Chemokine/ITGA4 Interaction Directs iPSC-Derived Myogenic Progenitor Migration to Injury Sites in Aging Muscle for Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human iPSC Culture
2.2. Generation of iPSC-Derived Muscle Progenitor Cells
2.3. Generation of ITGA4 Knockdown Muscle Progenitor Cells
2.4. Cell Migration
2.5. Immunofluorescence Staining for Cells
2.6. RNA Extraction and Realtime-PCR
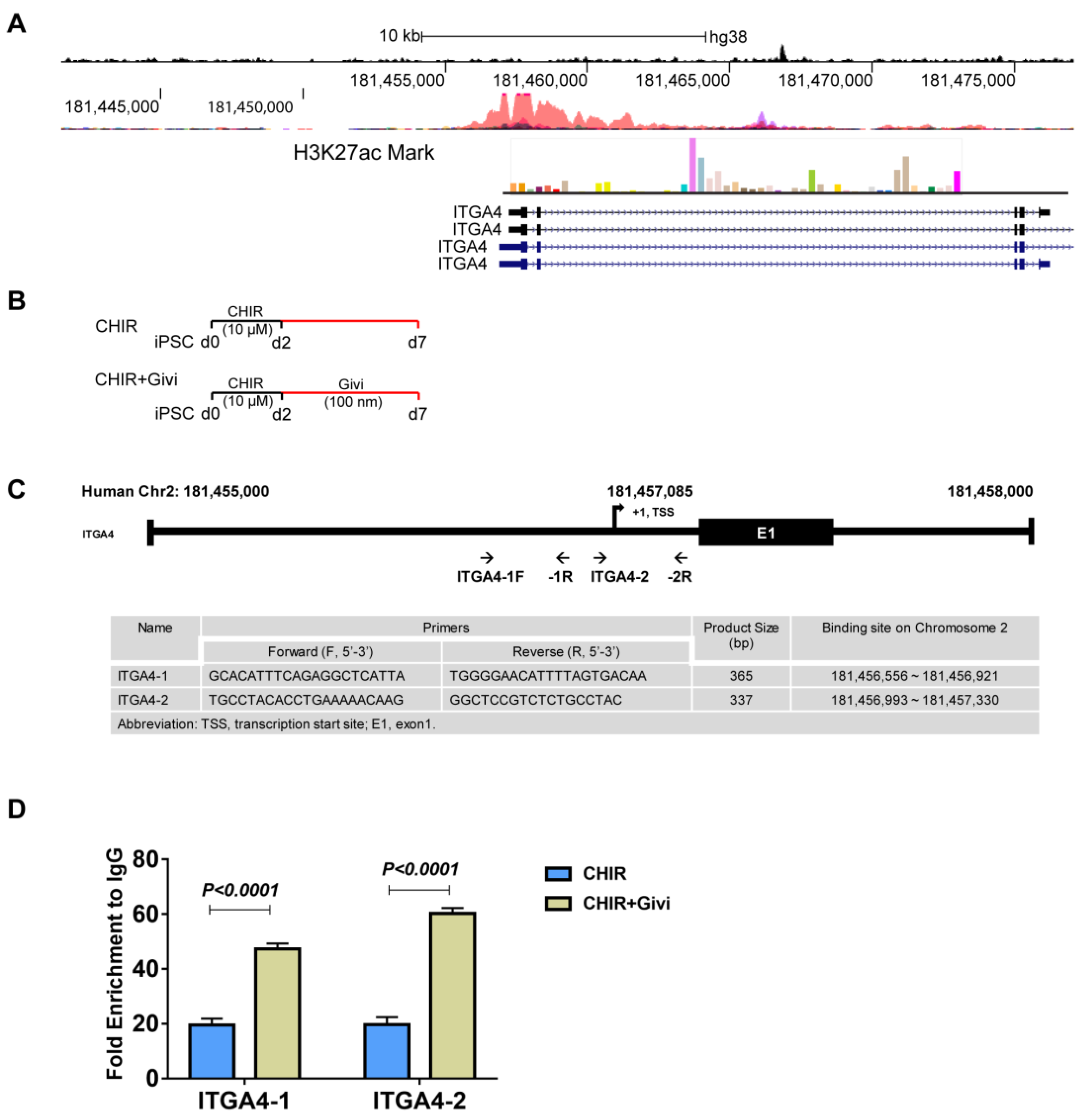
2.7. Chromatin Immunoprecipitation (ChIP)
2.8. Muscle Injury Model and Cell Transplantation
2.9. Histology
2.10. Immunofluorescence Staining for Muscle Tissue
2.11. Grip Strength Test
2.12. Statistical Analysis
3. Results
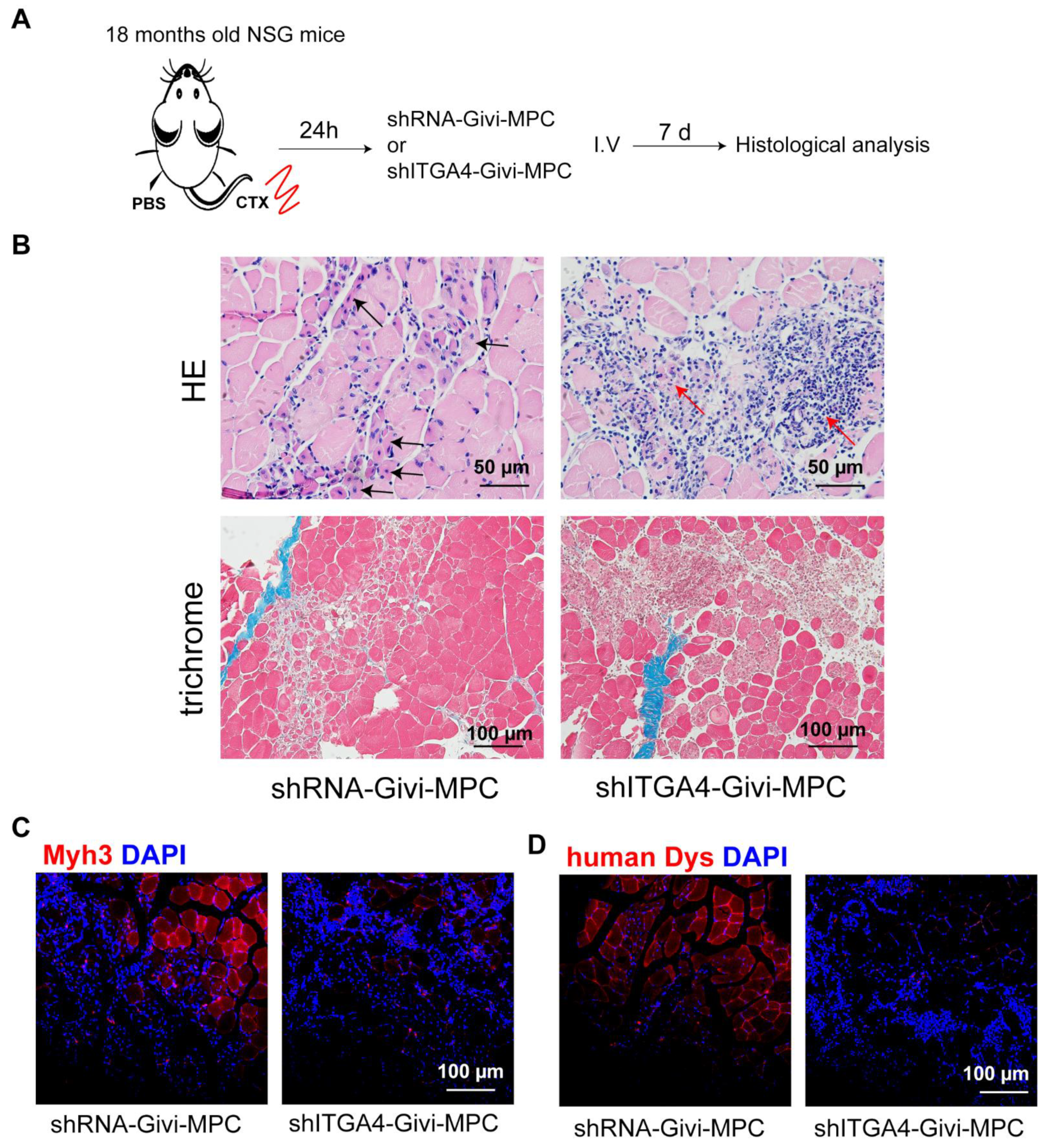
3.1. In Vitro and In Vivo Migration Characteristics of Givi-MPCs
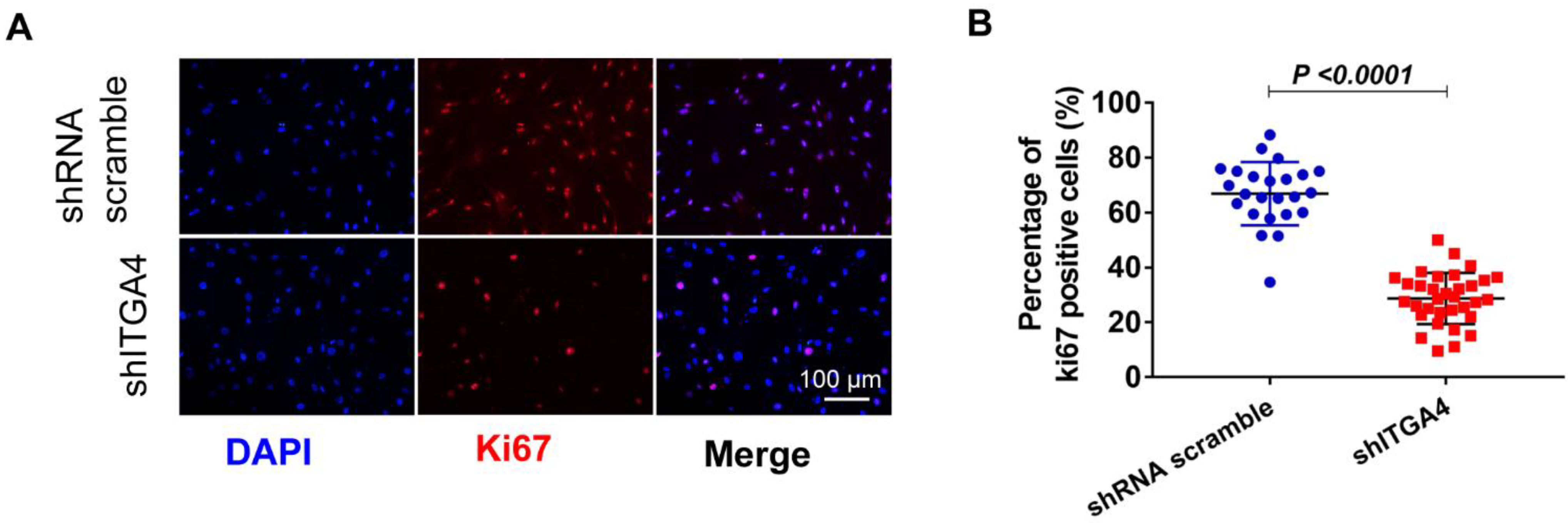
3.2. Knockdown of ITGA4 Impaired Proliferation and Differentiation of Givi-MPCs
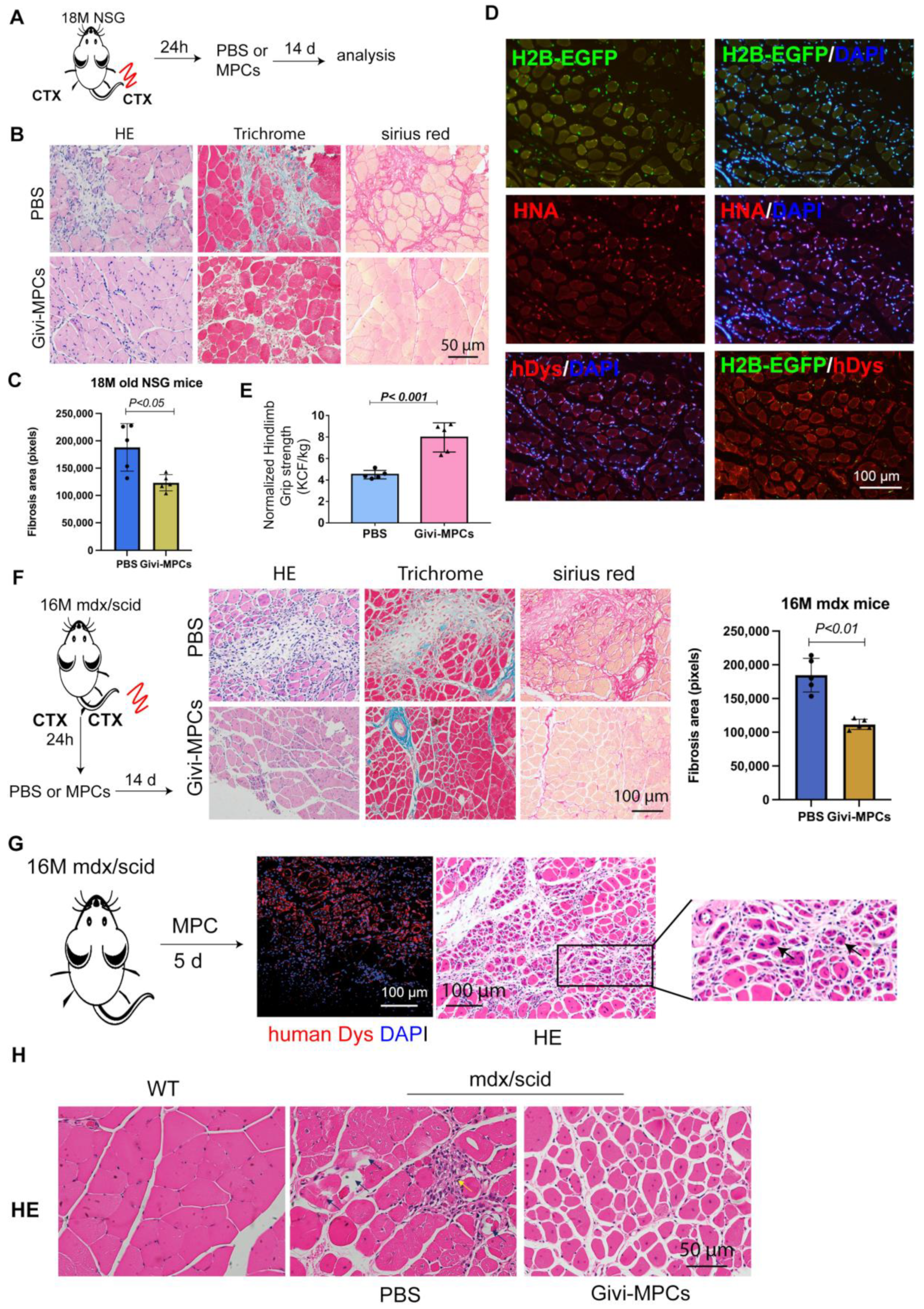
3.3. Both Local and Systemic Delivery of Givi-MPCs Improved Muscle Regeneration in Aging Muscle
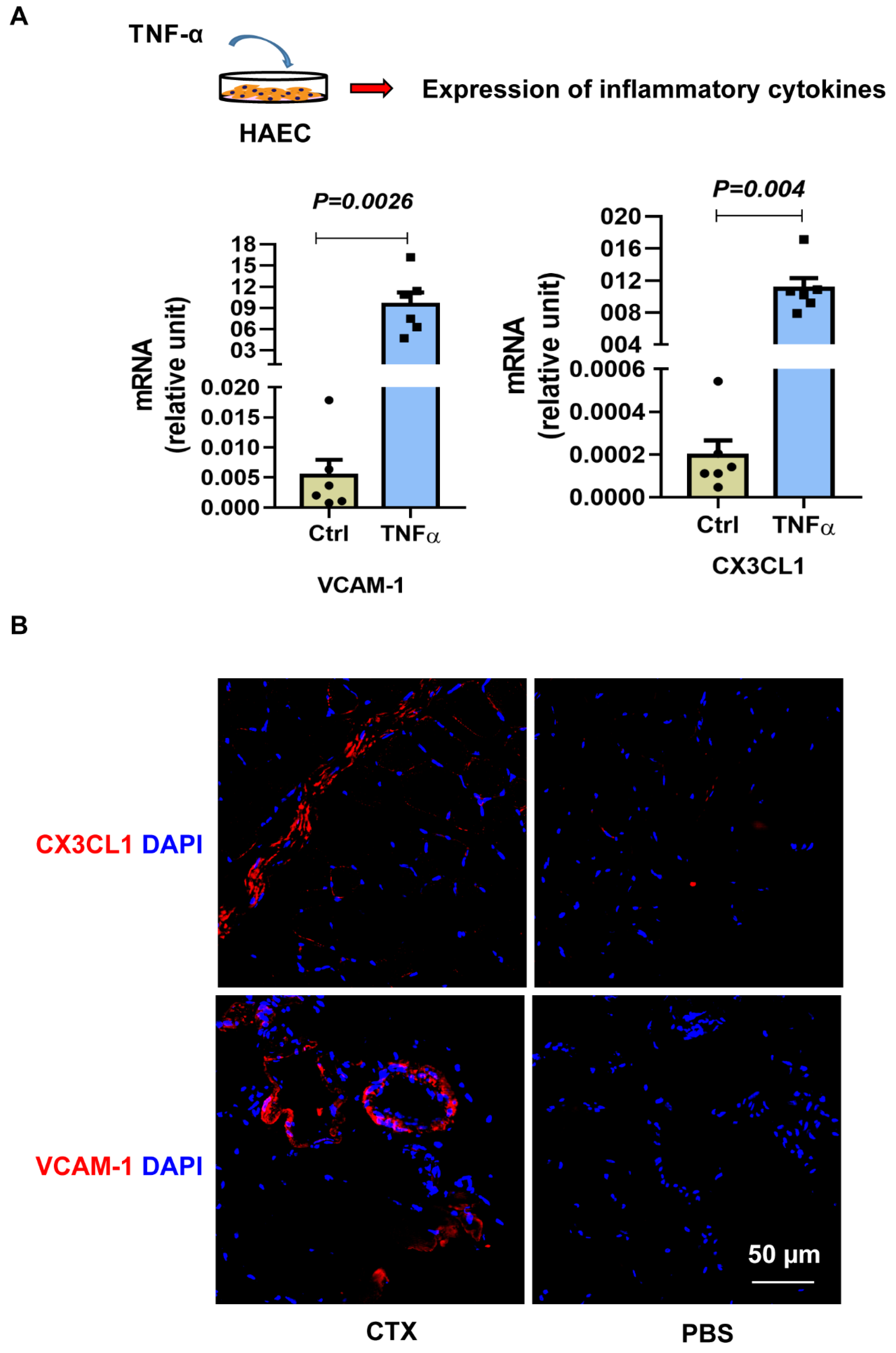
3.4. Mechanism of ITGA4 Expression in Givi-MPCs
