Chronic Intermittent Hypoxia-Induced Diaphragm Muscle Weakness Is NADPH Oxidase-2 Dependent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Chronic Intermittent Hypoxia Animal Model
2.3. Ex Vivo Muscle Function Analysis
2.3.1. Muscle Dissection and Preparation
2.3.2. Isometric Muscle Function
2.3.3. Isotonic Muscle Function
2.4. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.4.1. RNA Extraction and Preparation
2.4.2. cDNA Synthesis
2.4.3. qRT-PCR
2.5. Western Blotting
2.5.1. Protein Extraction and Quantification
2.5.2. Gel Electrophoresis
2.6. Spectrophotometric Assays
2.6.1. Protein Extraction and Quantification
2.6.2. NADPH Oxidase Activity
2.6.3. Citrate Synthase Activity
2.6.4. Thiobarbituric Acid Reactive Substances
2.7. Cell Signalling Assays
2.7.1. Protein Extraction and Quantification
2.7.2. Hypertrophy, Atrophy, and HIF Signalling Assays
2.8. Statistical Analysis
3. Results
3.1. Diaphragm Muscle Contractile Function Ex Vivo
3.2. NOX mRNA and Protein Expression in Diaphragm Muscle
3.3. Indices of Redox Balance, Protein Synthesis and Degradation in Diaphragm Homogenates from Sham and CIH-Exposed Mice
3.4. mRNA Expression of Genes Relating to Myogenesis in Diaphragm Muscle
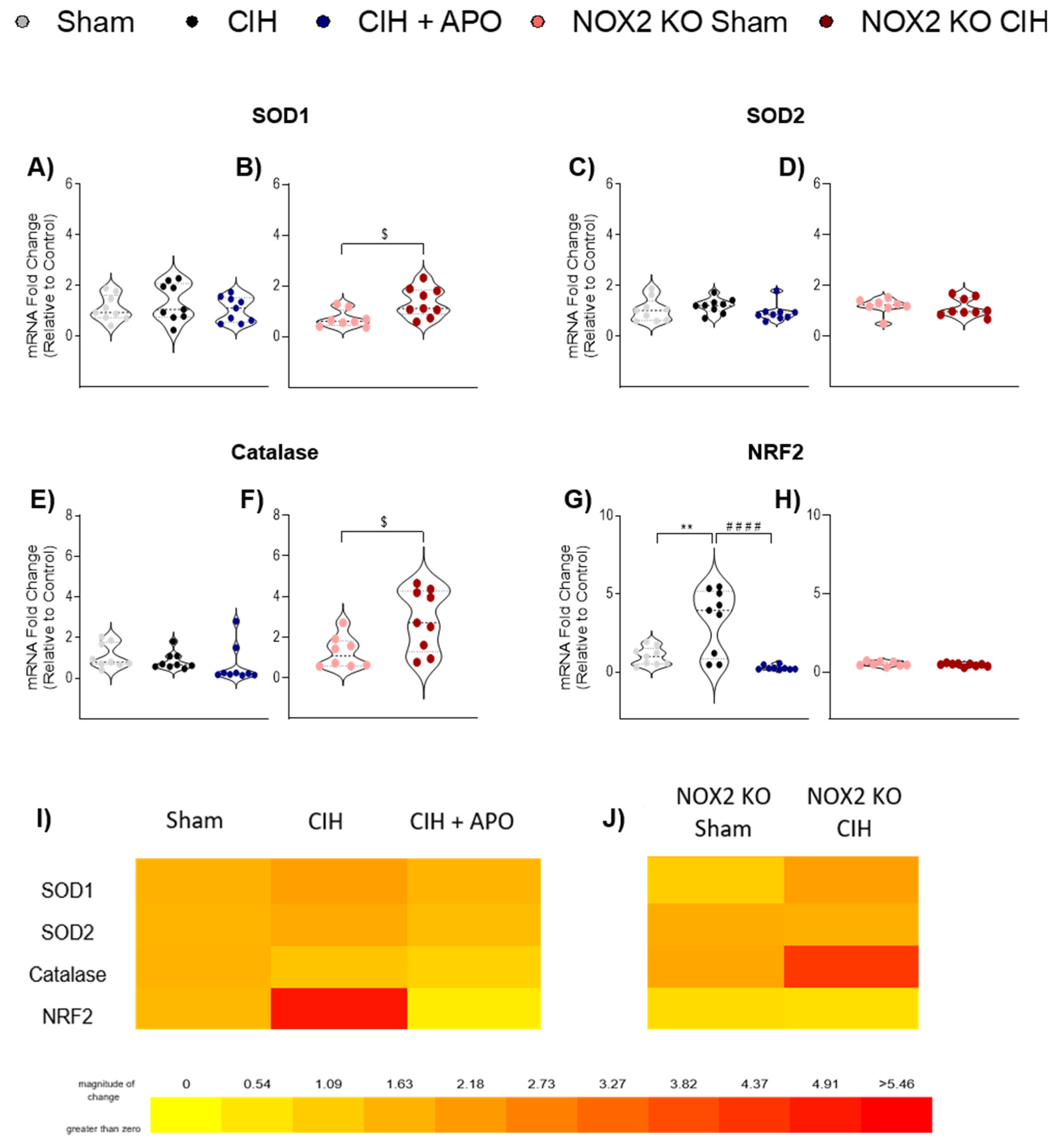
3.5. mRNA Expression of Genes Relating to Antioxidant Status in Diaphragm Muscle
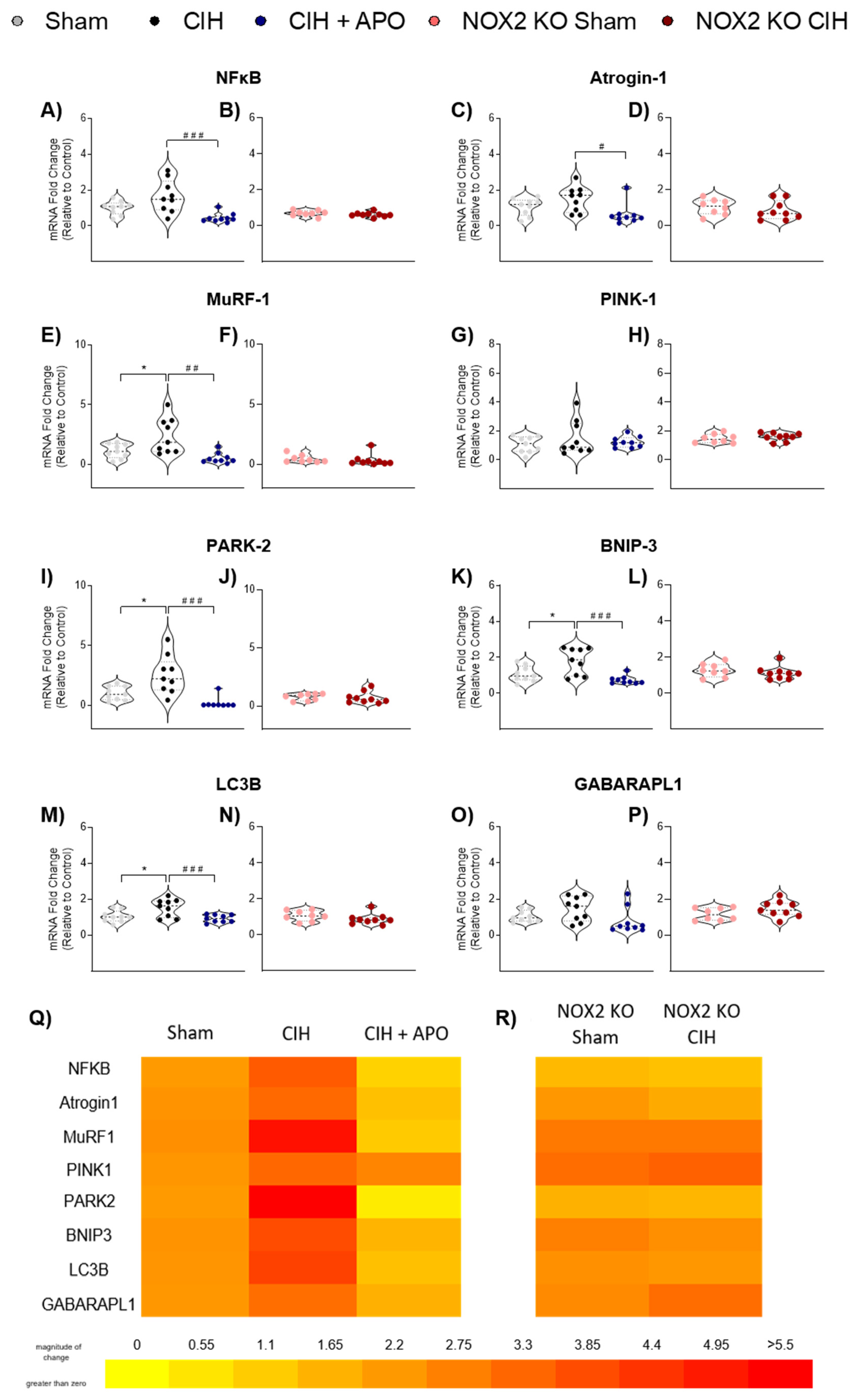
3.6. mRNA Expression of Genes Relating to Inflammation and Protein Degradation in Diaphragm Muscle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, D.P.; Younes, M.K. Obstructive sleep apnea. Compr. Physiol. 2012, 2, 2541–2594. [Google Scholar]
- Osman, A.M.; Carter, S.G.; Carberry, J.C.; Eckert, D.J. Obstructive sleep apnea: Current perspectives. Nat. Sci. Sleep. 2018, 10, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Veasey, S.C.; Rosen, I.M. Obstructive Sleep Apnea in Adults. N. Engl. J. Med. 2019, 380, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Griggs, G.A.; Findley, L.J.; Suratt, P.M.; Esau, S.A.; Wilhoit, S.C.; Rochester, D.F. Prolonged relaxation rate of inspiratory muscles in patients with sleep apnea. Am. Rev. Respir. Dis. 1989, 140, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.Y.; Wu, Y.T.; Lee, P.L.; Chang, Y.J.; Yang, P.C. Inspiratory muscle dysfunction in patients with severe obstructive sleep apnoea. Eur. Respir. J. 2010, 35, 373–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouillette, R.T.; Thach, B.T. A neuromuscular mechanism maintaining extrathoracic airway patency. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979, 46, 772–779. [Google Scholar] [CrossRef]
- de Carvalho, M.; Swash, M.; Pinto, S. Diaphragmatic Neurophysiology and Respiratory Markers in ALS. Front. Neurol. 2019, 10, 143. [Google Scholar] [CrossRef] [Green Version]
- Lavie, L. Obstructive sleep apnoea syndrome—An oxidative stress disorder. Sleep. Med. Rev. 2003, 7, 35–51. [Google Scholar] [CrossRef]
- O’Halloran, K.D. Chronic intermittent hypoxia creates the perfect storm with calamitous consequences for respiratory control. Respir. Physiol. Neurobiol. 2016, 226, 63–67. [Google Scholar] [CrossRef]
- Shortt, C.M.; Fredsted, A.; Chow, H.B.; Williams, R.; Skelly, J.R.; Edge, D.; Bradford, A.; O’Halloran, K.D. Reactive oxygen species mediated diaphragm fatigue in a rat model of chronic intermittent hypoxia. Exp. Physiol. 2014, 99, 688–700. [Google Scholar] [CrossRef] [Green Version]
- McGuire, M.; MacDermott, M.; Bradford, A. Effects of chronic intermittent asphyxia on rat diaphragm and limb muscle contractility. Chest 2003, 123, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Supinski, G.; Stofan, D.; Nethery, D.; Szweda, L.; DiMarco, A. Apocynin improves diaphragmatic function after endotoxin administration. J. Appl. Physiol. (1985) 1999, 87, 776–782. [Google Scholar] [CrossRef] [PubMed]
