Ex Vivo Preservation of Ovine Periosteum Using a Perfusion Bioreactor System
Abstract
1. Introduction
2. Materials and Methods
2.1. Periosteum Procurement
2.2. Perfusion Systems and Experimental Regimen for Testing Periosteal Perfusion
2.3. Perfusate
2.4. Live/Dead Cell Analysis
2.5. PrestoBlue Cell Viability and Metabolic Assay
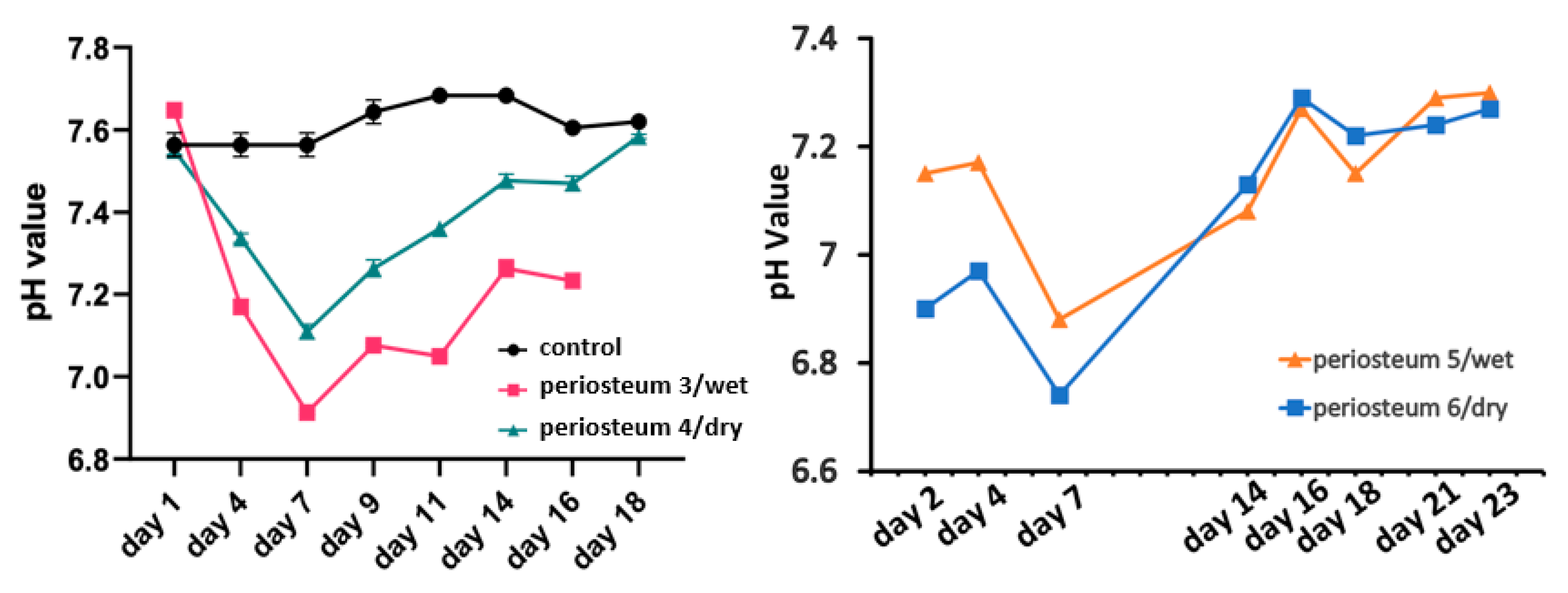
2.6. pH Measurement
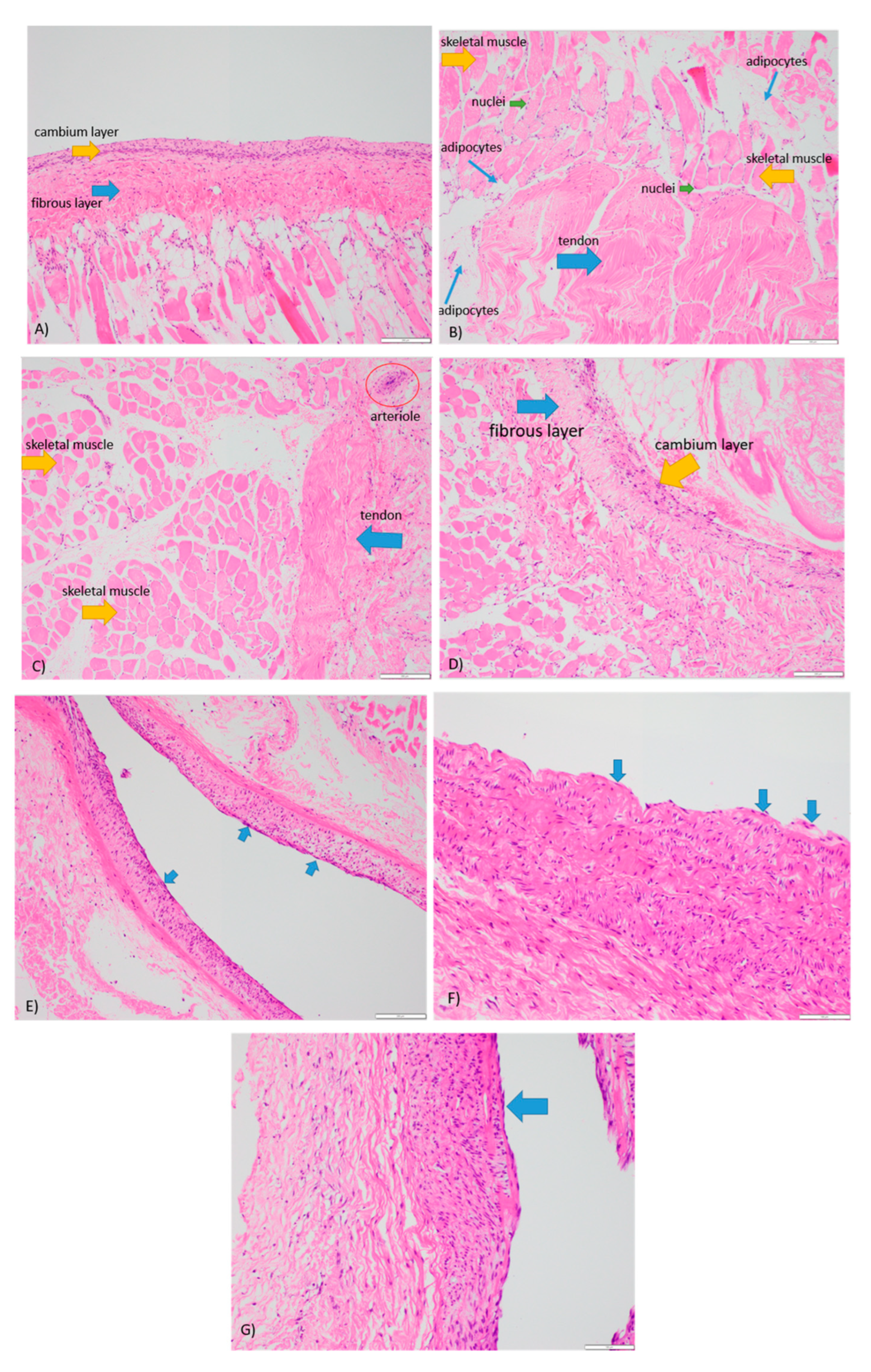
2.7. Histology
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D printing | Three-dimensional printing |
| PC2 laboratory | Physical contaminant level 2 laboratory |
| DMEM | Dulbecco’s modified Eagle’s medium |
| Calcein AM | Calcein acetoxymethyl |
| EthD-1 | Ethidium homodimer-1 |
| H&E staining | Hematoxylin and eosin staining |
References
- Accorona, R.; Gazzini, L.; Grigolato, R.; Fazio, E.; Nitro, L.; Abousiam, M.; Giorgetti, G.; Pignataro, L.; Capaccio, P.; Calabrese, L. Free Periosteal Flaps with Scaffold: An Overlooked Armamentarium for Maxillary and Mandibular Reconstruction. Cancers 2021, 13, 4373. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, F.; Smithers, F.; Cheng, K.; Mukherjee, P.; Hubert Low, T.-H.; Ch’Ng, S.; Palme, C.E.; Clark, J.R. Time and cost-analysis of virtual surgical planning for head and neck reconstruction: A matched pair analysis. Oral Oncol. 2020, 100, 104491. [Google Scholar] [CrossRef] [PubMed]
- Petrides, G.A.; Dunn, M.; Charters, E.; Venchiarutti, R.; Cheng, K.; Froggatt, C.; Mukherjee, P.; Wallace, C.; Howes, D.; Leinkram, D.; et al. Health-related quality of life in maxillectomy patients undergoing dentoalveolar rehabilitation. Oral Oncol. 2022, 126, 105757. [Google Scholar] [CrossRef] [PubMed]
