Xanol Promotes Apoptosis and Autophagy and Inhibits Necroptosis and Metastasis via the Inhibition of AKT Signaling in Human Oral Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and General Experimental Procedures
2.2. Xanol Compound and Stock Solution
2.3. Cell Culture
2.4. 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium Bromide (MTT) Assay
2.5. Western Blotting Analysis
2.6. Apoptosis Assay
2.7. Immunofluorescence Assay
2.8. Autophagosome Detection Assay
2.9. Cell Migration and Invasion Assays
2.10. Soft Agar Colony Formation Assay
2.11. Statistical Analysis
3. Results
3.1. Xanol Isolated from MeOH Extract of Dried A. keiskei Inhibits Cell Proliferation in Human OSCC
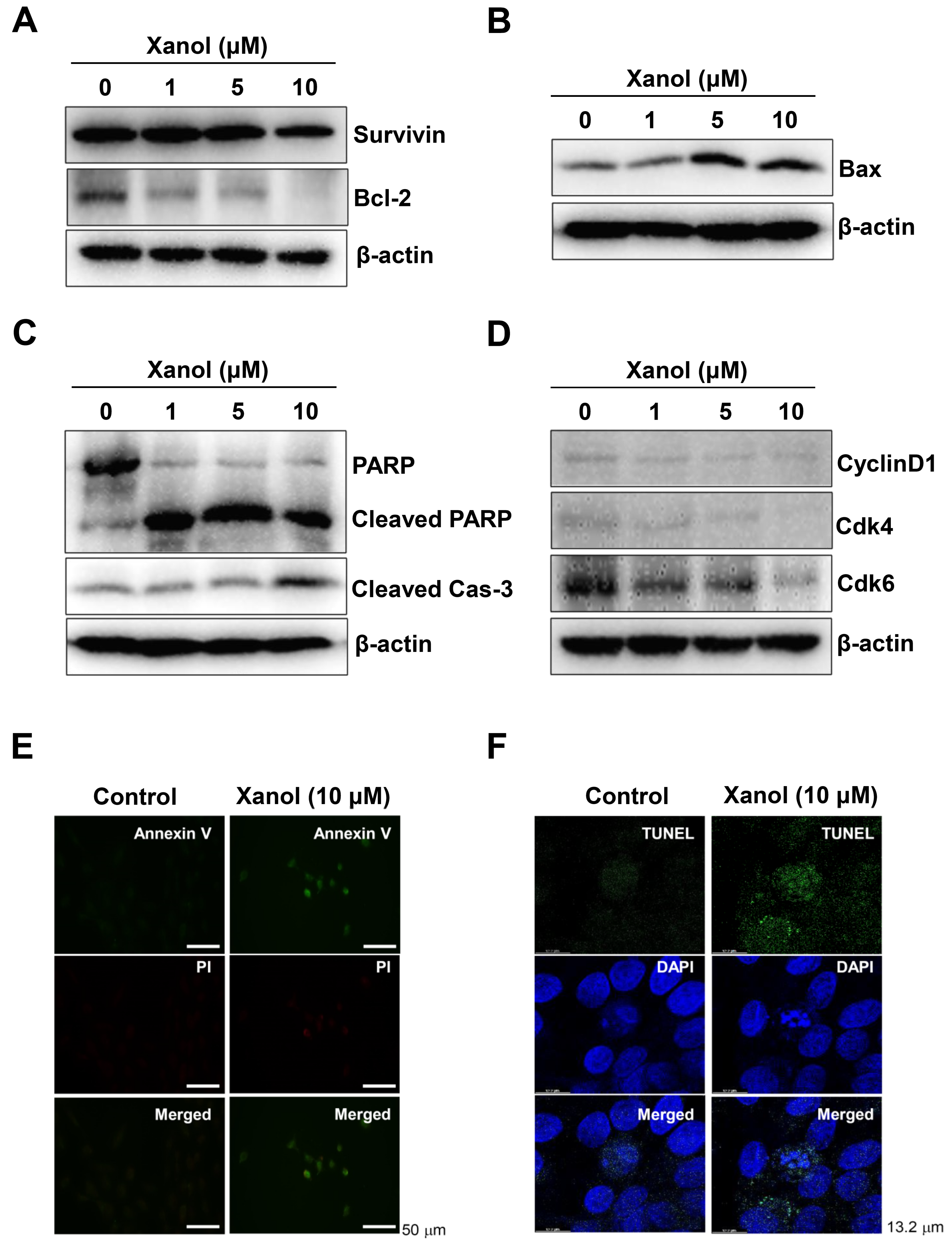
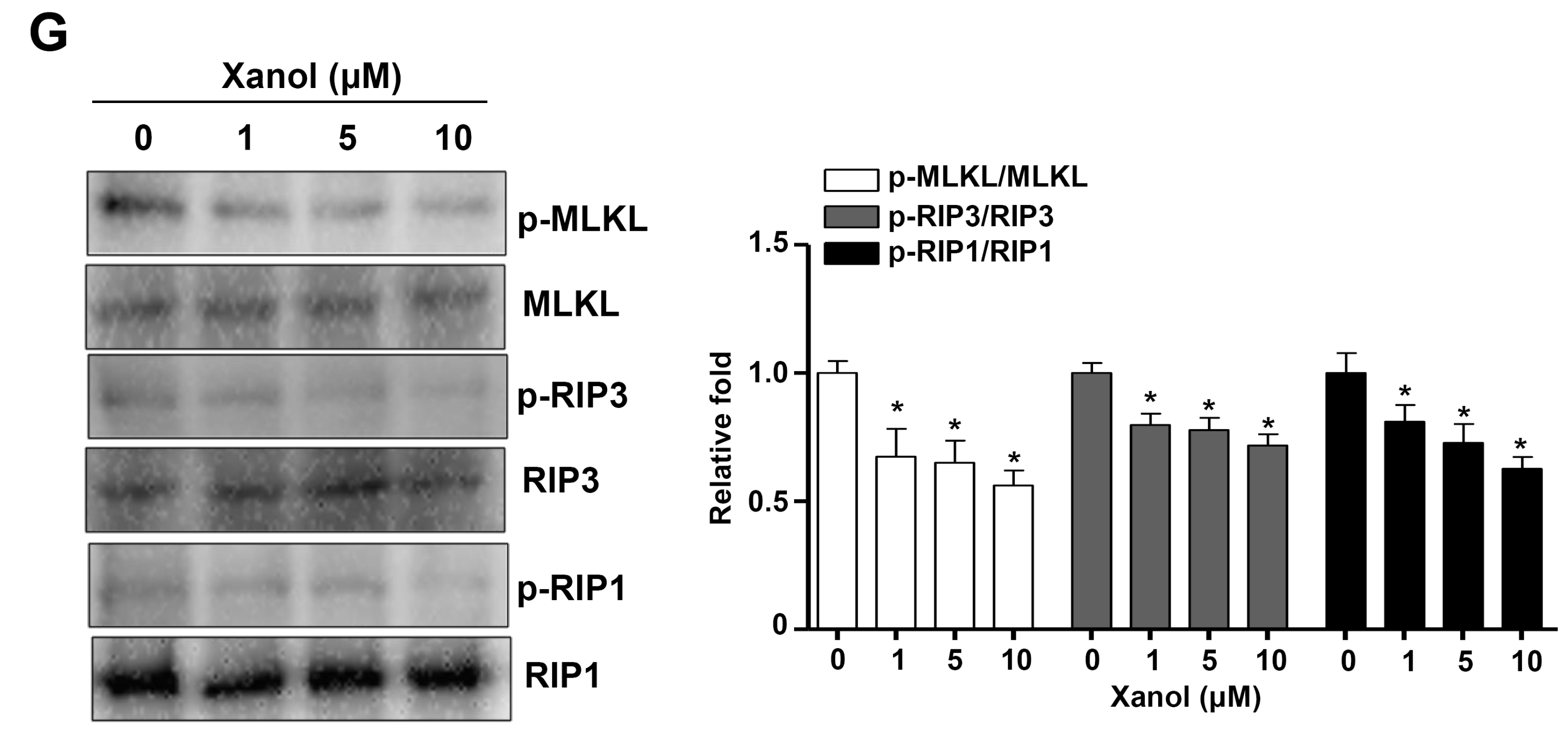
3.2. Xanol Triggers Apoptotic Cell Death and Inhibits Necroptotic Cell Death in Human OSCC
3.3. Xanol Inhibits the PI3K-AKT-mTOR-p70S6K Pathway and Induces Autophagy in Human OSCC
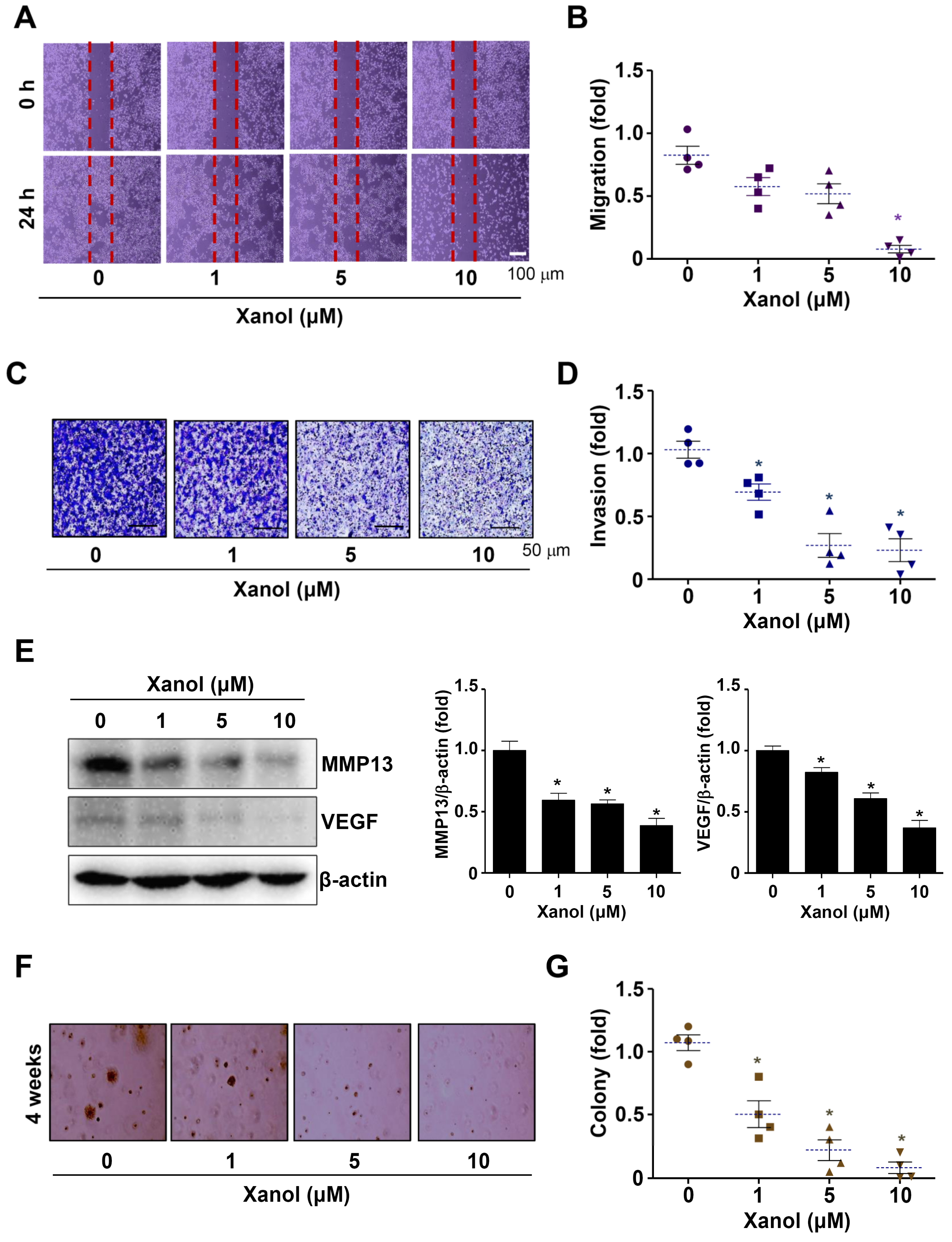
3.4. Xanol Suppresses the Migration, Invasion, and Tumorigenicity of Human OSCC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPLC | high-performance liquid chromatography |
| LC3A/B | microtubule-associated protein light chain 3A/B |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide |
| OSCC | oral squamous cell carcinoma |
| xanol | xanthoangelol |
References
- Flugge, T.; Gaudin, R.; Sabatakakis, A.; Troltzsch, D.; Heiland, M.; van Nistelrooij, N.; Vinayahalingam, S. Detection of oral squamous cell carcinoma in clinical photographs using a vision transformer. Sci. Rep. 2023, 13, 2296. [Google Scholar] [CrossRef]
