EpoR Activation Stimulates Erythroid Precursor Proliferation by Inducing Phosphorylation of Tyrosine-88 of the CDK-Inhibitor p27Kip1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Transfection
2.2. SDS–PAGE/Immunoblotting and Antibodies
2.3. pY88p27 Immunoprecipitation (IP)
2.4. Co–Immunoprecipitation (Co–IP)
2.5. Recombinant Protein Production and Glutathione–S–Transferase (GST) Pull–Down
2.6. In Vivo Experiments
2.7. CFU–E Assay
2.8. Antibody Staining and Fluorescence–Activated Cell Sorting (FACS)
2.9. Statistical Analysis
3. Results
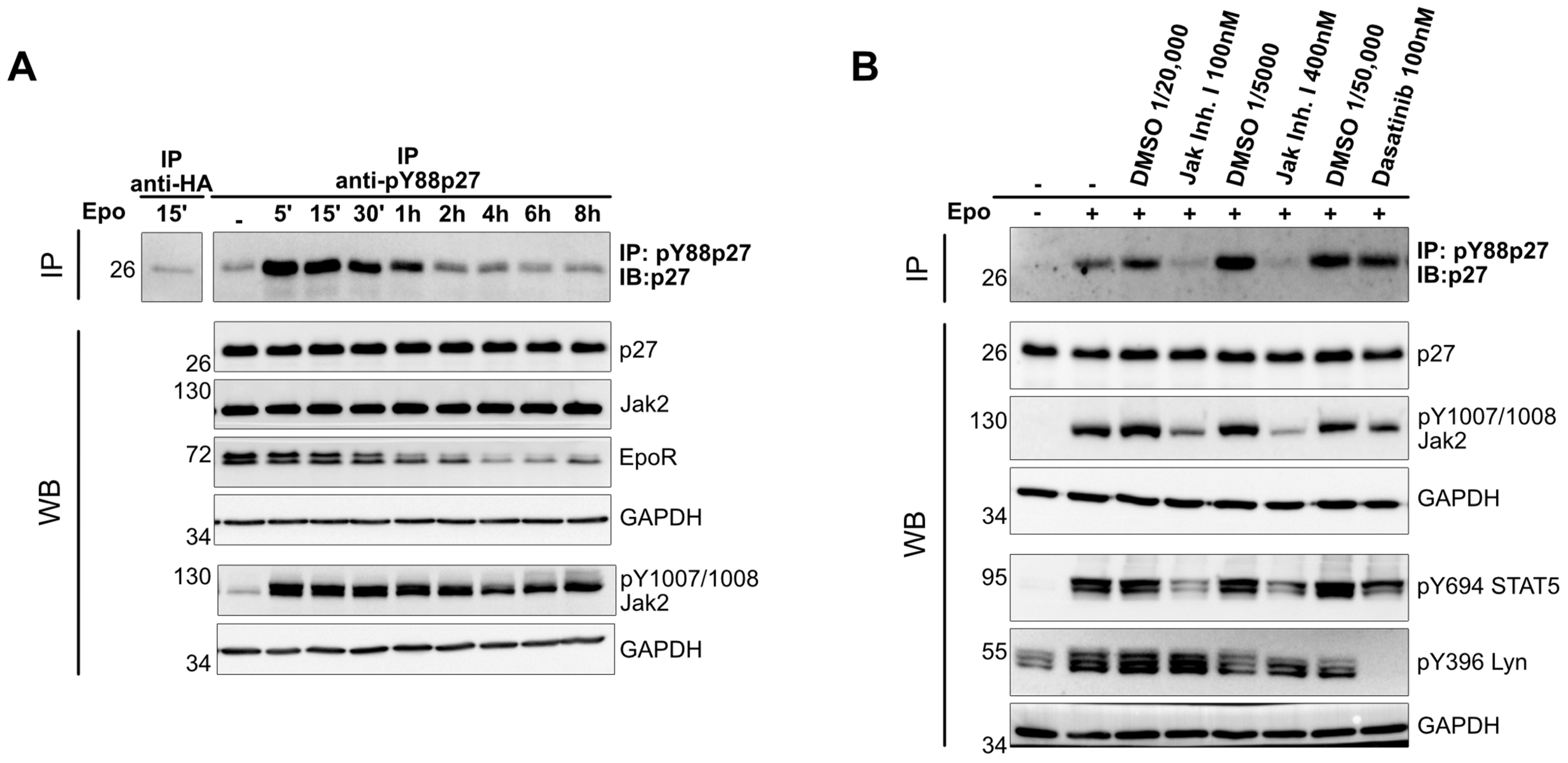
3.1. Epo Stimulation Causes Rapid Phosphorylation of p27 on Y88
3.2. Phosphorylation of p27 upon Epo Stimulation Depends on Jak2 Activation
3.3. p27 Can Bind to EpoR
3.4. p27 Directly Interacts with EpoR In Vitro

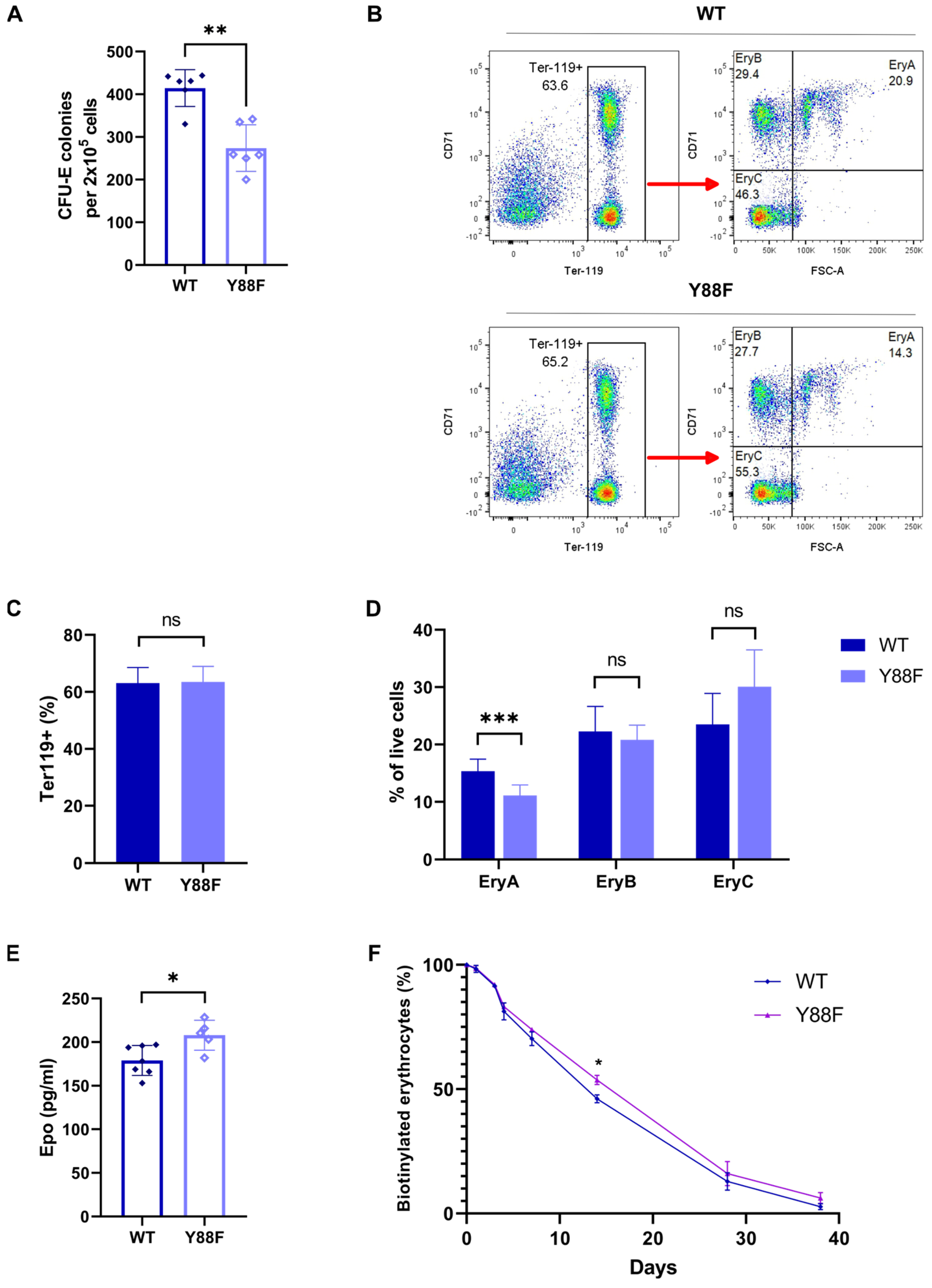
3.5. p27Y88F/Y88F Knock–In Mice Are Characterized by Reduced Red Blood Cell Counts and Lower Hematocrit Levels
3.6. Impaired CFU–E Colony–Forming Capacity of BM Cells from p27Y88F/Y88F Mice
3.7. p27Y88F/Y88F Mice Have Fewer Early Basophilic Erythroblasts in the BM
3.8. Epo Plasma Levels Are Increased in p27Y88F/Y88F Knock–In Mice
3.9. The Lifespan of Erythrocytes in p27Y88F/Y88F Mice Is Slightly Increased
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.; Liu, X.; Jaenisch, R.; Lodish, H.F. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell 1995, 83, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.S.; Lim, S.K.; D’Agati, V.; Costantini, F. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 1996, 10, 154–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witthuhn, B.A.; Quelle, F.W.; Silvennoinen, O.; Yi, T.; Tang, B.; Miura, O.; Ihle, J.N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell 1993, 74, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.J.; Constantinescu, S.N.; Lodish, H.F. The N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoietin receptor. Mol. Cell 2001, 8, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.F.; Barnett, L.A.; Green, W.F.; Freedman, K.; Matushansky, I.; Skoultchi, A.I.; Kelley, L.L. Cell cycle exit during terminal erythroid differentiation is associated with accumulation of p27Kip1 and inactivation of cdk2 kinase. Blood 2000, 96, 2746–2754. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jia, N.; Kapur, R.; Chun, K.T. Cul4A targets p27 for degradation and regulates proliferation, cell cycle exit, and differentiation during erythropoiesis. Blood 2006, 107, 4291–4299. [Google Scholar] [CrossRef] [Green Version]
