Inflammasomes: Mechanisms of Action and Involvement in Human Diseases
Abstract
1. Introduction
2. Inflammasome Structures and Mechanisms of Action
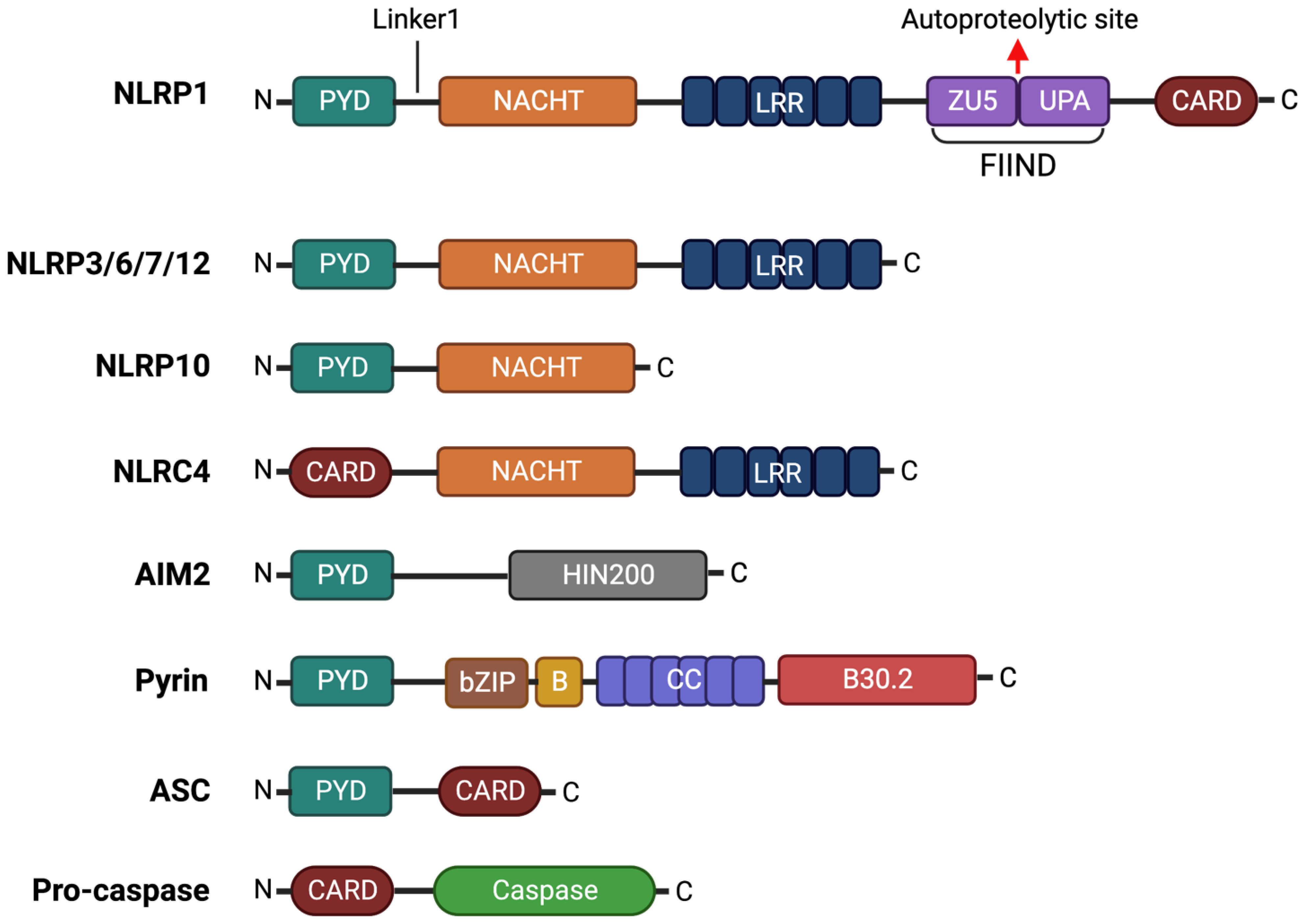
2.1. NLRP1
2.2. NLRP3
2.3. NLRP6
2.4. NLRP7
2.5. NLRP10
2.6. NLRP12
2.7. Pyrin
2.8. NLRC4
2.9. AIM2
3. Role of Inflammasomes in Human Disease
3.1. Autoinflammatory Diseases
3.1.1. Inflammasomopathies
3.1.2. Atherosclerosis
| Disease | Inflammasome | Cell Type/Tissue | Activator in Human Disease | References |
|---|---|---|---|---|
| Atherosclerosis | NLRP1 | Endothelial cells (in vitro) | Cholesterol Triglycerides (in vitro) | [151] |
| NLRP3 | Macrophages, foam cells, endothelial cells | Cholesterol Triglycerides ATP (from necrotic cells) | [147,150,152,153] | |
| AIM2 | Necrotic lesions | dsDNA | [154] | |
| Psoriasis | NLRP1 | PBMCs, keratinocytes, psoriatic lesions | Psoriasin (S100A7) | [156,157] |
| NLRP3 | Psoriatic biopsies, keratinocytes, whole blood | CD100 IL-17, IL-22, TNF-α | [156,157,158] | |
| AIM2 | Lesional and non-lesional skin, keratinocytes | dsDNA | [159,160] | |
| Inflammatory bowel disease | NLRP3 | PBMCs, colonic biopsies, intestinal mucosal cells | Intestinal microbiota | [161,162,163] |
| Rheumatoid arthritis | NLRP1 | PBMCs, synovial cells | P2X4 agonist | [164,165] |
| NLRP3 | PBMCs, monocytes | Unknown | [166] | |
| Sjogren’s syndrome | NLRP3 | PBMCs, salivary glands circulating monocytes | ATP, circulating free DNA | [167,168,169] |
| AIM2 | PBMCs, salivary glands | Circulating free DNA | [169,170] | |
| Systemic Lupus Erythematosus | NLRP3 | Mononuclear cells, monocytes | Neutrophil extracellular traps, anti-dsDNA antibodies, reactive oxygen species, K+ efflux | [171,172] |
| AIM2 | Renal tissue | Neutrophil extracellular traps | [173] | |
| Alzheimer’s disease | NLRP1 | Monocytes, neurons | Amyloid-β K+/Ca2+ imbalance | [174,175,176] |
| NLRP3 | Monocytes, microglia, astrocytes | Amyloid-β | [174,177,178,179] | |
| NLRC4 | Brain samples | Unknown | [180] | |
| Parkinson’s disease | NLRP3 | Monocytes, microglia | α-synuclein (Lewy bodies), reactive oxygen species | [181,182] |
| Multiple sclerosis | NLRP3 | Macrophages, microglia, astrocytes, CNS tissue | Unknown | [183,184] |
| NLRC4 | Astrocyte-rich brain tissue, regions of demyelination | Unknown | [179] |
3.1.3. Psoriasis
3.1.4. Inflammatory Bowel Disease
3.2. Autoimmune Diseases
3.2.1. Rheumatoid Arthritis
3.2.2. Sjogren’s Syndrome
3.2.3. Systemic Lupus Erythematosus
3.3. Neuroinflammatory and Neurodegenerative Diseases
3.3.1. Alzheimer’s Disease
3.3.2. Parkinson’s Disease
3.3.3. Multiple Sclerosis
4. Inflammasomes as Therapeutic Target for Inflammatory Diseases
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skeldon, A.; Saleh, M. The Inflammasomes: Molecular Effectors of Host Resistance Against Bacterial, Viral, Parasitic, and Fungal Infections. Front. Microbiol. 2011, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Christgen, S.; Place, D.E.; Kanneganti, T.D. Toward Targeting Inflammasomes: Insights into Their Regulation and Activation. Cell Res. 2020, 30, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Finger, J.N.; Lich, J.D.; Dare, L.C.; Cook, M.N.; Brown, K.K.; Duraiswami, C.; Bertin, J.J.; Gough, P.J. Autolytic Proteolysis within the Function to Find Domain (FIIND) Is Required for NLRP1 Inflammasome Activity. J. Biol. Chem. 2012, 287, 25030–25037. [Google Scholar] [CrossRef]
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey Daniel, R.; Latz, E.; Fitzgerald, K.A. AIM2 Recognizes Cytosolic dsDNA and Forms a Caspase-1-Activating Inflammasome with ASC. Nature 2009, 458, 514–518. [Google Scholar] [CrossRef]
- Chae, J.J.; Wood, G.; Masters, S.L.; Richard, K.; Park, G.; Smith, B.J.; Kastner, D.L. The B30.2 Domain of Pyrin, the Familial Mediterranean Fever Protein, Interacts Directly with Caspase-1 to Modulate IL-1β Production. Proc. Natl. Acad. Sci. USA 2006, 103, 9982–9987. [Google Scholar] [CrossRef]
- Mathur, A.; Hayward, J.A.; Man, S.M. Molecular Mechanisms of Inflammasome Signaling. J. Leukoc. Biol. 2017, 103, 233–257. [Google Scholar] [CrossRef]
- Man, S.M.; Kanneganti, T.-D. Regulation of Inflammasome Activation. Immunol. Rev. 2015, 265, 6–21. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Sastalla, I.; Crown, D.; Masters, S.L.; McKenzie, A.; Leppla, S.H.; Moayeri, M. Transcriptional Analysis of the Three Nlrp1 Paralogs in Mice. BMC Genom. 2013, 14, 188. [Google Scholar] [CrossRef]
- Chavarría-Smith, J.; Vance, R.E. The NLRP1 Inflammasomes. Immunol. Rev. 2015, 265, 22–34. [Google Scholar] [CrossRef] [PubMed]
- D’Osualdo, A.; Weichenberger, C.X.; Wagner, R.N.; Godzik, A.; Wooley, J.; Reed, J.C. CARD8 and NLRP1 Undergo Autoproteolytic Processing through a ZU5-Like Domain. PLoS ONE 2011, 6, e27396. [Google Scholar] [CrossRef] [PubMed]
- Frew, B.C.; Joag, V.R.; Mogridge, J. Proteolytic Processing of Nlrp1b Is Required for Inflammasome Activity. PLoS Pathog. 2012, 8, e1002659. [Google Scholar] [CrossRef] [PubMed]
- van Opdenbosch, N.; Gurung, P.; vande Walle, L.; Fossoul, A.; Kanneganti, T.-D.; Lamkanfi, M. Activation of the NLRP1b Inflammasome Independently of ASC-Mediated Caspase-1 Autoproteolysis and Speck Formation. Nat. Commun. 2014, 5, 3209. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, J.; Liu, C.; Qu, Y.; Wang, W.; Xiao, M.Z.X.; Zhu, F.; Liu, Z.; Liang, Q. KSHV-Encoded ORF45 Activates Human NLRP1 Inflammasome. Nat. Immunol. 2022, 23, 916–926. [Google Scholar] [CrossRef]
- Hollingsworth, L.R.; Sharif, H.; Griswold, A.R.; Fontana, P.; Mintseris, J.; Dagbay, K.B.; Paulo, J.A.; Gygi, S.P.; Bachovchin, D.A.; Wu, H. DPP9 Sequesters the C Terminus of NLRP1 to Repress Inflammasome Activation. Nature 2021, 592, 778–783. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, X.; Toh, G.A.; Gong, Q.; Wang, J.; Han, Z.; Wu, B.; Zhong, F.; Chai, J. Structural and Biochemical Mechanisms of NLRP1 Inhibition by DPP9. Nature 2021, 592, 773–777. [Google Scholar] [CrossRef]
- Chui, A.J.; Okondo, M.C.; Rao, S.D.; Gai, K.; Griswold, A.R.; Johnson, D.C.; Ball, D.P.; Taabazuing, C.Y.; Orth, E.L.; Vittimberga, B.A.; et al. N-Terminal Degradation Activates the NLRP1B Inflammasome. Science (1979) 2019, 364, 82–85. [Google Scholar] [CrossRef]
- Sandstrom, A.; Mitchell, P.S.; Goers, L.; Mu, E.W.; Lesser, C.F.; Vance, R.E. Functional Degradation: A Mechanism of NLRP1 Inflammasome Activation by Diverse Pathogen Enzymes. Science (1979) 2019, 364, eaau1330. [Google Scholar] [CrossRef]
- Xu, H.; Shi, J.; Gao, H.; Liu, Y.; Yang, Z.; Shao, F.; Dong, N. The N-end Rule Ubiquitin Ligase UBR2 Mediates NLRP1B Inflammasome Activation by Anthrax Lethal Toxin. EMBO J. 2019, 38, e101996. [Google Scholar] [CrossRef]
- Zhong, F.L.; Robinson, K.; Teo, D.E.T.; Tan, K.-Y.; Lim, C.; Harapas, C.R.; Yu, C.-H.; Xie, W.H.; Sobota, R.M.; Au, V.B.; et al. Human DPP9 Represses NLRP1 Inflammasome and Protects against Autoinflammatory Diseases via Both Peptidase Activity and FIIND Domain Binding. J. Biol. Chem. 2018, 293, 18864–18878. [Google Scholar] [CrossRef]
- Bauernfried, S.; Scherr, M.J.; Pichlmair, A.; Duderstadt, K.E.; Hornung, V. Human NLRP1 Is a Sensor for Double-Stranded RNA. Science (1979) 2021, 371, eabd0811. [Google Scholar] [CrossRef]
