Microvesicle-Mediated Tissue Regeneration Mitigates the Effects of Cellular Ageing
Abstract
1. Introduction
2. Materials and Methods
2.1. Extracellular Vesicle (EV) Isolation
2.2. Cell Culture
2.3. Pathfinder Cells (PCs)
2.4. Human Dermal Fibroblasts (HDFs)
2.5. Mesenchymal Stem Cells (MSCs)
2.6. Vascular Smooth Muscle Cells (VSMCs)
2.7. Co-Cultures
2.8. Effect of EVs Relative to Cell Age
2.9. Flow Cytometry
2.10. Senescence-Associated β-Galactosidase (SA β-gal) Assay
2.11. γH2A.X Staining
2.12. CDKN2A and CDKN1A Expression
2.13. Real-Time Cell Analysis (RTCA)
2.14. 5-Bromo-2′-Deoxyuridine (BrdU) Labelling
2.15. Time Lapse Fluorescence Microscopy
2.16. Statistical Analysis
3. Results
3.1. Only PC-Derived MVs and Not Exos Are Able to Accelerate Wound Healing
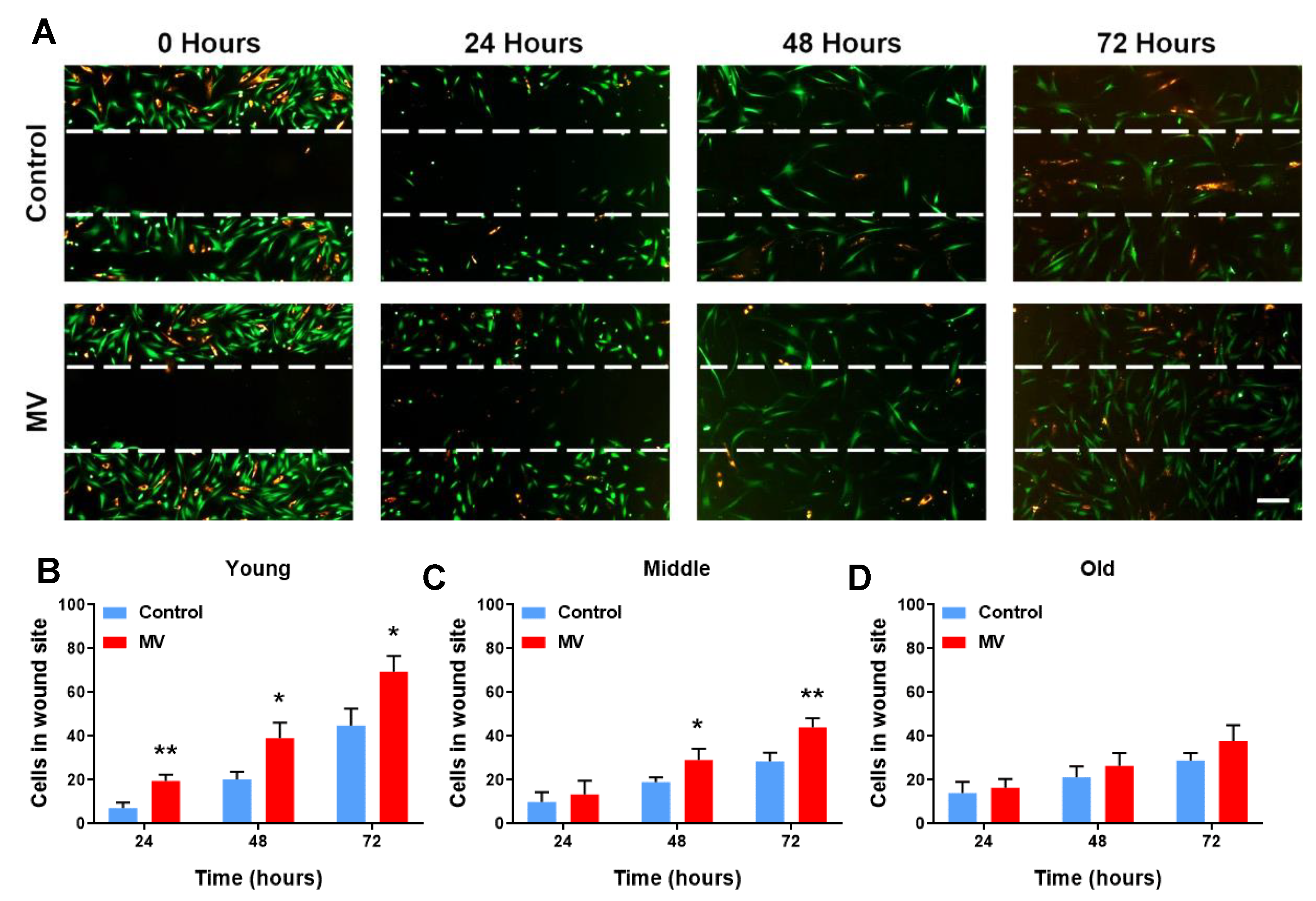
3.2. MV-Mediated Wound Healing Capacity Declines in HDF–MSC Co-Cultures with Increased Cellular Age
3.3. Cellular Age Does Not Impact MV Efficacy under Morbid Conditions
3.4. MV Treatment in the Context of Cellular Abnormality
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gems, D. The aging-disease false dichotomy: Understanding senescence as pathology. Front. Genet. 2015, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.J.; Martinez, C.H.; Mannino, D.M. Ageing and the epidemiology of multimorbidity. Eur. Respir. J. 2014, 44, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Gould, L.; Abadir, P.; Brem, H.; Carter, M.; Conner-Kerr, T.; Davidson, J.; DiPietro, L.A.; Falanga, V.; Fife, C.E.; Gardner, S.E.; et al. Chronic Wound Repair and Healing in Older Adults: Current Status and Future Research. J. Am. Geriatr. Soc. 2015, 63, 427–438. [Google Scholar] [CrossRef]
- Shiels, P.G.; Painer, J.; Natterson-Horowitz, B.; Johnson, R.J.; Miranda, J.J.; Stenvinkel, P. Manipulating the exposome to enable better ageing. Biochem. J. 2021, 478, 2889–2898. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Painer, J.; Kuro-o, M.; Lanaspa, M.; Arnold, W.; Ruf, T.; Shiels, P.G.; Johnson, R.J. Novel treatment strategies for chronic kidney disease: Insights from the animal kingdom. Nat. Rev. Nephrol. 2018, 14, 265–284. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Shiels, P.G. Long-lived animals with negligible senescence: Clues for ageing research. Biochem. Soc. Trans. 2019, 47, 1157–1164. [Google Scholar] [CrossRef]
- Shiels, P.G.; Stenvinkel, P.; Kooman, J.P.; McGuinness, D. Circulating markers of ageing and allostatic load: A slow train coming. Pract. Lab. Med. 2016, 7, 49–54. [Google Scholar] [CrossRef]