- Randle, S.J.; Nelson, D.E.; Patel, S.P.; Laman, H. Defective erythropoiesis in a mouse model of reduced Fbxo7 expression due to decreased p27 expression. J. Pathol. 2015, 237, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Gnanapragasam, M.N.; Bieker, J.J. Orchestration of late events in erythropoiesis by KLF1/EKLF. Curr. Opin. Hematol. 2017, 24, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Jakel, H.; Peschel, I.; Kunze, C.; Weinl, C.; Hengst, L. Regulation of p27 (Kip1) by mitogen-induced tyrosine phosphorylation. Cell Cycle 2012, 11, 1910–1917. [Google Scholar] [CrossRef] [Green Version]
- Guiley, K.Z.; Stevenson, J.W.; Lou, K.; Barkovich, K.J.; Kumarasamy, V.; Wijeratne, T.U.; Bunch, K.L.; Tripathi, S.; Knudsen, E.S.; Witkiewicz, A.K.; et al. p27 allosterically activates cyclin-dependent kinase 4 and antagonizes palbociclib inhibition. Science 2019, 366, eaaw2106. [Google Scholar] [CrossRef]
- Larrea, M.D.; Liang, J.; Da Silva, T.; Hong, F.; Shao, S.H.; Han, K.; Dumont, D.; Slingerland, J.M. Phosphorylation of p27Kip1 regulates assembly and activation of cyclin D1-Cdk4. Mol. Cell. Biol. 2008, 28, 6462–6472. [Google Scholar] [CrossRef] [Green Version]
- Vlach, J.; Hennecke, S.; Amati, B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 1997, 16, 5334–5344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrano, A.C.; Eytan, E.; Hershko, A.; Pagano, M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell. Biol. 1999, 1, 193–199. [Google Scholar] [CrossRef]
- Grimmler, M.; Wang, Y.; Mund, T.; Cilenšek, Z.; Keidel, E.-M.; Waddell, M.B.; Jäkel, H.; Kullmann, M.; Kriwacki, R.W.; Hengst, L. Cdk-Inhibitory Activity and Stability of p27Kip1 Are Directly Regulated by Oncogenic Tyrosine Kinases. Cell 2007, 128, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Jäkel, H.; Weinl, C.; Hengst, L. Phosphorylation of p27Kip1 by JAK2 directly links cytokine receptor signaling to cell cycle control. Oncogene 2011, 30, 3502–3512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okutani, Y.; Kitanaka, A.; Tanaka, T.; Kamano, H.; Ohnishi, H.; Kubota, Y.; Ishida, T.; Takahara, J. Src directly tyrosine-phosphorylates STAT5 on its activation site and is involved in erythropoietin-induced signaling pathway. Oncogene 2001, 20, 6643–6650. [Google Scholar] [CrossRef] [Green Version]
