Pattern Distribution of Connexins in the Ortho- and Parakeratinized Epithelium of the Lingual Mucosa in Birds
Abstract
1. Introduction
2. Materials and Methods
3. Results
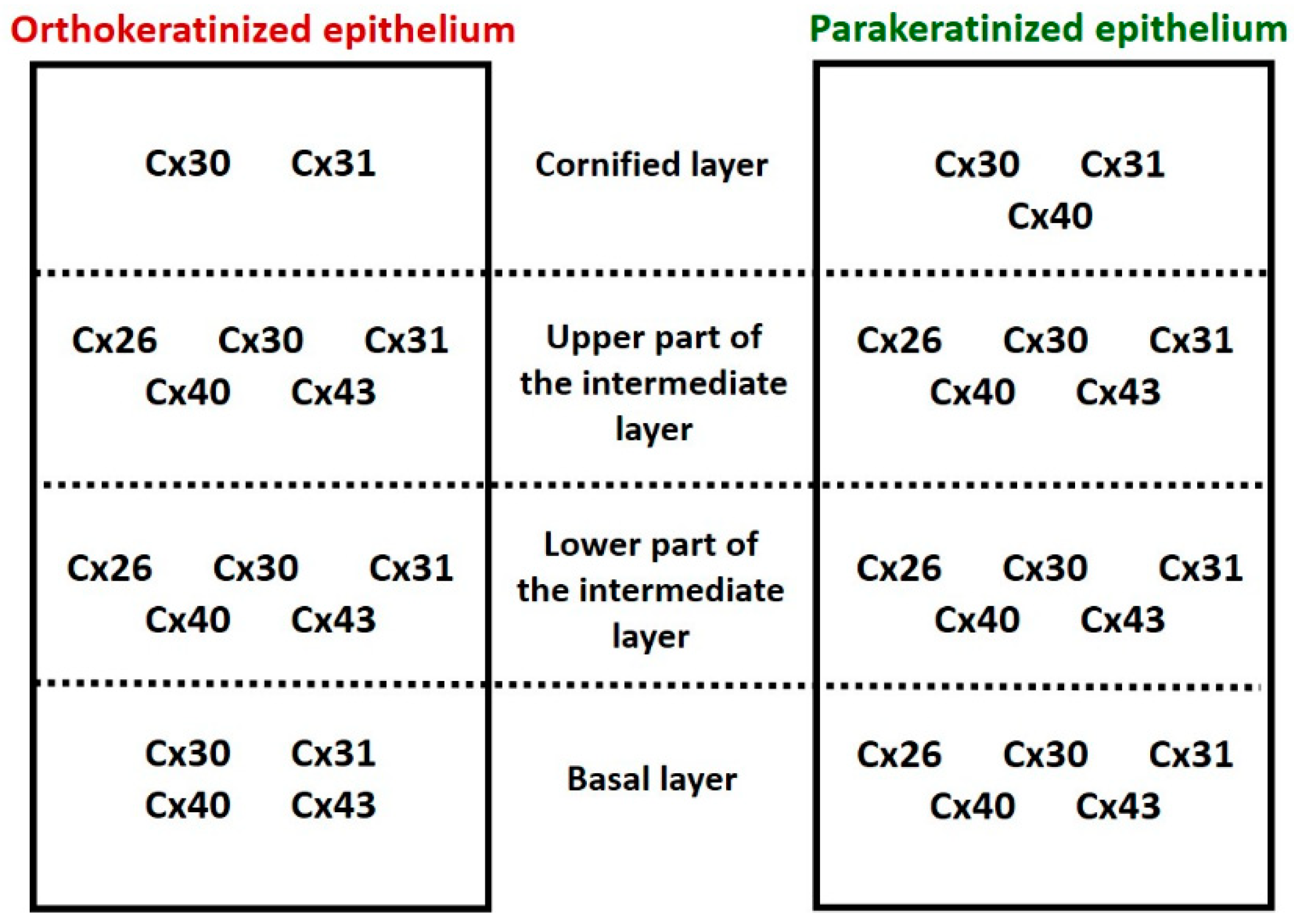
3.1. Immunohistochemical Analysis
3.1.1. Cx26 Analysis
3.1.2. Cx30 Analysis
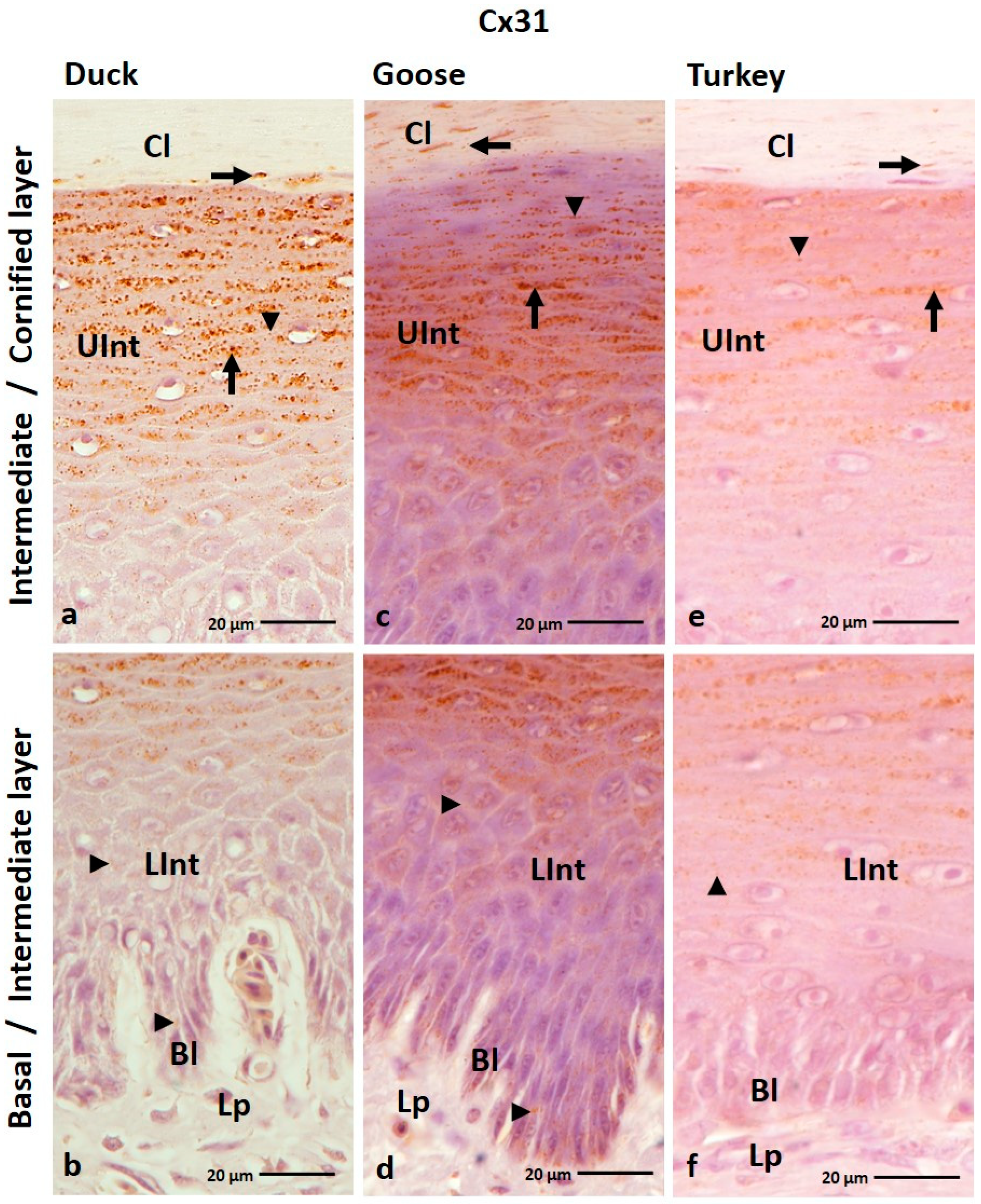
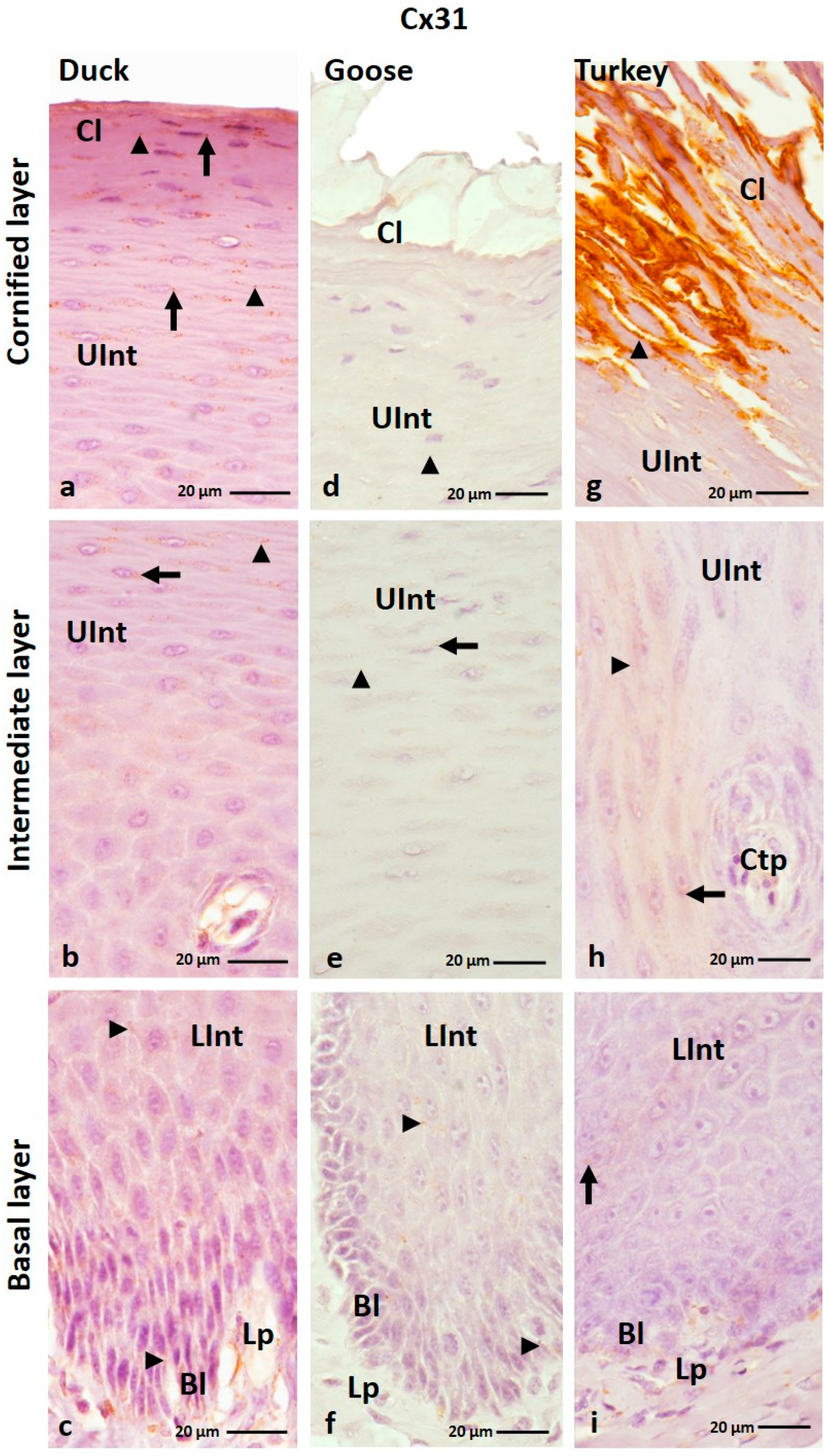
3.1.3. Cx31 Analysis
3.1.4. Cx40 Analysis
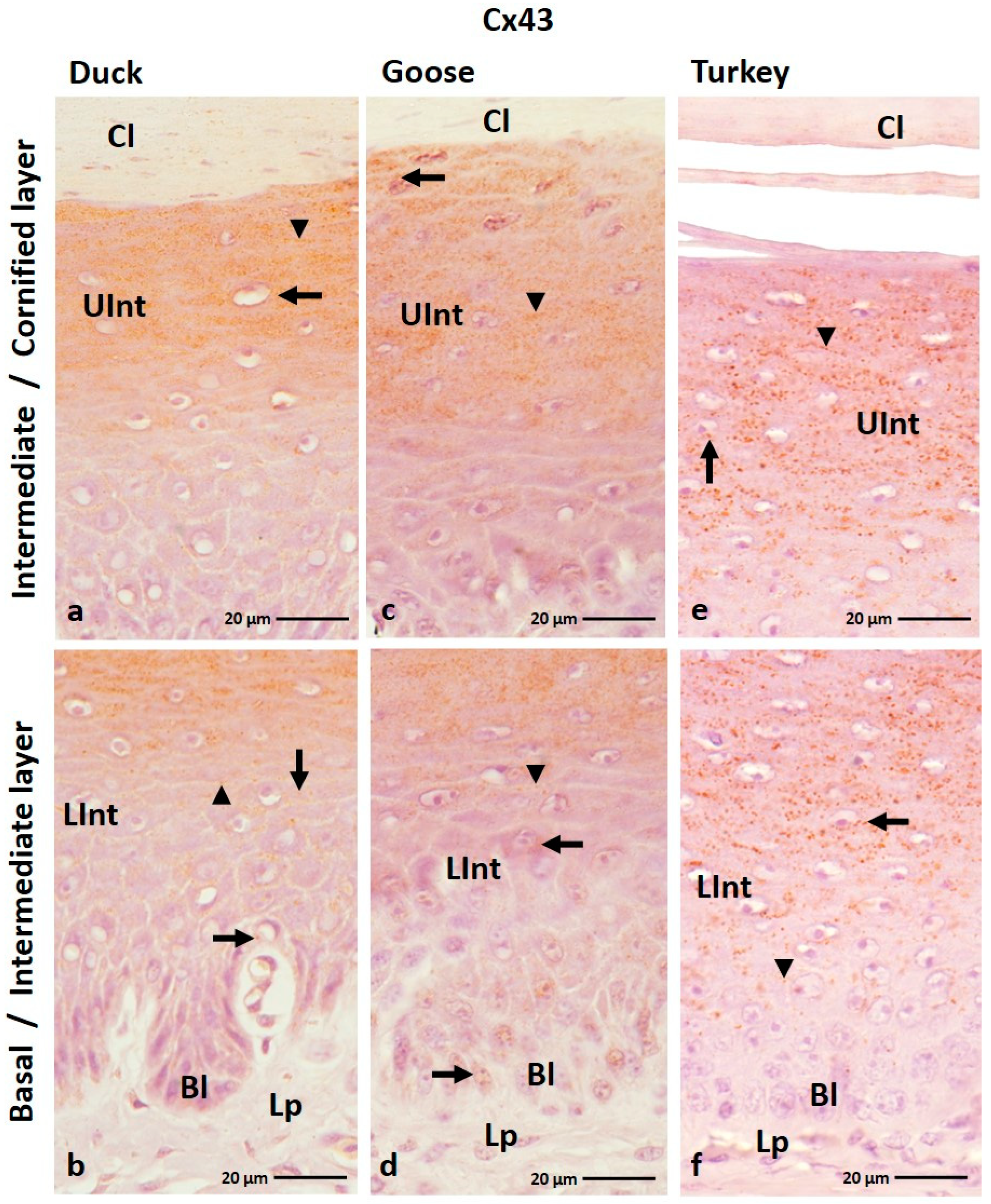
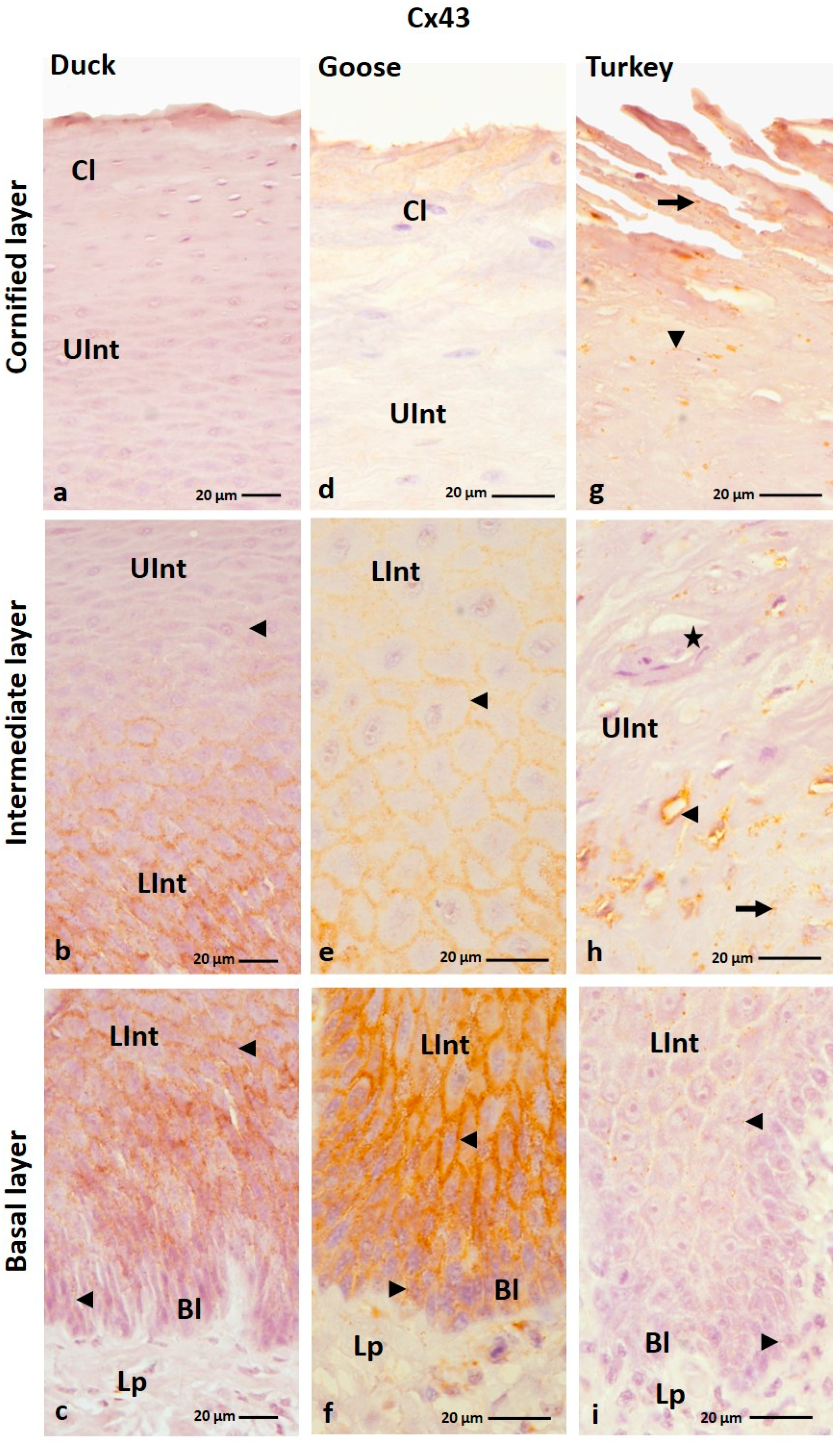
3.1.5. Cx43 Analysis
4. Discussion
4.1. The Pattern of Connexin Distribution in the Ortho- and Parakeratinized Epithelium of the Tongue of the Studied Bird Species
4.2. The Process of Cornification of the Ortho- and Parakeratinized Epithelium
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Risek, B.; Klier, F.G.; Gilula, N.B. Multiple gap junction genes are utilized during rat skin and hair development. Development 1992, 116, 639–651. [Google Scholar] [CrossRef]
- Brissette, J.L.; Kumar, N.M.; Gilula, N.B.; Hall, J.E.; Dotto, G.P. Switch in gap junction protein expression is associated with selective changes in junctional permeability during keratinocyte differentiation. Proc. Natl. Acad. Sci. USA 1994, 91, 6453–6457. [Google Scholar] [CrossRef]
- Dahl, E.; Winterhager, E.; Reuss, B.; Traub, O.; Butterweck, A.; Willecke, K. Expression of the gap junction proteins connexin 31 and connexin 43 correlates with communication compartments in extraembryonic tissues and in the gastrulating mouse embryo, respectively. J. Cell Sci. 1996, 109, 191–197. [Google Scholar] [CrossRef]
- Scott, C.A.; Tattersall, D.; O’Toole, E.A.; Kelsell, D.P. Connexins in epidermal homeostasis and skin disease. Biochim. et Biophys. Acta 2012, 1818, 1952–1961. [Google Scholar] [CrossRef]
- Evans, W.H.; Martin, P.E. Gap junctions: Structure and function (Review). Mol. Membr. Biol. 2002, 19, 121–136. [Google Scholar] [CrossRef]
- Kamibayashi, Y.; Oyamada, M.; Oyamada, Y.; Mori, M. Expression of gap junction proteins connexin 26 and 43 is modulated during differentiation of keratinocytes in newborn mouse epidermis. J. Investig. Dermatol. 1993, 101, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, W.R. Junctional intercellular communication and the control of growth. Biochim. Biophys. Acta 1979, 560, 1–65. [Google Scholar] [CrossRef] [PubMed]
