Immunochemical Identification of the Main Cell Wall Polysaccharides of the Early Land Plant Marchantia polymorpha
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Extraction of Cell Wall Polysaccharide Fractions
2.3. Polysaccharide Arrays
2.4. Immunocytochemistry of Cell Wall Epitopes
3. Results
3.1. Low-Methylesterified Homogalacturonans Are Major Pectins in M. polymorpha Thalli Cell Walls
3.2. Xyloglucans and Mannans Are Present in All the Cell Walls of M. polymorpha Thalli
3.3. Mannans and XXLG Xyloglucan Epitopes Are Accessible in All the Cell Walls of M. polymorpha Thalli
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowman, J.L. The liverwort Marchantia polymorpha, a model for all ages. Curr. Top. Dev. Biol. 2022, 147, 1–32. [Google Scholar]
- Delwiche, C.F.; Goodman, C.A.; Chang, C. Land plant model systems branch out. Cell 2017, 171, 265–266. [Google Scholar] [CrossRef] [Green Version]
- Shimamura, M. Marchantia polymorpha: Taxonomy, phylogeny and morphology of a model system. Plant Cell Physiol. 2016, 57, 230–256. [Google Scholar] [CrossRef] [Green Version]
- Bowman, J.L.; Kohchi, T.; Yamato, K.T.; Jenkins, J.; Shu, S.; Ishizaki, K.; Yamaoka, S. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 2017, 171, 287–304. [Google Scholar] [CrossRef] [Green Version]
- Dauphin, B.; Ranocha, P.; Dunand, C.; Burlat, V. Cell-wall microdomain remodeling controls crucial developmental processes. Trends Plant Sci. 2022, 27, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Franková, L.; Fry, S.C. Biochemistry and physiological roles of enzymes that ‘cut and paste’ plant cell-wall polysaccharides. J. Exp. Bot. 2013, 64, 3519–3550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampugnani, E.R.; Khan, G.A.; Somssich, I.E.; Persson, S. Building a plant cell wall at a glance. J. Cell Sci. 2018, 131, jcs207373. [Google Scholar] [CrossRef] [Green Version]
- Novaković, L.; Guo, L.T.; Bacic, A.; Sampathkumar, A.; Johnson, K.L. Hitting the wall-Sensing and signaling pathways involved in plant cell wall remodeling in response to abiotic stress. Plants 2018, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Tenhaken, R. Cell wall remodeling under biotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef] [Green Version]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants, consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef]
- San Clemente, H.; Kolkas, H.; Jamet, E. Plant cell wall proteomes: The core of conserved protein families and the case of non-canonical proteins. Int. J. Biol. Sci. 2022, 23, 4273. [Google Scholar] [CrossRef] [PubMed]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Zhong, R.; Ye, Z.-H. Secondary cell walls: Biosynthesis, patterned deposition and transcriptional regulation. Plant Cell Physiol. 2015, 56, 195–214. [Google Scholar] [CrossRef] [Green Version]
- Cohen, H.; Szymanski, J.; Aharoni, A. Assimilation of ‘omics’ strategies to study the cuticle layer and suberin lamellae in plants. J. Exp. Bot. 2017, 68, 5389–5400. [Google Scholar] [CrossRef]
