Impaired Insulin Signaling Alters Mediators of Hippocampal Synaptic Dynamics/Plasticity: A Possible Mechanism of Hyperglycemia-Induced Cognitive Impairment
Abstract
:Simple Summary
Abstracts
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Animal Groups and Experimental Design
2.4. Surgical Procedure for ICV- STZ/Vehicle Injection
2.5. Cognitive Behavior Tests
2.5.1. Novel Object Recognition (NOR) Test
2.5.2. T-Maze Test
2.6. Isolation of Hippocampal Synaptosomal Fractions
2.7. Estimation of Protein
2.8. Estimation of Acetylcholinesterase (AChE) and Na+/K+-ATPase Activity
2.9. Western Blot Analysis for Proteins of Synaptic Dynamics/Plasticity
2.10. Statistical Analysis
3. Results
3.1. IP-STZ and ICV-STZ Treatments Attenuated Rats’ Responses in Novel Object Recognition (NOR)
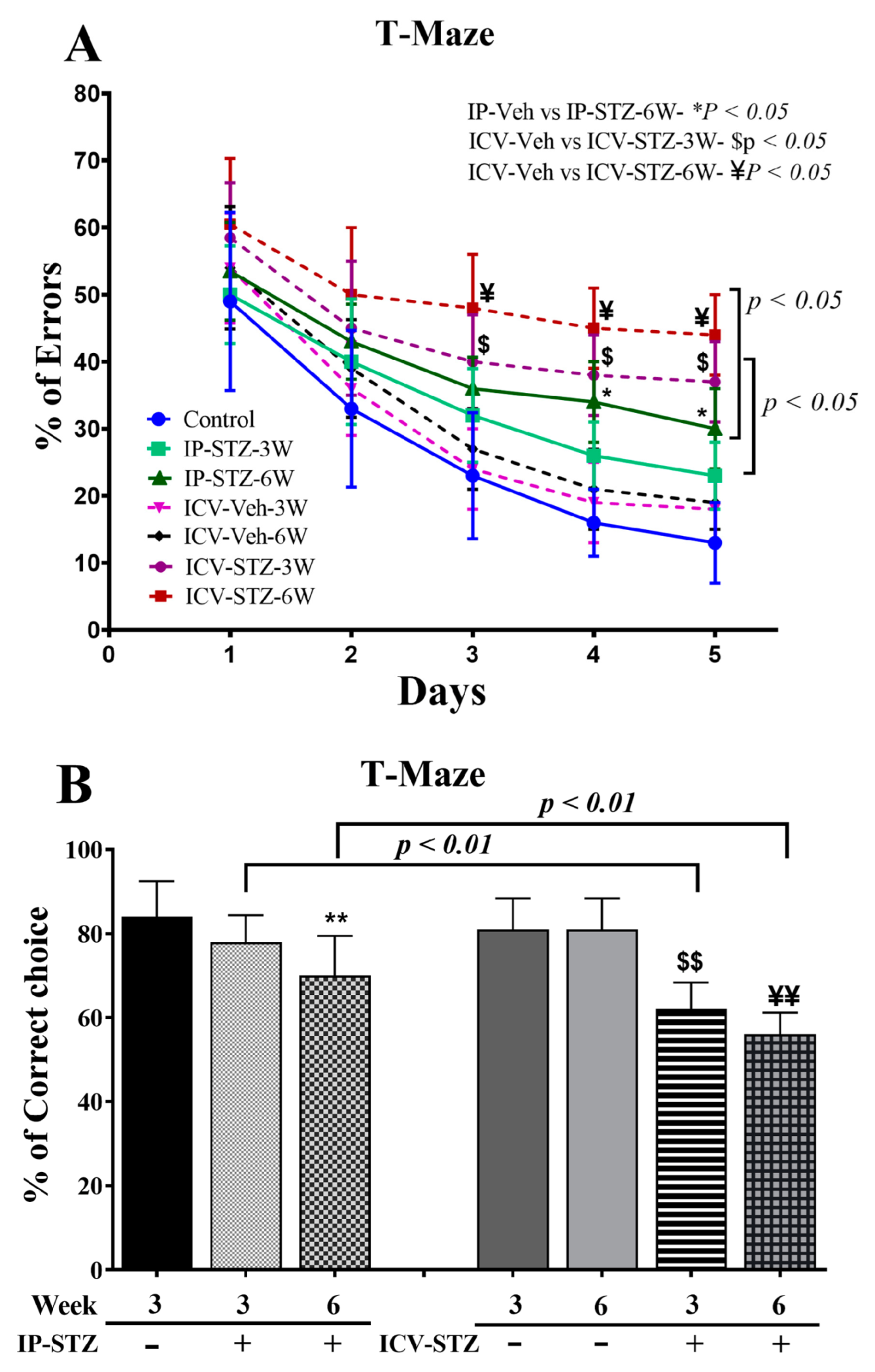
3.2. IP-STZ and ICV-STZ Treatments Attenuated Rats’ Cognitive Behavior in the T-Maze Test
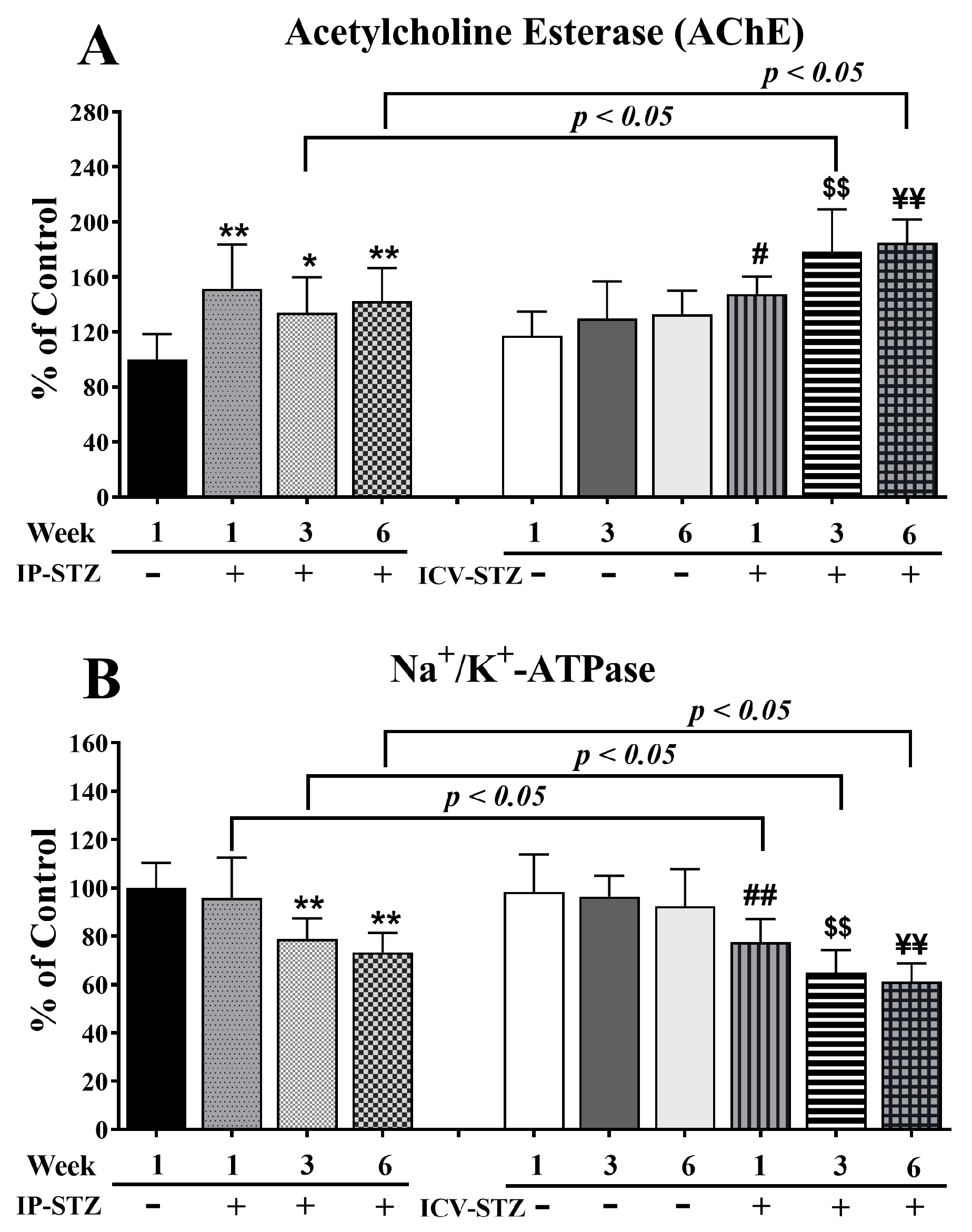
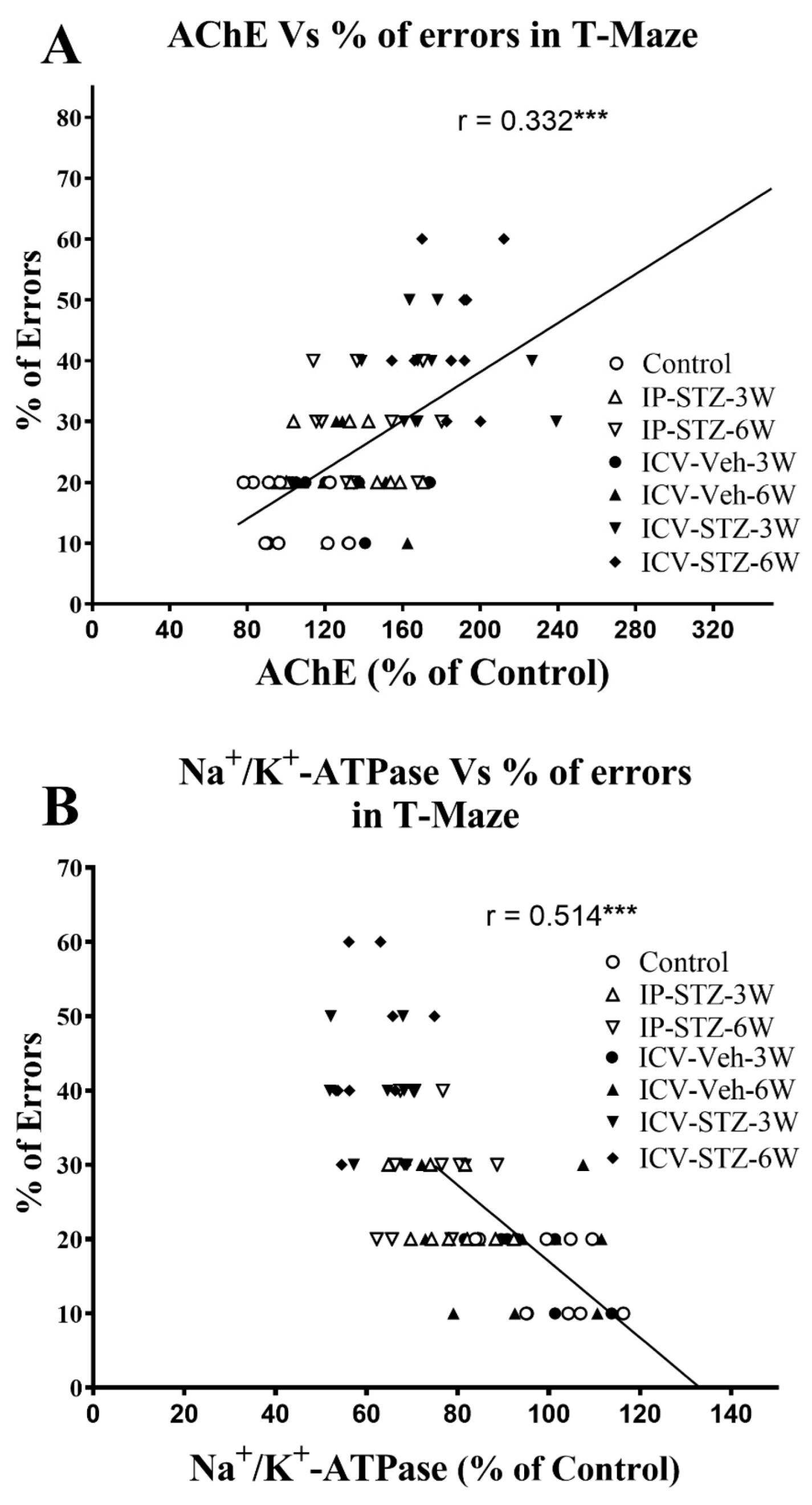
3.3. IP-STZ and ICV-STZ Induced Early Changes in the Activity of AChE and Na+/K+-ATPase
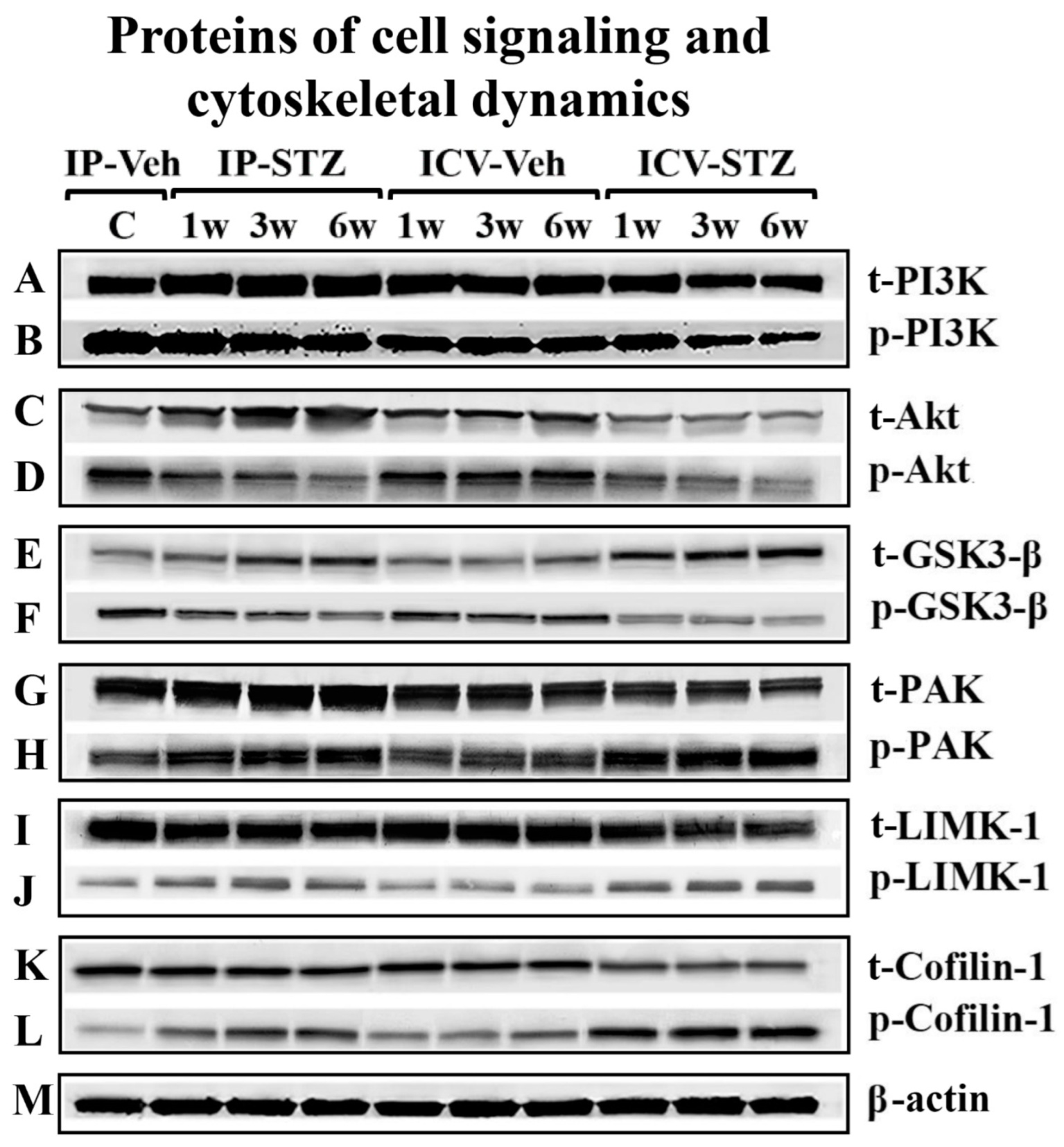
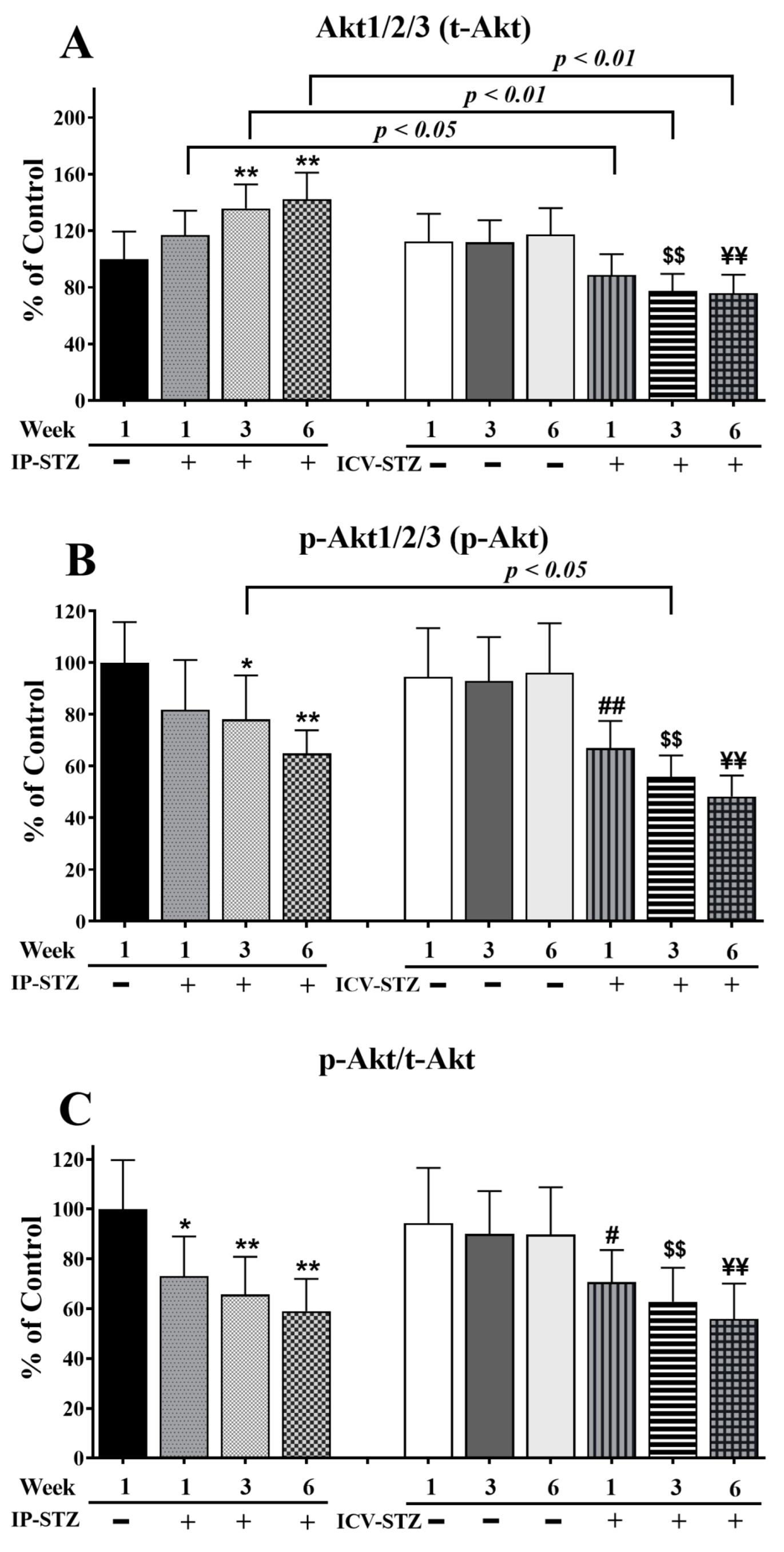
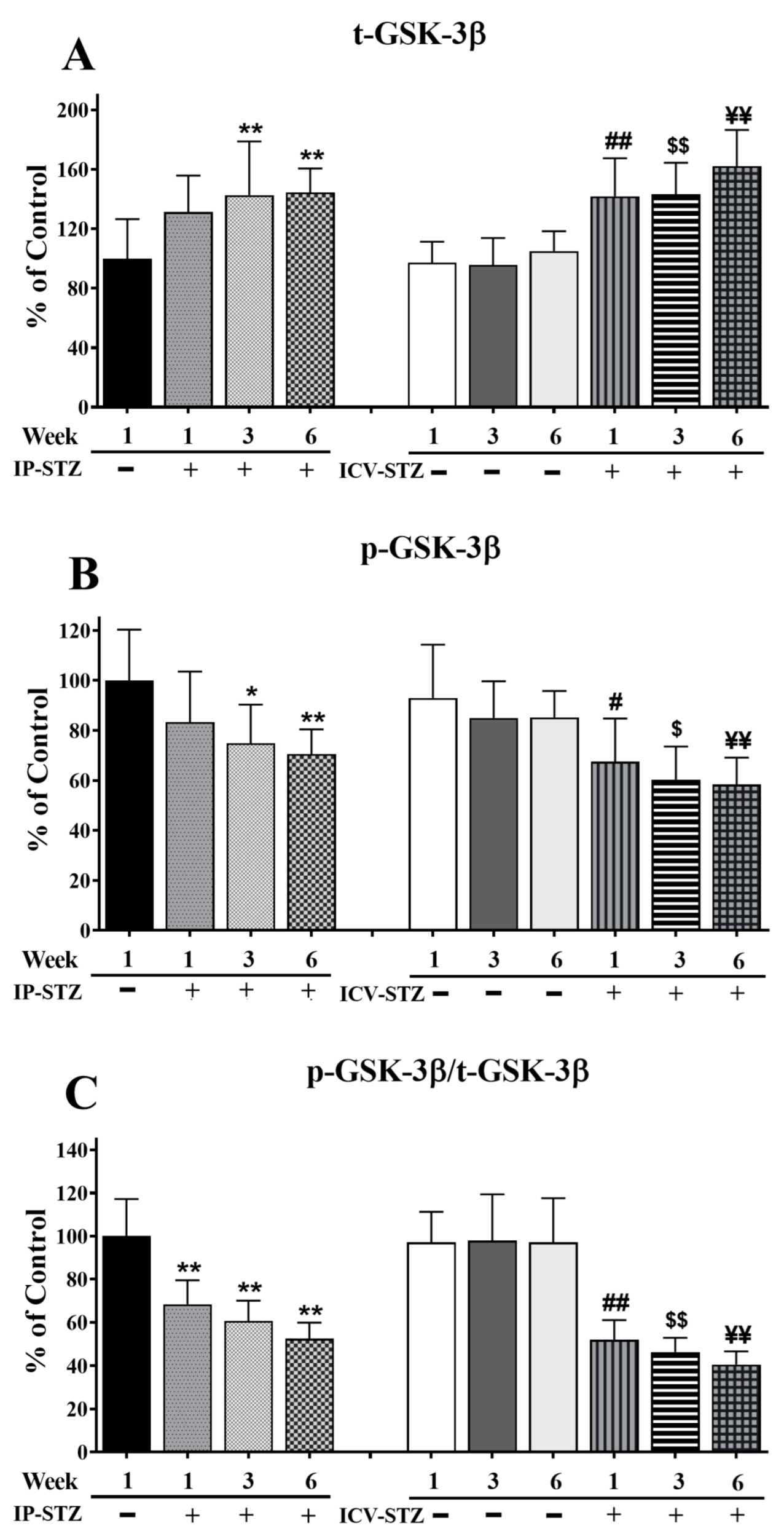
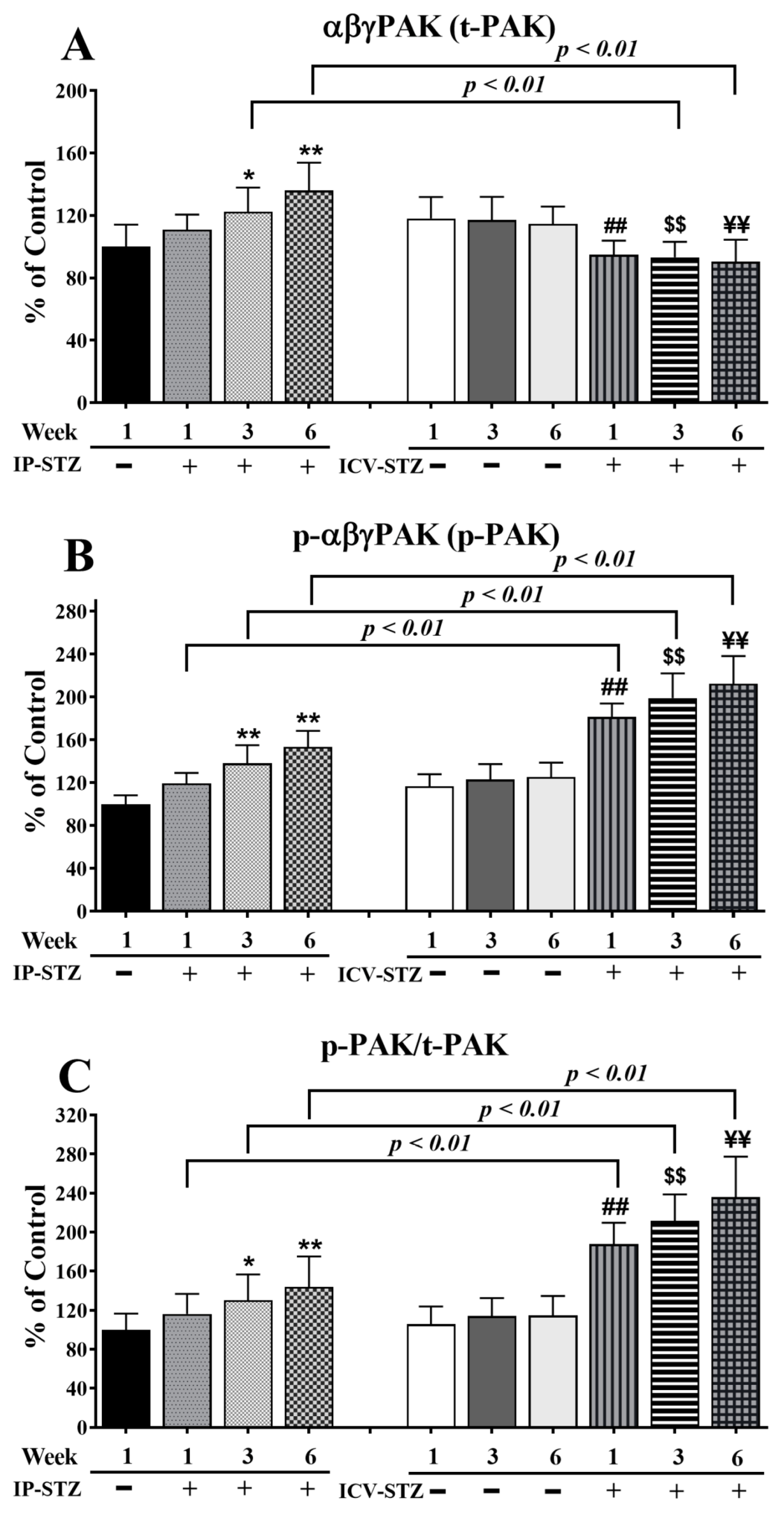
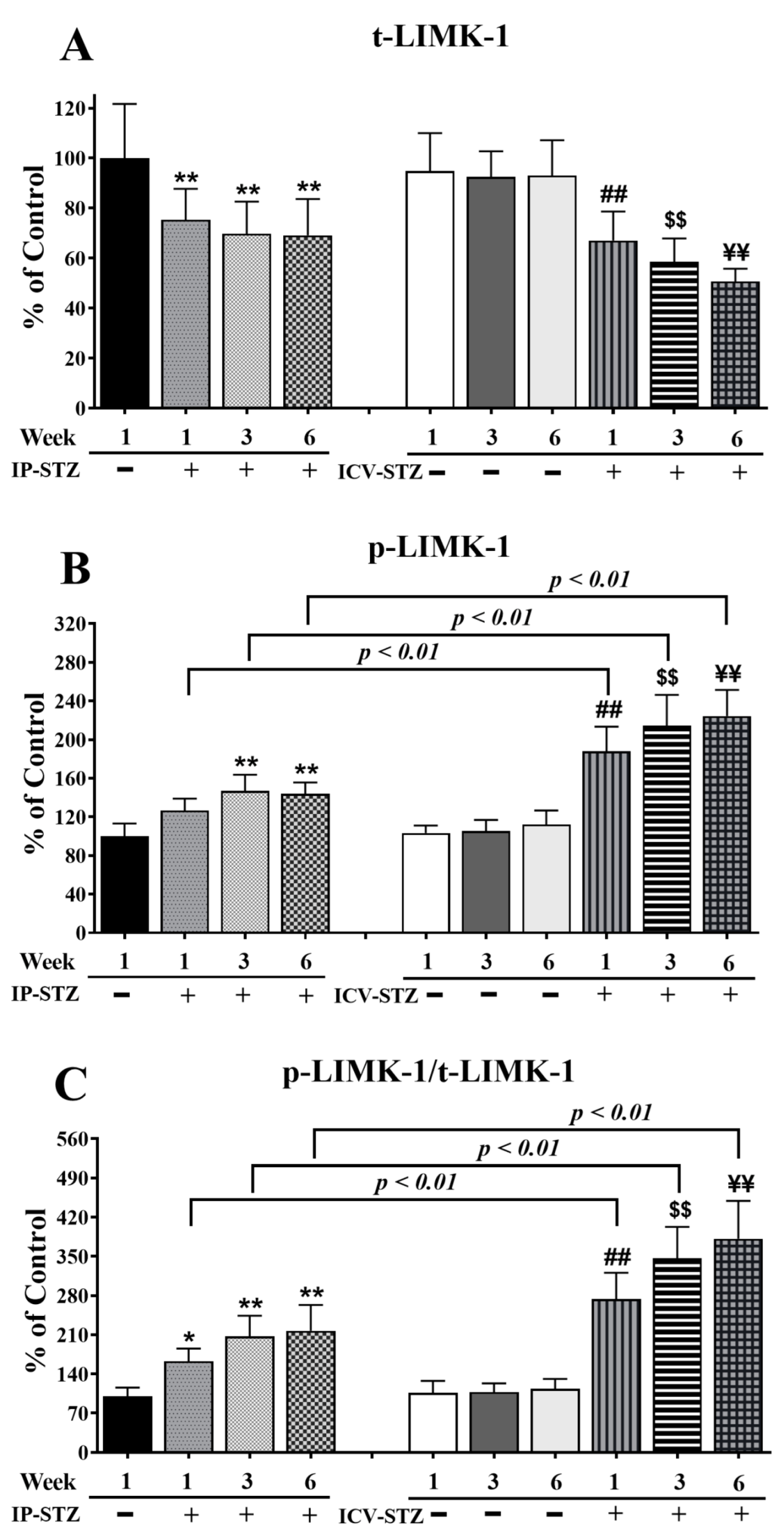
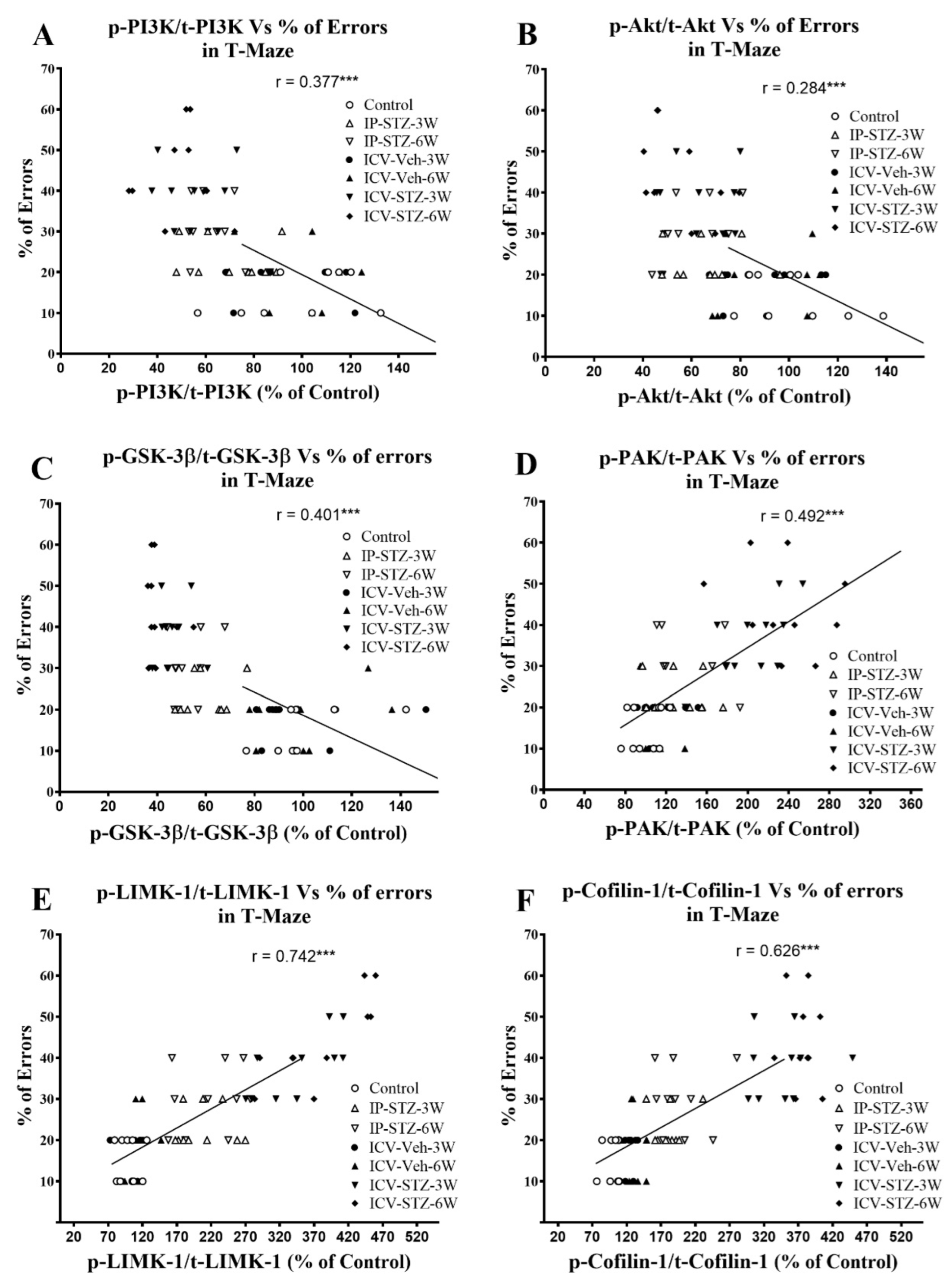
3.4. IP-STZ and ICV-STZ Induced Early Changes in the Expression Levels and Activation States of PI3K/Akt/GSK-3β and PAK/LIMK-1/Cofilin-1 Signaling Pathways
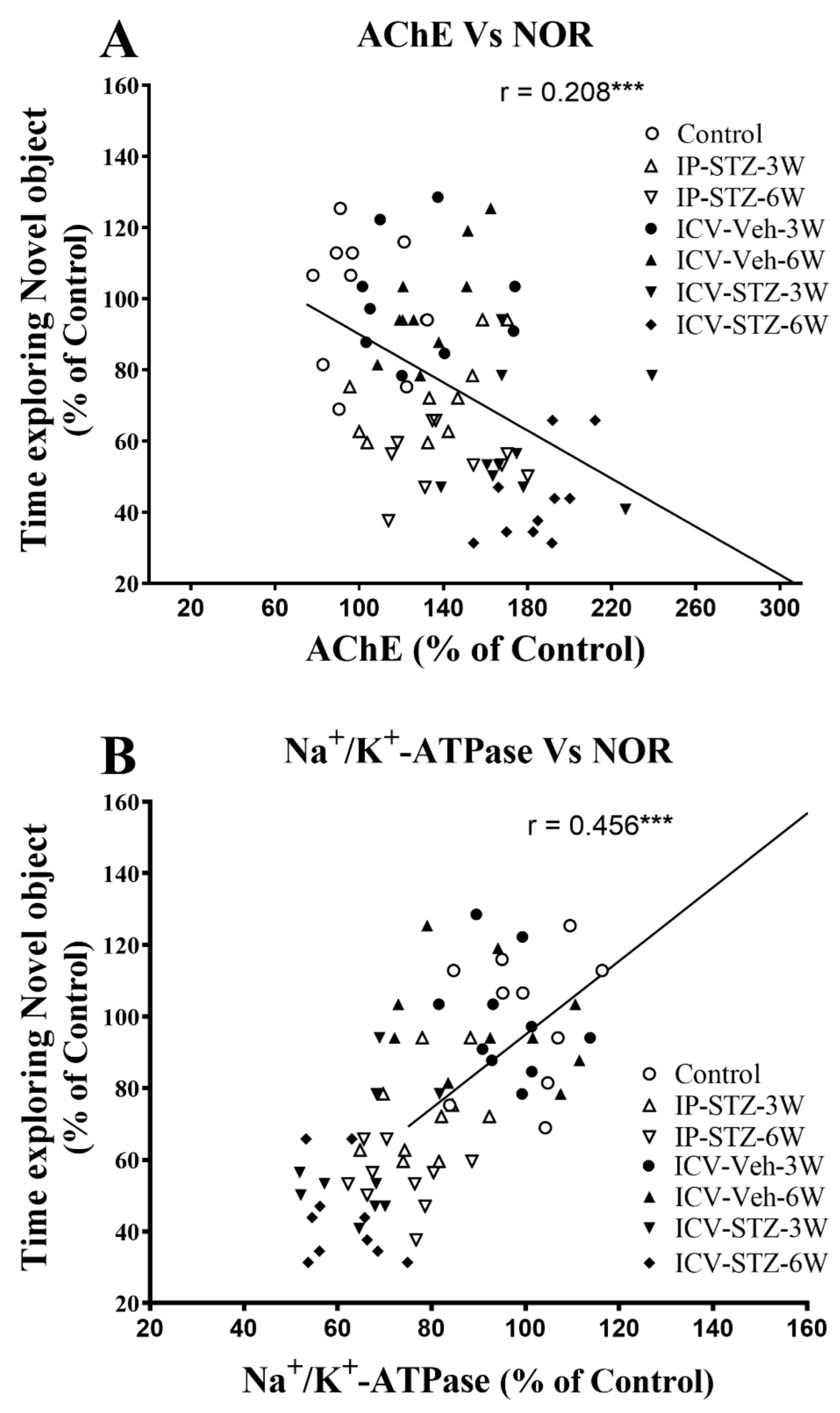
3.5. IP-STZ and ICV-STZ Induced Changes in Mediators of Neuronal Survival and Synaptic Dynamics/Plasticity Correlated with Cognitive Impairments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jayaraj, R.L.; Azimullah, S.; Beiram, R. Diabetes as a risk factor for Alzheimer’s disease in the Middle East and its shared pathological mediators. Saudi J. Biol. Sci. 2020, 27, 736–750. [Google Scholar] [CrossRef] [PubMed]
- Latina, V.; Giacovazzo, G.; Calissano, P.; Atlante, A.; La Regina, F.; Malerba, F.; Dell’Aquila, M.; Stigliano, E.; Balzamino, B.O.; Micera, A.; et al. Tau Cleavage Contributes to Cognitive Dysfunction in Strepto-Zotocin-Induced Sporadic Alzheimer’s Disease (sAD) Mouse Model. Int. J. Mol. Sci. 2021, 22, 2158. [Google Scholar] [CrossRef]
- Ansari, M.A.; Rao, M.S.; Al-Jarallah, A.; Babiker, F.M. Early time course of oxidative stress in hippocampal synaptosomes and cognitive loss following impaired insulin signaling in rats: Development of sporadic Alzheimer’s disease. Brain Res. 2023, 1798, 148134. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, N.; Nath, C.; Hanif, K.; Shukla, R. Intranasal insulin improves cerebral blood flow, Nrf-2 expression and BDNF in STZ (ICV)-induced memory impaired rats. Life Sci. 2017, 173, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Kang, Y.; Zhang, L.; Li, H.; Qu, C.; Luan, X.; Liu, L.; Zhang, S. Troxerutin attenuates cognitive decline in the hippocampus of male diabetic rats by inhibiting NADPH oxidase and activating the Nrf2/ARE signaling pathway. Int. J. Mol. Med. 2020, 46, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Gocmez, S.S.; Sahin, T.D.; Yazir, Y.; Duruksu, G.; Eraldemir, F.C.; Polat, S.; Utkan, T. Resveratrol prevents cognitive deficits by attenuating oxidative damage and inflammation in rat model of streptozotocin diabetes induced vascular dementia. Physiol. Behav. 2019, 201, 198–207. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Zhang, X.; Tang, J. Oxymatrine Ameliorates Memory Impairment in Diabetic Rats by Regulating Oxidative Stress and Apoptosis: Involvement of NOX2/NOX4. Oxid. Med. Cell. Longev. 2020, 2020, 3912173. [Google Scholar] [CrossRef] [PubMed]
- Ide, M.; Sonoda, N.; Inoue, T.; Kimura, S.; Minami, Y.; Makimura, H.; Hayashida, E.; Hyodo, F.; Yamato, M.; Takayanagi, R.; et al. The dipeptidyl peptidase-4 inhibitor, linagliptin, improves cognitive impairment in streptozotocin-induced diabetic mice by inhibiting oxidative stress and microglial activation. PLoS ONE 2020, 15, e0228750. [Google Scholar] [CrossRef] [Green Version]