- Bevans, C.G.; Harris, A.L. Direct high affinity modulation of connexin channel activity by cyclic nucleotides. J. Biol. Chem. 1999, 274, 3720–3725. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, G.S.; Valiunas, V.; Brink, P.R. Selective permeability of gap junction channels. Biochim. Biophys. Acta 2004, 1662, 96–101. [Google Scholar] [CrossRef]
- Bedner, P.; Niessen, H.; Odermatt, B.; Kretz, M.; Willecke, K.; Harz, H. Selective Permeability of Different Connexin Channels to the Second Messenger Cyclic AMP. J. Biol. Chem. 2006, 281, 6673–6681. [Google Scholar] [CrossRef]
- Segretain, D.; Falk, M.M. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim. Biophys. Acta 2004, 1662, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Z.; Veenstra, R.D. Monovalent ion selectivity sequences of the rat connexin43 gap junction channel. J. Gen. Physiol. 1997, 109, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.Q.; Nicholson, B.J. Size selectivity between gap junction channels composed of different connexins. Cell Commun. Adhes. 2001, 8, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Nakagawa, S.; Suga, M.; Yamashita, E.; Oshima, A.; Fujiyoshi, Y.; Tsukihara, T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature 2009, 458, 597–602. [Google Scholar] [CrossRef]
- Laird, D.W. Life cycle of connexins in health and disease. Biochem. J. 2006, 394, 527–543. [Google Scholar] [CrossRef]
- Thomas, T.; Jordan, K.; Simek, J.; Shao, Q.; Jedeszko, C.; Walton, P.; Laird, D.W. Mechanisms of Cx43 and Cx26 transport to the plasma membrane and gap junction regeneration. J. Cell Sci. 2005, 118, 4451–4462. [Google Scholar] [CrossRef]
- Musil, L.S.; Goodenough, D.A. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell 1993, 74, 1065–1077. [Google Scholar] [CrossRef]
- Koval, M.; Harley, J.E.; Hick, E.; Steinberg, T.H. Connexin46 is retained as monomers in a trans-Golgi compartment of osteoblastic cells. J. Cell Biol. 1997, 137, 847–857. [Google Scholar] [CrossRef]
- Fallon, R.F.; Goodenough, D.A. Five-hour half-life of mouse liver gap-junction protein. J. Cell Biol. 1981, 90, 521–526. [Google Scholar] [CrossRef]
- Beardslee, M.A.; Laing, J.G.; Beyer, E.C.; Saffitz, J.E. Rapid turnover of connexin43 in the adult rat heart. Circ. Res. 1998, 83, 629–635. [Google Scholar] [CrossRef]