- Plancot, B.; Gügi, B.; Mollet, J.C.; Loutelier-Bourhis, C.; Giovind, S.R.; Lerouge, P.; Follet-Gueye, M.L.; Vicré, M.; Alfonso, C.; Nguema-Ona, E.; et al. Dessication tolerance in plants: Structural charactérization of the cell wall hemicellulosic polysaccharides in three Selaginella species. Carbohydr. Polym. 2019, 208, 180–190. [Google Scholar] [CrossRef]
- Philippe, F.; Sørensen, I.; Jiao, C.; Sun, X.; Fei, Z.; Domozych, D.S.; Ros, J.K.C. Cutin and suberin: Assembly and origins of specialized lipidic cell wall scaffolds. Curr. Opin. Plant Biol. 2020, 55, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Soriano, G.; Del-Castillo-Alonso, M.A.; Monforte, L.; Núñez-Olivera, E.; Martínez-Abaigar, J. Phenolic compounds from different bryophyte species and cell compartments respond specifically to ultraviolet radiation, but not particularly quickly. Plant Physiol. Biochem. 2019, 134, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Niklas, K.J.; Cobb, E.D.; Matas, A.J. The evolution of hydrophobic cell wall biopolymers: From algae to angiosperms. J. Exp. Bot. 2017, 68, 5261–5269. [Google Scholar] [CrossRef] [Green Version]
- Popper, Z.; Michel, G.; Hervé, C.; Domozych, D.S.; Willats, W.G.T.; Tuohy, M.G.; Kloareg, B.; Stengel, D.B. Evolution and diversity of plant cell walls: From algae to flowering plants. Ann. Rev. Plant Biol. 2011, 62, 567–590. [Google Scholar] [CrossRef] [Green Version]
- Nothnagel, A.L.; Nothnagel, E.A. Primary cell wall structure in the evolution of land plants. J. Integr. Plant Biol. 2007, 49, 1271–1278. [Google Scholar] [CrossRef]
- Pfeifer, L.; Mueller, K.-K.; Classen, B. The cell wall of hornworts and liverworts: Innovations in early land plant evolution? J. Exp. Bot. 2022, 73, 4454–4472. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Bosneaga, E.; Auer, M. Plant cell walls throughout evolution: Towards a molecular understanding of their design principle. J. Exp. Bot. 2009, 60, 3615–3635. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, I.; Pettolino, F.A.; Bacic, A.; Ralph, J.; Lu, F.; O’Neill, M.A.; Fei, Z.; Rose, J.K.C.; Domozych, D.S.; Willats, W.G.T. The charophycean green algae provide insignt into the early origins of plant cell walls. Plant J. 2011, 68, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Popper, Z.; Fry, S.C. Primary cell wall composition of Bryophytes and Charophytes. Ann. Bot. 2003, 91, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Peña, M.J.; Darvill, A.G.; Eberhard, S.; York, W.S.; O’Neill, M.A. Moss and liverwort xyloglucans contain galacturonic acid and are structurally distinct from the xyloglucans synthesized by hornworts and vascular plants. Glycobiology 2008, 18, 891–904. [Google Scholar] [CrossRef]
- Kolkas, H.; Balliau, T.; Chourré, J.; Zivy, M.; Canut, H.; Jamet, E. The cell wall proteome of Marchantia polymorpha reveals specificities compared to those of flowering plants. Front. Plant Sci. 2022, 12, 765846. [Google Scholar] [CrossRef]