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lo, J.H.; U, K.P.; Yiu, T.; Ong, M.T.; Lee, W.Y. Sarcopenia: Current treatments and new regenerative therapeutic approaches. J. Orthop. Translat. 2020, 23, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, D.; Yang, Y.; Xie, W.; He, M.; Yu, D.; Wu, Y.; Wang, X.; Xiao, W.; Li, Y. The role and therapeutic potential of stem cells in skeletal muscle in sarcopenia. Stem. Cell Res. Ther. 2022, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Brotto, M.; Abreu, E.L. Sarcopenia: Pharmacology of today and tomorrow. J. Pharmacol. Exp. Ther. 2012, 343, 540–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckwee, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J. Nutr. Health Aging 2019, 23, 494–502. [Google Scholar] [CrossRef]
- Brooks, S.V.; Faulkner, J.A. Contractile properties of skeletal muscles from young, adult and aged mice. J. Physiol. 1988, 404, 71–82. [Google Scholar] [CrossRef]
- Rader, E.P.; Faulkner, J.A. Recovery from contraction-induced injury is impaired in weight-bearing muscles of old male mice. J. Appl. Physiol. 2006, 100, 656–661. [Google Scholar] [CrossRef] [Green Version]
- Greiwe, J.S.; Cheng, B.; Rubin, D.C.; Yarasheski, K.E.; Semenkovich, C.F. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J. 2001, 15, 475–482. [Google Scholar] [CrossRef]