- Gasparini, L.; Netzer, W.J.; Greengard, P.; Xu, H. Does insulin dysfunction play a role in Alzheimer’s disease? Trends. Pharmacol. Sci. 2002, 23, 288–293. [Google Scholar] [CrossRef]
- Ishrat, T.; Khan, M.B.; Hoda, M.N.; Yousuf, S.; Ahmad, M.; Ansari, M.A.; Ahmad, A.S.; Islam, F. Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav. Brain Res. 2006, 171, 9–16. [Google Scholar] [CrossRef]
- Ishrat, T.; Parveen, K.; Hoda, M.N.; Khan, M.B.; Yousuf, S.; Ansari, M.A.; Saleem, S.; Islam, F. Effects of Pycnogenol and vitamin E on cognitive deficits and oxidative damage induced by intracerebroventricular streptozotocin in rats. Behav. Pharmacol. 2009, 20, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Shoham, S.; Bejar, C.; Kovalev, E.; Weinstock, M. Intracerebroventricular injection of streptozotocin causes neurotoxicity to myelin that contributes to spatial memory deficits in rats. Exp. Neurol. 2003, 184, 1043–1052. [Google Scholar] [CrossRef]
- Dou, J.T.; Chen, M.; Dufour, F.; Alkon, D.L.; Zhao, W.Q. Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn. Mem. 2005, 12, 646–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzoubi, K.H.; Khabour, O.F.; Alhaidar, I.A.; Aleisa, A.M.; Alkadhi, K.A. Diabetes impairs synaptic plasticity in the superior cervical ganglion: Possible role for BDNF and oxidative stress. J. Mol. Neurosci. 2013, 51, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Massol, J.; Pichat, P.; Puech, A.J. Decreased central GABA B receptor binding sites in diabetic rats. Neuropsychobiology 1988, 19, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo-Solleiro, D.; Araiza, L.F.O.; Broccoli, L.; Hansson, A.C.; Rocha-Arrieta, L.L.; Aguilar-Roblero, R.; Crespo-Ramirez, M.; Fuxe, K.; Perez de la Mora, M. Dopamine D1 receptor activity is involved in the increased anxiety levels observed in STZ-induced diabetes in rats. Behav. Brain Res. 2016, 313, 293–301. [Google Scholar] [CrossRef]
- Mundinger, T.O.; Cooper, E.; Coleman, M.P.; Taborsky, G.J., Jr. Short-term diabetic hyperglycemia suppresses celiac ganglia neurotransmission, thereby impairing sympathetically mediated glucagon responses. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E246–E255. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.; Garg, M.L.; Smith, D.W. Altered expression of histone and synaptic plasticity associated genes in the hippocampus of streptozotocin-induced diabetic mice. Metab. Brain Dis. 2013, 28, 613–618. [Google Scholar] [CrossRef]
- Momeni, Z.; Bautista, M.; Neapetung, J.; Urban, R.; Yamamoto, Y.; Krishnan, A.; Campanucci, V.A. RAGE signaling is required for AMPA receptor dysfunction in the hippocampus of hyperglycemic mice. Physiol. Behav. 2021, 229, 113255. [Google Scholar] [CrossRef]
- Abdallah, H.M.; El Sayed, N.S.; Sirwi, A.; Ibrahim, S.R.M.; Mohamed, G.A.; Abdel Rasheed, N.O. Mangostanaxanthone IV Ameliorates Streptozotocin-Induced Neuro-Inflammation, Amyloid Deposition, and Tau Hyperphosphorylation via Modulating PI3K/Akt/GSK-3beta Pathway. Biology 2021, 10, 1298. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L. Calycosin ameliorates diabetes-induced cognitive impairments in rats by reducing oxidative stress via the PI3K/Akt/GSK-3beta signaling pathway. Biochem. Biophys. Res. Commun. 2016, 473, 428–434. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Dickerson, F.; Smith, T.L. Lipid order and composition of synaptic membranes in experimental diabetes mellitus. Neurochem. Res. 1990, 15, 981–985. [Google Scholar] [CrossRef]