- Chin, H.; Arai, A.; Wakao, H.; Kamiyama, R.; Miyasaka, N.; Miura, O. Lyn physically associates with the erythropoietin receptor and may play a role in activation of the Stat5 pathway. Blood 1998, 91, 3734–3745. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Tanaka, T.; Kitanaka, A.; Ohnishi, H.; Okutani, Y.; Waki, M.; Ishida, T.; Kamano, H. Src transduces erythropoietin-induced differentiation signals through phosphatidylinositol 3-kinase. EMBO J. 2001, 20, 5666–5677. [Google Scholar] [CrossRef] [Green Version]
- Livnah, O.; Stura, E.A.; Middleton, S.A.; Johnson, D.L.; Jolliffe, L.K.; Wilson, I.A. Crystallographic Evidence for Preformed Dimers of Erythropoietin Receptor Before Ligand Activation. Science 1999, 283, 987–990. [Google Scholar] [CrossRef]
- Jakel, H.; Taschler, M.; Jung, K.; Weinl, C.; Pegka, F.; Kullmann, M.K.; Podmirseg, S.R.; Dutta, S.; Moser, M.; Hengst, L. Inability to phosphorylate Y88 of p27(Kip1) enforces reduced p27 protein levels and accelerates leukemia progression. Leukemia 2022, 36, 1916–1925. [Google Scholar] [CrossRef]
- Komatsu, N.; Yamamoto, M.; Fujita, H.; Miwa, A.; Hatake, K.; Endo, T.; Okano, H.; Katsube, T.; Fukumaki, Y.; Sassa, S.; et al. Establishment and Characterization of an Erythropoietin-Dependent Subline, UT-7/Epo, Derived from Human Leukemia Cell Line, UT-7. Blood 1993, 82, 456–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxwell, P.; Melendez-Rodriguez, F.; Matchett, K.B.; Aragones, J.; Ben-Califa, N.; Jaekel, H.; Hengst, L.; Lindner, H.; Bernardini, A.; Brockmeier, U.; et al. Novel antibodies directed against the human erythropoietin receptor: Creating a basis for clinical implementation. Br. J. Haematol. 2015, 168, 429–442. [Google Scholar] [CrossRef] [Green Version]
- Hengst, L.; Dulic, V.; Slingerland, J.M.; Lees, E.; Reed, S.I. A cell cycle-regulated inhibitor of cyclin-dependent kinases. Proc. Natl. Acad. Sci. USA 1994, 91, 5291–5295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, M.; Fukada, S.; Yoshikuni, M.; Bulet, P.; Hirai, T.; Yamaguchi, A.; Yasuda, H.; Ohba, Y.; Nagahama, Y. M-phase-specific histone H1 kinase in fish oocytes. Eur. J. Biochem. 1992, 205, 537–543. [Google Scholar] [CrossRef]
- Lee, L.G.; Chen, C.H.; Chiu, L.A. Thiazole orange: A new dye for reticulocyte analysis. Cytometry 1986, 7, 508–517. [Google Scholar] [CrossRef]