- Dolgin, E. Send in the senolytics. Nat. Biotechnol. 2020, 38, 1371–1377. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; Lebrasseur, N.K.; Childs, B.G.; Van De Sluis, B.; Kirkland, J.L.; Van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Zhu, Y.I.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Kirkland, J.L. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Y.; Shao, L.; Laberge, R.-M.; DeMaria, M.; Campisi, J.; Janakiraman, K.; Sharpless, N.E.; Ding, S.; Feng, W.; et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016, 22, 78–83. [Google Scholar] [CrossRef]
- Shiels, P.G.; Buchanan, S.; Selman, C.; Stenvinkel, P. Allostatic load and ageing: Linking the microbiome and nutrition with age-related health. Biochem. Soc. Trans. 2019, 47, 1165–1172. [Google Scholar] [CrossRef]
- Li, Y.-C.; Zhu, K.; Young, T.-H. Induced pluripotent stem cells, form in vitro tissue engineering to in vivo allogeneic transplantation. J. Thorac. Dis. 2017, 9, 455–459. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar]
- Chen, W.; Kimura, M.; Kim, S.; Cao, X.; Srinivasan, S.R.; Berenson, G.S.; Kark, J.D.; Aviv, A. Longitudinal versus Cross-sectional Evaluations of Leukocyte Telomere Length Dynamics: Age-Dependent Telomere Shortening is the Rule. J. Gerontol. Ser. A 2011, 66, 312–319. [Google Scholar] [CrossRef]
- Otto, W.R.; Wright, N.A. Mesenchymal stem cells: From experiment to clinic. Fibrogenes. Tissue Repair 2011, 4, 20. [Google Scholar] [CrossRef]
- Zhuo, W.; Liao, L.; Xu, T.; Wu, W.; Yang, S.; Tan, J. Mesenchymal Stem Cells Ameliorate Ischemia-Reperfusion-Induced Renal Dysfunction by Improving the Antioxidant/Oxidant Balance in the Ischemic Kidney. Urol. Int. 2011, 86, 191–196. [Google Scholar] [CrossRef]
- Ezquer, F.E.; Ezquer, M.E.; Parrau, D.B.; Carpio, D.; Yañez, A.J.; Conget, P.A. Systemic Administration of Multipotent Mesenchymal Stromal Cells Reverts Hyperglycemia and Prevents Nephropathy in Type 1 Diabetic Mice. Biol. Blood Marrow Transplant. 2008, 14, 631–640. [Google Scholar] [CrossRef]
- Havens, A.M.; Shiozawa, Y.; Jung, Y.; Sun, H.; Wang, J.; McGee, S.; Mishra, A.; Taichman, L.S.; Danciu, T.; Jiang, Y.; et al. Human Very Small Embryonic-Like Cells Generate Skeletal Structures, In Vivo. Stem Cells Dev. 2013, 22, 622–630. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Liu, R.; Ratajczak, J.; Kucia, M.; Shin, D.-M. The role of pluripotent embryonic-like stem cells residing in adult tissues in regeneration and longevity. Differentiation 2011, 81, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, J.A.; Studer, L. Moving Stem Cells to the Clinic: Potential and Limitations for Brain Repair. Neuron 2015, 86, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy—Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.G.; Kwon, Y.W.; Lee, T.W.; Park, G.T.; Kim, J.H. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater. Res. 2018, 22, 36. [Google Scholar]