- Brooks, S.V.; Opiteck, J.A.; Faulkner, J.A. Conditioning of skeletal muscles in adult and old mice for protection from contraction-induced injury. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B163–B171. [Google Scholar] [CrossRef] [Green Version]
- Relaix, F.; Bencze, M.; Borok, M.J.; Der Vartanian, A.; Gattazzo, F.; Mademtzoglou, D.; Perez-Diaz, S.; Prola, A.; Reyes-Fernandez, P.C.; Rotini, A.; et al. Perspectives on skeletal muscle stem cells. Nat. Commun. 2021, 12, 692. [Google Scholar] [CrossRef]
- Huo, F.; Liu, Q.; Liu, H. Contribution of muscle satellite cells to sarcopenia. Front. Physiol. 2022, 13, 892749. [Google Scholar] [CrossRef]
- Hong, X.; Campanario, S.; Ramirez-Pardo, I.; Grima-Terren, M.; Isern, J.; Munoz-Canoves, P. Stem cell aging in the skeletal muscle: The importance of communication. Ageing Res. Rev. 2022, 73, 101528. [Google Scholar] [CrossRef]
- Naranjo, J.D.; Dziki, J.L.; Badylak, S.F. Regenerative Medicine Approaches for Age-Related Muscle Loss and Sarcopenia: A Mini-Review. Gerontology 2017, 63, 580–589. [Google Scholar] [CrossRef] [Green Version]