- Chinn, S.B.; Myers, J.N. Oral Cavity Carcinoma: Current Management, Controversies, and Future Directions. J. Clin. Oncol. 2015, 33, 3269–3276. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Liang, J.; Yang, Y.; Liang, H.; Jia, H.; Li, D. Current Trends of Targeted Drug Delivery for Oral Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 618931. [Google Scholar] [CrossRef]
- Almangush, A.; Leivo, I.; Makitie, A.A. Biomarkers for Immunotherapy of Oral Squamous Cell Carcinoma: Current Status and Challenges. Front. Oncol. 2021, 11, 616629. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Pang, X.; Hua, P.; Gao, X.; Li, Q.; Li, Z. Simultaneous Optimization of Ultrasound-Assisted Extraction for Flavonoids and Antioxidant Activity of Angelica Keiskei using Response Surface Methodology (RSM). Molecules 2019, 24, 3461. [Google Scholar] [CrossRef] [Green Version]
- Kil, Y.S.; Pham, S.T.; Seo, E.K.; Jafari, M. Angelica Keiskei, an emerging medicinal herb with various bioactive constituents and biological activities. Arch. Pharmacal Res. 2017, 40, 655–675. [Google Scholar] [CrossRef]
- Syed, Z.; Shal, B.; Azhar, A.; Amanat, S.; Khan, A.; Ali, H.; Kil, Y.S.; Seo, E.K.; Khan, S. Pharmacological mechanism of xanthoangelol underlying Nrf-2/TRPV1 and anti-apoptotic pathway against scopolamine-induced amnesia in mice. Biomed. Pharmacother. 2022, 150, 113073. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Taniguchi, M.; Baba, K. Antitumor and antimetastatic activities of 4-hydroxyderricin isolated from Angelica Keiskei roots. Planta Med. 2004, 70, 211–219. [Google Scholar] [PubMed]
- Kimura, Y.; Baba, K. Antitumor and antimetastatic activities of Angelica Keiskei roots, part 1: Isolation of an active substance, xanthoangelol. Int. J. Cancer 2003, 106, 429–437. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, L.; Liu, H.; Yuan, Y.; Zhang, Q.; Wu, M.; Wang, L.; Wang, H.; Liu, Z.; Yu, P. 3′-Geranyl-mono-substituted chalcone Xanthoangelovl induces apoptosis in human leukemia K562 cells via activation of mitochondrial pathway. Chem. Biol. Interact. 2017, 261, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Enoki, T.; Ohnogi, H.; Nagamine, K.; Kudo, Y.; Sugiyama, K.; Tanabe, M.; Kobayashi, E.; Sagawa, H.; Kato, I. Antidiabetic activities of chalcones isolated from a Japanese Herb, Angelica Keiskei. J. Agric. Food Chem. 2007, 55, 6013–6017. [Google Scholar] [CrossRef]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Samukawa, Y.; Yamashita, Y.; Ashida, H. 4-Hydroxyderricin and xanthoangelol isolated from Angelica Keiskei prevent dexamethasone-induced muscle loss. Food Funct. 2020, 11, 5498–5512. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.; Gao, C.; Huang, Y. Xanthoangelol alleviates cerebral ischemia reperfusion injury in rats. Anat. Rec. 2021, 304, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Park, J.E.; Lee, J.Y.; Park, K.R. Latifolin, a Natural Flavonoid, Isolated from the Heartwood of Dalbergia odorifera Induces Bioactivities through Apoptosis, Autophagy, and Necroptosis in Human Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 13629. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Kim, E.C.; Hong, J.T.; Yun, H.M. Dysregulation of 5-hydroxytryptamine 6 receptor accelerates maturation of bone-resorbing osteoclasts and induces bone loss. Theranostics 2018, 8, 3087–3098. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Yun, H.M.; Hong, J.T. G721-0282 inhibits cell growth and induces apoptosis in human osteosarcoma through down-regulation of the STAT3 pathway. Int. J. Biol. Sci. 2020, 16, 330–341. [Google Scholar] [CrossRef] [Green Version]