- Anthony, D.F.; Shiels, P.G. Exploiting paracrine mechanisms of tissue regeneration to repair damaged organs. Transplant. Res. 2013, 2, 10. [Google Scholar] [CrossRef]
- McGlynn, L.M.; Eller, K.; MacDonald, A.I.; MacIntyre, A.; Russell, D.; Koppelstaetter, C.; Davies, R.; Shiels, P.G. Pathfinder Cells Provide a Novel Therapeutic Intervention for Acute Kidney Injury. Rejuvenation Res. 2013, 16, 11–20. [Google Scholar] [CrossRef]
- Stevenson, K.S.; Chen, D.; MacIntyre, A.; McGlynn, L.M.; Montague, P.; Charif, R.; Subramaniam, M.; George, W.D.; Payne, A.P.; Davies, R.W.; et al. Pancreatic-Derived Pathfinder Cells Enable Regeneration of Critically Damaged Adult Pancreatic Tissue and Completely Reverse Streptozotocin-Induced Diabetes. Rejuvenation Res. 2011, 14, 163–171. [Google Scholar] [CrossRef]
- McGuinness, D.; Anthony, D.F.; Moulisova, V.; MacDonald, A.I.; MacIntyre, A.; Thomson, J.; Nag, A.; Davies, R.W.; Shiels, P.G. Microvesicles but Not Exosomes from Pathfinder Cells Stimulate Functional Recovery of the Pancreas in a Mouse Streptozotocin-Induced Diabetes Model. Rejuvenation Res. 2016, 19, 223–232. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Ratajczak, J. Horizontal transfer of RNA and proteins between cells by extracellular microvesicles: 14 years later. Clin. Transl. Med. 2016, 5, 7. [Google Scholar] [CrossRef]
- Turturici, G.; Tinnirello, R.; Sconzo, G.; Geraci, F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: Advantages and disadvantages. Am. J. Physiol. Physiol. 2014, 306, C621–C633. [Google Scholar] [CrossRef]
- Panagiotou, N.; Neytchev, O.; Selman, C.; Shiels, P. Extracellular Vesicles, Ageing, and Therapeutic Interventions. Cells 2018, 7, 110. [Google Scholar]
- Holm, M.M.; Kaiser, J.; Schwab, M.E. Extracellular Vesicles: Multimodal Envoys in Neural Maintenance and Repair. Trends Neurosci. 2018, 41, 360–372. [Google Scholar] [CrossRef]
- Chen, W.X.; Yan, Y.B.; Song, C.D.; Ding, Y.; Du, T. Microvesicles derived from human Wharton’s Jelly mesenchymal stem cells ameliorate ischemia-reperfusion-induced renal fibrosis by releasing from G2/M cell cycle arrest. Biochem. J. 2017, 474, 4207–4218. [Google Scholar]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef]
- Jeong, D.; Jo, W.; Yoon, J.; Kim, J.; Gianchandani, S.; Gho, Y.S.; Park, J. Nanovesicles engineered from ES cells for enhanced cell proliferation. Biomaterials 2014, 35, 9302–9310. [Google Scholar] [CrossRef]