- McClung, J.M.; Van Gammeren, D.; Whidden, M.A.; Falk, D.J.; Kavazis, A.N.; Hudson, M.B.; Gayan-Ramirez, G.; Decramer, M.; DeRuisseau, K.C.; Powers, S.K. Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Crit. Care Med. 2009, 37, 1373–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, M.B.; Haack, K.E.; Franchek, K.M.; Valberg, P.A.; Kobzik, L.; West, M.S. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J. Appl. Physiol. (1985) 1992, 73, 1797–1804. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Zhan, G.; Serrano, F.; Fenik, P.; Hsu, R.; Kong, L.; Pratico, D.; Klann, E.; Veasey, S.C. NADPH Oxidase Mediates Hypersomnolence and Brain Oxidative Injury in a Murine Model of Sleep Apnea. Am. J. Respir. Crit. Care Med. 2005, 172, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Yamashita, C.; Matsumoto, C.; Kwak, C.J.; Fujii, K.; Hirata, T.; Miyamura, M.; Mori, T.; Ukimura, A.; Okada, Y.; et al. Role of gp91phox-containing NADPH oxidase in left ventricular remodeling induced by intermittent hypoxic stress. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2197–H2203. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.-L.; Dai, D.-Z.; Zhang, C.; Dai, Y. Apocynin and raisanberine alleviate intermittent hypoxia induced abnormal StAR and 3β-HSD and low testosterone by suppressing endoplasmic reticulum stress and activated p66Shc in rat testes. Reprod. Toxicol. 2013, 36, 60–70. [Google Scholar] [CrossRef]
- Xia, R.; Webb, J.A.; Gnall, L.L.; Cutler, K.; Abramson, J.J. Skeletal muscle sarcoplasmic reticulum contains a NADH-dependent oxidase that generates superoxide. Am. J. Physiol. Cell Physiol. 2003, 285, C215–C221. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, C.; Sanchez, G.; Barrientos, G.; Aracena-Parks, P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S-glutathionylation. J. Biol. Chem. 2006, 281, 26473–26482. [Google Scholar] [CrossRef] [Green Version]
- Loehr, J.A.; Wang, S.; Cully, T.R.; Pal, R.; Larina, I.V.; Larin, K.V.; Rodney, G.G. NADPH oxidase mediates microtubule alterations and diaphragm dysfunction in dystrophic mice. Elife 2018, 7, e31732. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.; Beharry, A.W.; Frye, G.S.; Judge, A.R.; Ferreira, L.F. NAD(P)H oxidase subunit p47phox is elevated, and p47phox knockout prevents diaphragm contractile dysfunction in heart failure. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L497–L505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucking, E.F.; O’Halloran, K.D.; Jones, J.F. Increased cardiac output contributes to the development of chronic intermittent hypoxia-induced hypertension. Exp. Physiol. 2014, 99, 1312–1324. [Google Scholar] [CrossRef]
- Drummond, S.E.; Burns, D.P.; O’Connor, K.M.; Clarke, G.; O’Halloran, K.D. The role of NADPH oxidase in chronic intermittent hypoxia-induced respiratory plasticity in adult male mice. Respir. Physiol. Neurobiol. 2021, 292, 103713. [Google Scholar] [CrossRef]
- Drummond, S.E.; Burns, D.P.; Maghrani, S.E.; Ziegler, O.; Healy, V.; O’Halloran, K.D. NADPH oxidase 2 is necessary for chronic intermittent hypoxia-induced sternohyoid muscle weakness in adult male mice. Exp. Physiol. 2022, 107, 946–964. [Google Scholar] [CrossRef]
- O’Leary, A.J.; O’Halloran, K.D. Diaphragm muscle weakness and increased UCP-3 gene expression following acute hypoxic stress in the mouse. Respir. Physiol. Neurobiol. 2016, 226, 76–80. [Google Scholar] [CrossRef]
- Burns, D.P.; Roy, A.; Lucking, E.F.; McDonald, F.B.; Gray, S.; Wilson, R.J.; Edge, D.; O’Halloran, K.D. Sensorimotor control of breathing in the mdx mouse model of Duchenne muscular dystrophy. J. Physiol. 2017, 595, 6653–6672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, D.P.; Canavan, L.; Rowland, J.; O’Flaherty, R.; Brannock, M.; Drummond, S.E.; O’Malley, D.; Edge, D.; O’Halloran, K.D. Recovery of respiratory function in mdx mice co-treated with neutralizing interleukin-6 receptor antibodies and urocortin-2. J. Physiol. 2018, 596, 5175–5197. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, A.J.; Drummond, S.E.; Edge, D.; O’Halloran, K.D. Diaphragm Muscle Weakness Following Acute Sustained Hypoxic Stress in the Mouse Is Prevented by Pretreatment with N-Acetyl Cysteine. Oxid. Med. Cell Longev. 2018, 2018, 4805493. [Google Scholar] [CrossRef] [Green Version]