- Söhl, G.; Willecke, K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004, 62, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.M.; Gilula, N.B. The gap junction communication channel. Cell 1996, 84, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Eiberger, J.; Degen, J.; Romualdi, A.; Deutsch, U.; Willecke, K.; Söhl, G. Connexin genes in the mouse and human genome. Cell Commun. Adhes. 2001, 8, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Salomon, D.; Masgrau, E.; Vischer, S.; Ullrich, S.; Dupont, E.; Sappino, P.; Saurat, J.-H.; Medat, P. Topography of Mammalian Connexins in Human Skin. J. Investig. Dermatol. 1994, 103, 240–247. [Google Scholar] [CrossRef]
- Saitoh, M.; Oyamada, M.; Oyamada, Y.; Kaku, T.; Mori, M. Changes in the expression of gap junction proteins (connexins) in hamster tongue epithelium during wound healing and carcinogenesis. Carcinogenesis 1997, 18, 1319–1328. [Google Scholar] [CrossRef]
- Di, W.L.; Rugg, E.L.; Leigh, I.M.; Kelsell, D.P. Multiple epidermal connexins are expressed in different keratinocyte subpopulations including connexin 31. J. Investig. Dermatol. 2001, 117, 958–964. [Google Scholar] [CrossRef]
- Goliger, J.A.; Paul, D.L. Expression of gap junction proteins Cx26, Cx31.1, Cx37, and Cx43 in developing and mature rat epidermis. Dev. Dyn. 1994, 200, 1–13. [Google Scholar] [CrossRef]
- Lucke, T.; Choudhry, R.; Thom, R.; Selmer, I.S.; Burden, A.D.; Hodgins, M.B. Upregulation of connexin 26 is a feature of keratinocyte differentiation in hyperproliferative epidermis, vaginal epithelium, and buccal epithelium. J. Investig. Dermatol. 1999, 112, 354–361. [Google Scholar] [CrossRef]
- Kretz, M.; Maass, K.; Willecke, K. Expression and function of connexins in the epidermis, analyzed with transgenic mouse mutants. Eur. J. Cell Biol. 2004, 83, 647–654. [Google Scholar] [CrossRef]
- Davis, N.G.; Phillips, A.; Becker, D.L. Connexin dynamics in the privileged wound healing of the buccal mucosa. Wound Repair. Regen. 2013, 21, 571–578. [Google Scholar] [CrossRef]
- Meyer, W.; Oberthuer, A.; Ngezahayo, A.; Neumann, U.; Jacob, R. Immunohistochemical demonstration of connexins in the developing feather follicle of the chicken. Acta Histochem. 2014, 116, 639–645. [Google Scholar] [CrossRef]