- Gagne, J.; Milot, M.; Gelinas, S.; Lahsaini, A.; Trudeau, F.; Martinoli, M.G.; Massicotte, G. Binding properties of glutamate receptors in streptozotocin-induced diabetes in rats. Diabetes 1997, 46, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Franzon, R.; Chiarani, F.; Mendes, R.H.; Bello-Klein, A.; Wyse, A.T. Dietary soy prevents brain Na+, K(+)-ATPase reduction in streptozotocin diabetic rats. Diabetes Res. Clin. Pract. 2005, 69, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Chabot, C.; Massicotte, G.; Milot, M.; Trudeau, F.; Gagne, J. Impaired modulation of AMPA receptors by calcium-dependent processes in streptozotocin-induced diabetic rats. Brain Res. 1997, 768, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, L.; Li, J.; Fang, D.; Zhong, C.; Chen, J.X.; Yan, S.S. Synergistic exacerbation of mitochondrial and synaptic dysfunction and resultant learning and memory deficit in a mouse model of diabetic Alzheimer’s disease. J. Alzheimers Dis. 2015, 43, 451–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Liang, Z.; Tian, Z.; Blanchard, J.; Dai, C.L.; Chalbot, S.; Iqbal, K.; Liu, F.; Gong, C.X. Intracerebroventricular streptozotocin exacerbates Alzheimer-like changes of 3xTg-AD mice. Mol. Neurobiol. 2014, 49, 547–562. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Liu, H.P.; Hwang, S.L.; Hsu, L.F.; Lin, W.Y.; Tsai, F.J. Dystonin/BPAG1 modulates diabetes and Alzheimer’s disease cross-talk: A meta-analysis. Neurol. Sci. 2019, 40, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Shonesy, B.C.; Thiruchelvam, K.; Parameshwaran, K.; Rahman, E.A.; Karuppagounder, S.S.; Huggins, K.W.; Pinkert, C.A.; Amin, R.; Dhanasekaran, M.; Suppiramaniam, V. Central insulin resistance and synaptic dysfunction in intracerebroventricular-streptozotocin injected rodents. Neurobiol. Aging 2012, 33, 430-e5–430-e18. [Google Scholar] [CrossRef]
- Rai, S.; Kamat, P.K.; Nath, C.; Shukla, R. Glial activation and post-synaptic neurotoxicity: The key events in Streptozotocin (ICV) induced memory impairment in rats. Pharmacol. Biochem. Behav. 2014, 117, 104–117. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Li, S.; Wang, C. Effects of paeoniflorin on neurobehavior, oxidative stress, brain insulin signaling, and synaptic alterations in intracerebroventricular streptozotocin-induced cognitive impairment in mice. Physiol. Behav. 2018, 191, 12–20. [Google Scholar] [CrossRef]
- Hashemi-Firouzi, N.; Komaki, A.; Soleimani Asl, S.; Shahidi, S. The effects of the 5-HT7 receptor on hippocampal long-term potentiation and apoptosis in a rat model of Alzheimer’s disease. Brain Res. Bull. 2017, 135, 85–91. [Google Scholar] [CrossRef]
- Gao, C.; Liu, Y.; Jiang, Y.; Ding, J.; Li, L. Geniposide ameliorates learning memory deficits, reduces tau phosphorylation and decreases apoptosis via GSK3beta pathway in streptozotocin-induced alzheimer rat model. Brain Pathol. 2014, 24, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Li, X.; Li, W.; Wei, K.; Yao, Y.; Zhang, Q.; Liang, X.; Zhang, J. Tetramethylpyrazine reverses intracerebroventricular streptozotocin-induced memory deficits by inhibiting GSK-3beta. Acta Biochim. Biophys. Sin. 2017, 49, 722–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.; Xiang, Y.; Qu, X.; Liu, H.; Liu, C.; Li, G.; Han, L.; Qin, X. Apelin-13 Suppresses Neuroinflammation Against Cognitive Deficit in a Streptozotocin-Induced Rat Model of Alzheimer’s Disease Through Activation of BDNF-TrkB Signaling Pathway. Front. Pharmacol. 2019, 10, 395. [Google Scholar] [CrossRef]
- Gao, W.L.; Li, X.H.; Dun, X.P.; Jing, X.K.; Yang, K.; Li, Y.K. Grape Seed Proanthocyanidin Extract Ameliorates Streptozotocin-induced Cognitive and Synaptic Plasticity Deficits by Inhibiting Oxidative Stress and Preserving AKT and ERK Activities. Curr. Med. Sci. 2020, 40, 434–443. [Google Scholar] [CrossRef] [PubMed]
- da Costa, M.; Bernardi, J.; Costa, L.; Fiuza, T.; Brandao, R.; Ribeiro, M.F.; Amaral, J.D.; Rodrigues, C.M.P.; Pereira, M.E. N-acetylcysteine treatment attenuates the cognitive impairment and synaptic plasticity loss induced by streptozotocin. Chem. Biol. Interact. 2017, 272, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.C.; Chen, X.X.; Gao, X.R.; Xu, J.X.; Liu, S.; Ge, J.F. Impaired Learning and Memory Ability Induced by a Bilaterally Hippocampal Injection of Streptozotocin in Mice: Involved With the Adaptive Changes of Synaptic Plasticity. Front. Aging Neurosci. 2021, 13, 633495. [Google Scholar] [CrossRef]