- Burns, D.P.; Drummond, S.E.; Bolger, D.; Coiscaud, A.; Murphy, K.H.; Edge, D.; O’Halloran, K.D. N-acetylcysteine Decreases Fibrosis and Increases Force-Generating Capacity of mdx Diaphragm. Antioxidants 2019, 8, 581. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.K.; Martin, A.D.; Vandenborne, K.; Darragh, B.D.; Davenport, P.W. Chronic intrinsic transient tracheal occlusion elicits diaphragmatic muscle fiber remodeling in conscious rodents. PLoS ONE 2012, 7, e49264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montserrat, J.M.; Kosmas, E.N.; Cosio, M.G.; Kimoff, R.J. Lack of evidence for diaphragmatic fatigue over the course of the night in obstructive sleep apnoea. Eur. Respir. J. 1997, 10, 133–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clanton, T.L.; Wright, V.P.; Reiser, P.J.; Klawitter, P.F.; Prabhakar, N.R. Selected Contribution: Improved anoxic tolerance in rat diaphragm following intermittent hypoxia. J. Appl. Physiol. 2001, 90, 2508–2513. [Google Scholar] [CrossRef] [PubMed]
- Vincken, W.; Guilleminault, C.; Silvestri, L.; Cosio, M.; Grassino, A. Inspiratory muscle activity as a trigger causing the airways to open in obstructive sleep apnea. Am. Rev. Respir. Dis. 1987, 135, 372–377. [Google Scholar]
- Pae, E.K.; Wu, J.; Nguyen, D.; Monti, R.; Harper, R.M. Geniohyoid muscle properties and myosin heavy chain composition are altered after short-term intermittent hypoxic exposure. J. Appl. Physiol. (1985) 2005, 98, 889–894. [Google Scholar] [CrossRef]
- Giordano, C.; Lemaire, C.; Li, T.; Kimoff, R.J.; Petrof, B.J. Autophagy-associated atrophy and metabolic remodeling of the mouse diaphragm after short-term intermittent hypoxia. PLoS ONE 2015, 10, e0131068. [Google Scholar] [CrossRef]
- Lewis, P.; Sheehan, D.; Soares, R.; Coelho, A.V.; O’Halloran, K.D. Redox Remodeling Is Pivotal in Murine Diaphragm Muscle Adaptation to Chronic Sustained Hypoxia. Am. J. Respir. Cell Mol. Biol. 2016, 55, 12–23. [Google Scholar] [CrossRef]
- McDonald, F.B.; Dempsey, E.M.; O’Halloran, K.D. Effects of Gestational and Postnatal Exposure to Chronic Intermittent Hypoxia on Diaphragm Muscle Contractile Function in the Rat. Front. Physiol. 2016, 7, 276. [Google Scholar] [CrossRef] [Green Version]
- Attal, P.; Lambert, F.; Marchand-Adam, S.; Bobin, S.; Pourny, J.C.; Chemla, D.; Lecarpentier, Y.; Coirault, C. Severe mechanical dysfunction in pharyngeal muscle from adult mdx mice. Am. J. Respir. Crit. Care Med. 2000, 162, 278–281. [Google Scholar] [CrossRef]
- Burns, D.P.; Rowland, J.; Canavan, L.; Murphy, K.H.; Brannock, M.; O’Malley, D.; O’Halloran, K.D.; Edge, D. Restoration of pharyngeal dilator muscle force in dystrophin-deficient (mdx) mice following co-treatment with neutralizing interleukin-6 receptor antibodies and urocortin 2. Exp. Physiol. 2017, 102, 1177–1193. [Google Scholar] [CrossRef] [Green Version]
- Skelly, J.R.; Bradford, A.; Jones, J.F.; O’Halloran, K.D. Superoxide scavengers improve rat pharyngeal dilator muscle performance. Am. J. Respir. Cell Mol. Biol. 2010, 42, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Skelly, J.R.; Edge, D.; Shortt, C.M.; Jones, J.F.; Bradford, A.; O’Halloran, K.D. Tempol ameliorates pharyngeal dilator muscle dysfunction in a rodent model of chronic intermittent hypoxia. Am. J. Respir. Cell Mol. Biol. 2012, 46, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.; Lemaire, P.; Lewis, P.; McDonald, F.B.; Lucking, E.; Hogan, S.; Sheehan, D.; Healy, V.; O’Halloran, K.D. Chronic intermittent hypoxia increases rat sternohyoid muscle NADPH oxidase expression with attendant modest oxidative stress. Front. Physiol. 2015, 6, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piao, Y.J.; Seo, Y.H.; Hong, F.; Kim, J.H.; Kim, Y.J.; Kang, M.H.; Kim, B.S.; Jo, S.A.; Jo, I.; Jue, D.M.; et al. Nox 2 stimulates muscle differentiation via NF-kappaB/iNOS pathway. Free Radic. Biol. Med. 2005, 38, 989–1001. [Google Scholar] [CrossRef]
- Sun, Q.A.; Hess, D.T.; Nogueira, L.; Yong, S.; Bowles, D.E.; Eu, J.; Laurita, K.R.; Meissner, G.; Stamler, J.S. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc. Natl. Acad. Sci. USA 2011, 108, 16098–16103. [Google Scholar] [CrossRef]
- Hutchinson, D.S.; Csikasz, R.I.; Yamamoto, D.L.; Shabalina, I.G.; Wikström, P.; Wilcke, M.; Bengtsson, T. Diphenylene iodonium stimulates glucose uptake in skeletal muscle cells through mitochondrial complex I inhibition and activation of AMP-activated protein kinase. Cell Signal 2007, 19, 1610–1620. [Google Scholar] [CrossRef]