- Tatara, A.M.; Shah, S.R.; Demian, N.; Ho, T.; Shum, J.; van den Beucken, J.J.J.P.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. Reconstruction of large mandibular defects using autologous tissues generated from in vivo bioreactors. Acta Biomater. 2016, 45, 72–84. [Google Scholar] [CrossRef]
- Baba, S.; Yamada, Y.; Komuro, A.; Yotsui, Y.; Umeda, M.; Shimuzutani, K.; Nakamura, S. Phase I/II Trial of Autologous Bone Marrow Stem Cell Transplantation with a Three-Dimensional Woven-Fabric Scaffold for Periodontitis. Stem Cells Int. 2016, 2016, 6205910. [Google Scholar] [CrossRef]
- Talaat, W.M.; Ghoneim, M.M.; Salah, O.; Adly, O.A. Autologous Bone Marrow Concentrates and Concentrated Growth Factors Accelerate Bone Regeneration After Enucleation of Mandibular Pathologic Lesions. J. Craniofacial Surg. 2018, 29, 992–997. [Google Scholar] [CrossRef]
- Al Maruf, D.S.A.; Ghosh, Y.A.; Xin, H.; Cheng, K.; Mukherjee, P.; Crook, J.M.; Wallace, G.G.; Klein, T.J.; Clark, J.R. Hydrogel: A Potential Material for Bone Tissue Engineering Repairing the Segmental Mandibular Defect. Polymers 2022, 14, 4186. [Google Scholar] [CrossRef]
- Al Maruf, D.S.A.; Parthasarathi, K.; Cheng, K.; Mukherjee, P.; McKenzie, D.R.; Crook, J.M.; Wallace, G.G.; Clark, J.R. Current and future perspectives on biomaterials for segmental mandibular defect repair. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 725–737. [Google Scholar] [CrossRef]
- Dwek, J.R. The periosteum: What is it, where is it, and what mimics it in its absence? Skelet. Radiol. 2010, 39, 319–323. [Google Scholar] [CrossRef]
- Lin, Z.; Fateh, A.; Salem, D.; Intini, G. Periosteum: Biology and Applications in Craniofacial Bone Regeneration. J. Dent. Res. 2014, 93, 109–116. [Google Scholar] [CrossRef]