- Chu, I.; Sun, J.; Arnaout, A.; Kahn, H.; Hanna, W.; Narod, S.; Sun, P.; Tan, C.K.; Hengst, L.; Slingerland, J. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell 2007, 128, 281–294. [Google Scholar] [CrossRef] [Green Version]
- Peschel, I.; Podmirseg, S.R.; Taschler, M.; Duyster, J.; Gotze, K.S.; Sill, H.; Nachbaur, D.; Jakel, H.; Hengst, L. FLT3 and FLT3-ITD phosphorylate and inactivate the cyclin-dependent kinase inhibitor p27(Kip1) in acute myeloid leukemia. Haematologica 2017, 102, 1378–1390. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Witthuhn, B.A.; Matsuda, T.; Kohlhuber, F.; Kerr, I.M.; Ihle, J.N. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol. Cell. Biol. 1997, 17, 2497–2501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantinescu, S.N.; Huang, L.J.; Nam, H.; Lodish, H.F. The erythropoietin receptor cytosolic juxtamembrane domain contains an essential, precisely oriented, hydrophobic motif. Mol. Cell 2001, 7, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Guerrouahen, B.S.; Futami, M.; Vaklavas, C.; Kanerva, J.; Whichard, Z.L.; Nwawka, K.; Blanchard, E.G.; Lee, F.Y.; Robinson, L.J.; Arceci, R.; et al. Dasatinib inhibits the growth of molecularly heterogeneous myeloid leukemias. Clin. Cancer Res. 2010, 16, 1149–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheaff, R.J.; Groudine, M.; Gordon, M.; Roberts, J.M.; Clurman, B.E. Cyclin E-CDK2 is a regulator of p27(Kip1). Gene Dev. 1997, 11, 1464–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blain, S.W.; Montalvo, E.; Massague, J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27(Kip1) with cyclin A-Cdk2 and cyclin D2-Cdk4. J. Biol. Chem. 1997, 272, 25863–25872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, A.; James, M.K.; Larochelle, S.; Fisher, R.P.; Blain, S.W. p27(Kip1) Inhibits Cyclin D-Cyclin-Dependent Kinase 4 by Two Independent Modes. Mol. Cell. Biol. 2009, 29, 986–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besson, A.; Hwang, H.C.; Cicero, S.; Donovan, S.L.; Gurian-West, M.; Johnson, D.; Clurman, B.E.; Dyer, M.A.; Roberts, J.M. Discovery of an oncogenic activity in p27(Kip1) that causes stem cell expansion and a multiple tumor phenotype. Gene Dev. 2007, 21, 1731–1746. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, Y.; Tomoda, K.; Tanaka, T.; Arata, Y.; Yoneda-Kato, N.; Kato, J. Direct binding of the signal-transducing adaptor Grb2 facilitates down-regulation of the cyclin-dependent kinase inhibitor p27Kip1. J. Biol. Chem. 2001, 276, 12084–12090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moeller, S.J.; Head, E.D.; Sheaff, R.J. p27Kip1 inhibition of GRB2-SOS formation can regulate Ras activation. Mol. Cell. Biol. 2003, 23, 3735–3752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirito, K.; Nakajima, K.; Watanabe, T.; Uchida, M.; Tanaka, M.; Ozawa, K.; Komatsu, N. Identification of the human erythropoietin receptor region required for Stat1 and Stat3 activation. Blood 2002, 99, 102–110. [Google Scholar] [CrossRef]
- Arai, A.; Kanda, E.; Nosaka, Y.; Miyasaka, N.; Miura, O. CrkL is recruited through its SH2 domain to the erythropoietin receptor and plays a role in Lyn-mediated receptor signaling. J. Biol. Chem. 2001, 276, 33282–33290. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Xie, Y.; Ma, J.; Luo, X.; Nie, P.; Zuo, Z.; Lahrmann, U.; Zhao, Q.; Zheng, Y.; Zhao, Y.; et al. IBS: An illustrator for the presentation and visualization of biological sequences. Bioinformatics 2015, 31, 3359–3361. [Google Scholar] [CrossRef] [Green Version]
- Chu, I.M.; Hengst, L.; Slingerland, J.M. The Cdk inhibitor p27 in human cancer: Prognostic potential and relevance to anticancer therapy. Nat. Rev. Cancer 2008, 8, 253–267. [Google Scholar] [CrossRef]
- Polenakovic, M.; Sikole, A. Is erythropoietin a survival factor for red blood cells? J. Am. Soc. Nephrol. 1996, 7, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- Foller, M.; Huber, S.M.; Lang, F. Erythrocyte programmed cell death. IUBMB Life 2008, 60, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, B.; Wang, J.; Wang, L.; Tao, M.; Lu, J.; Lin, J.; Sun, J.; Wang, R. Red blood cell lifespan in long-term hemodialysis patients treated with roxadustat or recombinant human erythropoietin. Ren. Fail. 2021, 43, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Fero, M.L.; Randel, E.; Gurley, K.E.; Roberts, J.M.; Kemp, C.J. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 1998, 396, 177–180. [Google Scholar] [CrossRef] [Green Version]
- Soos, T.J.; Kiyokawa, H.; Yan, J.S.; Rubin, M.S.; Giordano, A.; DeBlasio, A.; Bottega, S.; Wong, B.M.; Mendelsohn, J.; Koff, A. Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ. 1996, 7, 135–146. [Google Scholar] [PubMed]