- Moulin, V.J.; Mayrand, D.; Messier, H.; Martinez, M.C.; Lopez-Vallé, C.A.; Genest, H. Shedding of microparticles by myofibroblasts as mediator of cellular cross-talk during normal wound healing. J. Cell. Physiol. 2010, 225, 734–740. [Google Scholar] [CrossRef]
- Li, X.; Jiang, C.; Zhao, J. Human endothelial progenitor cells-derived exosomes accelerate cutaneous wound healing in diabetic rats by promoting endothelial function. J. Diabetes Complicat. 2016, 30, 986–992. [Google Scholar] [CrossRef]
- Leoni, G.; Neumann, P.-A.; Kamaly, N.; Quiros, M.; Nishio, H.; Jones, H.R.; Sumagin, R.; Hilgarth, R.S.; Alam, A.; Fredman, G.; et al. Annexin A1–containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J. Clin. Investig. 2015, 125, 1215–1227. [Google Scholar] [CrossRef]
- Cheng, C.F.; Fan, J.; Fedesco, M.; Guan, S.; Li, Y.; Bandyopadhyay, B.; Li, W. Transforming growth factor alpha (TGFalpha)-stimulated secretion of HSP90alpha: Using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing. Mol. Cell. Biol. 2008, 28, 3344–3358. [Google Scholar]
- Guo, S.-C.; Tao, S.-C.; Yin, W.-J.; Qi, X.; Yuan, T.; Zhang, C.-Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017, 7, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Vrijsen, K.R.; Maring, J.A.; Chamuleau, S.A.J.; Verhage, V.; Mol, E.A.; Deddens, J.C.; Metz, C.H.G.; Lodder, K.; Van Eeuwijk, E.C.M.; Van Dommelen, S.M.; et al. Exosomes from Cardiomyocyte Progenitor Cells and Mesenchymal Stem Cells Stimulate Angiogenesis Via EMMPRIN. Adv. Health Mater. 2016, 5, 2555–2565. [Google Scholar] [CrossRef]
- Gerstein, A.D.; Phillips, T.; Rogers, G.S.; Gilchrest, B.A. Wound healing and aging. Dermatol. Clin. 1993, 11, 749–757. [Google Scholar]
- Kook, Y.-M.; Jeong, Y.; Lee, K.; Koh, W.-G. Design of biomimetic cellular scaffolds for co-culture system and their application. J. Tissue Eng. 2017, 8, 2041731417724640. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, N.; Davies, R.W.; Selman, C.; Shiels, P.G. Microvesicles as Vehicles for Tissue Regeneration: Changing of the Guards. Curr. Pathobiol. Rep. 2016, 4, 181–187. [Google Scholar] [CrossRef]
- Shah, T.; Qin, S.; Vashi, M.; Predescu, D.N.; Jeganathan, N.; Bardita, C.; Predescu, S.A. Alk5/Runx1 signaling mediated by extracellular vesicles promotes vascular repair in acute respiratory distress syndrome. Clin. Transl. Med. 2018, 7, 19. [Google Scholar]
- Van Der Pol, E.; Coumans, F.A.W.; Grootemaat, A.E.; Gardiner, C.; Sargent, I.L.; Harrison, P.; Sturk, A.; van Leeuwen, T.G.; Nieuwland, R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J. Thromb. Haemost. 2014, 12, 1182–1192. [Google Scholar] [CrossRef]
- Shiels, P.G.; Ritzau-Reid, K. Biological Ageing, Inflammation and Nutrition: How Might They Impact on Systemic Sclerosis? Curr. Aging Sci. 2015, 8, 123–130. [Google Scholar] [CrossRef]