- Handayaningsih, A.E.; Iguchi, G.; Fukuoka, H.; Nishizawa, H.; Takahashi, M.; Yamamoto, M.; Herningtyas, E.H.; Okimura, Y.; Kaji, H.; Chihara, K.; et al. Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Endocrinology 2011, 152, 912–921. [Google Scholar] [CrossRef] [Green Version]
- Hori, Y.S.; Kuno, A.; Hosoda, R.; Tanno, M.; Miura, T.; Shimamoto, K.; Horio, Y. Resveratrol ameliorates muscular pathology in the dystrophic mdx mouse, a model for Duchenne muscular dystrophy. J. Pharmacol. Exp. Ther. 2011, 338, 784–794. [Google Scholar] [CrossRef] [Green Version]
- Spurney, C.F.; Knoblach, S.; Pistilli, E.E.; Nagaraju, K.; Martin, G.R.; Hoffman, E.P. Dystrophin-deficient cardiomyopathy in mouse: Expression of Nox4 and Lox are associated with fibrosis and altered functional parameters in the heart. Neuromuscul. Disord. 2008, 18, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, N.P.; Yeung, E.W.; Froehner, S.C.; Allen, D.G. Skeletal muscle NADPH oxidase is increased and triggers stretch-induced damage in the mdx mouse. PLoS ONE 2010, 5, e15354. [Google Scholar] [CrossRef] [Green Version]
- Sullivan-Gunn, M.J.; Lewandowski, P.A. Elevated hydrogen peroxide and decreased catalase and glutathione peroxidase protection are associated with aging sarcopenia. BMC Geriatr. 2013, 13, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, R.; Palmieri, M.; Loehr, J.A.; Li, S.; Abo-Zahrah, R.; Monroe, T.O.; Thakur, P.B.; Sardiello, M.; Rodney, G.G. Src-dependent impairment of autophagy by oxidative stress in a mouse model of Duchenne muscular dystrophy. Nat. Commun. 2014, 5, 4425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henríquez-Olguín, C.; Altamirano, F.; Valladares, D.; López, J.R.; Allen, P.D.; Jaimovich, E. Altered ROS production, NF-κB activation and interleukin-6 gene expression induced by electrical stimulation in dystrophic mdx skeletal muscle cells. Biochim. Biophys. Acta 2015, 1852, 1410–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, T.; Abe, M.; Lee, J.; Tatebayashi, D.; Himori, K.; Kanzaki, K.; Wada, M.; Bruton, J.D.; Westerblad, H.; Lanner, J.T. Muscle dysfunction associated with adjuvant-induced arthritis is prevented by antioxidant treatment. Skelet. Muscle 2015, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.J.; Yuan, G.; Jacono, F.J.; Kumar, G.K.; Prabhakar, N.R. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J. Physiol. 2006, 576 Pt 1, 289–295. [Google Scholar] [CrossRef]
- Jun, J.; Savransky, V.; Nanayakkara, A.; Bevans, S.; Li, J.; Smith, P.L.; Polotsky, V.Y. Intermittent hypoxia has organ-specific effects on oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1274–R1281. [Google Scholar] [CrossRef] [Green Version]
- Nisbet, R.E.; Graves, A.S.; Kleinhenz, D.J.; Rupnow, H.L.; Reed, A.L.; Fan, T.H.; Mitchell, P.O.; Sutliff, R.L.; Hart, C.M. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am. J. Respir. Cell Mol. Biol. 2009, 40, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.J.; Nanduri, J.; Yuan, G.; Wang, N.; Deneris, E.; Pendyala, S.; Natarajan, V.; Kumar, G.K.; Prabhakar, N.R. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J. Neurosci. 2009, 29, 4903–4910. [Google Scholar] [CrossRef] [Green Version]
- Hui-guo, L.; Kui, L.; Yan-ning, Z.; Yong-jian, X. Apocynin attenuate spatial learning deficits and oxidative responses to intermittent hypoxia. Sleep. Med. 2010, 11, 205–212. [Google Scholar] [CrossRef]
- Peng, Y.J.; Raghuraman, G.; Khan, S.A.; Kumar, G.K.; Prabhakar, N.R. Angiotensin II evokes sensory long-term facilitation of the carotid body via NADPH oxidase. J. Appl. Physiol. (1985) 2011, 111, 964–970. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Ding, X.S.; Zhang, Y.M.; Dai, D.Z.; Liu, M.; Zhang, C.; Dai, Y. Hypoxia/oxidative stress alters the pharmacokinetics of CPU86017-RS through mitochondrial dysfunction and NADPH oxidase activation. Acta Pharmacol. Sin. 2013, 34, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, A.C.C.; do Rêgo-Monteiro, I.C.; Louzada, R.A.; Ortenzi, V.H.; de Aguiar, A.P.; de Abreu, E.S.; Cavalcanti-de-Albuquerque, J.P.A.; Hecht, F.; de Oliveira, A.C.; Ceccatto, V.M.; et al. Differential Expression of NADPH Oxidases Depends on Skeletal Muscle Fiber Type in Rats. Oxidative Med. Cell. Longev. 2016, 2016, 6738701. [Google Scholar] [CrossRef] [Green Version]