- Ghosh, Y.A.; Gupta, R.; Al Maruf, D.A.; Cheng, K.; Mukherjee, P.; Clark, J.R. Surgical technique: A novel pedicled periosteal scapular flap to facilitate bone growth in an Ovine model. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 1497–1520. [Google Scholar] [CrossRef]
- Hurrell, M.J.L.; Low, T.-H.; Ch’Ng, S.; Clark, J.R. Fascio-cutaneous and fascio-periosteal free flaps for treatment of intermediate stage osteoradionecrosis of the jaws. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, L.; Yabe, T.; Wykes, J.; McAndrew, D.J.; Clark, J.R.; Ashford, B.G. Defining the Dimensions of Periosteal Free Tissue Transfer Harvest Sites. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3846. [Google Scholar] [CrossRef] [PubMed]
- Kelley, P.; Klebuc, M.; Hollier, L. Complex Midface Reconstruction: Maximizing Contour and Bone Graft Survival Utilizing Periosteal Free Flaps. J. Craniofacial Surg. 2003, 14, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Saimbi, C.; Khan, M.; Goel, A. Use of periosteal membrane as a barrier membrane for the treatment of buccal Grade II furcation defects in lower molars: A novel technique. Indian J. Dent. Res. 2011, 22, 511–516. [Google Scholar] [CrossRef]
- Tobon-Arroyave, S.I.; Dominguez-Mejia, J.S.; Florez-Moreno, G.A. Periosteal grafts as barriers in periradicular surgery: Report of two cases. Int. Endod. J. 2004, 37, 632–642. [Google Scholar] [CrossRef]
- Kim, C.-S.; Jang, Y.-J.; Choi, S.-H.; Cho, K.-S. Long-Term Results from Soft and Hard Tissue Augmentation by a Modified Vascularized Interpositional Periosteal-Connective Tissue Technique in the Maxillary Anterior Region. J. Oral Maxillofac. Surg. 2012, 70, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulos, L.; Karring, T. Role of periosteum in the formation of jaw bone. J. Clin. Periodontol. 