- Li, A.; Cho, J.H.; Reid, B.; Tseng, C.C.; He, L.; Tan, P.; Yeh, C.Y.; Wu, P.; Li, Y.; Widelitz, R.B.; et al. Calcium oscillations coordinate feather mesenchymal cell movement by SHH dependent modulation of gap junction networks. Nat. Commun. 2018, 9, 537. [Google Scholar] [CrossRef]
- Iwasaki, S.; Asami, T.; Chiba, A. Ultrastructural study of the keratinization of the dorsal epithelium of the tongue of Middendorff’s bean goose, Anser fabalis middendorfii (Anseres, Antidae). Anat. Rec. 1997, 247, 147–163. [Google Scholar] [CrossRef]
- Jackowiak, H.; Godynicki, S. Light and scanning electron microscopic study of the tongue in the white-tailed eagle (Haliaeetus albicilla, Accipitriadae, Aves). Ann. Anat. 2005, 187, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Jackowiak, H.; Skieresz-Szewczyk, K.; Kwieciński, Z.; Trzcielińska-Lorych, J.; Godynicki, S. Functional morphology of the tongue in the Nutcracker (Nucifraga caryocatactes). Zool. Sci. 2010, 27, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Jackowiak, H.; Skieresz-Szewczyk, K.; Godynicki, S.; Iwasaki, S.; Meyer, W. Functional morphology of the tongue in the domestic goose (Anseranser f. domestica). Anat. Rec. 2011, 294, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Skieresz-Szewczyk, K.; Jackowiak, H. Morphofunctional study of the tongue in the domestic duck (Anas platyrhynchos f. domestica, Anatidae): LM and SEM study. Zoomorphology 2016, 135, 255–268. [Google Scholar] [CrossRef]
- Skieresz-Szewczyk, K.; Jackowiak, H.; Ratajczak, M. LM and TEM study of the orthokeratinized and parakeratinized epithelium of the tongue in the domestic duck (Anas platyrhynchos f. domestica). Micron 2014, 67, 117–124. [Google Scholar] [CrossRef]
- Skieresz-Szewczyk, K.; Plewa, B.; Jackowiak, H. Functional morphology of the tongue in the domestic turkey (Meleagris gallopavo gallopavo var. domesticus). Poultr. Sci. 2021, 100, 101038. [Google Scholar] [CrossRef]
- Skieresz-Szewczyk, K.; Jackowiak, H.; Buchwald, T.; Szybowicz, M. Localization of alpha-keratin and beta-keratin (corneous beta protein) in the epithelium on the ventral surface of the lingual apex and its lingual nail in the domestic goose (Anser anser f. domestica) by using immunohistochemistry (IHC) and Raman microspectroscopy analysis. Anat. Rec. 2017, 300, 1361–1368. [Google Scholar] [CrossRef]