- Minamide, L.S.; Striegl, A.M.; Boyle, J.A.; Meberg, P.J.; Bamburg, J.R. Neurodegenerative stimuli induce persistent ADF/cofilin-actin rods that disrupt distal neurite function. Nat. Cell. Biol. 2000, 2, 628–636. [Google Scholar] [CrossRef]
- Antonipillai, J.; Mittelstaedt, K.; Rigby, S.; Bassler, N.; Bernard, O. LIM kinase 2 (LIMK2) may play an essential role in platelet function. Exp. Cell. Res. 2020, 388, 111822. [Google Scholar] [CrossRef]
- Antonipillai, J.; Rigby, S.; Bassler, N.; Peter, K.; Bernard, O. Pharmacological inhibition of LIM kinase pathway impairs platelets functionality and facilitates thrombolysis. Exp. Cell. Res. 2019, 382, 111458. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, F. High glucose increases phosphocofilin via phosphorylation of LIM kinase due to Rho/Rho kinase activation in cultured pig proximal tubular epithelial cells. Diabetes Res. Clin Pract. 2008, 80, 24–33. [Google Scholar] [CrossRef]
- Arsenault, D.; Dal-Pan, A.; Tremblay, C.; Bennett, D.A.; Guitton, M.J.; De Koninck, Y.; Tonegawa, S.; Calon, F. PAK inactivation impairs social recognition in 3xTg-AD Mice without increasing brain deposition of tau and Abeta. J. Neurosci. 2013, 33, 10729–10740. [Google Scholar] [CrossRef] [Green Version]
- Ishizuka, Y.; Hanamura, K. Drebrin in Alzheimer’s Disease. Adv. Exp. Med. Biol. 2017, 1006, 203–223. [Google Scholar] [CrossRef]
- Gautam, S.; Ishrat, N.; Singh, R.; Narender, T.; Srivastava, A.K. Aegeline from Aegle marmelos stimulates glucose transport via Akt and Rac1 signaling, and contributes to a cytoskeletal rearrangement through PI3K/Rac1. Eur. J. Pharmacol. 2015, 762, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Rachubik, P.; Szrejder, M.; Rogacka, D.; Audzeyenka, I.; Rychlowski, M.; Angielski, S.; Piwkowska, A. The TRPC6-AMPK Pathway is Involved in Insulin-Dependent Cytoskeleton Reorganization and Glucose Uptake in Cultured Rat Podocytes. Cell. Physiol. Biochem. 2018, 51, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhuang, T.T.; Chen, J.J.; Zhu, X.L.; Cai, Y.F.; Lu, Y.P. Novel derivative of Paeonol, Paeononlsilatie sodium, alleviates behavioral damage and hippocampal dendritic injury in Alzheimer’s disease concurrent with cofilin1/phosphorylated-cofilin1 and RAC1/CDC42 alterations in rats. PLoS ONE 2017, 12, e0185102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.; Vidal, G.S.; Djurisic, M.; William, C.M.; Birnbaum, M.E.; Garcia, K.C.; Hyman, B.T.; Shatz, C.J. Human LilrB2 is a beta-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer’s model. Science 2013, 341, 1399–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, A.M.; de Maturana, R.L.; Ricobaraza, A.; Escribano, L.; Schiapparelli, L.; Cuadrado-Tejedor, M.; Perez-Mediavilla, A.; Avila, J.; Del Rio, J.; Frechilla, D. Early changes in hippocampal Eph receptors precede the onset of memory decline in mouse models of Alzheimer’s disease. J. Alzheimers Dis. 2009, 17, 773–786. [Google Scholar] [CrossRef] [Green Version]
- Kojima, N.; Shirao, T. Synaptic dysfunction and disruption of postsynaptic drebrin-actin complex: A study of neurological disorders accompanied by cognitive deficits. Neurosci. Res. 2007, 58, 1–5. [Google Scholar] [CrossRef]
- Munsie, L.N.; Truant, R. The role of the cofilin-actin rod stress response in neurodegenerative diseases uncovers potential new drug targets. Bioarchitecture 2012, 2, 204–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, A.E.; Bamburg, J.R. Peptide regulation of cofilin activity in the CNS: A novel therapeutic approach for treatment of multiple neurological disorders. Pharmacol. Ther 2017, 175, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Kommaddi, R.P.; Das, D.; Karunakaran, S.; Nanguneri, S.; Bapat, D.; Ray, A.; Shaw, E.; Bennett, D.A.; Nair, D.; Ravindranath, V. Abeta mediates F-actin disassembly in dendritic spines leading to cognitive deficits in Alzheimer’s disease. J. Neurosci. 2018, 38, 1085–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Rong, X.; Lu, W.; Peng, Y.; Li, J.; Xu, S.; Wang, L.; Wang, X. Translational study of Alzheimer’s disease (AD) biomarkers from brain tissues in AbetaPP/PS1 mice and serum of AD patients. J. Alzheimers Dis. 2015, 45, 269–282. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, W.; Cao, X.; Wang, Y.; Zhu, C.; Guan, J. Identification of Serum Biomarkers in Patients with Alzheimer’s Disease by 2D-DIGE Proteomics. Gerontology 2022, 68, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, B.; Kowluru, A. Phagocytic NADPH oxidase links ARNO-Arf6 signaling pathway in glucose-stimulated insulin secretion from the pancreatic beta-cell. Cell. Physiol. Biochem. 2012, 30, 1351–1362. [Google Scholar] [CrossRef]