- Xu, J.; Núñez, G. The NLRP3 Inflammasome: Activation and Regulation. Trends Biochem. Sci. 2023, 48, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; McManus, R.M.; Latz, E. Inflammasome Signalling in Brain Function and Neurodegenerative Disease. Nat. Rev. Neurosci. 2018, 19, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting Edge: NF-ΚB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Eigenbrod, T.; Núñez, G. Cutting Edge: TNF-α Mediates Sensitization to ATP and Silica via the NLRP3 Inflammasome in the Absence of Microbial Stimulation. J. Immunol. 2009, 183, 792–796. [Google Scholar] [CrossRef]
- Mariathasan, S.; Weiss, D.S.; Newton, K.; McBride, J.; O’Rourke, K.; Roose-Girma, M.; Lee, W.P.; Weinrauch, Y.; Monack, D.M.; Dixit, V.M. Cryopyrin Activates the Inflammasome in Response to Toxins and ATP. Nature 2006, 440, 228–232. [Google Scholar] [CrossRef]
- Muñoz-Planillo, R.; Kuffa, P.; Martínez-Colón, G.; Smith, B.L.; Rajendiran, T.M.; Núñez, G. K+ Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-Associated Uric Acid Crystals Activate the NALP3 Inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Hornung, V.; Bauernfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica Crystals and Aluminum Salts Activate the NALP3 Inflammasome through Phagosomal Destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef]
- Dostert, C.; Pétrilli, V.; Van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate Immune Activation Through Nalp3 Inflammasome Sensing of Asbestos and Silica. Science (1979) 2008, 320, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrod, T.; Dalpke, A.H. Bacterial RNA: An Underestimated Stimulus for Innate Immune Responses. J. Immunol. 2015, 195. [Google Scholar] [CrossRef] [PubMed]
- Greaney, A.J.; Leppla, S.H.; Moayeri, M. Bacterial Exotoxins and the Inflammasome. Front. Immunol. 2015, 6, 570. [Google Scholar] [CrossRef] [PubMed]
- Seoane, P.I.; Lee, B.; Hoyle, C.; Yu, S.; Lopez-Castejon, G.; Lowe, M.; Brough, D. The NLRP3–Inflammasome as a Sensor of Organelle Dysfunction. J. Cell Biol. 2020, 219, e202006194. [Google Scholar] [CrossRef]
- Perregaux, D.; Gabel, C.A. Interleukin-1 Beta Maturation and Release in Response to ATP and Nigericin. Evidence That Potassium Depletion Mediated by These Agents Is a Necessary and Common Feature of Their Activity. J. Biol. Chem. 1994, 269, 15195–15203. [Google Scholar] [CrossRef]
- Walev, I.; Klein, J.; Husmann, M.; Valeva, A.; Strauch, S.; Wirtz, H.; Weichel, O.; Bhakdi, S. Potassium Regulates IL-1β Processing Via Calcium-Independent Phospholipase A2. J. Immunol. 2000, 164, 5120–5124. [Google Scholar] [CrossRef]
- Walev, I.; Reske, K.; Palmer, M.; Valeva, A.; Bhakdi, S. Potassium-Inhibited Processing of IL-1 Beta in Human Monocytes. EMBO J. 1995, 14, 1607–1614. [Google Scholar] [CrossRef]
- Pétrilli, V.; Papin, S.; Dostert, C.; Mayor, A.; Martinon, F.; Tschopp, J. Activation of the NALP3 Inflammasome Is Triggered by Low Intracellular Potassium Concentration. Cell Death Differ. 2007, 14, 1583–1589. [Google Scholar] [CrossRef]
- Groß, C.J.; Mishra, R.; Schneider, K.S.; Médard, G.; Wettmarshausen, J.; Dittlein, D.C.; Shi, H.; Gorka, O.; Koenig, P.-A.; Fromm, S.; et al. K + Efflux-Independent NLRP3 Inflammasome Activation by Small Molecules Targeting Mitochondria. Immunity 2016, 45, 761–773. [Google Scholar] [CrossRef]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 Cleaves Gasdermin D for Non-Canonical Inflammasome Signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef]
- Yang, D.; He, Y.; Muñoz-Planillo, R.; Liu, Q.; Núñez, G. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity 2015, 43, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Pelegrin, P.; Surprenant, A. Pannexin-1 Mediates Large Pore Formation and Interleukin-1β Release by the ATP-Gated P2X7 Receptor. EMBO J. 2006, 25, 5071–5082. [Google Scholar] [CrossRef]
- Piccini, A.; Carta, S.; Tassi, S.; Lasiglié, D.; Fossati, G.; Rubartelli, A. ATP Is Released by Monocytes Stimulated with Pathogen-Sensing Receptor Ligands and Induces IL-1β and IL-18 Secretion in an Autocrine Way. Proc. Natl. Acad. Sci. USA 2008, 105, 8067–8072. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by Inflammatory Caspases Determines Pyroptotic Cell Death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Gaidt, M.M.; Ebert, T.S.; Chauhan, D.; Schmidt, T.; Schmid-Burgk, J.L.; Rapino, F.; Robertson, A.A.B.; Cooper, M.A.; Graf, T.; Hornung, V. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity 2016, 44, 833–846. [Google Scholar] [CrossRef]
- Laudisi, F.; Viganò, E.; Mortellaro, A. Tyrosine Kinases: The Molecular Switch for Inflammasome Activation. Cell Mol. Immunol. 2014, 11, 129–131. [Google Scholar] [CrossRef]
- He, Y.; Franchi, L.; Núñez, G. TLR Agonists Stimulate Nlrp3-Dependent IL-1β Production Independently of the Purinergic P2X7 Receptor in Dendritic Cells and In Vivo. J. Immunol. 2013, 190, 334–339. [Google Scholar] [CrossRef]
- Song, N.; Liu, Z.-S.; Xue, W.; Bai, Z.-F.; Wang, Q.-Y.; Dai, J.; Liu, X.; Huang, Y.-J.; Cai, H.; Zhan, X.-Y.; et al. NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Mol. Cell 2017, 68, 185–197.e6. [Google Scholar] [CrossRef]
- Seok, J.K.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Regulation of the NLRP3 Inflammasome by Post-Translational Modifications and Small Molecules. Front. Immunol. 2021, 11, 618231. [Google Scholar] [CrossRef]
- He, Y.; Zeng, M.Y.; Yang, D.; Motro, B.; Núñez, G. NEK7 Is an Essential Mediator of NLRP3 Activation Downstream of Potassium Efflux. Nature 2016, 530, 354–357. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Li, X.; Zhan, X.; Tang, M.; Fina, M.; Su, L.; Pratt, D.; Bu, C.H.; Hildebrand, S.; et al. NLRP3 Activation and Mitosis Are Mutually Exclusive Events Coordinated by NEK7, a New Inflammasome Component. Nat. Immunol. 2016, 17, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Kern, L.; Elinav, E. The NLRP6 Inflammasome. Immunology 2021, 162, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Seregin, S.S.; Yang, D.; Fukase, K.; Chamaillard, M.; Alnemri, E.S.; Inohara, N.; Chen, G.Y.; Núñez, G. The NLRP6 Inflammasome Recognizes Lipoteichoic Acid and Regulates Gram-Positive Pathogen Infection. Cell 2018, 175, 1651–1664.e14. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Yin, H.; Qin, S.; Zhang, K.; Guan, Y.; Fang, R.; Wang, H.; Li, G.; Jiang, Z.; Sun, F.; et al. NLRP6 Self-Assembles into a Linear Molecular Platform Following LPS Binding and ATP Stimulation. Sci. Rep. 2020, 10, 198. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, S.; Yang, L.; Cui, S.; Pan, W.; Jackson, R.; Zheng, Y.; Rongvaux, A.; Sun, Q.; Yang, G.; et al. Nlrp6 Regulates Intestinal Antiviral Innate Immunity. Science (1979) 2015, 350, 826–830. [Google Scholar] [CrossRef]
- Li, G.; Tian, X.; Lv, D.; Zhang, L.; Zhang, Z.; Wang, J.; Yang, M.; Tao, J.; Ma, T.; Wu, H.; et al. NLRP7 Is Expressed in the Ovine Ovary and Associated with in Vitro Pre-Implantation Embryo Development. Reproduction 2019, 158, 415–427. [Google Scholar] [CrossRef]
- Khare, S.; Dorfleutner, A.; Bryan, N.B.; Yun, C.; Radian, A.D.; de Almeida, L.; Rojanasakul, Y.; Stehlik, C. An NLRP7-Containing Inflammasome Mediates Recognition of Microbial Lipopeptides in Human Macrophages. Immunity 2012, 36, 464–476. [Google Scholar] [CrossRef]
- Radian, A.D.; Khare, S.; Chu, L.H.; Dorfleutner, A.; Stehlik, C. ATP Binding by NLRP7 Is Required for Inflammasome Activation in Response to Bacterial Lipopeptides. Mol. Immunol. 2015, 67, 294–302. [Google Scholar] [CrossRef]