- Skieresz-Szewczyk, K.; Buchwald, T.; Szybowicz, M.; Jackowiak, H. Alpha-keratin and corneous beta protein in the parakeratinized epithelium of the tongue in the domestic goose (Anser anser f. domestica). J. Exp. Zool. B. Mol. Dev. Evol. 2019, 332, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Skieresz-Szewczyk, K.; Jackowiak, H.; Skrzypski, M. Alpha-keratin, keratin-associated proteins and transglutaminase 1 are present in the ortho- and parakeratinized epithelium of the avian tongue. Cells 2022, 11, 1899. [Google Scholar] [CrossRef] [PubMed]
- Sachslehner, A.P.; Surbek, M.; Golabi, B.; Geiselhofer, M.; Jäger, K.; Hess, C.; Kuchler, U.; Gruber, R.; Eckhart, L. Transglutaminase Activity Is Conserved in Stratified Epithelia and Skin Appendages of Mammals and Birds. Int. J. Mol. Sci. 2023, 24, 2193. [Google Scholar] [CrossRef] [PubMed]
- He, D.S.; Jiang, J.X.; Taffet, S.M.; Burt, J.M. Formation of heteromeric gap junction channels by connexins 40 and 43 in vascular smooth muscle cells. Proc. Natl. Acad. Sci. USA 1999, 96, 6495–6500. [Google Scholar] [CrossRef]
- Mlitz, V.; Strasser, B.; Jaeger, K.; Hermann, M.; Ghannadan, M.; Buchberger, M.; Alibardi, L.; Tschachler, E.; Eckhart, L. Trichohyalin-like proteins have evolutionarily conserved roles in the morphogenesis of skin appendages. J. Investig. Dermatol. 2014, 134, 2685–2692. [Google Scholar] [CrossRef]
- Wiszniewski, L.; Limat, A.; Saurat, J.-H.; Meda, P.; Salomon, D. Differential expression of connexins during stratification of human keratinocytes. J. Investig. Dermatol. 2000, 115, 278–285. [Google Scholar] [CrossRef]
- Brockmeyer, P.; Jung, K.; Perske, C.; Schliephake, H.; Hemmerlein, B. Membrane connexin 43 acts as an independent prognostic marker in oral squamous cell carcinoma. Int. J. Oncol. 2014, 45, 273–281. [Google Scholar] [CrossRef]
- Brockmeyer, P.; Hemmerlein, B.; Jung, K.; Fialka, F.; Brodmann, T.; Gruber, R.M.; Schliephake, H.; Kramer, F.J. Connexin subtype expression during oral carcinogenesis: A pilot study in patients with oral squamous cell carcinoma. Mol. Clin. Oncol. 2016, 4, 298–302. [Google Scholar] [CrossRef]
- Segura, I.G.; Secchi, D.G.; Galíndez, M.F.; Carrica, A.; Bologna-Molina, R.; Brunotto, M.; Centeno, V.A. Connexin 43, Bcl-2, Bax, Ki67, and E-cadherin patterns in oral squamous cell carcinoma and its relationship with GJA1 rs12197797 C/G. Med. Oral. Patol. Oral. Cir. Bucal 2022, 27, 366–374. [Google Scholar] [CrossRef]