- Qazi, T.H.; Duda, G.N.; Ort, M.J.; Perka, C.; Geissler, S.; Winkler, T. Cell therapy to improve regeneration of skeletal muscle injuries. J. Cachexia Sarcopenia Muscle 2019, 10, 501–516. [Google Scholar] [CrossRef] [Green Version]
- Xuan, W.; Khan, M.; Ashraf, M. Pluripotent stem cell-induced skeletal muscle progenitor cells with givinostat promote myoangiogenesis and restore dystrophin in injured Duchenne dystrophic muscle. Stem. Cell Res. Ther. 2021, 12, 131. [Google Scholar] [CrossRef]
- Iberite, F.; Gruppioni, E.; Ricotti, L. Skeletal muscle differentiation of human iPSCs meets bioengineering strategies: Perspectives and challenges. NPJ Regen. Med. 2022, 7, 23. [Google Scholar] [CrossRef]
- Lapasset, L.; Milhavet, O.; Prieur, A.; Besnard, E.; Babled, A.; Ait-Hamou, N.; Leschik, J.; Pellestor, F.; Ramirez, J.M.; De Vos, J.; et al. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011, 25, 2248–2253. [Google Scholar] [CrossRef] [Green Version]
- Chal, J.; Oginuma, M.; Al Tanoury, Z.; Gobert, B.; Sumara, O.; Hick, A.; Bousson, F.; Zidouni, Y.; Mursch, C.; Moncuquet, P.; et al. Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nat. Biotechnol. 2015, 33, 962–969. [Google Scholar] [CrossRef] [Green Version]