- Connor, M.K.; Kotchetkov, R.; Cariou, S.; Resch, A.; Lupetti, R.; Beniston, R.G.; Melchior, F.; Hengst, L.; Slingerland, J.M. CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear export signal and links p27 export and proteolysis. Mol. Biol. Cell. 2003, 14, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Rath, S.L.; Senapati, S. Mechanism of p27 Unfolding for CDK2 Reactivation. Sci. Rep. 2016, 6, 26450. [Google Scholar] [CrossRef] [Green Version]
- Montagnoli, A.; Fiore, F.; Eytan, E.; Carrano, A.C.; Draetta, G.F.; Hershko, A.; Pagano, M. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Gene Dev. 1999, 13, 1181–1189. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.; Wittig, J.G.; Ghamari, A.; Maeda, M.; Dailey, T.A.; Bergonia, H.; Kafina, M.D.; Coughlin, E.E.; Minogue, C.E.; Hebert, A.S.; et al. Erythropoietin signaling regulates heme biosynthesis. Elife 2017, 6, e24767. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, O.; Hradilek, A.; Borova, J.; Neuwirt, J.; Travnicek, M. Effect of heme on globin messenger RNA synthesis. Acta. Biol. Med. Ger. 1981, 40, 915–925. [Google Scholar]
- Mense, S.M.; Zhang, L. Heme: A versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell Res. 2006, 16, 681–692. [Google Scholar] [CrossRef] [Green Version]
- Dailey, H.A.; Meissner, P.N. Erythroid heme biosynthesis and its disorders. Cold Spring Harb. Perspect. Med. 2013, 3, a011676. [Google Scholar] [CrossRef] [Green Version]
- Liao, R.; Bresnick, E.H. Heme as a differentiation-regulatory transcriptional cofactor. Int. J. Hematol. 2022, 116, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Tsiftsoglou, A.S.; Vizirianakis, I.S.; Strouboulis, J. Erythropoiesis: Model systems, molecular regulators, and developmental programs. IUBMB Life 2009, 61, 800–830. [Google Scholar] [CrossRef] [PubMed]
- Bhoopalan, S.V.; Huang, L.J.; Weiss, M.J. Erythropoietin regulation of red blood cell production: From bench to bedside and back. F1000Res 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Grillot, D.; Benito, A.; Richard, C.; Nunez, G.; Fernandez-Luna, J.L. Erythropoietin can promote erythroid progenitor survival by repressing apoptosis through Bcl-XL and Bcl-2. Blood 1996, 88, 1576–1582. [Google Scholar] [CrossRef]
- Silva, M.; Benito, A.; Sanz, C.; Prosper, F.; Ekhterae, D.; Nunez, G.; Fernandez-Luna, J.L. Erythropoietin can induce the expression of bcl-x(L) through Stat5 in erythropoietin-dependent progenitor cell lines. J. Biol. Chem. 1999, 274, 22165–22169. [Google Scholar] [CrossRef] [Green Version]