- Serrander, L.; Cartier, L.; Bedard, K.; Banfi, B.; Lardy, B.; Plastre, O.; Sienkiewicz, A.; Fórró, L.; Schlegel, W.; Krause, K.-H. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem. J. 2007, 406, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diebold, I.; Petry, A.; Hess, J.; Gorlach, A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol. Biol. Cell 2010, 21, 2087–2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, B.; Beharry, A.W.; Coblentz, P.D.; Patel, N.; Judge, A.R.; Bonnell, M.R.; Hoopes, C.W. Diaphragm Abnormalities in Heart Failure Patients: Upregulation of NAD(P)H Oxidase Subunits and Heightened Protein Oxidation. In C73 OXIDANTS; American Thoracic Society: New York, NY, USA, 2016; p. A5916. [Google Scholar]
- Liu, X.H.; Harlow, L.; Graham, Z.A.; Bauman, W.A.; Cardozo, C. Spinal Cord Injury Leads to Hyperoxidation and Nitrosylation of Skeletal Muscle Ryanodine Receptor-1 Associated with Upregulation of Nicotinamide Adenine Dinucleotide Phosphate Oxidase 4. J. Neurotrauma 2017, 34, 2069–2074. [Google Scholar] [CrossRef]
- Regan, J.N.; Mikesell, C.; Reiken, S.; Xu, H.; Marks, A.R.; Mohammad, K.S.; Guise, T.A.; Waning, D.L. Osteolytic Breast Cancer Causes Skeletal Muscle Weakness in an Immunocompetent Syngeneic Mouse Model. Front. Endocrinol. 2017, 8, 358. [Google Scholar] [CrossRef] [Green Version]
- Cully, T.R.; Rodney, G.G. Nox4-RyR1-Nox2: Regulators of micro-domain signaling in skeletal muscle. Redox Biol. 2020, 36, 101557. [Google Scholar] [CrossRef]
- Javeshghani, D.; Magder, S.A.; Barreiro, E.; Quinn, M.T.; Hussain, S.N. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am. J. Respir. Crit. Care Med. 2002, 165, 412–418. [Google Scholar]
- Adams, V.; Linke, A.; Kränkel, N.; Erbs, S.; Gielen, S.; Möbius-Winkler, S.; Gummert, J.F.; Mohr, F.W.; Schuler, G.; Hambrecht, R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation 2005, 111, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Bowen, T.S.; Mangner, N.; Werner, S.; Glaser, S.; Kullnick, Y.; Schrepper, A.; Doenst, T.; Oberbach, A.; Linke, A.; Steil, L.; et al. Diaphragm muscle weakness in mice is early-onset post-myocardial infarction and associated with elevated protein oxidation. J. Appl. Physiol. 2015, 118, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Bowen, T.S.; Rolim, N.P.; Fischer, T.; Baekkerud, F.H.; Medeiros, A.; Werner, S.; Brønstad, E.; Rognmo, O.; Mangner, N.; Linke, A.; et al. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur. J. Heart Fail. 2015, 17, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Rezende, F.; Löwe, O.; Helfinger, V.; Prior, K.K.; Walter, M.; Zukunft, S.; Fleming, I.; Weissmann, N.; Brandes, R.P.; Schröder, K. Unchanged NADPH Oxidase Activity in Nox1-Nox2-Nox4 Triple Knockout Mice: What Do NADPH-Stimulated Chemiluminescence Assays Really Detect? Antioxid. Redox Signal 2016, 24, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Shiose, A.; Kuroda, J.; Tsuruya, K.; Hirai, M.; Hirakata, H.; Naito, S.; Hattori, M.; Sakaki, Y.; Sumimoto, H. A Novel Superoxide-producing NAD(P)H Oxidase in Kidney. J. Biol. Chem. 2001, 276, 1417–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of oxidative stress in skeletal muscle injury and regeneration: Focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–393. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Yin, X.; Wang, Y.; Tan, Y.; Cai, L.; Wang, B.; Cai, J.; Fu, Y. Intermittent hypoxia-induced renal antioxidants and oxidative damage in male mice: Hormetic dose response. Dose Response 2012, 11, 385–400. [Google Scholar] [CrossRef]
- Zhou, S.; Yin, X.; Jin, J.; Tan, Y.; Conklin, D.J.; Xin, Y.; Zhang, Z.; Sun, W.; Cui, T.; Cai, J.; et al. Intermittent hypoxia-induced cardiomyopathy and its prevention by Nrf2 and metallothionein. Free Radic. Biol. Med. 2017, 112, 224–239. [Google Scholar] [CrossRef]