- Stout, C.E.; Costantin, J.L.; Naus, C.C.; Charles, A.C. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 2002, 277, 10482–104881. [Google Scholar] [CrossRef]
- Sáez, J.C.; Schalper, K.A.; Retamal, M.A.; Orellana, J.A.; Shoji, K.F.; Bennett, M.V. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp. Cell Res. 2010, 316, 2377–2389. [Google Scholar] [CrossRef] [PubMed]
- Taruno, A. ATP Release Channels. Int. J. Mol. Sci. 2018, 19, 808. [Google Scholar] [CrossRef] [PubMed]
- Tovar, L.M.; Burgos, C.F.; Yévenes, G.E.; Moraga-Cid, G.; Fuentealba, J.; Coddou, C.; Bascunan-Godoy, L.; Catrupay, C.; Torres, A.; Castro, P.A. Understanding the Role of ATP Release through Connexins Hemichannels during Neurulation. Int. J. Mol. Sci. 2023, 24, 2159. [Google Scholar] [CrossRef]
- Finger, T.E.; Danilova, V.; Barrows, J.; Bartel, D.L.; Vigers, A.J.; Stonem, L.; Hellekant, G.; Kinnamon, S.C. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 2005, 310, 1495–1499. [Google Scholar] [CrossRef]
- Romanov, R.A.; Rogachevskaja, O.A.; Bystrova, M.F.; Jiang, P.; Margolskee, R.F.; Kolesnikov, S.S. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007, 26, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Romanov, R.A.; Rogachevskaja, O.A.; Khokhlov, A.A.; Kolesnikov, S.S. Voltage dependence of ATP secretion in mammalian taste cells. J. Gen. Physiol. 2008, 132, 731–744. [Google Scholar] [CrossRef]
- Ganchrow, D.; Ganchrow, J.R. Number and distribution of taste buds in the oral cavity of hatchling chicks. Physiol. Behav. 1985, 34, 889–894. [Google Scholar] [CrossRef]
- Kudo, K.; Nishimura, S.; Tabata, S. Distribution of taste buds in layer-type chickens: Scanning electron microscopic observations. Anim. Sci. J. 2008, 79, 680–685. [Google Scholar] [CrossRef]
- Kudo, K.; Shiraishi, J.; Nishimura, S.; Bungo, T.; Tabata, S. The number of taste buds is related to bitter taste sensitivity in layer and broiler chickens. Anim. Sci. J. 2010, 81, 240–244. [Google Scholar] [CrossRef]
- Menon, G.K.; Grayson, S.; Elias, P.M. Ionic calcium reservoirs in mammalian epidermis: Ultrastructural localization by ion-capture cytochemistry. J. Investig. Dermatol. 1985, 84, 508–512. [Google Scholar] [CrossRef]