- Jayasinghe, L.; Balasooriya, B.A.; Padmini, W.C.; Hara, N.; Fujimoto, Y. Geranyl chalcone derivatives with antifungal and radical scavenging properties from the leaves of Artocarpus nobilis. Phytochemistry 2004, 65, 1287–1290. [Google Scholar] [CrossRef]
- Simpson, D.R.; Mell, L.K.; Cohen, E.E. Targeting the PI3K/AKT/mTOR pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2015, 51, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Harsha, C.; Banik, K.; Ang, H.L.; Girisa, S.; Vikkurthi, R.; Parama, D.; Rana, V.; Shabnam, B.; Khatoon, E.; Kumar, A.P.; et al. Targeting AKT/mTOR in Oral Cancer: Mechanisms and Advances in Clinical Trials. Int. J. Mol. Sci. 2020, 21, 3285. [Google Scholar] [CrossRef]
- Almangush, A.; Heikkinen, I.; Makitie, A.A.; Coletta, R.D.; Laara, E.; Leivo, I.; Salo, T. Prognostic biomarkers for oral tongue squamous cell carcinoma: A systematic review and meta-analysis. Br. J. Cancer 2017, 117, 856–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, Z.; Cheng, B.; Tao, X. Epithelial-to-mesenchymal transition in oral squamous cell carcinoma: Challenges and opportunities. Int. J. Cancer 2021, 148, 1548–1561. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J.; de Wit, J.G.; Voskuil, F.J.; Witjes, M.J.H. Improving oral cavity cancer diagnosis and treatment with fluorescence molecular imaging. Oral Dis. 2021, 27, 21–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [Green Version]
- Tewari, M.; Quan, L.T.; O’Rourke, K.; Desnoyers, S.; Zeng, Z.; Beidler, D.R.; Poirier, G.G.; Salvesen, G.S.; Dixit, V.M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell 1995, 81, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.T.; Moles, R.; Chaib-Mezrag, H.; Nicot, C. Small PARP inhibitor PJ-34 induces cell cycle arrest and apoptosis of adult T-cell leukemia cells. J. Hematol. Oncol. 2015, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Huang, S.; Shen, Z. Biomarkers for the detection of necroptosis. Cell. Mol. Life Sci. 2016, 73, 2177–2181. [Google Scholar] [CrossRef]