- Bednash, J.S.; Weathington, N.; Londino, J.; Rojas, M.; Gulick, D.L.; Fort, R.; Han, S.; McKelvey, A.C.; Chen, B.B.; Mallampalli, R.K. Targeting the Deubiquitinase STAMBP Inhibits NALP7 Inflammasome Activity. Nat. Commun. 2017, 8, 15203. [Google Scholar] [CrossRef]
- Kinoshita, T.; Wang, Y.; Hasegawa, M.; Imamura, R.; Suda, T. PYPAF3, a PYRIN-Containing APAF-1-like Protein, Is a Feedback Regulator of Caspase-1-Dependent Interleukin-1β Secretion. J. Biol. Chem. 2005, 280, 21720–21725. [Google Scholar] [CrossRef]
- Grenier, J.M.; Wang, L.; Manji, G.A.; Huang, W.-J.; Al-Garawi, A.; Kelly, R.; Carlson, A.; Merriam, S.; Lora, J.M.; Briskin, M.; et al. Functional Screening of Five PYPAF Family Members Identifies PYPAF5 as a Novel Regulator of NF-ΚB and Caspase-1. FEBS Lett. 2002, 530, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Messaed, C.; Chebaro, W.; Roberto, R.B.D.; Rittore, C.; Cheung, A.; Arseneau, J.; Schneider, A.; Chen, M.F.; Bernishke, K.; Surti, U.; et al. NLRP7 in the Spectrum of Reproductive Wastage: Rare Non-Synonymous Variants Confer Genetic Susceptibility to Recurrent Reproductive Wastage. J. Med. Genet. 2011, 48, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Lech, M.; Avila-Ferrufino, A.; Skuginna, V.; Susanti, H.E.; Anders, H.J. Quantitative Expression of RIG-like Helicase, NOD-like Receptor and Inflammasome-Related mRNAs in Humans and Mice. Int. Immunol. 2010, 22, 717–728. [Google Scholar] [CrossRef]
- Imamura, R.; Wang, Y.; Kinoshita, T.; Suzuki, M.; Noda, T.; Sagara, J.; Taniguchi, S.; Okamoto, H.; Suda, T. Anti-Inflammatory Activity of PYNOD and Its Mechanism in Humans and Mice. J. Immunol. 2010, 184, 5874–5884. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Böhme, J.; Zambetti, L.P.; Khameneh, H.J.; Paleja, B.S.; Laudisi, F.; Ho, A.W.S.; Neo, K.; Leong, K.W.K.; Marzuki, M.; et al. NLRP10 Enhances CD4+ T-Cell-Mediated IFNγ Response via Regulation of Dendritic Cell-Derived IL-12 Release. Front. Immunol. 2017, 8, 1462. [Google Scholar] [CrossRef]
- Clay, G.M.; Valadares, D.G.; Graff, J.W.; Ulland, T.K.; Davis, R.E.; Scorza, B.M.; Zhanbolat, B.S.; Chen, Y.; Sutterwala, F.S.; Wilson, M.E. An Anti-Inflammatory Role for NLRP10 in Murine Cutaneous Leishmaniasis. J. Immunol. 2017, 199, 2823–2833. [Google Scholar] [CrossRef]
- Wang, Y.; Hasegawa, M.; Imamura, R.; Kinoshita, T.; Kondo, C.; Konaka, K.; Suda, T. PYNOD, a Novel Apaf-1/CED4-like Protein Is an Inhibitor of ASC and Caspase-1. Int. Immunol. 2004, 16, 777–786. [Google Scholar] [CrossRef]
- Próchnicki, T.; Vasconcelos, M.B.; Robinson, K.S.; Mangan, M.S.J.; De Graaf, D.; Shkarina, K.; Lovotti, M.; Standke, L.; Kaiser, R.; Stahl, R.; et al. Mitochondrial Damage Activates the NLRP10 Inflammasome. Nat. Immunol. 2023, 24, 595–603. [Google Scholar] [CrossRef]
- Zheng, D.; Mohapatra, G.; Kern, L.; He, Y.; Shmueli, M.D.; Valdés-Mas, R.; Kolodziejczyk, A.A.; Próchnicki, T.; Vasconcelos, M.B.; Schorr, L.; et al. Epithelial Nlrp10 Inflammasome Mediates Protection against Intestinal Autoinflammation. Nat. Immunol. 2023, 24, 585–594. [Google Scholar] [CrossRef]
- Allen, I.C.; Wilson, J.E.; Schneider, M.; Lich, J.D.; Roberts, R.A.; Arthur, J.C.; Woodford, R.-M.T.; Davis, B.K.; Uronis, J.M.; Herfarth, H.H.; et al. NLRP12 Suppresses Colon Inflammation and Tumorigenesis through the Negative Regulation of Noncanonical NF-ΚB Signaling. Immunity 2012, 36, 742–754. [Google Scholar] [CrossRef]
- Williams, K.L.; Taxman, D.J.; Linhoff, M.W.; Reed, W.; Ting, J.P.-Y. Cutting Edge: Monarch-1: A Pyrin/Nucleotide-Binding Domain/Leucine-Rich Repeat Protein That Controls Classical and Nonclassical MHC Class I Genes. J. Immunol. 2003, 170, 5354–5358. [Google Scholar] [CrossRef]
- Williams, K.L.; Lich, J.D.; Duncan, J.A.; Reed, W.; Rallabhandi, P.; Moore, C.; Kurtz, S.; Coffield, V.M.; Accavitti-Loper, M.A.; Su, L.; et al. The Caterpillar Protein Monarch-1 Is an Antagonist of Toll-like Receptor-, Tumor Necrosis Factor α-, and Mycobacterium Tuberculosis-Induced Pro-Inflammatory Signals. J. Biol. Chem. 2005, 280, 39914–39924. [Google Scholar] [CrossRef]
- Lich, J.D.; Williams, K.L.; Moore, C.B.; Arthur, J.C.; Davis, B.K.; Taxman, D.J.; Ting, J.P.-Y. Cutting Edge: Monarch-1 Suppresses Non-Canonical NF-ΚB Activation and P52-Dependent Chemokine Expression in Monocytes. J. Immunol. 2007, 178, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Hornick, E.E.; Banoth, B.; Miller, A.M.; Zacharias, Z.R.; Jain, N.; Wilson, M.E.; Gibson-Corley, K.N.; Legge, K.L.; Bishop, G.A.; Sutterwala, F.S.; et al. Nlrp12 Mediates Adverse Neutrophil Recruitment during Influenza Virus Infection. J. Immunol. 2018, 200, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Ulland, T.K.; Jain, N.; Hornick, E.E.; Elliott, E.I.; Clay, G.M.; Sadler, J.J.; Mills, K.A.M.; Janowski, A.M.; Volk, A.P.D.; Wang, K.; et al. Nlrp12 Mutation Causes C57BL/6J Strain-Specific Defect in Neutrophil Recruitment. Nat. Commun. 2016, 7, 13180. [Google Scholar] [CrossRef] [PubMed]
- Zamoshnikova, A.; Groß, C.J.; Schuster, S.; Chen, K.W.; Wilson, A.; Tacchini-Cottier, F.; Schroder, K. NLRP12 Is a Neutrophil-Specific, Negative Regulator of in Vitro Cell Migration but Does Not Modulate LPS- or Infection-Induced NF-ΚB or ERK Signalling. Immunobiology 2016, 221, 341–346. [Google Scholar] [CrossRef]
- Lukens, J.R.; Gurung, P.; Shaw, P.J.; Barr, M.J.; Zaki, M.H.; Brown, S.A.; Vogel, P.; Chi, H.; Kanneganti, T.-D. The NLRP12 Sensor Negatively Regulates Autoinflammatory Disease by Modulating Interleukin-4 Production in T Cells. Immunity 2015, 42, 654–664. [Google Scholar] [CrossRef]
- Gurung, P.; Kanneganti, T.-D. NLRP12 in Autoimmune Diseases. Oncotarget 2015, 6, 19950–19951. [Google Scholar] [CrossRef] [PubMed]
- Vladimer, G.I.; Weng, D.; Paquette, S.W.M.; Vanaja, S.K.; Rathinam, V.A.K.; Aune, M.H.; Conlon, J.E.; Burbage, J.J.; Proulx, M.K.; Liu, Q.; et al. The NLRP12 Inflammasome Recognizes Yersinia Pestis. Immunity 2012, 37, 96–107. [Google Scholar] [CrossRef]
- Tuladhar, S.; Kanneganti, T.-D. NLRP12 in Innate Immunity and Inflammation. Mol. Asp. Med. 2020, 76, 100887. [Google Scholar] [CrossRef]
- The International FMF Consortium Ancient Missense Mutations in a New Member of the RoRet Gene Family Are Likely to Cause Familial Mediterranean Fever. Cell 1997, 90, 797–807. [CrossRef] [PubMed]
- Mansfield, E.; Chae, J.J.; Komarow, H.D.; Brotz, T.M.; Frucht, D.M.; Aksentijevich, I.; Kastner, D.L. The Familial Mediterranean Fever Protein, Pyrin, Associates with Microtubules and Colocalizes with Actin Filaments. Blood 2001, 98, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Papin, S.; Cuenin, S.; Agostini, L.; Martinon, F.; Werner, S.; Beer, H.-D.; Grütter, C.; Grütter, M.; Tschopp, J. The SPRY Domain of Pyrin, Mutated in Familial Mediterranean Fever Patients, Interacts with Inflammasome Components and Inhibits ProIL-1β Processing. Cell Death Differ. 2007, 14, 1457–1466. [Google Scholar] [CrossRef]
- Xu, H.; Yang, J.; Gao, W.; Li, L.; Li, P.; Zhang, L.; Gong, Y.-N.; Peng, X.; Xi, J.J.; Chen, S.; et al. Innate Immune Sensing of Bacterial Modifications of Rho GTPases by the Pyrin Inflammasome. Nature 2014, 513, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Just, I.; Selzer, J.; Wilm, M.; von Eichel-Streiber, C.; Mann, M.; Aktories, K. Glucosylation of Rho Proteins by Clostridium Difficile Toxin B. Nature 1995, 375, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Mostowy, S.; Shenoy, A.R. The Cytoskeleton in Cell-Autonomous Immunity: Structural Determinants of Host Defence. Nat. Rev. Immunol. 2015, 15, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Kamanova, J.; Kofronova, O.; Masin, J.; Genth, H.; Vojtova, J.; Linhartova, I.; Benada, O.; Just, I.; Sebo, P. Adenylate Cyclase Toxin Subverts Phagocyte Function by RhoA Inhibition and Unproductive Ruffling. J. Immunol. 2008, 181, 5587–5597. [Google Scholar] [CrossRef]