- Tabur, S.; Oztuzcu, S.; Oguz, E.; Demiryurek, S.; Dagli, H.; Alasehirli, B.; Ozkaya, M.; Demiryurek, A.T. Evidence for elevated (LIMK2 and CFL1) and suppressed (ICAM1, EZR, MAP2K2, and NOS3) gene expressions in metabolic syndrome. Endocrine 2016, 53, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Gao, J.; Bi, X.; He, H.; Shi, X.; Weng, S.; Chen, Y.; Yang, Y.; Ye, Y.; Fu, G. The Rho kinase inhibitor, fasudil, ameliorates diabetes-induced cardiac dysfunction by improving calcium clearance and actin remodeling. J. Mol. Med. 2017, 95, 155–165. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Di, H.; Zhao, D.; Wang, J. The role of actin depolymerizing factor in advanced glycation endproducts-induced impairment in mouse brain microvascular endothelial cells. Mol. Cell. Biochem. 2017, 433, 103–112. [Google Scholar] [CrossRef]
- Morales-Quinones, M.; Ramirez-Perez, F.I.; Foote, C.A.; Ghiarone, T.; Ferreira-Santos, L.; Bloksgaard, M.; Spencer, N.; Kimchi, E.T.; Manrique-Acevedo, C.; Padilla, J.; et al. LIMK (LIM Kinase) Inhibition Prevents Vasoconstriction- and Hypertension-Induced Arterial Stiffening and Remodeling. Hypertension 2020, 76, 393–403. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, C.; Liu, D.; Yang, Q.; Tang, L.; Wang, T.; Tian, H.; Lu, L.; Xu, J.Y.; Gao, F.; et al. Erythropoietin protects the inner blood-retinal barrier by inhibiting microglia phagocytosis via Src/Akt/cofilin signalling in experimental diabetic retinopathy. Diabetologia 2021, 64, 211–225. [Google Scholar] [CrossRef]
- Xie, M.; Wang, M.; Liu, W.; Xu, M.; Shang, P.; Jiang, D.; Ju, L.; Wu, F.; Sun, A.; Yu, S.; et al. Lipin1 Is Involved in the Pathogenesis of Diabetic Encephalopathy through the PKD/Limk/Cofilin Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 1723423. [Google Scholar] [CrossRef] [PubMed]
- Bamburg, J.R.; Bernstein, B.W. Actin dynamics and cofilin-actin rods in alzheimer disease. Cytoskeleton 2016, 73, 477–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Zhou, L.; Yang, Q.D.; Du, X.P.; Li, M.; Yuan, M.; Zhou, Z.W. Changes in hippocampal synapses and learning-memory abilities in a streptozotocin-treated rat model and intervention by using fasudil hydrochloride. Neuroscience 2012, 200, 120–129. [Google Scholar] [CrossRef]
- Shankar, G.M.; Bloodgood, B.L.; Townsend, M.; Walsh, D.M.; Selkoe, D.J.; Sabatini, B.L. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 2007, 27, 2866–2875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiteman, I.T.; Gervasio, O.L.; Cullen, K.M.; Guillemin, G.J.; Jeong, E.V.; Witting, P.K.; Antao, S.T.; Minamide, L.S.; Bamburg, J.R.; Goldsbury, C. Activated actin-depolymerizing factor/cofilin sequesters phosphorylated microtubule-associated protein during the assembly of alzheimer-like neuritic cytoskeletal striations. J. Neurosci. 2009, 29, 12994–13005. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.; Low, J.K.; Logge, W.; Garner, B.; Karl, T. Novel behavioural characteristics of female APPSwe/PS1DeltaE9 double transgenic mice. Behav. Brain Res. 2014, 260, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Espana, J.; Gimenez-Llort, L.; Valero, J.; Minano, A.; Rabano, A.; Rodriguez-Alvarez, J.; LaFerla, F.M.; Saura, C.A. Intraneuronal beta-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer’s disease transgenic mice. Biol. Psychiatry 2010, 67, 513–521. [Google Scholar] [CrossRef]