- Seo, J.; Nam, Y.W.; Kim, S.; Oh, D.B.; Song, J. Necroptosis molecular mechanisms: Recent findings regarding novel necroptosis regulators. Exp. Mol. Med. 2021, 53, 1007–1017. [Google Scholar] [CrossRef]
- Tai, Y.L.; Chen, L.C.; Shen, T.L. Emerging roles of focal adhesion kinase in cancer. BioMed Res. Int. 2015, 2015, 690690. [Google Scholar] [CrossRef] [Green Version]
- Xi, W.H.; Yang, L.Y.; Cao, Z.Y.; Qian, Y. Tivantinib (ARQ-197) exhibits anti-tumor activity with down-regulation of FAK in oral squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2015, 457, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Vander Broek, R.; Mohan, S.; Eytan, D.F.; Chen, Z.; Van Waes, C. The PI3K/Akt/mTOR axis in head and neck cancer: Functions, aberrations, cross-talk, and therapies. Oral Dis. 2015, 21, 815–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, Y.; Kanzawa, T.; Sawaya, R.; Kondo, S. The role of autophagy in cancer development and response to therapy. Nat. Rev. Cancer 2005, 5, 726–734. [Google Scholar] [CrossRef]
- Xu, Z.; Han, X.; Ou, D.; Liu, T.; Li, Z.; Jiang, G.; Liu, J.; Zhang, J. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl. Microbiol. Biotechnol. 2020, 104, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Hsieh, M.J.; Chen, P.N.; Weng, C.J.; Yang, S.F.; Lin, C.W. Erianin Induces Apoptosis and Autophagy in Oral Squamous Cell Carcinoma Cells. Am. J. Chin. Med. 2020, 48, 183–200. [Google Scholar] [CrossRef]
- Levine, B. Cell biology: Autophagy and cancer. Nature 2007, 446, 745–747. [Google Scholar] [CrossRef]