1995, 22, 247–254. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Bikle, D.D. Osteogenic Differentiation of Periosteal Cells During Fracture Healing. J. Cell. Physiol. 2017, 232, 913–921. [Google Scholar] [CrossRef]
- Li, Z.; Pan, J.; Ma, J.; Zhang, Z.; Bai, Y. Microarray gene expression of periosteum in spontaneous bone regeneration of mandibular segmental defects. Sci. Rep. 2017, 7, 13535. [Google Scholar] [CrossRef]
- Han, D.; Dai, K. Prefabrication of a Vascularized Bone Graft with Beta Tricalcium Phosphate Using an In Vivo Bioreactor. Artif. Organs 2013, 37, 884–893. [Google Scholar] [CrossRef]
- Huang, R.-L.; Tremp, M.; Ho, C.-K.; Sun, Y.; Liu, K.; Li, Q. Prefabrication of a functional bone graft with a pedicled periosteal flap as an in vivo bioreactor. Sci. Rep. 2017, 7, 18038. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M.; Marini, R.P.; Schaefer, D.; Aronson, J.; Langer, R.; Shastri, V.P. In vivo engineering of organs: The bone bioreactor. Proc. Natl. Acad. Sci. USA 2005, 102, 11450–11455. [Google Scholar] [CrossRef] [PubMed]
- Kruse, H.V.; Lewin, W.T.; Suchowerska, N.; Al Maruf, D.S.A.; Cheng, K.; Clark, J.R.; McKenzie, D.R. Plasma immersion ion-implanted 3D-printed PEEK bone implants: In vivo sheep study shows strong osseointegration. Plasma Process. Polym. 2022, 19, 2100244. [Google Scholar] [CrossRef]
- Won, N.; Castillo-Prado, J.; Tan, X.; Ford, J.; Heath, D.; Mazilescu, L.I.; Selzner, M.; Rogers, I.M. Ex Vivo Perfusion Using a Mathematical Modeled, Controlled Gas Exchange Self-Contained Bioreactor Can Maintain a Mouse Kidney for Seven Days. Cells 2022, 11, 1822. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; McCanna, D.J.; Sivak, J.G. Use of the viability reagent PrestoBlue in comparison with alamarBlue and MTT to assess the viability of human corneal epithelial cells. J. Pharmacol. Toxicol. Methods 2015, 71, 1–7. [Google Scholar] [CrossRef]
- Tiefenthaler, M.; Amberger, A.; Bacher, N.; Hartmann, B.L.; Margreiter, R.; Kofler, R.; Konwalinka, G. Increased lactate production follows loss of mitochondrial membrane potential during apoptosis of human leukaemia cells. Br. J. Haematol. 2001, 114, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Sittinger, M. Periosteal Cells in Bone Tissue Engineering. Tissue Eng. 2003, 9 (Suppl. S1), 45–64. [Google Scholar] [CrossRef]
- Grayson, W.L.; Marolt, D.; Bhumiratana, S.; Fröhlich, M.; Guo, X.E.; Vunjak-Novakovic, G. Optimizing the medium perfusion rate in bone tissue engineering bioreactors. Biotechnol. Bioeng. 2011, 108, 1159–1170. [Google Scholar] [CrossRef]
- Dua, R.; Jones, H.; Noble, P.C. Designing and validation of an automated ex-vivo bioreactor system for long term culture of bone. Bone Rep. 2021, 14, 101074. [Google Scholar] [CrossRef]
- Cartmell, S.H.; Porter, B.D.; García, A.J.; Guldberg, R.E. Effects of Medium Perfusion Rate on Cell-Seeded Three-Dimensional Bone Constructs in Vitro. Tissue Eng. 2003, 9, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Birru, B.; Mekala, N.K.; Parcha, S.R. Mechanistic role of perfusion culture on bone regeneration. J. Biosci. 2019, 44, 23. [Google Scholar] [CrossRef] [PubMed]
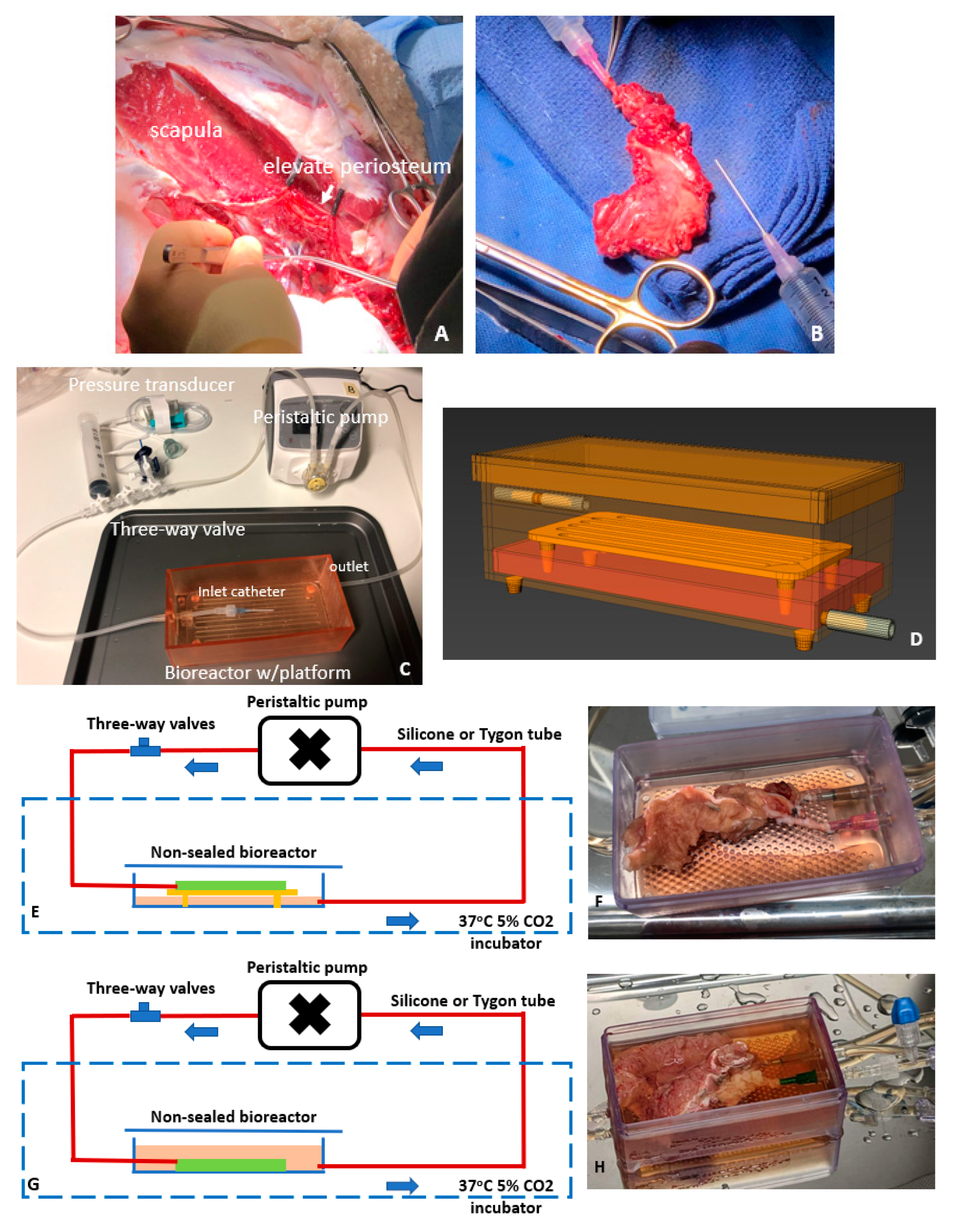



| Case Number | Procurement Site (Weight) | Perfusion Mode | Tubing | Frequency of Medium Change | System Duration (Days) |
|---|---|---|---|---|---|