- Koppel, J.; Jimenez, H.; Azose, M.; D’Abramo, C.; Acker, C.; Buthorn, J.; Greenwald, B.S.; Lewis, J.; Lesser, M.; Liu, Z.; et al. Pathogenic tau species drive a psychosis-like phenotype in a mouse model of Alzheimer’s disease. Behav. Brain Res. 2014, 275, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.O.; Mazucanti, C.H.; Xavier, G.F.; Torrao, A.S. Early and late neurodegeneration and memory disruption after intracerebroventricular streptozotocin. Physiol. Behav. 2012, 107, 401–413. [Google Scholar] [CrossRef]
- Yassine, N.; Lazaris, A.; Dorner-Ciossek, C.; Despres, O.; Meyer, L.; Maitre, M.; Mensah-Nyagan, A.G.; Cassel, J.C.; Mathis, C. Detecting spatial memory deficits beyond blindness in tg2576 Alzheimer mice. Neurobiol. Aging 2013, 34, 716–730. [Google Scholar] [CrossRef]
- Bartolini, L.; Casamenti, F.; Pepeu, G. Aniracetam restores object recognition impaired by age, scopolamine, and nucleus basalis lesions. Pharmacol. Biochem. Behav. 1996, 53, 277–283. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Bevins, R.A.; Besheer, J. Object recognition in rats and mice: A one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat. Protoc. 2006, 1, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Jabbarpour, Z.; Shahidi, S.; Saidijam, M.; Sarihi, A.; Hassanzadeh, T.; Esmaeili, R. Effect of tempol on the passive avoidance and novel object recognition task in diabetic rats. Brain Res. Bull. 2014, 101, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M.; Rawlins, J.N. T-maze alternation in the rodent. Nat. Protoc 2006, 1, 7–12. [Google Scholar] [CrossRef]
- Lalonde, R. The neurobiological basis of spontaneous alternation. Neurosci. Biobehav. Rev. 2002, 26, 91–104. [Google Scholar] [CrossRef]
- Sharma, S.; Rakoczy, S.; Brown-Borg, H. Assessment of spatial memory in mice. Life Sci. 2010, 87, 521–536. [Google Scholar] [CrossRef]
- Nagayach, A.; Bhaskar, R.; Patro, I. Microglia activation and inflammation in hippocampus attenuates memory and mood functions during experimentally induced diabetes in rat. J. Chem. Neuroanat. 2022, 125, 102160. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, P.; Mosinger, B. Catecholamines and the brain microsomal Na, K-adenosinetriphosphatase--I. Protection against lipoperoxidative damage. Biochem. Pharmacol. 1981, 30, 427–432. [Google Scholar] [CrossRef]
- Ansari, M.A.; Roberts, K.N.; Scheff, S.W. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic. Biol. Med. 2008, 45, 443–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, M.A.; Roberts, K.N.; Scheff, S.W. A time course of NADPH-oxidase up-regulation and endothelial nitric oxide synthase activation in the hippocampus following neurotrauma. Free Radic. Biol. Med. 2014, 77, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braszko, J.J.; Wisniewski, K.; Kupryszewski, G.; Witczuk, B. Psychotropic effects of angiotensin II and III in rats: Locomotor and exploratory vs cognitive behaviour. Behav. Brain Res. 1987, 25, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zeng, Y.; Xiao, W.; Zhang, L.; Shu, Y. LC-MS-Based Untargeted Metabolomics Reveals Early Biomarkers in STZ-Induced Diabetic Rats With Cognitive Impairment. Front. Endocrinol. 2021, 12, 665309. [Google Scholar] [CrossRef]
- Grzeda, E.; Wisniewska, R.J.; Wisniewski, K. Effect of an NMDA receptor agonist on T-maze and passive avoidance test in 12-week streptozotocin-induced diabetic rats. Pharmacol. Rep. 2007, 59, 656–663. [Google Scholar] [PubMed]
- Orduna, V.; Hong, E.; Bouzas, A. Timing behavior in streptozotocin-induced diabetic rats. Behav. Brain Res. 2011, 224, 189–194. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, J.; Xu, M.; Wang, Y.; Zhang, M.; Zhou, Y. Resveratrol Improves Cognitive Impairment by Regulating Apoptosis and Synaptic Plasticity in Streptozotocin-Induced Diabetic Rats. Cell. Physiol. Biochem. 2016, 40, 1670–1677. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Ling, S.; Zhang, X. Expression changes of growth-associated protein-43 (GAP-43) and mitogen-activated protein kinase phosphatase-1 (MKP-1) and in hippocampus of streptozotocin-induced diabetic cognitive impairment rats. Exp. Neurol. 2007, 206, 201–208. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, B.S.; Sun, G.C.; Sun, X.; Sun, F.Y. Diabetes synergistically exacerbates poststroke dementia and tau abnormality in brain. Neurochem. Int. 2010, 56, 955–961. [Google Scholar] [CrossRef]