- Dolznig, H.; Habermann, B.; Stangl, K.; Deiner, E.M.; Moriggl, R.; Beug, H.; Mullner, E.W. Apoptosis protection by the Epo target Bcl-X(L) allows factor-independent differentiation of primary erythroblasts. Curr. Biol. 2002, 12, 1076–1085. [Google Scholar] [CrossRef] [Green Version]
- Ghezzi, P.; Brines, M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004, 11 (Suppl. S1), S37–S44. [Google Scholar] [CrossRef] [Green Version]
- Haseyama, Y.; Sawada, K.; Oda, A.; Koizumi, K.; Takano, H.; Tarumi, T.; Nishio, M.; Handa, M.; Ikeda, Y.; Koike, T. Phosphatidylinositol 3-kinase is involved in the protection of primary cultured human erythroid precursor cells from apoptosis. Blood 1999, 94, 1568–1577. [Google Scholar] [CrossRef]
- Socolovsky, M.; Nam, H.; Fleming, M.D.; Haase, V.H.; Brugnara, C.; Lodish, H.F. Ineffective erythropoiesis in Stat5a−/−5b−/− mice due to decreased survival of early erythroblasts. Blood 2001, 98, 3261–3273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Maria, R.; Testa, U.; Luchetti, L.; Zeuner, A.; Stassi, G.; Pelosi, E.; Riccioni, R.; Felli, N.; Samoggia, P.; Peschle, C. Apoptotic role of Fas/Fas ligand system in the regulation of erythropoiesis. Blood 1999, 93, 796–803. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pegka, F.; Ben-Califa, N.; Neumann, D.; Jäkel, H.; Hengst, L. EpoR Activation Stimulates Erythroid Precursor Proliferation by Inducing Phosphorylation of Tyrosine-88 of the CDK-Inhibitor p27Kip1. Cells 2023, 12, 1704. https://doi.org/10.3390/cells12131704
Pegka F, Ben-Califa N, Neumann D, Jäkel H, Hengst L. EpoR Activation Stimulates Erythroid Precursor Proliferation by Inducing Phosphorylation of Tyrosine-88 of the CDK-Inhibitor p27Kip1. Cells. 2023; 12(13):1704. https://doi.org/10.3390/cells12131704
Chicago/Turabian StylePegka, Fragka, Nathalie Ben-Califa, Drorit Neumann, Heidelinde Jäkel, and Ludger Hengst. 2023. "EpoR Activation Stimulates Erythroid Precursor Proliferation by Inducing Phosphorylation of Tyrosine-88 of the CDK-Inhibitor p27Kip1" Cells 12, no. 13: 1704. https://doi.org/10.3390/cells12131704
APA StylePegka, F., Ben-Califa, N., Neumann, D., Jäkel, H., & Hengst, L. (2023). EpoR Activation Stimulates Erythroid Precursor Proliferation by Inducing Phosphorylation of Tyrosine-88 of the CDK-Inhibitor p27Kip1. Cells, 12(13), 1704. https://doi.org/10.3390/cells12131704