- Aubert, D.F.; Xu, H.; Yang, J.; Shi, X.; Gao, W.; Li, L.; Bisaro, F.; Chen, S.; Valvano, M.A.; Shao, F. A Burkholderia Type VI Effector Deamidates Rho GTPases to Activate the Pyrin Inflammasome and Trigger Inflammation. Cell Host Microbe 2016, 19, 664–674. [Google Scholar] [CrossRef]
- Park, Y.H.; Wood, G.; Kastner, D.L.; Chae, J.J. Pyrin Inflammasome Activation and RhoA Signaling in the Autoinflammatory Diseases FMF and HIDS. Nat. Immunol. 2016, 17, 914–921. [Google Scholar] [CrossRef]
- Lee, S.; Karki, R.; Wang, Y.; Nguyen, L.N.; Kalathur, R.C.; Kanneganti, T.-D. AIM2 Forms a Complex with Pyrin and ZBP1 to Drive PANoptosis and Host Defence. Nature 2021, 597, 415–419. [Google Scholar] [CrossRef]
- Li, Y.; Fu, T.-M.; Lu, A.; Witt, K.; Ruan, J.; Shen, C.; Wu, H. Cryo-EM Structures of ASC and NLRC4 CARD Filaments Reveal a Unified Mechanism of Nucleation and Activation of Caspase-1. Proc. Natl. Acad. Sci. USA 2018, 115, 10845–10852. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xuan, B.; Liu, Y.; Wang, L.; He, L.; Meng, X.; Zhou, T.; Wang, Y. Updating the NLRC4 Inflammasome: From Bacterial Infections to Autoimmunity and Cancer. Front. Immunol. 2021, 12, 702527. [Google Scholar] [CrossRef]
- Broz, P.; Newton, K.; Lamkanfi, M.; Mariathasan, S.; Dixit, V.M.; Monack, D.M. Redundant Roles for Inflammasome Receptors NLRP3 and NLRC4 in Host Defense against Salmonella. J. Exp. Med. 2010, 207, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Tourlomousis, P.; Hopkins, L.; Monie, T.P.; Fitzgerald, K.A.; Bryant, C.E. Salmonella Infection Induces Recruitment of Caspase-8 to the Inflammasome To Modulate IL-1β Production. J. Immunol. 2013, 191, 5239–5246. [Google Scholar] [CrossRef]
- Man, S.M.; Hopkins, L.J.; Nugent, E.; Cox, S.; Glück, I.M.; Tourlomousis, P.; Wright, J.A.; Cicuta, P.; Monie, T.P.; Bryant, C.E. Inflammasome Activation Causes Dual Recruitment of NLRC4 and NLRP3 to the Same Macromolecular Complex. Proc. Natl. Acad. Sci. USA 2014, 111, 7403–7408. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, B.; Kanneganti, T.-D. Advances in Understanding Activation and Function of the NLRC4 Inflammasome. Int. J. Mol. Sci. 2021, 22, 1048. [Google Scholar] [CrossRef]
- Barnett, K.C.; Li, S.; Liang, K.; Ting, J.P.-Y. A 360° View of the Inflammasome: Mechanisms of Activation, Cell Death, and Diseases. Cell 2023, 186, 2288–2312. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Zamboni, D.S.; Roy, C.R.; Dietrich, W.F.; Vance, R.E. Flagellin-Deficient Legionella Mutants Evade Caspase-1- and Naip5-Mediated Macrophage Immunity. PLoS Pathog. 2006, 2, e18. [Google Scholar] [CrossRef]
- Franchi, L.; Amer, A.; Body-Malapel, M.; Kanneganti, T.-D.; Özören, N.; Jagirdar, R.; Inohara, N.; Vandenabeele, P.; Bertin, J.; Coyle, A.; et al. Cytosolic Flagellin Requires Ipaf for Activation of Caspase-1 and Interleukin 1β in Salmonella-Infected Macrophages. Nat. Immunol. 2006, 7, 576–582. [Google Scholar] [CrossRef]
- Miao, E.A.; Alpuche-Aranda, C.M.; Dors, M.; Clark, A.E.; Bader, M.W.; Miller, S.I.; Aderem, A. Cytoplasmic Flagellin Activates Caspase-1 and Secretion of Interleukin 1β via Ipaf. Nat. Immunol. 2006, 7, 569–575. [Google Scholar] [CrossRef]
- Kofoed, E.M.; Vance, R.E. Innate Immune Recognition of Bacterial Ligands by NAIPs Determines Inflammasome Specificity. Nature 2011, 477, 592–595. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Shi, J.; Gong, Y.-N.; Lu, Q.; Xu, H.; Liu, L.; Shao, F. The NLRC4 Inflammasome Receptors for Bacterial Flagellin and Type III Secretion Apparatus. Nature 2011, 477, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, D.S.; Kobayashi, K.S.; Kohlsdorf, T.; Ogura, Y.; Long, E.M.; Vance, R.E.; Kuida, K.; Mariathasan, S.; Dixit, V.M.; Flavell, R.A.; et al. The Birc1e Cytosolic Pattern-Recognition Receptor Contributes to the Detection and Control of Legionella Pneumophila Infection. Nat. Immunol. 2006, 7, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Franchi, L.; Kanneganti, T.-D.; Body-Malapel, M.; Özören, N.; Brady, G.; Meshinchi, S.; Jagirdar, R.; Gewirtz, A.; Akira, S.; et al. Regulation of Legionella Phagosome Maturation and Infection through Flagellin and Host Ipaf. J. Biol. Chem. 2006, 281, 35217–35223. [Google Scholar] [CrossRef]
- Karki, R.; Lee, E.; Place, D.; Samir, P.; Mavuluri, J.; Sharma, B.R.; Balakrishnan, A.; Malireddi, R.K.S.; Geiger, R.; Zhu, Q.; et al. IRF8 Regulates Transcription of Naips for NLRC4 Inflammasome Activation. Cell 2018, 173, 920–933.e13. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, Q.; Zhang, C.; Fan, S.; Cheng, W.; Zhao, Y.; Shao, F.; Wang, H.-W.; Sui, S.-F.; Chai, J. Structural and Biochemical Basis for Induced Self-Propagation of NLRC4. Science (1979) 2015, 350, 399–404. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, S.; Ruan, J.; Wu, J.; Tong, A.B.; Yin, Q.; Li, Y.; David, L.; Lu, A.; Wang, W.L.; et al. Cryo-EM Structure of the Activated NAIP2-NLRC4 Inflammasome Reveals Nucleated Polymerization. Science (1979) 2015, 350, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Radha, V.; Swarup, G. Interaction with Sug1 Enables Ipaf Ubiquitination Leading to Caspase 8 Activation and Cell Death. Biochem. J. 2010, 427, 91–104. [Google Scholar] [CrossRef]
- Raghawan, A.K.; Sripada, A.; Gopinath, G.; Pushpanjali, P.; Kumar, Y.; Radha, V.; Swarup, G. A Disease-Associated Mutant of NLRC4 Shows Enhanced Interaction with SUG1 Leading to Constitutive FADD-Dependent Caspase-8 Activation and Cell Death. J. Biol. Chem. 2017, 292, 1218–1230. [Google Scholar] [CrossRef]
- von Moltke, J.; Trinidad, N.J.; Moayeri, M.; Kintzer, A.F.; Wang, S.B.; van Rooijen, N.; Brown, C.R.; Krantz, B.A.; Leppla, S.H.; Gronert, K.; et al. Rapid Induction of Inflammatory Lipid Mediators by the Inflammasome in Vivo. Nature 2012, 490, 107–111. [Google Scholar] [CrossRef]
- Muruve, D.A.; Pétrilli, V.; Zaiss, A.K.; White, L.R.; Clark, S.A.; Ross, P.J.; Parks, R.J.; Tschopp, J. The Inflammasome Recognizes Cytosolic Microbial and Host DNA and Triggers an Innate Immune Response. Nature 2008, 452, 103–107. [Google Scholar] [CrossRef]
- Fernandes-Alnemri, T.; Yu, J.-W.; Datta, P.; Wu, J.; Alnemri, E.S. AIM2 Activates the Inflammasome and Cell Death in Response to Cytoplasmic DNA. Nature 2009, 458, 509–513. [Google Scholar] [CrossRef]
- Wang, B.; Yin, Q. AIM2 Inflammasome Activation and Regulation: A Structural Perspective. J. Struct. Biol. 2017, 200, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, A.; Frera, G.; Lugrin, J.; Jamilloux, Y.; Hsu, E.-T.; Tardivel, A.; De Gassart, A.; Zaffalon, L.; Bujisic, B.; Siegert, S.; et al. AIM2 Inflammasome Is Activated by Pharmacological Disruption of Nuclear Envelope Integrity. Proc. Natl. Acad. Sci. USA 2016, 113, E4671–E4680. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Martinon, F. The AIM2 Inflammasome: Sensor of Pathogens and Cellular Perturbations. Immunol. Rev. 2018, 281, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, L.; Byrd, K.M.; Ko, C.-C.; Zhao, Z.; Fang, J. AIM2 Inflammasome’s First Decade of Discovery: Focus on Oral Diseases. Front. Immunol. 2020, 11, 1487. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T.-D. AIM2 Inflammasome in Infection, Cancer, and Autoimmunity: Role in DNA Sensing, Inflammation, and Innate Immunity. Eur. J. Immunol. 2016, 46, 269–280. [Google Scholar] [CrossRef]