- Happ, K.; Classen, B. Arabinogalactan-proteins from the liverwort Marchantia polymorpha L., a member of a basal land plant lineage, are structurally different to those of angiosperms. Plants 2019, 8, 460. [Google Scholar] [CrossRef] [Green Version]
- Mueller, K.-K.; Pfeifer, L.; Schuldt, L.; Szövényi, P.; de Vries, S.; de Vries, J.; Johnson, K.L.; Classen, B.C. Fern cell walls and the evolution of arabinogalactan proteins in streptophytes. Plant J. 2023, 114, 875–894. [Google Scholar] [CrossRef]
- Jayme, G.; Neuschäffer, K. Tri-(en)-Cadmiumhydroxyd als neues farbloses, wäβriges Lösungsmittel für Cellulose. Naturwissenschaften 1957, 44, 62–63. [Google Scholar] [CrossRef]
- Moller, I.; Sørensen, I.; Bernal, A.J.; Blaukopf, C.; Lee, K.; Øbro, J.; Pettolino, F.; Roberts, A.; Mikkelsen, J.D.; Knox, J.P.; et al. High-throughput mapping of cell-wall polymers within and between plants using novel microarrays. Plant J. 2007, 50, 1118–1128. [Google Scholar] [CrossRef]
- Ralet, M.-C.; Tranquet, O.; Poulain, D.; Moïse, A.; Guillon, F. Monoclonal antibodies to rhamnogalacturonan I backbone. Planta 2010, 231, 1373–1383. [Google Scholar] [CrossRef]
- Pauly, M.; Keegstra, K. Biosynthesis of the plant cell wall matrix polysaccharide xyloglucan. Annu. Rev.Plant Biol. 2017, 67, 235–259. [Google Scholar] [CrossRef]
- Francoz, E.; Ranocha, P.; Pernot, C.; Le Ru, A.; Pacquit, V.; Dunand, C.; Burlat, V. Complementarity of medium-throughput in situ RNA hybridization and tissue-specific transcriptomics: Case study of Arabidopsis seed development kinetics. Sci. Rep. 2016, 6, e24644. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Melton, R.D.; Brasch, D.J. Cell-wall polysaccharides of kiwifruit (Actinidia deliciosa): Chemical features in different tissue zones of the fruit at harvest. Carbohydr. Res. 1988, 182, 241–258. [Google Scholar] [CrossRef]
- Kaczmarska, A.; Pieczywek, P.; Cybulska, J.; Zdunek, A. Structure and functionality of Rhamnogalacturonan I in the cell wall and in solution: A review. Carbohydr. Polym. 2022, 278, 118909. [Google Scholar] [CrossRef]
- Konno, H.; Yamasaki, Y.; Katoh, K. Fractionation and partial characterization of pectic polysaccharides in cell walls from liverwort (Marchantia polymorpha) cell cultures. J. Exp. Bot. 1987, 38, 711–722. [Google Scholar] [CrossRef]
- Ligrone, R.; Vaughn, K.; Renzaglia, K.S.; Knox, J.P.; Duckett, J.G. Diversity of the distribution of polysaccharides and glycoprotein epitopes in the cell walls of bryophytes: New evidence for the multiple evolution of water-conducting cells. New Phytol. 2002, 156, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Hocq, L.; Pelloux, J.; Lefebvre, V. Connecting homogalacturonan-type pectin remodeling to acid growth. Trends Plant Sci. 2017, 22, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Zablackis, E.; Huang, J.; Müller, B.; Darvill, A.; Albersheim, P. Characterization of the cell-wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol. 1995, 107, 1129–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, L.; Milne, J.L.; Ashford, D.; McQueen-Mason, S.J. Cell wall arabinan is essential for guard cell function. Proc. Natl. Acad. Sci. USA 2003, 100, 11783–11788. [Google Scholar] [CrossRef]
