging | Peripheral neuronal cells (pain receptors) | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candelise, N.; Santilli, F.; Fabrizi, J.; Caissutti, D.; Spinello, Z.; Moliterni, C.; Lancia, L.; Delle Monache, S.; Mattei, V.; Misasi, R. The Importance of Stem Cells Isolated from Human Dental Pulp and Exfoliated Deciduous Teeth as Therapeutic Approach in Nervous System Pathologies. Cells 2023, 12, 1686. https://doi.org/10.3390/cells12131686
Candelise N, Santilli F, Fabrizi J, Caissutti D, Spinello Z, Moliterni C, Lancia L, Delle Monache S, Mattei V, Misasi R. The Importance of Stem Cells Isolated from Human Dental Pulp and Exfoliated Deciduous Teeth as Therapeutic Approach in Nervous System Pathologies. Cells. 2023; 12(13):1686. https://doi.org/10.3390/cells12131686
Chicago/Turabian StyleCandelise, Niccolò, Francesca Santilli, Jessica Fabrizi, Daniela Caissutti, Zaira Spinello, Camilla Moliterni, Loreto Lancia, Simona Delle Monache, Vincenzo Mattei, and Roberta Misasi. 2023. "The Importance of Stem Cells Isolated from Human Dental Pulp and Exfoliated Deciduous Teeth as Therapeutic Approach in Nervous System Pathologies" Cells 12, no. 13: 1686. https://doi.org/10.3390/cells12131686
APA StyleCandelise, N., Santilli, F., Fabrizi, J., Caissutti, D., Spinello, Z., Moliterni, C., Lancia, L., Delle Monache, S., Mattei, V., & Misasi, R. (2023). The Importance of Stem Cells Isolated from Human Dental Pulp and Exfoliated Deciduous Teeth as Therapeutic Approach in Nervous System Pathologies. Cells, 12(13), 1686. https://doi.org/10.3390/cells12131686