- Marino, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Bendas, G.; Borsig, L. Cancer cell adhesion and metastasis: Selectins, integrins, and the inhibitory potential of heparins. Int. J. Cell Biol. 2012, 2012, 676731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.Y.; Liu, J.F.; Tsai, H.C.; Tzeng, H.E.; Hsieh, T.H.; Wang, M.; Lin, Y.F.; Lu, C.C.; Lien, M.Y.; Tang, C.H. Interleukin-11/gp130 upregulates MMP-13 expression and cell migration in OSCC by activating PI3K/Akt and AP-1 signaling. J. Cell Physiol. 2022, 237, 4551–4562. [Google Scholar] [CrossRef]
- Shibuya, C.M.; Tjioe, K.C.; Oliveira, S.H.P.; Bernabe, D.G. Propranolol inhibits cell viability and expression of the pro-tumorigenic proteins Akt, NF-kB, and VEGF in oral squamous cell carcinoma. Arch. Oral Biol. 2022, 136, 105383. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, H.-M.; Kim, B.; Kim, S.H.; Kwon, S.-H.; Park, K.-R. Xanol Promotes Apoptosis and Autophagy and Inhibits Necroptosis and Metastasis via the Inhibition of AKT Signaling in Human Oral Squamous Cell Carcinoma. Cells 2023, 12, 1768. https://doi.org/10.3390/cells12131768
Yun H-M, Kim B, Kim SH, Kwon S-H, Park K-R. Xanol Promotes Apoptosis and Autophagy and Inhibits Necroptosis and Metastasis via the Inhibition of AKT Signaling in Human Oral Squamous Cell Carcinoma. Cells. 2023; 12(13):1768. https://doi.org/10.3390/cells12131768
Chicago/Turabian StyleYun, Hyung-Mun, Bomi Kim, Soo Hyun Kim, Seung-Hae Kwon, and Kyung-Ran Park. 2023. "Xanol Promotes Apoptosis and Autophagy and Inhibits Necroptosis and Metastasis via the Inhibition of AKT Signaling in Human Oral Squamous Cell Carcinoma" Cells 12, no. 13: 1768. https://doi.org/10.3390/cells12131768