- Shrivastava, G.; León-Juárez, M.; García-Cordero, J.; Meza-Sánchez, D.E.; Cedillo-Barrón, L. Inflammasomes and Its Importance in Viral Infections. Immunol. Res. 2016, 64, 1101–1117. [Google Scholar] [CrossRef]
- Ma, Z.; Ni, G.; Damania, B. Innate Sensing of DNA Virus Genomes. Annu. Rev. Virol. 2018, 5, 341–362. [Google Scholar] [CrossRef]
- Hayward, J.A.; Mathur, A.; Ngo, C.; Man, S.M. Cytosolic Recognition of Microbes and Pathogens: Inflammasomes in Action. Microbiol. Mol. Biol. Rev. 2018, 82, e00015-18. [Google Scholar] [CrossRef]
- Storek, K.M.; Gertsvolf, N.A.; Ohlson, M.B.; Monack, D.M. CGAS and Ifi204 Cooperate To Produce Type I IFNs in Response to Francisella Infection. J. Immunol. 2015, 194, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Karki, R.; Sasai, M.; Place, D.E.; Kesavardhana, S.; Temirov, J.; Frase, S.; Zhu, Q.; Malireddi, R.K.S.; Kuriakose, T.; et al. IRGB10 Liberates Bacterial Ligands for Sensing by the AIM2 and Caspase-11-NLRP3 Inflammasomes. Cell 2016, 167, 382–396.e17. [Google Scholar] [CrossRef]
- Meunier, E.; Wallet, P.; Dreier, R.F.; Costanzo, S.; Anton, L.; Rühl, S.; Dussurgey, S.; Dick, M.S.; Kistner, A.; Rigard, M.; et al. Guanylate-Binding Proteins Promote Activation of the AIM2 Inflammasome during Infection with Francisella Novicida. Nat. Immunol. 2015, 16, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Bürckstümmer, T.; Baumann, C.; Blüml, S.; Dixit, E.; Dürnberger, G.; Jahn, H.; Planyavsky, M.; Bilban, M.; Colinge, J.; Bennett, K.L.; et al. An Orthogonal Proteomic-Genomic Screen Identifies AIM2 as a Cytoplasmic DNA Sensor for the Inflammasome. Nat. Immunol. 2009, 10, 266–272. [Google Scholar] [CrossRef]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Bhattacharya, M.; Roy, S.; Tian, Y.; Yin, Q. Immunobiology and Structural Biology of AIM2 Inflammasome. Mol. Asp. Med. 2020, 76, 100869. [Google Scholar] [CrossRef]
- Dorfleutner, A.; Bryan, N.B.; Talbott, S.J.; Funya, K.N.; Rellick, S.L.; Reed, J.C.; Shi, X.; Rojanasakul, Y.; Flynn, D.C.; Stehlik, C. Cellular Pyrin Domain-Only Protein 2 Is a Candidate Regulator of Inflammasome Activation. Infect. Immun. 2007, 75, 1484–1492. [Google Scholar] [CrossRef]
- Khare, S.; Ratsimandresy, R.A.; de Almeida, L.; Cuda, C.M.; Rellick, S.L.; Misharin, A.V.; Wallin, M.C.; Gangopadhyay, A.; Forte, E.; Gottwein, E.; et al. The PYRIN Domain–Only Protein POP3 Inhibits ALR Inflammasomes and Regulates Responses to Infection with DNA Viruses. Nat. Immunol. 2014, 15, 343–353. [Google Scholar] [CrossRef]
- Stehlik, C.; Krajewska, M.; Welsh, K.; Krajewski, S.; Godzik, A.; Reed, J.C. The PAAD/PYRIN-Only Protein POP1/ASC2 Is a Modulator of ASC-Mediated Nuclear-Factor-KappaB and pro-Caspase-1 Regulation. Biochem. J. 2003, 373, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ma, D.; Huang, H.; Lu, Y.; Liao, Y.; Liu, L.; Liu, X.; Fang, F. Interaction between HCMV PUL83 and Human AIM2 Disrupts the Activation of the AIM2 Inflammasome. Virol. J. 2017, 14, 34. [Google Scholar] [CrossRef]
- Bauernfeind, F.; Hornung, V. Of Inflammasomes and Pathogens – Sensing of Microbes by the Inflammasome. EMBO Mol. Med. 2013, 5, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, H.E.; Özen, S. A Clinical Update on Inflammasomopathies. Int. Immunol. 2017, 29, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Menu, P.; Vince, J.E.; Vince, J.; Menu, P. The NLRP3 Inflammasome in Health and Disease: The Good, the Bad and the Ugly. Clin. Exp. Immunol. 2011, 166, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Rigante, D.; Maggio, M.C.; Emmi, G.; Romano, M.; Silvestri, E.; Lucherini, O.M.; Emmi, L.; Gerloni, V.; Cantarini, L. Rare NLRP12 Variants Associated with the NLRP12-Autoinflammatory Disorder Phenotype: An Italian Case Series. Clin. Exp. Rheumatol. 2013, 31, 155–156. [Google Scholar] [PubMed]
- Chen, S.; Li, Z.; Hu, X.; Zhang, H.; Chen, W.; Xu, Q.; Tang, L.; Ge, H.; Zhen, Q.; Yong, L.; et al. Rare Mutations in NLRP3 and NLRP12 Associated with Familial Cold Autoinflammatory Syndrome: Two Chinese Pedigrees. Clin. Rheumatol. 2022, 41, 3461–3470. [Google Scholar] [CrossRef] [PubMed]
- Borghini, S.; Tassi, S.; Chiesa, S.; Caroli, F.; Carta, S.; Caorsi, R.; Fiore, M.; Delfino, L.; Lasigliè, D.; Ferraris, C.; et al. Clinical Presentation and Pathogenesis of Cold-Induced Autoinflammatory Disease in a Family with Recurrence of an NLRP12 Mutation. Arthritis Rheum. 2011, 63, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, A.; Sasaki, Y.; Abe, T.; Kano, H.; Yasutomo, K. An Inherited Mutation in NLRC4 Causes Autoinflammation in Human and Mice. J. Exp. Med. 2014, 211, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.M.; Mueller, J.L.; Broide, D.H.; Wanderer, A.A.; Kolodner, R.D. Mutation of a New Gene Encoding a Putative Pyrin-like Protein Causes Familial Cold Autoinflammatory Syndrome and Muckle–Wells Syndrome. Nat. Genet. 2001, 29, 301–305. [Google Scholar] [CrossRef]
- Martorana, D.; Bonatti, F.; Mozzoni, P.; Vaglio, A.; Percesepe, A. Monogenic Autoinflammatory Diseases with Mendelian Inheritance: Genes, Mutations, and Genotype/Phenotype Correlations. Front. Immunol. 2017, 8, 344. [Google Scholar] [CrossRef]
- Welzel, T.; Kuemmerle-Deschner, J.B. Diagnosis and Management of the Cryopyrin-Associated Periodic Syndromes (CAPS): What Do We Know Today? J. Clin. Med. 2021, 10, 128. [Google Scholar] [CrossRef]
- Canna, S.W.; De Jesus, A.A.; Gouni, S.; Brooks, S.R.; Marrero, B.; Liu, Y.; Dimattia, M.A.; Zaal, K.J.M.; Sanchez, G.A.M.; Kim, H.; et al. An Activating NLRC4 Inflammasome Mutation Causes Autoinflammation with Recurrent Macrophage Activation Syndrome. Nat. Genet. 2014, 46, 1140–1146. [Google Scholar] [CrossRef]
- Romberg, N.; Al Moussawi, K.; Nelson-Williams, C.; Stiegler, A.L.; Loring, E.; Choi, M.; Overton, J.; Meffre, E.; Khokha, M.K.; Huttner, A.J.; et al. Mutation of NLRC4 Causes a Syndrome of Enterocolitis and Autoinflammation. Nat. Genet. 2014, 46, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Canna, S.W.; Girard, C.; Malle, L.; de Jesus, A.; Romberg, N.; Kelsen, J.; Surrey, L.F.; Russo, P.; Sleight, A.; Schiffrin, E.; et al. Life-Threatening NLRC4-Associated Hyperinflammation Successfully Treated with IL-18 Inhibition. J. Allergy Clin. Immunol. 2017, 139, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Alfano, D.N.; Squires, J.E.; Riley, M.M.; Parks, W.T.; Kofler, J.; El-Gharbawy, A.; Madan-Kheterpal, S.; Acquaro, R.; Picarsic, J. Novel NLRC4 Mutation Causes a Syndrome of Perinatal Autoinflammation With Hemophagocytic Lymphohistiocytosis, Hepatosplenomegaly, Fetal Thrombotic Vasculopathy, and Congenital Anemia and Ascites. Pediatr. Dev. Pathol. 2017, 20, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Grandemange, S.; Sanchez, E.; Louis-Plence, P.; Tran Mau-Them, F.; Bessis, D.; Coubes, C.; Frouin, E.; Seyger, M.; Girard, M.; Puechberty, J.; et al. A New Autoinflammatory and Autoimmune Syndrome Associated with NLRP1 Mutations: NAIAD (NLRP1- Associated Autoinflammation with Arthritis and Dyskeratosis). Ann. Rheum. Dis. 2017, 76, 1191–1198. [Google Scholar] [CrossRef]
- Infevers. Available online: https://infevers.umai-montpellier.fr/web/index.php (accessed on 4 June 2023).