- Chandran, R.; Kim, T.; Mehta, S.L.; Udho, E.; Chanana, V.; Cengiz, P.; Kim, H.; Kim, C.; Vemuganti, R. A combination antioxidant therapy to inhibit NOX2 and activate Nrf2 decreases secondary brain damage and improves functional recovery after traumatic brain injury. J. Cereb. Blood Flow. Metab. 2018, 38, 1818–1827. [Google Scholar] [CrossRef]
- Hopps, E.; Canino, B.; Calandrino, V.; Montana, M.; Lo Presti, R.; Caimi, G. Lipid peroxidation and protein oxidation are related to the severity of OSAS. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3773–3778. [Google Scholar]
- Kent, B.D.; Ryan, S.; McNicholas, W.T. Obstructive sleep apnea and inflammation: Relationship to cardiovascular co-morbidity. Respir. Physiol. Neurobiol. 2011, 178, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, J.L.; Andrade, F.H. Mitochondrial content and distribution changes specific to mouse diaphragm after chronic normobaric hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R575–R583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shortt, C.M.; Fredsted, A.; Bradford, A.; O’Halloran, K.D. Diaphragm muscle remodeling in a rat model of chronic intermittent hypoxia. J. Histochem. Cytochem. 2013, 61, 487–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, A.; Van der Meulen, J.H.; Defour, A.; Hogarth, M.; Sreetama, S.C.; Reed, A.; Scheffer, L.; Chandel, N.S.; Jaiswal, J.K. Mitochondrial redox signaling enables repair of injured skeletal muscle cells. Sci. Signal 2017, 10, eaaj1978. [Google Scholar] [CrossRef] [Green Version]
- Narendra, D.P.; Youle, R.J. Targeting mitochondrial dysfunction: Role for PINK1 and Parkin in mitochondrial quality control. Antioxid. Redox Signal 2011, 14, 1929–1938. [Google Scholar] [CrossRef]
- Lee, S.H.; Du, J.; Stitham, J.; Atteya, G.; Lee, S.; Xiang, Y.; Wang, D.; Jin, Y.; Leslie, K.L.; Spollett, G.; et al. Inducing mitophagy in diabetic platelets protects against severe oxidative stress. EMBO Mol. Med. 2016, 8, 779–795. [Google Scholar] [CrossRef]
- Tang, Y.C.; Tian, H.X.; Yi, T.; Chen, H.B. The critical roles of mitophagy in cerebral ischemia. Protein Cell 2016, 7, 699–713. [Google Scholar] [CrossRef] [Green Version]
- Nah, J.; Miyamoto, S.; Sadoshima, J. Mitophagy as a Protective Mechanism against Myocardial Stress. Compr. Physiol. 2017, 7, 1407–1424. [Google Scholar]
- Tan, V.P.; Smith, J.M.; Tu, M.; Yu, J.D.; Ding, E.Y.; Miyamoto, S. Dissociation of mitochondrial HK-II elicits mitophagy and confers cardioprotection against ischemia. Cell Death Dis. 2019, 10, 730. [Google Scholar] [CrossRef] [Green Version]
- Borgia, D.; Malena, A.; Spinazzi, M.; Desbats, M.A.; Salviati, L.; Russell, A.P.; Miotto, G.; Tosatto, L.; Pegoraro, E.; Sorarù, G.; et al. Increased mitophagy in the skeletal muscle of spinal and bulbar muscular atrophy patients. Hum. Mol. Genet. 2017, 26, 1087–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, Y.; Ohtsubo, H.; Nihei, N.; Kaneko, T.; Adachi, S.I.; Kondo, S.; Nakamura, M.; Mizunoya, W.; Iida, H.; Tatsumi, R.; et al. Apobec2 deficiency causes mitochondrial defects and mitophagy in skeletal muscle. Faseb J. 2018, 32, 1428–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalov, S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011, 51, 1289–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.M.; Kim, S.J.; Tatsunami, R.; Yamamura, H.; Fukai, T.; Ushio-Fukai, M. ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis. Am. J. Physiol. Cell Physiol. 2017, 312, C749–C764. [Google Scholar] [CrossRef] [Green Version]
- Dobrowolny, G.; Aucello, M.; Rizzuto, E.; Beccafico, S.; Mammucari, C.; Boncompagni, S.; Belia, S.; Wannenes, F.; Nicoletti, C.; Del Prete, Z.; et al. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 2008, 8, 425–436. [Google Scholar] [CrossRef]
- Kadoguchi, T.; Takada, S.; Yokota, T.; Furihata, T.; Matsumoto, J.; Tsuda, M.; Mizushima, W.; Fukushima, A.; Okita, K.; Kinugawa, S. Deletion of NAD(P)H Oxidase 2 Prevents Angiotensin II-Induced Skeletal Muscle Atrophy. BioMed Res. Int. 2018, 2018, 3194917. [Google Scholar] [CrossRef] [Green Version]