- Kooman, J.P.; Kotanko, P.; Schols, A.M.W.J.; Shiels, P.; Stenvinkel, P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 2014, 10, 732–742. [Google Scholar] [CrossRef]
- Fuchs, Y.; Steller, H. Live to die another way: Modes of programmed cell death and the signals emanating from dying cells. Nat. Rev. Mol. Cell Biol. 2015, 16, 329–344. [Google Scholar] [CrossRef]
- Fan, Y.; Bergmann, A. Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell! Trends Cell Biol. 2008, 18, 467–473. [Google Scholar] [CrossRef]
- Gupta, K.H.; Goldufsky, J.W.; Wood, S.J.; Tardi, N.J.; Moorthy, G.S.; Gilbert, D.Z.; Shafikhani, S.H. Apoptosis and Compensatory Proliferation Signaling Are Coupled by CrkI-Containing Microvesicles. Dev. Cell 2017, 41, 674–684.e5. [Google Scholar]
- Ranghino, A.; Bruno, S.; Bussolati, B.; Moggio, A.; DiMuccio, V.; Tapparo, M.; Biancone, L.; Gontero, P.; Frea, B.; Camussi, G. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res. Ther. 2017, 8, 24. [Google Scholar] [CrossRef]
- Bruno, S.; Camussi, G. Role of mesenchymal stem cell-derived microvesicles in tissue repair. Pediatr. Nephrol. 2013, 28, 2249–2254. [Google Scholar] [CrossRef]
- Camussi, G.; Deregibus, M.C.; Cantaluppi, V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem. Soc. Trans. 2013, 41, 283–287. [Google Scholar] [CrossRef]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef]
- Chen, H.-Z.; Wang, F.; Gao, P.; Pei, J.-F.; Liu, Y.; Xu, T.-T.; Tang, X.; Fu, W.-Y.; Lu, J.; Yan, Y.-F.; et al. Age-Associated Sirtuin 1 Reduction in Vascular Smooth Muscle Links Vascular Senescence and Inflammation to Abdominal Aortic Aneurysm. Circ. Res. 2016, 119, 1076–1088. [Google Scholar] [CrossRef]
- Zhang, W.-M.; Liu, Y.; Li, T.-T.; Piao, C.-M.; Liu, O.; Liu, J.-L.; Qi, Y.-F.; Jia, L.-X.; Du, J. Sustained activation of ADP/P2ry12 signaling induces SMC senescence contributing to thoracic aortic aneurysm/dissection. J. Mol. Cell. Cardiol. 2016, 99, 76–86. [Google Scholar] [CrossRef]
- Riches, K.; Angelini, T.G.; Mudhar, G.S.; Kaye, J.; Clark, E.; Bailey, M.A.; Sohrabi, S.; Korossis, S.; Walker, P.G.; Scott, D.J.A.; et al. Exploring smooth muscle phenotype and function in a bioreactor model of abdominal aortic aneurysm. J. Transl. Med. 2013, 11, 208. [Google Scholar] [CrossRef]
- Lacolley, P.; Regnault, V.; Nicoletti, A.; Li, Z.; Michel, J.-B. The vascular smooth muscle cell in arterial pathology: A cell that can take on multiple roles. Cardiovasc. Res. 2012, 95, 194–204. [Google Scholar] [CrossRef]