- Shi, X.; Xie, W.L.; Kong, W.W.; Chen, D.; Qu, P. Expression of the NLRP3 Inflammasome in Carotid Atherosclerosis. J. Stroke Cerebrovasc. Dis. 2015, 24, 2455–2466. [Google Scholar] [CrossRef]
- Ramji, D.P.; Davies, T.S. Cytokines in Atherosclerosis: Key Players in All Stages of Disease and Promising Therapeutic Targets. Cytokine Growth Factor Rev. 2015, 26, 673–685. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. New Eng. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nũez, G.; Schnurr, M.; et al. NLRP3 Inflammasomes Are Required for Atherogenesis and Activated by Cholesterol Crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef]
- Bleda, S.; De Haro, J.; Varela, C.; Ferruelo, A.; Acin, F. Elevated Levels of Triglycerides and Vldl-Cholesterol Provoke Activation of Nlrp1 Inflammasome in Endothelial Cells. Int. J. Cardiol. 2016, 220, 52–55. [Google Scholar] [CrossRef]
- Folco, E.J.; Sukhova, G.K.; Quillard, T.; Libby, P. Moderate Hypoxia Potentiates Interleukin-1β Production in Activated Human Macrophages. Circ. Res. 2014, 115, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Stachon, P.; Heidenreich, A.; Merz, J.; Hilgendorf, I.; Wolf, D.; Willecke, F.; Von Garlen, S.; Albrecht, P.; Härdtner, C.; Ehrat, N.; et al. P2X7 Deficiency Blocks Lesional Inflammasome Activity and Ameliorates Atherosclerosis in Mice. Circulation 2017, 135, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, M.; Peters, A.; Becker, A.; Böckler, D.; Dihlmann, S. Inflammation-Related Induction of Absent in Melanoma 2 (AIM2) in Vascular Cells and Atherosclerotic Lesions Suggests a Role in Vascular Pathogenesis. J. Vasc. Surg. 2014, 59, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Fiolet, A.T.L.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; Bax, W.A.; Budgeon, C.A.; Tijssen, J.G.P.; Mosterd, A.; Cornel, J.H.; et al. The Effect of Low-Dose Colchicine in Patients with Stable Coronary Artery Disease: The LoDoCo2 Trial Rationale, Design, and Baseline Characteristics. Am. Heart J. 2019, 218, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Xia, Y.; Huang, M.; Zhang, L.; Chen, L. Expression of NLPR3 in Psoriasis Is Associated with Enhancement of Interleukin-1β and Caspase-1. Med. Sci. Monit. 2018, 24, 7909–7913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiao, C.; Dang, E.; Cao, J.; Zhu, Z.; Fu, M.; Yao, X.; Liu, Y.; Jin, B.; Wang, G.; et al. CD100–Plexin-B2 Promotes the Inflammation in Psoriasis by Activating NF-ΚB and the Inflammasome in Keratinocytes. J. Investig. Dermatol. 2018, 138, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Fekri, S.Z.; Sigurdardottir, G.; Bivik Eding, C.; Sandin, C.; Enerbäck, C. Enhanced Inflammasome Activity in Patients with Psoriasis Promotes Systemic Inflammation. J. Investig. Dermatol. 2021, 141, 586–595.e5. [Google Scholar] [CrossRef]
- De Koning, H.D.; Bergboer, J.G.M.; Van den Bogaard, E.H.; Van Vlijmen-Willems, I.M.J.J.; Rodijk-Olthuis, D.; Simon, A.; Zeeuwen, P.L.J.M.; Schalkwijk, J. Strong Induction of AIM2 Expression in Human Epidermis in Acute and Chronic Inflammatory Skin Conditions. Exp. Dermatol. 2012, 21, 961–964. [Google Scholar] [CrossRef]
- Dombrowski, Y.; Peric, M.; Koglin, S.; Kammerbauer, C.; Göß, C.; Anz, D.; Simanski, M.; Gläser, R.; Harder, J.; Hornung, V.; et al. Cytosolic DNA Triggers Inflammasome Activation in Keratinocytes in Psoriatic Lesions. Sci. Transl. Med. 2011, 3, 82ra38. [Google Scholar] [CrossRef]
- Lazaridis, L.D.; Pistiki, A.; Giamarellos-Bourboulis, E.J.; Georgitsi, M.; Damoraki, G.; Polymeros, D.; Dimitriadis, G.D.; Triantafyllou, K. Activation of NLRP3 Inflammasome in Inflammatory Bowel Disease: Differences Between Crohn’s Disease and Ulcerative Colitis. Dig. Dis. Sci. 2017, 62, 2348–2356. [Google Scholar] [CrossRef]
- Mao, L.; Kitani, A.; Strober, W.; Fuss, I.J. The Role of NLRP3 and IL-1β in the Pathogenesis of Inflammatory Bowel Disease. Front. Immunol. 2018, 9, 2566. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, G.; Yuan, Y.; Wu, G.; Wang, S.; Yuan, L. NEK7 Interacts with NLRP3 to Modulate the Pyroptosis in Inflammatory Bowel Disease via NF-ΚB Signaling. Cell Death Dis. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Sui, J.; Li, H.; Fang, Y.; Liu, Y.; Li, M.; Zhong, B.; Yang, F.; Zou, Q.; Wu, Y. NLRP1 Gene Polymorphism Influences Gene Transcription and Is a Risk Factor for Rheumatoid Arthritis in Han Chinese. Arthritis Rheum. 2012, 64, 647–654. [Google Scholar] [CrossRef]
- Goh, L.L.; Yong, M.Y.; See, W.Q.; Chee, E.Y.W.; Lim, P.Q.; Koh, E.T.; Leong, K.P.; Chan, G.Y.L.; Chan, M.T.L.; Chia, F.L.A.; et al. NLRP1, PTPN22 and PADI4 Gene Polymorphisms and Rheumatoid Arthritis in ACPA-Positive Singaporean Chinese. Rheumatol. Int. 2017, 37, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Ruscitti, P.; Cipriani, P.; Di Benedetto, P.; Liakouli, V.; Berardicurti, O.; Carubbi, F.; Ciccia, F.; Alvaro, S.; Triolo, G.; Giacomelli, R. Monocytes from Patients with Rheumatoid Arthritis and Type 2 Diabetes Mellitus Display an Increased Production of Interleukin (IL)-1β via the Nucleotide-Binding Domain and Leucine-Rich Repeat Containing Family Pyrin 3(NLRP3)-Inflammasome Activation: A Possible Implication for Therapeutic Decision in These Patients. Clin. Exp. Immunol. 2015, 182, 35–44. [Google Scholar] [PubMed]
- Kim, S.K.; Choe, J.Y.; Lee, G.H. Enhanced Expression of NLRP3 Inflammasome-Related Inflammation in Peripheral Blood Mononuclear Cells in Sjögren’s Syndrome. Clin. Chim. Acta 2017, 474, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, Y.; Li, M.; Gao, Q.; Peng, Y.; Gong, Q.; Zhang, Z.; Wu, X. Paeoniflorin Down-Regulates ATP-Induced Inflammatory Cytokine Production and P2X7R Expression on Peripheral Blood Mononuclear Cells from Patients with Primary Sjögren’s Syndrome. Int. Immunopharmacol. 2015, 28, 115–120. [Google Scholar] [CrossRef]
- Vakrakou, A.G.; Boiu, S.; Ziakas, P.D.; Xingi, E.; Boleti, H.; Manoussakis, M.N. Systemic Activation of NLRP3 Inflammasome in Patients with Severe Primary Sjögren’s Syndrome Fueled by Inflammagenic DNA Accumulations. J. Autoimmun. 2018, 91, 23–33. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Vakrakou, A.G.; Papadopoulou, A.; Germenis, A.; Kanavakis, E.; Moutsopoulos, H.M.; Manoussakis, M.N. Impaired Degradation and Aberrant Phagocytosis of Necrotic Cell Debris in the Peripheral Blood of Patients with Primary Sjögren’s Syndrome. J. Autoimmun. 2015, 56, 12–22. [Google Scholar] [CrossRef]
- Shin, M.S.; Kang, Y.; Lee, N.; Wahl, E.R.; Kim, S.H.; Kang, K.S.; Lazova, R.; Kang, I. Self Double-Stranded (Ds)DNA Induces IL-1β Production from Human Monocytes by Activating NLRP3 Inflammasome in the Presence of Anti–DsDNA Antibodies. J. Immunol. 2013, 190, 1407–1415. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, R.; Guo, C.; Huang, Y.; Wang, H.; Wang, S.; Zhao, J.; Yang, N. Anti-DsDNA Antibodies Bind to TLR4 and Activate NLRP3 Inflammasome in Lupus Monocytes/Macrophages. J. Transl. Med. 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Antiochos, B.; Trejo-Zambrano, D.; Fenaroli, P.; Rosenberg, A.; Baer, A.; Garg, A.; Sohn, J.; Li, J.; Petri, M.; Goldman, D.W.; et al. The DNA Sensors AIM2 and IFI16 Are SLE Autoantigens That Bind Neutrophil Extracellular Traps. eLife 2022, 11, 72103. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; La Rosa, F.; Piancone, F.; Zoppis, M.; Marventano, I.; Calabrese, E.; Rainone, V.; Nemni, R.; Mancuso, R.; Clerici, M. The NLRP3 and NLRP1 Inflammasomes Are Activated in Alzheimer’s Disease. Mol. Neurodegener. 2016, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, V.; Dye, R.; Pakavathkumar, P.; Foveau, B.; Flores, J.; Hyman, B.; Ghetti, B.; Koller, B.H.; LeBlanc, A.C. Neuronal NLRP1 Inflammasome Activation of Caspase-1 Coordinately Regulates Inflammatory Interleukin-1-Beta Production and Axonal Degeneration-Associated Caspase-6 Activation. Cell Death Differ. 2015, 22, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
- Yaron, J.R.; Gangaraju, S.; Rao, M.Y.; Kong, X.; Zhang, L.; Su, F.; Tian, Y.; Glenn, H.L.; Meldrum, D.R. K(+) Regulates Ca(2+) to Drive Inflammasome Signaling: Dynamic Visualization of Ion Flux in Live Cells. Cell Death Dis. 2015, 6, e1954. [Google Scholar] [CrossRef] [PubMed]
- Halle, A.; Hornung, V.; Petzold, G.C.; Stewart, C.R.; Monks, B.G.; Reinheckel, T.; Fitzgerald, K.A.; Latz, E.; Moore, K.J.; Golenbock, D.T. The NALP3 Inflammasome Is Involved in the Innate Immune Response to Amyloid-β. Nat. Immunol. 2008, 9, 857–865. [Google Scholar] [CrossRef]
- Couturier, J.; Stancu, I.C.; Schakman, O.; Pierrot, N.; Huaux, F.; Kienlen-Campard, P.; Dewachter, I.; Octave, J.N. Activation of Phagocytic Activity in Astrocytes by Reduced Expression of the Inflammasome Component ASC and Its Implication in a Mouse Model of Alzheimer Disease. J. Neuroinflamm. 2016, 13, 20. [Google Scholar] [CrossRef]