- Yun, Z.; Lin, Q.; Giaccia, A.J. Adaptive myogenesis under hypoxia. Mol. Cell Biol. 2005, 25, 3040–3055. [Google Scholar] [CrossRef] [Green Version]
- Sakushima, K.; Yoshikawa, M.; Osaki, T.; Miyamoto, N.; Hashimoto, T. Moderate hypoxia promotes skeletal muscle cell growth and hypertrophy in C2C12 cells. Biochem. Biophys. Res. Commun. 2020, 525, 921–927. [Google Scholar] [CrossRef]
- Smith, L.R.; Barton, E.R. Regulation of fibrosis in muscular dystrophy. Matrix Biol. 2018, 68–69, 602–615. [Google Scholar] [CrossRef]
- Didier, M.; Rotenberg, C.; Marchant, D.; Sutton, A.; Valeyre, D.; Nunes, H.; Boncoeur, E.; Planes, C. Effect of Chronic Intermittent Hypoxia in a Murine Model of Bleomycin-Induced Pulmonary Fibrosis. In B57 FIBROSIS BIOLOGY; American Thoracic Society: New York, NY, USA, 2016; p. A4014. [Google Scholar]
- Kang, H.H.; Kim, I.K.; Lee, H.I.; Joo, H.; Lim, J.U.; Lee, J.; Lee, S.H.; Moon, H.S. Chronic intermittent hypoxia induces liver fibrosis in mice with diet-induced obesity via TLR4/MyD88/MAPK/NF-kB signaling pathways. Biochem. Biophys. Res. Commun. 2017, 490, 349–355. [Google Scholar] [CrossRef]
- Kang, H.H.; Kim, I.K.; Lee, S.H. Chronic intermittent hypoxia exacerbates lung fibrosis in bleomycin-induced lung injury mouse model. Eur. Respir. J. 2018, 52 (Suppl. S62), PA428. [Google Scholar]
- Wang, W.; Zhang, K.; Li, X.; Ma, Z.; Zhang, Y.; Yuan, M.; Suo, Y.; Liang, X.; Tse, G.; Goudis, C.A.; et al. Doxycycline attenuates chronic intermittent hypoxia-induced atrial fibrosis in rats. Cardiovasc. Ther. 2018, 36, e12321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Su, X.; Zou, F.; Xu, T.; Pan, P.; Hu, C. Toll-like receptor-4 deficiency alleviates chronic intermittent hypoxia-induced renal injury, inflammation, and fibrosis. Sleep. Breath. 2019, 23, 503–513. [Google Scholar] [CrossRef] [PubMed]
- de Paula Brotto, M.; van Leyen, S.A.; Brotto, L.S.; Jin, J.P.; Nosek, C.M.; Nosek, T.M. Hypoxia/fatigue-induced degradation of troponin I and troponin C: New insights into physiologic muscle fatigue. Pflugers Arch. 2001, 442, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Ottenheijm, C.A.; Heunks, L.M.; Sieck, G.C.; Zhan, W.Z.; Jansen, S.M.; Degens, H.; de Boo, T.; Dekhuijzen, P.N. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 172, 200–205. [Google Scholar] [CrossRef] [Green Version]
- Ottenheijm, C.A.; Heunks, L.M.; Geraedts, M.C.; Dekhuijzen, P.N. Hypoxia-induced skeletal muscle fiber dysfunction: Role for reactive nitrogen species. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L127–L135. [Google Scholar] [CrossRef] [Green Version]
- Coirault, C.; Guellich, A.; Barbry, T.; Samuel, J.L.; Riou, B.; Lecarpentier, Y. Oxidative stress of myosin contributes to skeletal muscle dysfunction in rats with chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1009–H1017. [Google Scholar] [CrossRef]
- Degens, H.; Bosutti, A.; Gilliver, S.F.; Slevin, M.; van Heijst, A.; Wüst, R.C. Changes in contractile properties of skinned single rat soleus and diaphragm fibres after chronic hypoxia. Pflugers Arch. 2010, 460, 863–873. [Google Scholar] [CrossRef]
- Smuder, A.J.; Kavazis, A.N.; Hudson, M.B.; Nelson, W.B.; Powers, S.K. Oxidation enhances myofibrillar protein degradation via calpain and caspase-3. Free Radic. Biol. Med. 2010, 49, 1152–1160. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.R.; de Sousa, V.P.; Sorenson, M.M. Redox state of troponin C cysteine in the D/E helix alters the C-domain affinity for the thin filament of vertebrate striated muscle. Biochim. Biophys. Acta 2011, 1810, 391–397. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, B.; Sakellariou, G.K.; Smith, N.T.; Brownridge, P.; Jackson, M.J. Differential cysteine labeling and global label-free proteomics reveals an altered metabolic state in skeletal muscle aging. J. Proteome Res. 2014, 13, 5008–5021. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.D.; Patial, K.; Vijayan, V.K.; Ravi, K. Oxidative stress and obstructive sleep apnoea syndrome. Indian J. Chest Dis. Allied Sci. 2009, 51, 217–224. [Google Scholar] [PubMed]
- Sadasivam, K.; Patial, K.; Vijayan, V.K.; Ravi, K. Anti-oxidant treatment in obstructive sleep apnoea syndrome. Indian J. Chest Dis. Allied Sci. 2011, 53, 153–162. [Google Scholar] [PubMed]