- Galloway, A.F.; Pedersen, M.J.; Merry, B.; Marcus, S.E.; Blacker, J.; Benning, L.G.; Field, K.J.; Knox, J.P. Xyloglucan is released by plants and promotes soil particle aggregation. New Phytol. 2018, 217, 1128–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domozych, D.S.; Sørensen, I.; Willats, W.G.T. The distribution of cell wall polymers during antheridium development and spermatogenesis in the Charophycean green alga, Chara corallina. Ann. Bot. 2009, 104, 1045–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carafa, A.; Duckett, J.G.; Knox, J.P.; Ligrone, R. Distribution of cell-wall xylans in bryophytes and tracheophytes: New insights into basal interrelationships of land plants. New Phytol. 2005, 168, 231–240. [Google Scholar] [CrossRef] [PubMed]
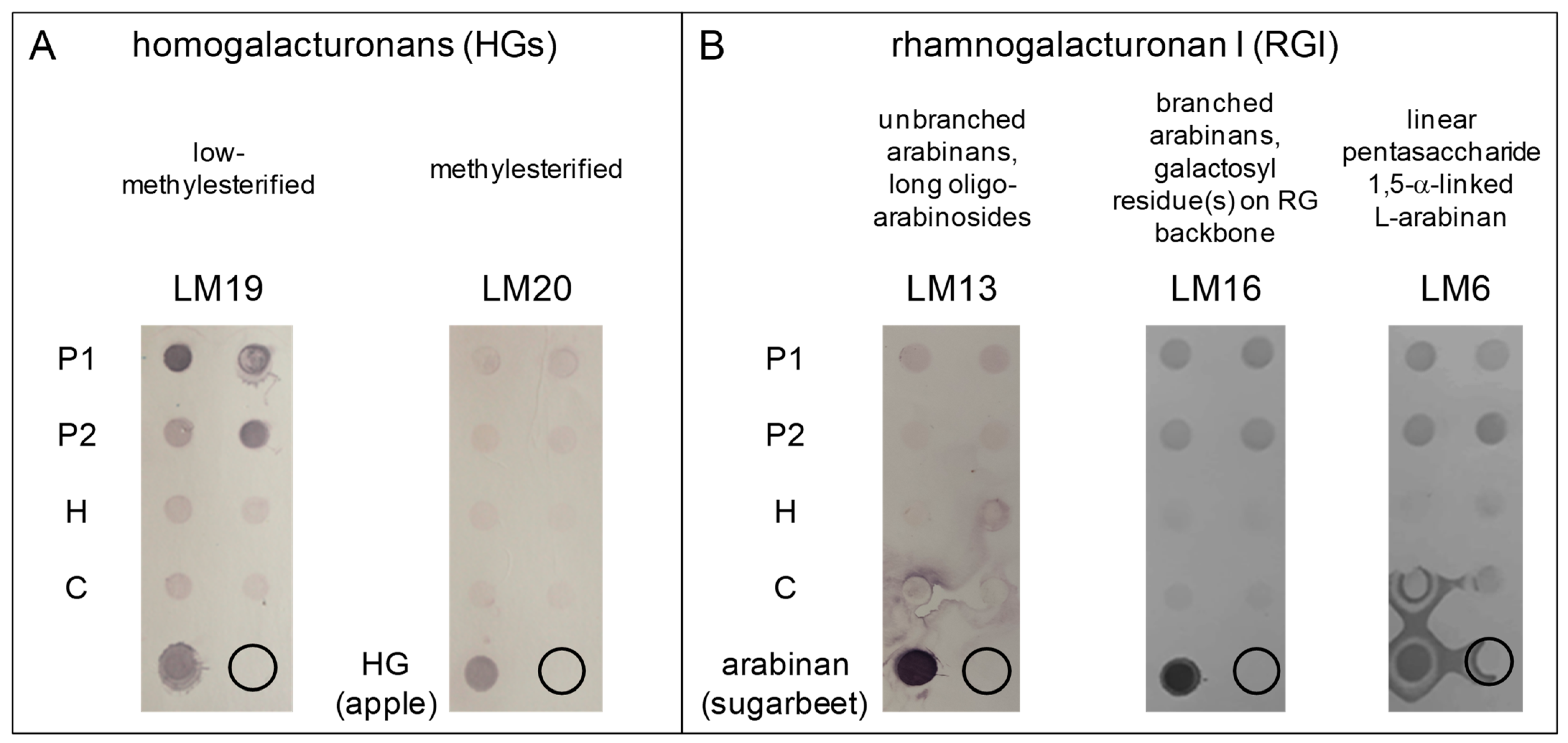


| Cell Wall Polysaccharides | Monoclonal Antibody | Specificity |
|---|---|---|
| pectins | LM19 | low-esterified homogalacturonans |
| LM20 | high-methylesterified homogalacturonans | |
| LM13 | unbranched arabinans, long oligo-arabinosides | |
| LM16 | branched arabinans, galactosyl residue(s) on RG backbones | |
| LM6 | linear pentasaccharide of 1,5-α-linked L-arabinan epitopes | |
| RU1 | [→2)-α-L-rhamnose p-(1→4)-α-D-galacturonic acid p-(1→]7, at least 6 disaccharide «rhamnose-galacturonic acid» | |
| RU2 | [→2)-α-L-rhamnose p-(1→4)-α-D-galacturonic acid p-(1→]7, at least 2 disaccharide «rhamnose-galacturonic acid» repeats, tolerates galactose substitutions | |
| hemicelluloses * | LM25 | xyloglucans (XLLG, XXLG, XXXG modules) * |
| LM24 | xyloglucans (XXLG module) * | |
| LM15 | xyloglucans (XXXG module) * | |
| LM21 | mannan, glucomannan, galactomannan, β-(1→4)-manno-oligosaccharides from DP2 to DP5 | |
| LM22 | mannan, glucomannan, β-(1→4)-manno-oligosaccharides from DP2 to DP5 | |
| LM10 | unsubstituted and relatively low-substituted xylans | |
| LM11 | unsubstituted and relatively low-substituted xylans, wheat arabinoxylan |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolkas, H.; Burlat, V.; Jamet, E. Immunochemical Identification of the Main Cell Wall Polysaccharides of the Early Land Plant Marchantia polymorpha. Cells 2023, 12, 1833. https://doi.org/10.3390/cells12141833
Kolkas H, Burlat V, Jamet E. Immunochemical Identification of the Main Cell Wall Polysaccharides of the Early Land Plant Marchantia polymorpha. Cells. 2023; 12(14):1833. https://doi.org/10.3390/cells12141833
Chicago/Turabian StyleKolkas, Hasan, Vincent Burlat, and Elisabeth Jamet. 2023. "Immunochemical Identification of the Main Cell Wall Polysaccharides of the Early Land Plant Marchantia polymorpha" Cells 12, no. 14: 1833. https://doi.org/10.3390/cells12141833