- Freeman, L.; Guo, H.; David, C.N.; Brickey, W.J.; Jha, S.; Ting, J.P.Y. NLR Members NLRC4 and NLRP3 Mediate Sterile Inflammasome Activation in Microglia and Astrocytes. J. Exp. Med. 2017, 214, 1351–1370. [Google Scholar] [CrossRef]
- Liu, L.; Chan, C. IPAF Inflammasome Is Involved in Interleukin-1β Production from Astrocytes, Induced by Palmitate; Implications for Alzheimer’s Disease. Neurobiol. Aging 2014, 35, 309–321. [Google Scholar] [CrossRef]
- Gustot, A.; Gallea, J.I.; Sarroukh, R.; Celej, M.S.; Ruysschaert, J.M.; Raussens, V. Amyloid Fibrils Are the Molecular Trigger of Inflammation in Parkinson’s Disease. Biochem. J. 2015, 471, 323–333. [Google Scholar] [CrossRef]
- Codolo, G.; Plotegher, N.; Pozzobon, T.; Brucale, M.; Tessari, I.; Bubacco, L.; de Bernard, M. Triggering of Inflammasome by Aggregated α-Synuclein, an Inflammatory Response in Synucleinopathies. PLoS ONE 2013, 8, e55375. [Google Scholar] [CrossRef] [PubMed]
- Kawana, N.; Yamamoto, Y.; Ishida, T.; Saito, Y.; Konno, H.; Arima, K.; Satoh, J.I. Reactive Astrocytes and Perivascular Macrophages Express NLRP3 Inflammasome in Active Demyelinating Lesions of Multiple Sclerosis and Necrotic Lesions of Neuromyelitis Optica and Cerebral Infarction. Clin. Exp. Neuroimmunol. 2013, 4, 296–304. [Google Scholar] [CrossRef]
- McKenzie, B.A.; Mamik, M.K.; Saito, L.B.; Boghozian, R.; Monaco, M.C.; Major, E.O.; Lu, J.Q.; Branton, W.G.; Power, C. Caspase-1 Inhibition Prevents Glial Inflammasome Activation and Pyroptosis in Models of Multiple Sclerosis. Proc. Natl. Acad. Sci. USA 2018, 115, E6065–E6074. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, M.; Kang, R.; Dai, Y.; Yu, Y.; Gao, F.; Wang, H.; Sun, X.; Li, X.; Li, J.; et al. HMGB1–DNA Complex-Induced Autophagy Limits AIM2 Inflammasome Activation through RAGE. Biochem. Biophys. Res. Commun. 2014, 450, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Tang, X.; Guo, Z.; Cheng, H.; Zheng, X.; Chen, G.; Huang, H.; Wang, W.; Gao, J.; Sheng, Y.; et al. AURKA Facilitates the Psoriasis-Related Inflammation by Impeding Autophagy-Mediated AIM2 Inflammasome Suppression. Immunol. Lett. 2021, 240, 98–105. [Google Scholar] [CrossRef]
- Cao, T.; Yuan, X.; Fang, H.; Chen, J.; Xue, K.; Li, Z.; Dang, E.; Wang, G.; Shao, S. Neutrophil Extracellular Traps Promote Keratinocyte Inflammation via AIM2 Inflammasome and AIM2-XIAP in Psoriasis. Exp. Dermatol. 2023, 32, 368–378. [Google Scholar] [CrossRef]
- Zwicker, S.; Hattinger, E.; Bureik, D.; Batycka-Baran, A.; Schmidt, A.; Gerber, P.A.; Rothenfusser, S.; Gilliet, M.; Ruzicka, T.; Wolf, R. Th17 Micro-Milieu Regulates NLRP1-Dependent Caspase-5 Activity in Skin Autoinflammation. PLoS ONE 2017, 12, e0175153. [Google Scholar] [CrossRef]
- Salskov-Iversen, M.L.; Johansen, C.; Kragballe, K.; Iversen, L. Caspase-5 Expression Is Upregulated in Lesional Psoriatic Skin. J. Investig. Dermatol. 2011, 131, 670–676. [Google Scholar] [CrossRef]
- Ekman, A.K.; Verma, D.; Fredrikson, M.; Bivik, C.; Enerbäck, C. Genetic Variations of NLRP1: Susceptibility in Psoriasis. Br. J. Dermatol 2014, 171, 1517–1520. [Google Scholar] [CrossRef]
- Cho, K.A.; Suh, J.W.; Ho Lee, K.; Kang, J.L.; Woo, S.Y. IL-17 and IL-22 Enhance Skin Inflammation by Stimulating the Secretion of IL-1β by Keratinocytes via the ROS-NLRP3-Caspase-1 Pathway. Int. Immunol. 2012, 24, 147–158. [Google Scholar] [CrossRef]
- Pizarro, T.T.; Michie, M.H.; Bentz, M.; Woraratanadharm, J.; Smith, M.F.; Foley, E.; Moskaluk, C.A.; Bickston, S.J.; Cominelli, F. IL-18, a Novel Immunoregulatory Cytokine, Is Up-Regulated in Crohn’s Disease: Expression and Localization in Intestinal Mucosal Cells. J. Immunol. 1999, 162, 6829–6835. [Google Scholar] [CrossRef] [PubMed]
- Ligumsky, M.; Simon, P.L.; Karmeli, F.; Rachmilewitz, D. Role of Interleukin 1 in Inflammatory Bowel Disease–Enhanced Production during Active Disease. Gut 1990, 31, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Mitsialis, V.; Wall, S.; Liu, P.; Ordovas-Montanes, J.; Parmet, T.; Vukovic, M.; Spencer, D.; Field, M.; McCourt, C.; Toothaker, J.; et al. Single-Cell Analyses of Colon and Blood Reveal Distinct Immune Cell Signatures of Ulcerative Colitis and Crohn’s Disease. Gastroenterology 2020, 159, 591–608.e10. [Google Scholar] [CrossRef] [PubMed]
- Ranson, N.; Veldhuis, M.; Mitchell, B.; Fanning, S.; Cook, A.L.; Kunde, D.; Eri, R. Nod-Like Receptor Pyrin-Containing Protein 6 (NLRP6) Is Up-Regulated in Ileal Crohn’s Disease and Differentially Expressed in Goblet Cells. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 110–112.e8. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Kolahi, A.A.; Hoy, D.; Smith, E.; Bettampadi, D.; Mansournia, M.A.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Moradi-Lakeh, M.; Qorbani, M.; et al. Global, Regional and National Burden of Rheumatoid Arthritis 1990–2017: A Systematic Analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2019, 78, 1463–1471. [Google Scholar] [CrossRef]
- Choulaki, C.; Papadaki, G.; Repa, A.; Kampouraki, E.; Kambas, K.; Ritis, K.; Bertsias, G.; Boumpas, D.T.; Sidiropoulos, P. Enhanced Activity of NLRP3 Inflammasome in Peripheral Blood Cells of Patients with Active Rheumatoid Arthritis. Arthritis Res. Ther. 2015, 17, 257. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Cao, J.; Yu, C.; Yang, Q.; Zhang, Y.; Han, L. Caspase-1 Mediated Interleukin-18 Activation in Neutrophils Promotes the Activity of Rheumatoid Arthritis in a NLRP3 Inflammasome Independent Manner. Joint Bone Spine 2016, 83, 282–289. [Google Scholar] [CrossRef]
- Jansen, J.P.; Buckley, F.; Dejonckheere, F.; Ogale, S. Comparative Efficacy of Biologics as Monotherapy and in Combination with Methotrexate on Patient Reported Outcomes (PROs) in Rheumatoid Arthritis Patients with an Inadequate Response to Conventional DMARDs--a Systematic Review and Network Meta-Analysis. Health Qual. Life Outcomes 2014, 12, 102. [Google Scholar] [CrossRef]
- Sun, R.; Huang, Y.; Zhang, H.; Liu, R. MMP-2, TNF-α and NLRP1 Polymorphisms in Chinese Patients with Ankylosing Spondylitis and Rheumatoid Arthritis. Mol. Biol. Rep. 2013, 40, 6303–6308. [Google Scholar] [CrossRef]
- Keystone, E.C.; Wang, M.M.; Layton, M.; Hollis, S.; McInnes, I.B. Clinical Evaluation of the Efficacy of the P2X7 Purinergic Receptor Antagonist AZD9056 on the Signs and Symptoms of Rheumatoid Arthritis in Patients with Active Disease despite Treatment with Methotrexate or Sulphasalazine. Ann. Rheum. Dis. 2012, 71, 1630–1635. [Google Scholar] [CrossRef]
- Stock, T.C.; Bloom, B.J.; Wei, N.; Ishaq, S.; Park, W.; Wang, X.; Gupta, P.; Mebus, C.A. Efficacy and Safety of CE-224,535, an Antagonist of P2X7 Receptor, in Treatment of Patients with Rheumatoid Arthritis Inadequately Controlled by Methotrexate. J. Rheumatol. 2012, 39, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.; Zhang, J.; Lin, Y.; Chen, W.; Fan, X.; Zhang, D. Pathogenesis and Treatment of Sjogren’s Syndrome: Review and Update. Front. Immunol. 2023, 14, 420. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Deng, F.; Zheng, J.; Yin, J.; Huang, R.; Liu, W.; Lin, Q.; Gao, Y.; Gao, X.; Yu, X.; et al. High Circulating Level of Interleukin-18 in Patients with Primary Sjögren’s Syndrome Is Associated with Disease Activity. Mod. Rheumatol. 2016, 26, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Khalafalla, M.G.; Woods, L.T.; Camden, J.M.; Khan, A.A.; Limesand, K.H.; Petris, M.J.; Erb, L.; Weisman, G.A. P2X7 Receptor Antagonism Prevents IL-1β Release from Salivary Epithelial Cells and Reduces Inflammation in a Mouse Model of Autoimmune Exocrinopathy. J. Biol. Chem. 2017, 292, 16626–16637. [Google Scholar] [CrossRef]
- Xie, B.; Chen, Y.; Zhang, S.; Wu, X.; Zhang, Z.; Peng, Y.; Huang, X. The Expression of P2X7 Receptors on Peripheral Blood Mononuclear Cells in Patients with Primary Sjögren’s Syndrome and Its Correlation with Anxiety and Depression. Clin. Exp. Rheumatol. 2014, 32, 354–360. [Google Scholar]
- Baldini, C.; Santini, E.; Rossi, C.; Donati, V.; Solini, A. The P2X7 Receptor–NLRP3 Inflammasome Complex Predicts the Development of Non-Hodgkin’s Lymphoma in Sjogren’s Syndrome: A Prospective, Observational, Single-Centre Study. J. Intern. Med. 2017, 282, 175–186. [Google Scholar] [CrossRef]
- Fava, A.; Petri, M. Systemic Lupus Erythematosus: Diagnosis and Clinical Management. J. Autoimmun. 2019, 96, 1–13. [Google Scholar] [CrossRef]
- Leffler, J.; Ciacma, K.; Gullstrand, B.; Bengtsson, A.A.; Martin, M.; Blom, A.M. A Subset of Patients with Systemic Lupus Erythematosus Fails to Degrade DNA from Multiple Clinically Relevant Sources. Arthritis Res. Ther. 2015, 17, 1–10. [Google Scholar] [CrossRef]
- Rönnblom, L.; Elkon, K.B. Cytokines as Therapeutic Targets in SLE. Nat. Rev. Rheumatol. 2010, 6, 339–347. [Google Scholar] [CrossRef]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; Loo, G. Inflammasomes in Neuroinflammatory and Neurodegenerative Diseases. EMBO Mol. Med. 2019, 11, e10248. [Google Scholar] [CrossRef]