| 1 | Femur (7~8 g) | Dry perfusion | Silicone | Every two days | 4 |
| 2 | Femur (7~8 g) | Dry perfusion | 4 | ||
| 3 | Scapula (18~21 g) | Wet perfusion | Silicone | 17 | |
| 4 | Scapula (18~21 g) | Dry perfusion | 18 | ||
| 5 | Scapula (18~21 g) | Wet perfusion | Tygon | 25 | |
| 6 | Scapula (18~21 g) | Dry perfusion | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, H.; Romanazzo, S.; Tomaskovic-Crook, E.; Mitchell, T.C.; Hung, J.C.; Wise, S.G.; Cheng, K.; Al Maruf, D.S.A.; Stokan, M.J.; Manzie, T.G.H.; et al. Ex Vivo Preservation of Ovine Periosteum Using a Perfusion Bioreactor System. Cells 2023, 12, 1724. https://doi.org/10.3390/cells12131724
Xin H, Romanazzo S, Tomaskovic-Crook E, Mitchell TC, Hung JC, Wise SG, Cheng K, Al Maruf DSA, Stokan MJ, Manzie TGH, et al. Ex Vivo Preservation of Ovine Periosteum Using a Perfusion Bioreactor System. Cells. 2023; 12(13):1724. https://doi.org/10.3390/cells12131724
Chicago/Turabian StyleXin, Hai, Sara Romanazzo, Eva Tomaskovic-Crook, Timothy C. Mitchell, Jui Chien Hung, Steven G. Wise, Kai Cheng, D S Abdullah Al Maruf, Murray J. Stokan, Timothy G. H. Manzie, and et al. 2023. "Ex Vivo Preservation of Ovine Periosteum Using a Perfusion Bioreactor System" Cells 12, no. 13: 1724. https://doi.org/10.3390/cells12131724
APA StyleXin, H., Romanazzo, S., Tomaskovic-Crook, E., Mitchell, T. C., Hung, J. C., Wise, S. G., Cheng, K., Al Maruf, D. S. A., Stokan, M. J., Manzie, T. G. H., Parthasarathi, K., Cheung, V. K. Y., Gupta, R., Ly, M., Pulitano, C., Wise, I. K., Crook, J. M., & Clark, J. R. (2023). Ex Vivo Preservation of Ovine Periosteum Using a Perfusion Bioreactor System. Cells, 12(13), 1724. https://doi.org/10.3390/cells12131724