- Liao, S.; Curci, J.A.; Kelley, B.J.; Sicard, G.A.; Thompson, R.W. Accelerated Replicative Senescence of Medial Smooth Muscle Cells Derived from Abdominal Aortic Aneurysms Compared to the Adjacent Inferior Mesenteric Artery. J. Surg. Res. 2000, 92, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Teti, G.; Chiarini, F.; Mazzotti, E.; Ruggeri, A.; Carano, F.; Falconi, M. Cellular senescence in vascular wall mesenchymal stromal cells, a possible contribution to the development of aortic aneurysm. Mech. Ageing Dev. 2021, 197, 111515. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-García, C.; Burillo, E.; Lindholt, J.S.; Martinez-Lopez, D.; Pilely, K.; Mazzeo, C.; Michel, J.; Egido, J.; Garred, P.; Blanco-Colio, L.M.; et al. Association of ficolin-3 with abdominal aortic aneurysm presence and progression. J. Thromb. Haemost. 2016, 15, 575–585. [Google Scholar] [CrossRef]
- Weintraub, N.L. Understanding Abdominal Aortic Aneurysm. N. Engl. J. Med. 2009, 361, 1114–1116. [Google Scholar] [CrossRef]
- Sakalihasan, N.; Limet, R.; Defawe, O.D. Abdominal aortic aneurysm. Lancet 2005, 365, 1577–1589. [Google Scholar]
- Bloom, D.E.; Chatterji, S.; Kowal, P.; Lloyd-Sherlock, P.; McKee, M.; Rechel, B.; Rosenberg, L.; Smith, J.P. Macroeconomic implications of population ageing and selected policy responses. Lancet 2015, 385, 649–657. [Google Scholar] [CrossRef]
- Beard, J.R.; Bloom, D.E. Towards a comprehensive public health response to population ageing. Lancet 2015, 385, 658–661. [Google Scholar]
- Selman, C. Dietary restriction and the pursuit of effective mimetics. Proc. Nutr. Soc. 2014, 73, 260–270. [Google Scholar] [CrossRef]
- Selman, C.; Lingard, S.; Choudhury, A.I.; Batterham, R.L.; Claret, M.; Clements, M.; Ramadani, F.; Okkenhaug, K.; Schuster, E.; Blanc, E.; et al. Evidence for lifespan extension and delayed age–related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008, 22, 807–818. [Google Scholar] [CrossRef]
- Selman, C.; Lingard, S.; Gems, D.; Partridge, L.; Withers, D.J. Comment on “Brain IRS2 Signaling Coordinates Life Span and Nutrient Homeostasis”. Science 2008, 320, 1012. [Google Scholar] [CrossRef]
- Kenyon, C. The Plasticity of Aging: Insights from Long-Lived Mutants. Cell 2005, 120, 449–460. [Google Scholar] [CrossRef]
- Stevenson, K.S.; Hodge, M.; McLinden, H.; George, W.D.; Davies, R.W.; Shiels, P.G.; McGuinness, D.; Anthony, D.F.; Moulisova, V.; MacDonald, A.I.; et al. Isolation, Characterization, and Differentiation of Thy1.1-Sorted Pancreatic Adult Progenitor Cell Populations. Stem Cells Dev. 2009, 18, 1389–1398. [Google Scholar] [CrossRef]
- Browder, K.C.; Reddy, P.; Yamamoto, M.; Haghani, A.; Guillen, I.G.; Sahu, S.; Wang, C.; Luque, Y.; Prieto, J.; Shi, L.; et al. In vivo partial reprogramming alters age-associated molecular changes during physiological aging in mice. Nat. Aging 2022, 2, 243–253. [Google Scholar] [CrossRef]