- Park, S.A. A common pathogenic mechanism linking type-2 diabetes and Alzheimer’s disease: Evidence from animal models. J. Clin Neurol. 2011, 7, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Huang, L.; Zheng, W.; An, J.; Zhan, Z.; Wang, L.; Chen, Z.; Liu, L. Recurrent nonsevere hypoglycemia exacerbates imbalance of mitochondrial homeostasis leading to synapse injury and cognitive deficit in diabetes. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E973–E986. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.X.; Correia, S.C.; Alves, M.G.; Oliveira, P.F.; Cardoso, S.; Carvalho, C.; Duarte, A.I.; Santos, M.S.; Moreira, P.I. Insulin therapy modulates mitochondrial dynamics and biogenesis, autophagy and tau protein phosphorylation in the brain of type 1 diabetic rats. Biochim. Biophys. Acta 2014, 1842, 1154–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.P.; Feng, L.; Zhang, M.H.; Ma, D.Y.; Wang, S.Y.; Gu, J.; Fu, Q.; Qu, R.; Ma, S.P. Neuroprotective effect of Liuwei Dihuang decoction on cognition deficits of diabetic encephalopathy in streptozotocin-induced diabetic rat. J. Ethnopharmacol. 2013, 150, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Datusalia, A.K.; Sharma, S.S. Amelioration of diabetes-induced cognitive deficits by GSK-3beta inhibition is attributed to modulation of neurotransmitters and neuroinflammation. Mol. Neurobiol. 2014, 50, 390–405. [Google Scholar] [CrossRef]
- Baltaci, S.B.; Unal, O.; Gulbahce-Mutlu, E.; Gumus, H.; Pehlivanoglu, S.; Yardimci, A.; Mogulkoc, R.; Baltaci, A.K. The Role of Zinc Status on Spatial Memory, Hippocampal Synaptic Plasticity, and Insulin Signaling in icv-STZ-Induced Sporadic Alzheimer’s-Like Disease in Rats. Biol. Trace Elem. Res. 2022, 200, 4068–4078. [Google Scholar] [CrossRef]
- Levenga, J.; Wong, H.; Milstead, R.; LaPlante, L.; Hoeffer, C.A. Immunohistological Examination of AKT Isoforms in the Brain: Cell-Type Specificity That May Underlie AKT’s Role in Complex Brain Disorders and Neurological Disease. Cereb. Cortex. Commun. 2021, 2, tgab036. [Google Scholar] [CrossRef]
- Lauterborn, J.C.; Cox, C.D.; Chan, S.W.; Vanderklish, P.W.; Lynch, G.; Gall, C.M. Synaptic actin stabilization protein loss in Down syndrome and Alzheimer disease. Brain Pathol. 2020, 30, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Hotulainen, P.; Hoogenraad, C.C. Actin in dendritic spines: Connecting dynamics to function. J. Cell Biol. 2010, 189, 619–629. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Lee, C.W.; Fan, Y.; Komlos, D.; Tang, X.; Sun, C.; Yu, K.; Hartzell, H.C.; Chen, G.; Bamburg, J.R.; et al. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat. Neurosci. 2010, 13, 1208–1215. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, J.; Hayama, T.; Watanabe, S.; Ucar, H.; Yagishita, S.; Takahashi, N.; Kasai, H. State-dependent diffusion of actin-depolymerizing factor/cofilin underlies the enlargement and shrinkage of dendritic spines. Sci. Rep. 2016, 6, 32897. [Google Scholar] [CrossRef]
- Swanger, S.A.; Mattheyses, A.L.; Gentry, E.G.; Herskowitz, J.H. ROCK1 and ROCK2 inhibition alters dendritic spine morphology in hippocampal neurons. Cell. Logist. 2015, 5, e1133266. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Meng, Y.; Asrar, S.; Todorovski, Z.; Jia, Z. A critical role of Rho-kinase ROCK2 in the regulation of spine and synaptic function. Neuropharmacology 2009, 56, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Barone, E.; Mosser, S.; Fraering, P.C. Inactivation of brain Cofilin-1 by age, Alzheimer’s disease and gamma-secretase. Biochim. Biophys. Acta 2014, 1842, 2500–2509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza-Naranjo, A.; Contreras-Vallejos, E.; Henriquez, D.R.; Otth, C.; Bamburg, J.R.; Maccioni, R.B.; Gonzalez-Billault, C. Fibrillar amyloid-beta1-42 modifies actin organization affecting the cofilin phosphorylation state: A role for Rac1/cdc42 effector proteins and the slingshot phosphatase. J. Alzheimers Dis. 2012, 29, 63–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rush, T.; Martinez-Hernandez, J.; Dollmeyer, M.; Frandemiche, M.L.; Borel, E.; Boisseau, S.; Jacquier-Sarlin, M.; Buisson, A. Synaptotoxicity in Alzheimer’s Disease Involved a Dysregulation of Actin Cytoskeleton Dynamics through Cofilin 1 Phosphorylation. J. Neurosci. 2018, 38, 10349–10361. [Google Scholar] [CrossRef] [Green Version]
- Bravo-Cordero, J.J.; Magalhaes, M.A.; Eddy, R.J.; Hodgson, L.; Condeelis, J. Functions of cofilin in cell locomotion and invasion. Nat. Rev. Mol. Cell. Biol. 2013, 14, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Namme, J.N.; Bepari, A.K.; Takebayashi, H. Cofilin Signaling in the CNS Physiology and Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 727. [Google Scholar] [CrossRef]
- Woo, J.A.; Liu, T.; Fang, C.C.; Cazzaro, S.; Kee, T.; LePochat, P.; Yrigoin, K.; Penn, C.; Zhao, X.; Wang, X.; et al. Activated cofilin exacerbates tau pathology by impairing tau-mediated microtubule dynamics. Commun Biol. 2019, 2, 112. [Google Scholar] [CrossRef] [Green Version]
- Bie, B.; Wu, J.; Foss, J.F.; Naguib, M. Amyloid fibrils induce dysfunction of hippocampal glutamatergic silent synapses. Hippocampus 2018, 28, 549–556. [Google Scholar] [CrossRef]
- Woo, J.A.; Zhao, X.; Khan, H.; Penn, C.; Wang, X.; Joly-Amado, A.; Weeber, E.; Morgan, D.; Kang, D.E. Slingshot-Cofilin activation mediates mitochondrial and synaptic dysfunction via Abeta ligation to beta1-integrin conformers. Cell. Death Differ. 2015, 22, 921–934. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Wei, J.; Cheng, J.; Zhong, P.; Xiong, Z.; Liu, A.; Lin, L.; Chen, S.; Yan, Z. Partial Amelioration of Synaptic and Cognitive Deficits by Inhibiting Cofilin Dephosphorylation in an Animal Model of Alzheimer’s Disease. J. Alzheimers Dis. 2016, 53, 1419–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, T.; Davies, D.S.; Tannenberg, R.K.; Fok, S.; Shepherd, C.; Dodd, P.R.; Cullen, K.M.; Goldsbury, C. Cofilin rods and aggregates concur with tau pathology and the development of Alzheimer’s disease. J. Alzheimers Dis. 2014, 42, 1443–1460. [Google Scholar] [CrossRef] [PubMed]
- Madineni, A.; Alhadidi, Q.; Shah, Z.A. Cofilin Inhibition Restores Neuronal Cell Death in Oxygen-Glucose Deprivation Model of Ischemia. Mol. Neurobiol. 2016, 53, 867–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Lu, J.; Peng, W.; Mak, M.S.; Yang, Y.; Zhu, Z.; Wang, S.; Hou, J.; Zhou, X.; Xin, W.; et al. Acrolein, an endogenous aldehyde induces Alzheimer’s disease-like pathologies in mice: A new sporadic AD animal model. Pharmacol. Res. 2022, 175, 106003. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, M.A.; Al-Jarallah, A.; Babiker, F.A. Impaired Insulin Signaling Alters Mediators of Hippocampal Synaptic Dynamics/Plasticity: A Possible Mechanism of Hyperglycemia-Induced Cognitive Impairment. Cells 2023, 12, 1728. https://doi.org/10.3390/cells12131728
Ansari MA, Al-Jarallah A, Babiker FA. Impaired Insulin Signaling Alters Mediators of Hippocampal Synaptic Dynamics/Plasticity: A Possible Mechanism of Hyperglycemia-Induced Cognitive Impairment. Cells. 2023; 12(13):1728. https://doi.org/10.3390/cells12131728
Chicago/Turabian StyleAnsari, Mubeen A., Aishah Al-Jarallah, and Fawzi A. Babiker. 2023. "Impaired Insulin Signaling Alters Mediators of Hippocampal Synaptic Dynamics/Plasticity: A Possible Mechanism of Hyperglycemia-Induced Cognitive Impairment" Cells 12, no. 13: 1728. https://doi.org/10.3390/cells12131728
APA StyleAnsari, M. A., Al-Jarallah, A., & Babiker, F. A. (2023). Impaired Insulin Signaling Alters Mediators of Hippocampal Synaptic Dynamics/Plasticity: A Possible Mechanism of Hyperglycemia-Induced Cognitive Impairment. Cells, 12(13), 1728. https://doi.org/10.3390/cells12131728