- Tsai, S.J. Effects of Interleukin-1beta Polymorphisms on Brain Function and Behavior in Healthy and Psychiatric Disease Conditions. Cytokine Growth Factor Rev. 2017, 37, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 Is Activated in Alzheimer’s Disease and Contributes to Pathology in APP/PS1 Mice. Nature 2012, 493, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Simard, A.R.; Soulet, D.; Gowing, G.; Julien, J.P.; Rivest, S. Bone Marrow-Derived Microglia Play a Critical Role in Restricting Senile Plaque Formation in Alzheimer’s Disease. Neuron 2006, 49, 489–502. [Google Scholar] [CrossRef]
- Akama, K.T.; Van Eldik, L.J. Beta-Amyloid Stimulation of Inducible Nitric-Oxide Synthase in Astrocytes Is Interleukin-1beta- and Tumor Necrosis Factor-Alpha (TNFalpha)-Dependent, and Involves a TNFalpha Receptor-Associated Factor- and NFkappaB-Inducing Kinase-Dependent Signaling Mechanism. J. Biol. Chem. 2000, 275, 7918–7924. [Google Scholar]
- Venegas, C.; Kumar, S.; Franklin, B.S.; Dierkes, T.; Brinkschulte, R.; Tejera, D.; Vieira-Saecker, A.; Schwartz, S.; Santarelli, F.; Kummer, M.P.; et al. Microglia-Derived ASC Specks Cross-Seed Amyloid-β in Alzheimer’s Disease. Nature 2017, 552, 355–361. [Google Scholar] [CrossRef]
- Tan, M.S.; Tan, L.; Jiang, T.; Zhu, X.C.; Wang, H.F.; Jia, C.D.; Yu, J.T. Amyloid-β Induces NLRP1-Dependent Neuronal Pyroptosis in Models of Alzheimer’s Disease. Cell Death Dis. 2014, 5, e1382. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, X.; Xu, G.; Zeng, X.; Li, L. Thioredoxin-1 Inhibits Amyloid-Β25-35-Induced Activation of NLRP1/Caspase-1/GSDMD Pyroptotic Pathway in PC12 Cells. Mol. Biol. Rep. 2022, 49, 3445–3452. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, M.; Du, R.H.; Qiao, C.; Jiang, C.Y.; Zhang, K.Z.; Ding, J.H.; Hu, G. MicroRNA-7 Targets Nod-like Receptor Protein 3 Inflammasome to Modulate Neuroinflammation in the Pathogenesis of Parkinson’s Disease. Mol. Neurodegener. 2016, 11, 28. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, L.; Luo, H.; Li, X.; Ruan, Y.; Fan, R.; Si, Z.; Chen, Y.; Li, L.; Liu, Y. The Significance of NLRP Inflammasome in Neuropsychiatric Disorders. Brain Sci. 2022, 12, 1057. [Google Scholar] [CrossRef]
- Wang, W.; Nguyen, L.T.T.; Burlak, C.; Chegini, F.; Guo, F.; Chataway, T.; Ju, S.; Fisher, O.S.; Miller, D.W.; Datta, D.; et al. Caspase-1 Causes Truncation and Aggregation of the Parkinson’s Disease-Associated Protein α-Synuclein. Proc. Natl. Acad. Sci. USA 2016, 113, 9587–9592. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Jedrychowski, M.P.; Moore, C.S.; Cialic, R.; Lanser, A.J.; Gabriely, G.; Koeglsperger, T.; Dake, B.; Wu, P.M.; Doykan, C.E.; et al. Identification of a Unique TGF-β–Dependent Molecular and Functional Signature in Microglia. Nat. Neurosci. 2013, 17, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Imani, D.; Azimi, A.; Salehi, Z.; Rezaei, N.; Emamnejad, R.; Sadr, M.; Izad, M. Association of Nod-like Receptor Protein-3 Single Nucleotide Gene Polymorphisms and Expression with the Susceptibility to Relapsing-Remitting Multiple Sclerosis. Int. J. Immunogenet. 2018, 45, 329–336. [Google Scholar] [CrossRef]
- Soares, J.L.; Oliveira, E.M.; Pontillo, A. Variants in NLRP3 and NLRC4 Inflammasome Associate with Susceptibility and Severity of Multiple Sclerosis. Mult. Scler. Relat. Disord. 2019, 29, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Vidmar, L.; Maver, A.; Drulović, J.; Sepčić, J.; Novaković, I.; Ristič, S.; Šega, S.; Peterlin, B. Multiple Sclerosis Patients Carry an Increased Burden of Exceedingly Rare Genetic Variants in the Inflammasome Regulatory Genes. Sci. Rep. 2019, 9, 9171. [Google Scholar] [CrossRef]
- Papagoras, C.; Lampropoulou, V.; Mavraki, E.; Chrysanthopoulou, A.; Deftereos, S.; Aróstegui, J.I.; Skendros, P.; Ritis, K. Multiple Sclerosis in a Patient with Cryopyrin-Associated Autoinflammatory Syndrome: Evidence That Autoinflammation Is the Common Link. Clin. Immunol. 2021, 227, 108750. [Google Scholar] [CrossRef]
- Ali, S.B.; Chaudhuri, D.; Field, D.; Hissaria, P. Familial Cold Autoinflammatory Syndrome and Multiple Sclerosis. Clin. Exp. Neuroimmunol. 2022, 13, 80–83. [Google Scholar] [CrossRef]
- Maver, A.; Lavtar, P.; Ristić, S.; Stopinšek, S.; Simčič, S.; Hočevar, K.; Sepčić, J.; Drulović, J.; Pekmezović, T.; Novaković, I.; et al. Identification of Rare Genetic Variation of NLRP1 Gene in Familial Multiple Sclerosis. Sci. Rep. 2017, 7, 3715. [Google Scholar] [CrossRef]
- Bernales, C.Q.; Encarnacion, M.; Criscuoli, M.G.; Yee, I.M.; Traboulsee, A.L.; Sadovnick, A.D.; Vilariño-Güell, C. Analysis of NOD-like Receptor NLRP1 in Multiple Sclerosis Families. Immunogenetics 2018, 70, 205–207. [Google Scholar] [CrossRef]
- Placha, D.; Jampilek, J. Chronic Inflammatory Diseases, Anti-Inflammatory Agents and Their Delivery Nanosystems. Pharmaceutics 2021, 13, 64. [Google Scholar] [CrossRef]
- Dinarello, C.A.; van der Meer, J.W.M. Treating Inflammation by Blocking Interleukin-1 in Humans. Semin. Immunol. 2013, 25, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Colantuoni, M.; Jofra Hernandez, R.; Pettinato, E.; Basso-Ricci, L.; Magnani, L.; Andolfi, G.; Rigamonti, C.; Finardi, A.; Romeo, V.; Soldi, M.; et al. Constitutive IL-1RA Production by Modified Immune Cells Protects against IL-1-Mediated Inflammatory Disorders. Sci. Transl. Med. 2023, 15, eade3856. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.C.; Hill, J.R.; Day, C.J.; Zamoshnikova, A.; Boucher, D.; Massey, N.L.; Chitty, J.L.; Fraser, J.A.; Jennings, M.P.; Robertson, A.A.B.; et al. MCC950 Directly Targets the NLRP3 ATP-Hydrolysis Motif for Inflammasome Inhibition. Nat. Chem. Biol. 2019, 15, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Nodthera. Available online: https://www.nodthera.com/news/nodthera-announces-positive-phase-1-study-readouts-for-the-nlrp3-inflammasome-inhibitors-nt-0796-and-nt-0249/ (accessed on 30 May 2023).
- Sharma, B.; Satija, G.; Madan, A.; Garg, M.; Alam, M.M.; Shaquiquzzaman, M.; Khanna, S.; Tiwari, P.; Parvez, S.; Iqubal, A.; et al. Role of NLRP3 Inflammasome and Its Inhibitors as Emerging Therapeutic Drug Candidate for Alzheimer’s Disease: A Review of Mechanism of Activation, Regulation, and Inhibition. Inflammation 2023, 46, 56–87. [Google Scholar] [CrossRef]
- Bertinaria, M.; Gastaldi, S.; Marini, E.; Giorgis, M. Development of Covalent NLRP3 Inflammasome Inhibitors: Chemistry and Biological Activity. Arch. Biochem. Biophys. 2019, 670, 116–139. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.F.; Yang, X.; Xiong, L.; Wu, M.; Chen, S.; Xu, H.; Gong, Y.; Zhang, L.; Zhang, Q.; Zhang, X. Exploring the Mechanism of Action of Dapansutrile in the Treatment of Gouty Arthritis Based on Molecular Docking and Molecular Dynamics. Front. Physiol. 2022, 13, 990469. [Google Scholar] [CrossRef] [PubMed]


| Gene | Disease | Mutations Linked to Disease Phenotype |
|---|---|---|
| NLRP1 | NAIAD | A59P, R726W, L813P, P1214R and L1214L |
| NLRP3 | FCAS | C259W, L305P, L353P, T436A, A439V, E525K, Y563N, E627G, M659K |
| MWS | R170S, R260L, L264V, D303A, E311K, H312P, R325W, T348M, A352V, K355T, A439T, F523C, E567K, E567A, G569R | |
| NOMID | R260P, V262A, L264F, L264H, L264R, D303H, E304K, G307S, G307V, F309S, G326E, A352T, E354D, H358R, A374D, T405P, M406V, M406I, T436P, T436N, A439P, F443L, N477K, F523Y, E525V, F566L, K568N, G569A, Y570C, Y570F, L571F | |
| NLRP12 | FCAS2 | R284X, D294E, H304Y, W408X, S578G, L591M, L710P, R753H, N940S, S979G, R754H, F402L, G448A |
| NLRC4 | FCAS4 | H443P, T177A |
| AIFEC | G172S, T177S, T337S, T337N, L339P, V341L, V341A, H443P, H443Q, W655C, Q657L, delexon5, Q880E | |
| MEFV | FMF | K25R, R39G, E84K, A89T, Q97X, E167D, 606_621dup, K224del, S242R C > G, T267I, P313H, R354W, L372P, L384P, D389V, L396F, E403K, Y471X, F479L, R501C, S503C, 1611-1G > C, S650Y, G668R, M680L, M680V, M680IGA, G687D, Y688F, Y688X, I692DEL, M694V, M694L, M694DEL, M694K, M694I, K695N, V726A, F743Y, Q753H, R761H, N766H, P769A, Q778Sfs*4 |
| PAAND | S242G, S242R C > A, E244K, S363N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulté, D.; Rigamonti, C.; Romano, A.; Mortellaro, A. Inflammasomes: Mechanisms of Action and Involvement in Human Diseases. Cells 2023, 12, 1766. https://doi.org/10.3390/cells12131766
Bulté D, Rigamonti C, Romano A, Mortellaro A. Inflammasomes: Mechanisms of Action and Involvement in Human Diseases. Cells. 2023; 12(13):1766. https://doi.org/10.3390/cells12131766
Chicago/Turabian StyleBulté, Dimitri, Chiara Rigamonti, Alessandro Romano, and Alessandra Mortellaro. 2023. "Inflammasomes: Mechanisms of Action and Involvement in Human Diseases" Cells 12, no. 13: 1766. https://doi.org/10.3390/cells12131766
APA StyleBulté, D., Rigamonti, C., Romano, A., & Mortellaro, A. (2023). Inflammasomes: Mechanisms of Action and Involvement in Human Diseases. Cells, 12(13), 1766. https://doi.org/10.3390/cells12131766