- Lu, Y.; Brommer, B.; Tian, X.; Krishnan, A.; Meer, M.; Wang, C.; Vera, D.L.; Zeng, Q.; Yu, D.; Bonkowski, M.S.; et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 2020, 588, 124–129. [Google Scholar] [CrossRef]
- Chen, B.; Li, Q.; Zhao, B.; Wang, Y. Stem Cell-Derived Extracellular Vesicles as a Novel Potential Therapeutic Tool for Tissue Repair. Stem Cells Transl. Med. 2017, 6, 1753–1758. [Google Scholar] [CrossRef]
- Lovisolo, F.; Carton, F.; Gino, S.; Migliario, M.; Renò, F. Platelet rich plasma-derived microvesicles increased in vitro wound healing. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9658–9664. [Google Scholar]
- Merjaneh, M.; Langlois, A.; Larochelle, S.; Cloutier, C.B.; Ricard-Blum, S.; Moulin, V.J. Pro-angiogenic capacities of microvesicles produced by skin wound myofibroblasts. Angiogenesis 2017, 20, 385–398. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef]
- Blunder, S.; Messner, B.; Scharinger, B.; Doppler, C.; Zeller, I.; Zierer, A.; Laufer, G.; Bernhard, D. Targeted gene expression analyses and immunohistology suggest a pro-proliferative state in tricuspid aortic valve-, and senescence and viral infections in bicuspid aortic valve-associated thoracic aortic aneurysms. Atherosclerosis 2018, 271, 111–119. [Google Scholar] [CrossRef]
- Raffaele, M.; Vinciguerra, M. The costs and benefits of senotherapeutics for human health. Lancet Health Longev. 2022, 3, e67–e77. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panagiotou, N.; McGuinness, D.; Jaminon, A.M.G.; Mees, B.; Selman, C.; Schurgers, L.; Shiels, P.G. Microvesicle-Mediated Tissue Regeneration Mitigates the Effects of Cellular Ageing. Cells 2023, 12, 1707. https://doi.org/10.3390/cells12131707
Panagiotou N, McGuinness D, Jaminon AMG, Mees B, Selman C, Schurgers L, Shiels PG. Microvesicle-Mediated Tissue Regeneration Mitigates the Effects of Cellular Ageing. Cells. 2023; 12(13):1707. https://doi.org/10.3390/cells12131707
Chicago/Turabian StylePanagiotou, Nikolaos, Dagmara McGuinness, Armand M. G. Jaminon, Barend Mees, Colin Selman, Leon Schurgers, and Paul G. Shiels. 2023. "Microvesicle-Mediated Tissue Regeneration Mitigates the Effects of Cellular Ageing" Cells 12, no. 13: 1707. https://doi.org/10.3390/cells12131707
APA StylePanagiotou, N., McGuinness, D., Jaminon, A. M. G., Mees, B., Selman, C., Schurgers, L., & Shiels, P. G. (2023). Microvesicle-Mediated Tissue Regeneration Mitigates the Effects of Cellular Ageing. Cells, 12(13), 1707. https://doi.org/10.3390/cells12131707