- Menon, G.K.; Elias, P.M.; Feingold, K.R. Integrity of the permeability barrier is crucial for maintenance of the epidermal calcium gradient. Br. J. Dermatol. 1994, 130, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Vicanová, J.; Boelsma, E.; Mommaas, A.M.; Kempenaar, J.A.; Forslind, B.; Pallon, J.; Egelrud, T.; Koerten, H.K.; Ponec, M. Normalization of epidermal calcium distribution profile in reconstructed human epidermis is related to improvement of terminal differentiation and stratum corneum barrier formation. J. Investig. Dermatol. 1998, 111, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Goliger, J.A.; Paul, D.L. Wounding alters epidermal connexin expression and gap junction-mediated intercellular communication. Mol. Biol. Cell 1995, 6, 1491–1501. [Google Scholar] [CrossRef]
- Hervé, J.-C.; Bourmeyster, N.; Sarrouilhe, D.; Duffy, H.S. Gap junctional complexes: From partners to functions. Prog. Biophys. Mol. Biol. 2007, 94, 29–65. [Google Scholar] [CrossRef] [PubMed]
- Giepmans, B.N.; Verlaan, I.; Hengeveld, T.; Janssen, H.; Calafat, J.; Falk, M.M.; Moolenaar, W.H. Gap junction protein connexin-43 interacts directly with microtubules. Curr. Biol. 2001, 11, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.E.; Easton, J.A.; Hodgins, M.B.; Wright, C.S. Connexins: Sensors of epidermal integrity that are therapeutic targets. FEBS Lett. 2014, 588, 1304–1314. [Google Scholar] [CrossRef]
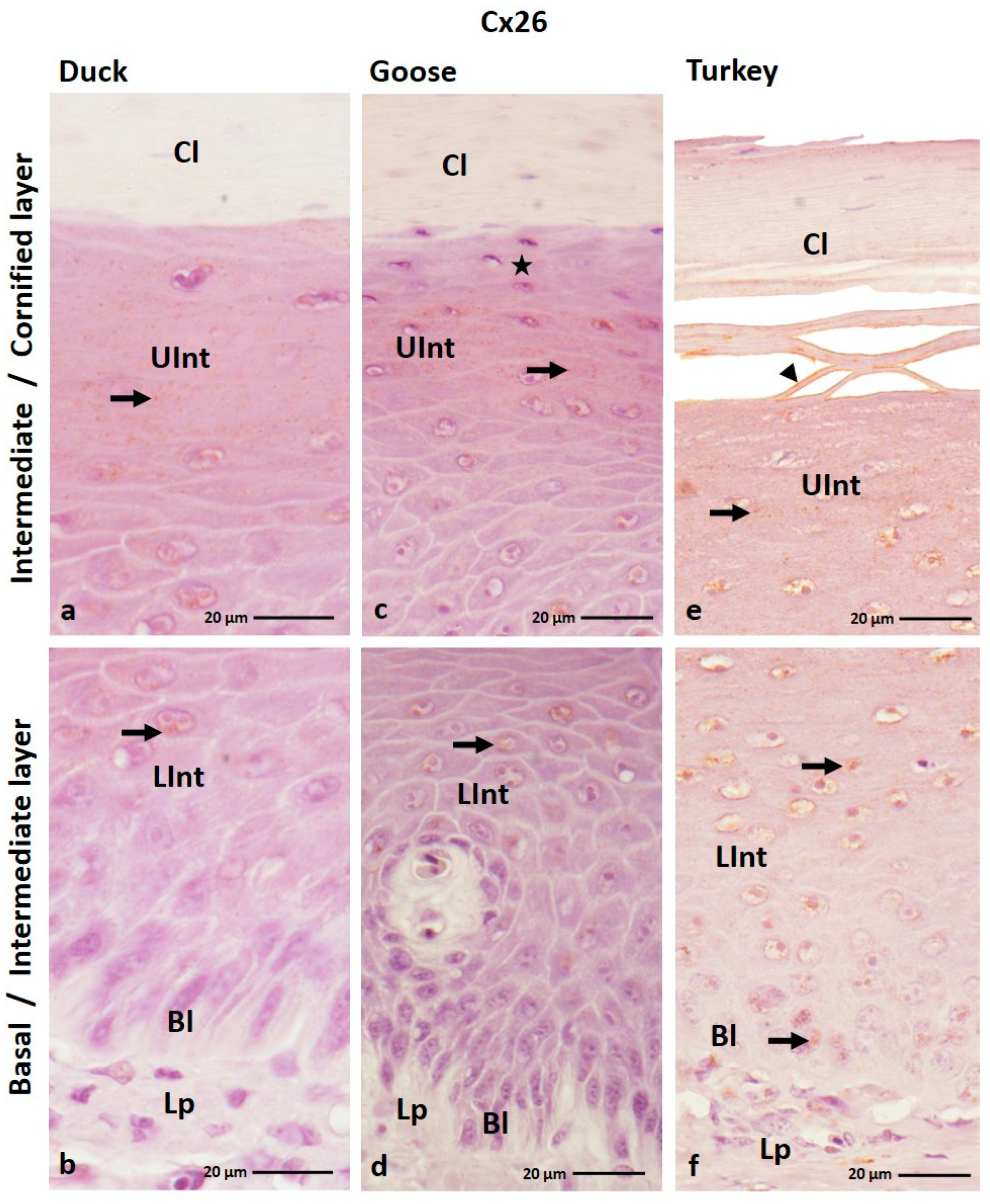
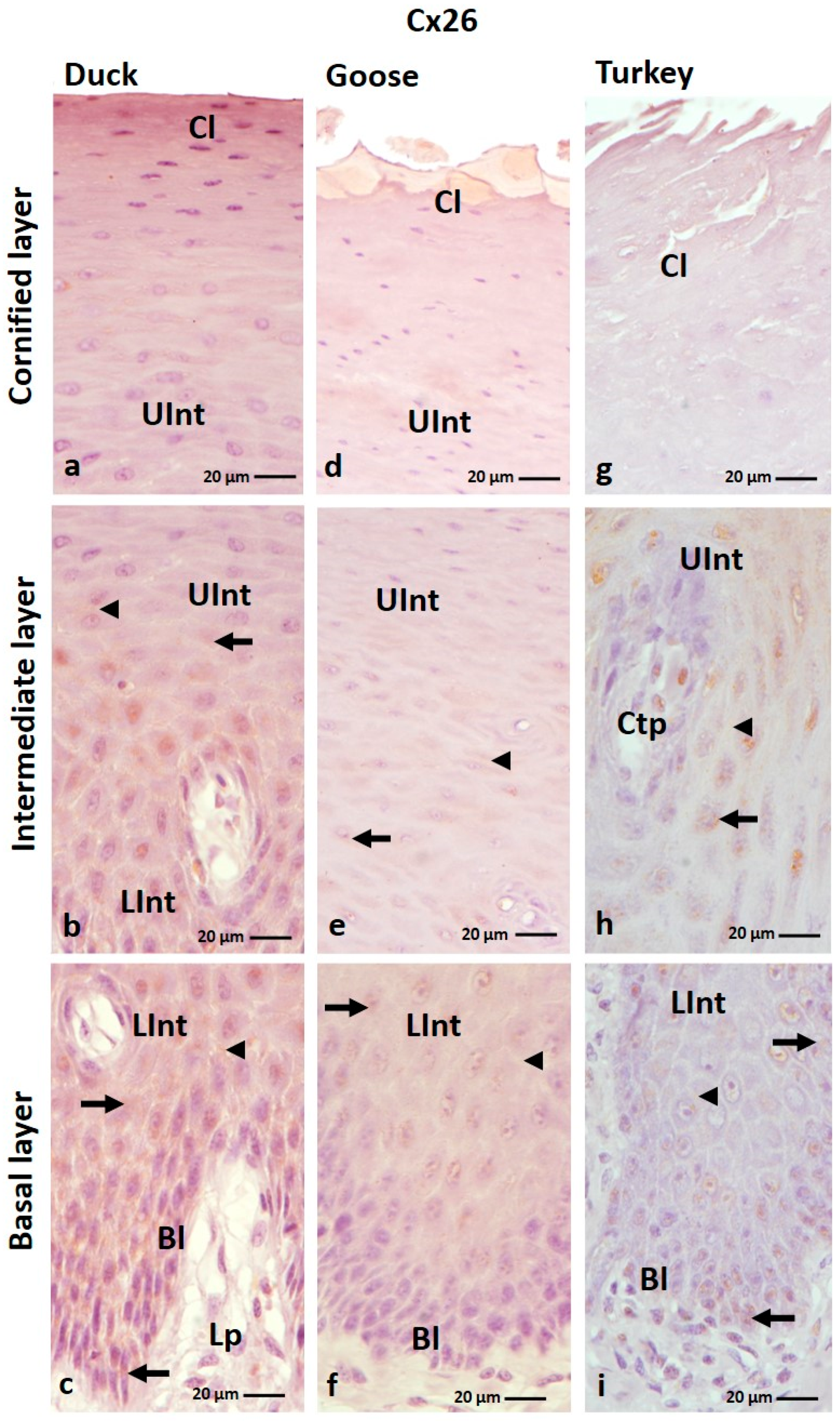
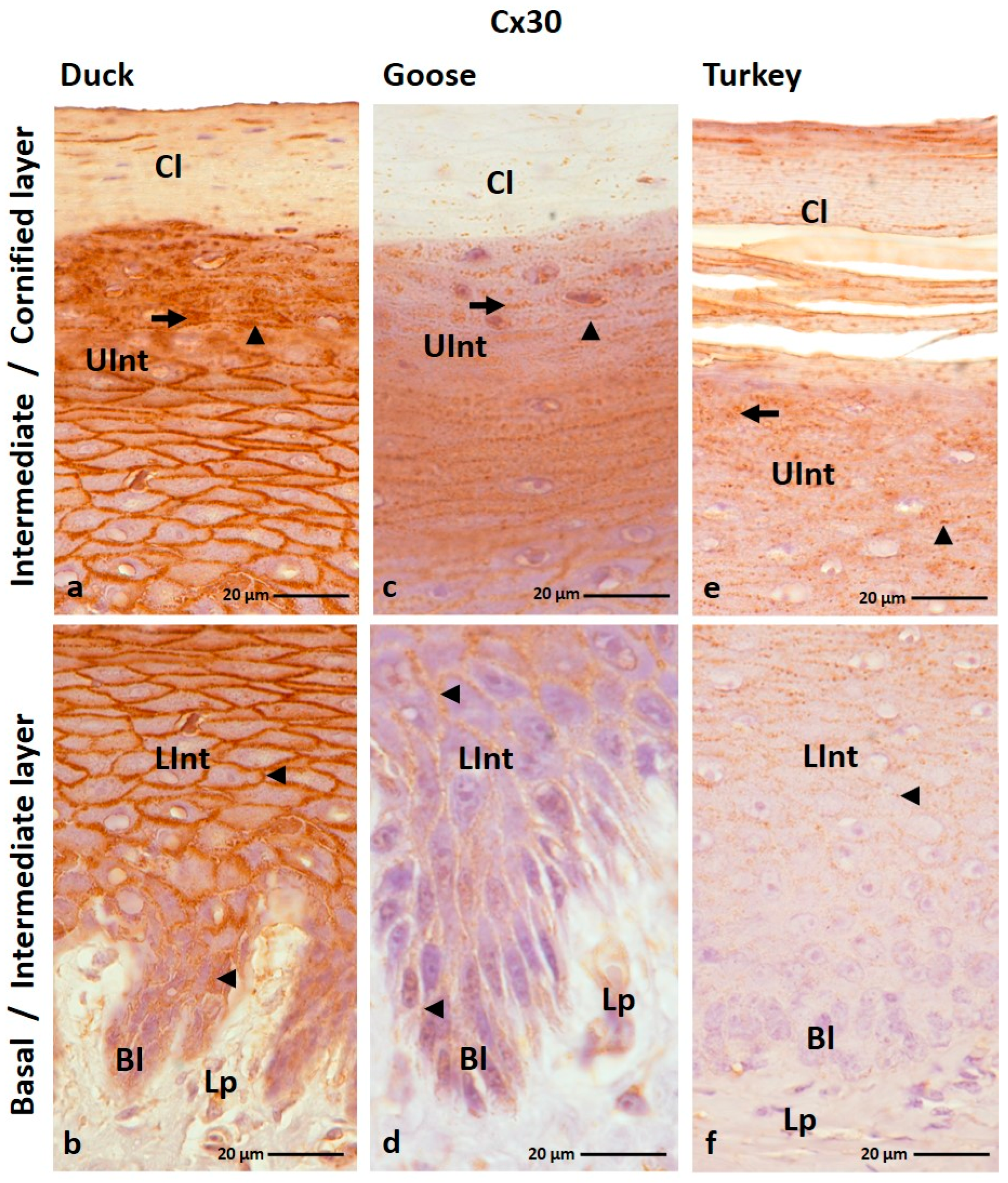
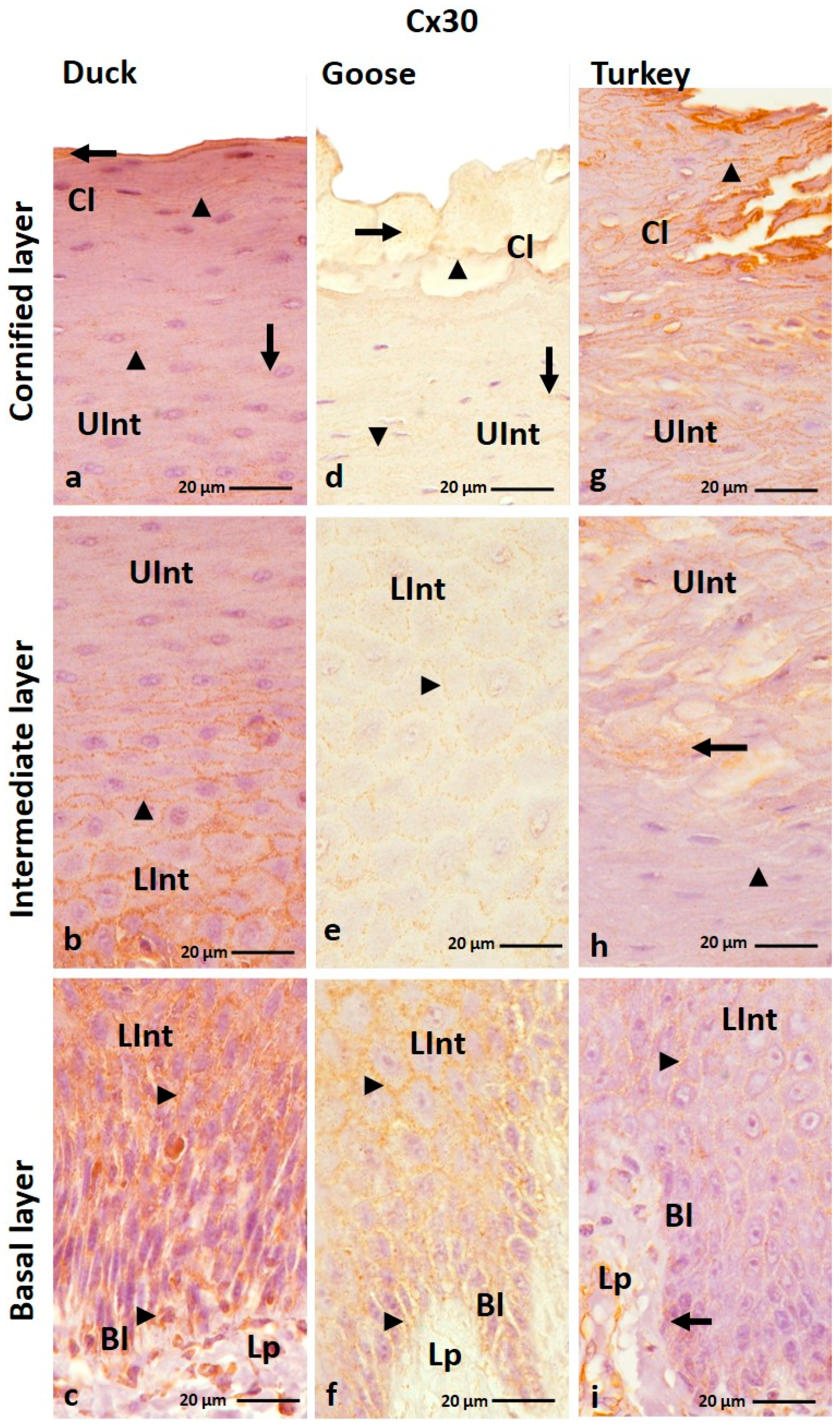


| Cx26 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Species | Basal Layer | Intermediate Layer -Lower Part | Intermediate Layer -Upper Part | Cornified Layer | ||||
| Ortho- | Para- | Ortho- | Para- | Ortho- | Para- | Ortho- | Para- | |
| Duck | - | ++ cc, cm | + cc | ++ cc, cm | + cc | + cc, cm | - | - |
| Goose | - | - | + cc | + cc, cm | −/+ cc | + cc, cm | - | - |
| Turkey | + cc | + cc | + cc | + cc, cm | + cc | + cc, cm | −/+ cm | - |
| Cx30 | ||||||||
| Species | Basal Layer | Intermediate Layer -Lower Part | Intermediate Layer -Upper Part | Cornified Layer | ||||
| Ortho- | Para- | Ortho- | Para- | Ortho- | Para- | Ortho- | Para- | |
| Duck | ++ cm | ++ cm | +++ cm | +/++ cm | +++ cc, cm | + cc, cm | −/+ cc | + cc, cm |
| Goose | ++ cm | ++ cm | +++ cm | +/++ cm | ++/+++ cc, cm | + cc, cm | −/+ cc | + cc, cm |
| Turkey | - | + cc | ++ cm | ++ cm | ++ cc, cm | + cc, cm | −/+ cc | ++ cc |