- Wu, K.; Su, X.; Li, G.; Zhang, N. Antioxidant Carbocysteine Treatment in Obstructive Sleep Apnea Syndrome: A Randomized Clinical Trial. PLoS ONE 2016, 11, e0148519. [Google Scholar] [CrossRef]



| Gene Name | Gene Symbol | Assay ID |
|---|---|---|
| NOX enzymes | ||
| NOX1 | NOX1 | 310986 |
| NOX2 | Cybb | 317885 |
| NOX4 | NOX4 | 300795 |
| p22phox | Cyba | 317890 |
| p47phox | Ncf1 | 301105 |
| p67phox | Ncf2 | 317897 |
| p40phox | Ncf4 | 317894 |
| Rac | Racgap1 | 310907 |
| Duox1 | Duox1 | 317891 |
| Duox2 | Duox2 | 317888 |
| Atrophy | ||
| Atrogin-1 | Fbxo32 | 317844 |
| MuRF-1 | Trim63 | 317843 |
| Autophagy | ||
| BNIP3 | Bnip3 | 311465 |
| LC3B | Map1lc3b | 317920 |
| GABARAPL1 | Gabarapl1 | 317923 |
| Mitophagy | ||
| PINK-1 | Pink1 | 331846 |
| PARK-2 | Park2 | 317264 |
| Inflammation | ||
| NFκB | Nfkb1 | 300085 |
| Antioxidant | ||
| SOD1 | Sod1 | 310738 |
| SOD2 | Sod2 | 310295 |
| Catalase | Cat | 310718 |
| Nrf2 | Nfe2l2 | 313377 |
| Muscle differentiation | ||
| Myogenin | Myog | 313501 |
| Myostatin | Mstn | 318626 |
| MyoD | Myod1 | 313570 |
| MEF2C | Mef2c | 318629 |
| IGF1 | Igf1 | 313359 |
| Sirtuin-1 | Sirt1 | 310480 |
| Reference | ||
| HPRT1 | Hprt1 | 307879 |
| Sham (n = 8) | CIH (n = 8) | CIH + APO (n = 9) | One-Way ANOVA | Sham vs. CIH (p Value) | CIH vs. CIH + APO (p Value) | |
|---|---|---|---|---|---|---|
| Pt (N/cm2) | 2.62 ± 0.90 | 1.63 ± 0.31 | 4.10 ± 1.86 | 0.0021 | 0.2738 | 0.0016 |
| CT (ms) | 15.70 ± 0.96 | 16.70 ± 3.87 | 18.17 ± 3.14 | 0.2378 | - | - |
| ½ RT (ms) | 19.69 ± 7.82 | 19.63 ± 6.39 | 17.50 ± 3.79 | 0.7045 | - | - |
| Wmax (J/cm2) | 1.60 ± 0.85 | 0.63 ± 0.21 | 1.69 ± 0.84 | 0.0012 | 0.0060 | 0.0030 |
| Pmax (W/cm2) | 14.70 ± 8.13 | 6.57 ± 2.35 | 13.60 ± 4.55 | 0.0142 | 0.0193 | 0.0392 |
| Smax (L/L0) | 0.35 ± 0.06 | 0.30 ± 0.08 | 0.37 ± 0.07 | 0.1374 | - | - |
| Vmax (L0/s) | 4.78 ± 1.57 | 3.59 ± 1.19 | 4.51 ± 0.95 | 0.1403 | - | - |
| Muscle mass (mg) | 1.48 ± 0.13 | 1.30 ± 0.21 | 1.47 ± 0.47 | 0.2680 | - | - |
| Body mass (g) | 23.6 ± 1.5 | 21.4 ± 0.7 | 23.5 ± 1.6 | 0.0014 | 0.0032 | 0.0047 |
| NOX2 KO Sham (n = 9) | NOX2 KO CIH (n = 9) | NOX2 KO Sham vs. NOX2 KO CIH (p Value) | |
|---|---|---|---|
| Pt (N/cm2) | 3.50 ± 2.30 | 5.52 ± 2.75 | 0.1107 |
| CT (ms) | 12.28 ± 2.00 | 12.44 ± 1.67 | 0.8502 |
| ½ RT (ms) | 13.39 ± 5.41 | 12.56 ± 5.04 | 0.8482 |
| Wmax (J/cm2) | 2.42 ± 0.67 | 2.16 ± 0.38 | 0.3923 |
| Pmax (W/cm2) | 18.74 ± 4.61 | 15.57 ± 5.02 | 0.1821 |
| Smax (L/L0) | 0.40 ± 0.05 | 0.36 ± 0.04 | 0.1148 |
| Vmax (L0/s) | 4.53 ± 1.39 | 3.98 ± 1.27 | 0.3958 |
| Muscle mass (mg) | 2.01 ± 0.89 | 1.66 ± 0.39 | 0.2866 |
| Body mass (g) | 28.1 ± 2.5 | 25.4 ± 0.9 | 0.0042 |
| Sham (n = 8) | CIH (n = 8) | Sham vs. CIH (p Value) | |
|---|---|---|---|
| NADPH Oxidase Activity (nmol/min/µg) | 0.26 ± 0.09 | 0.29 ± 0.06 | 0.5565 |
| TBARS (nM/mg) | 193.2 ± 93.18 | 151.6 ± 79.53 | 0.3536 |
| Citrate Synthase Activity (µmole/mg) | 0.68 ± 0.20 | 0.63 ± 0.22 | 0.5892 |
| HIF-1α (signal/µg) | 2.73 ± 0.48 | 2.62 ± 0.34 | 0.5982 |
| Phospho-FOXO-3a (signal/µg) | 72.81 ± 19.59 | 63.27 ± 18.09 | 0.3284 |
| Phopho-ERK1/2 (signal/µg) | 54.49 ± 18.05 | 41.43 ± 17.61 | 0.0379 |
| Phospho-JNK (signal/µg) | 61.15 ± 10.34 | 48.25 ± 10.82 | 0.0287 |
| Phospho-p38 (signal/µg) | 13.83 ± 1.18 | 11.36 ± 1.10 | 0.0007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drummond, S.E.; Burns, D.P.; El Maghrani, S.; Ziegler, O.; Healy, V.; O’Halloran, K.D. Chronic Intermittent Hypoxia-Induced Diaphragm Muscle Weakness Is NADPH Oxidase-2 Dependent. Cells 2023, 12, 1834. https://doi.org/10.3390/cells12141834
Drummond SE, Burns DP, El Maghrani S, Ziegler O, Healy V, O’Halloran KD. Chronic Intermittent Hypoxia-Induced Diaphragm Muscle Weakness Is NADPH Oxidase-2 Dependent. Cells. 2023; 12(14):1834. https://doi.org/10.3390/cells12141834
Chicago/Turabian StyleDrummond, Sarah E., David P. Burns, Sarah El Maghrani, Oscar Ziegler, Vincent Healy, and Ken D. O’Halloran. 2023. "Chronic Intermittent Hypoxia-Induced Diaphragm Muscle Weakness Is NADPH Oxidase-2 Dependent" Cells 12, no. 14: 1834. https://doi.org/10.3390/cells12141834