| Cx31 | ||||||||
| Species | Basal Layer | Intermediate Layer -Lower Part | Intermediate Layer -Upper Part | Cornified Layer | ||||
| Ortho- | Para- | Ortho- | Para- | Ortho- | Para- | Ortho- | Para- | |
| Duck | + cm | + cm | + cm | + cm | ++ cc, cm | + cc, cm | −/+ cc | + cc, cm |
| Goose | ++ cm | + cm | + cm | + cm | ++ cc, cm | + cc, cm | −/+ cc | - |
| Turkey | + cm | - | + cm | −/+ cc, cm | ++ cc, cm | −/+ cc, cm | −/+ cc | ++ cm |
| Cx40 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Species | Basal Layer | Intermediate Layer -Lower Part | Intermediate Layer -Upper Part | Cornified Layer | ||||
| Ortho- | Para- | Ortho- | Para- | Ortho- | Para- | Ortho- | Para- | |
| Duck | + cm | +++ cm | + cm | +++ cm | ++ cc, cm | + cm | - | + cc |
| Goose | + cm | +++ cm | ++ cc, cm | +++ cm | ++ cc, cm | + cc, cm | - | −/+ cc, cm |
| Turkey | + cm | ++ cm | + cm | ++ cm | ++ cc, cm | + cc, cm | - | + cc, cm |
| Cx43 | ||||||||
| Species | Basal Layer | Intermediate Layer -Lower Part | Intermediate Layer -Upper Part | Cornified Layer | ||||
| Ortho- | Para- | Ortho- | Para- | Ortho- | Para- | Ortho- | Para- | |
| Duck | + cc | + cm | + cc, cm | +++ cm | +++ cc, cm | −/+ cm | - | - |
| Goose | + cc | +++ cm | + cc, cm | +++ cm | +++ cc, cm | −/+ cm | - | - |
| Turkey | - | + cm | ++ cc, cm | ++ cm | +++ cc, cm | −/+ cc, cm | - | + cc, cm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skieresz-Szewczyk, K.; Jackowiak, H. Pattern Distribution of Connexins in the Ortho- and Parakeratinized Epithelium of the Lingual Mucosa in Birds. Cells 2023, 12, 1776. https://doi.org/10.3390/cells12131776
Skieresz-Szewczyk K, Jackowiak H. Pattern Distribution of Connexins in the Ortho- and Parakeratinized Epithelium of the Lingual Mucosa in Birds. Cells. 2023; 12(13):1776. https://doi.org/10.3390/cells12131776
Chicago/Turabian StyleSkieresz-Szewczyk, Kinga, and Hanna Jackowiak. 2023. "Pattern Distribution of Connexins in the Ortho- and Parakeratinized Epithelium of the Lingual Mucosa in Birds" Cells 12, no. 13: 1776. https://doi.org/10.3390/cells12131776
APA StyleSkieresz-Szewczyk, K., & Jackowiak, H. (2023). Pattern Distribution of Connexins in the Ortho- and Parakeratinized Epithelium of the Lingual Mucosa in Birds. Cells, 12(13), 1776. https://doi.org/10.3390/cells12131776