- Otto, A.; Collins-Hooper, H.; Patel, A.; Dash, P.R.; Patel, K. Adult skeletal muscle stem cell migration is mediated by a blebbing/amoeboid mechanism. Rejuvenation Res. 2011, 14, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.S.; Arpke, R.W.; Filareto, A.; Xie, N.; Pappas, M.P.; Penaloza, J.S.; Perlingeiro, R.C.R.; Kyba, M. Skeletal Muscle Stem Cells from PSC-Derived Teratomas Have Functional Regenerative Capacity. Cell Stem Cell 2018, 23, 74–85.e6. [Google Scholar] [CrossRef] [Green Version]
- Manickam, R.; Tur, J.; Badole, S.L.; Chapalamadugu, K.C.; Sinha, P.; Wang, Z.; Russ, D.W.; Brotto, M.; Tipparaju, S.M. Nampt activator P7C3 ameliorates diabetes and improves skeletal muscle function modulating cell metabolism and lipid mediators. J. Cachexia Sarcopenia Muscle 2022, 13, 1177–1196. [Google Scholar] [CrossRef]
- Jung, J.W.; Kwon, M.; Choi, J.C.; Shin, J.W.; Park, I.W.; Choi, B.W.; Kim, J.Y. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med. J. 2013, 54, 1293–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavakis, E.; Urbich, C.; Dimmeler, S. Homing and engraftment of progenitor cells: A prerequisite for cell therapy. J. Mol. Cell. Cardiol. 2008, 45, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Ganju, R.K.; Brubaker, S.A.; Meyer, J.; Dutt, P.; Yang, Y.; Qin, S.; Newman, W.; Groopman, J.E. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J. Biol. Chem. 1998, 273, 23169–23175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christopherson, K.W., 2nd; Hangoc, G.; Mantel, C.R.; Broxmeyer, H.E. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science 2004, 305, 1000–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hight-Warburton, W.; Parsons, M. Regulation of cell migration by alpha4 and alpha9 integrins. Biochem. J. 2019, 476, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Hight-Warburton, W.; Felix, R.; Burton, A.; Maple, H.; Chegkazi, M.S.; Steiner, R.A.; McGrath, J.A.; Parsons, M. alpha4/alpha9 Integrins Coordinate Epithelial Cell Migration Through Local Suppression of MAP Kinase Signaling Pathways. Front. Cell Dev. Biol. 2021, 9, 750771. [Google Scholar] [CrossRef]
- Bajanca, F.; Thorsteinsdottir, S. Integrin expression patterns during early limb muscle development in the mouse. Gene Expr. Patterns 2002, 2, 133–136. [Google Scholar] [CrossRef]
- Cachaco, A.S.; Pereira, C.S.; Pardal, R.G.; Bajanca, F.; Thorsteinsdottir, S. Integrin repertoire on myogenic cells changes during the course of primary myogenesis in the mouse. Dev. Dyn. 2005, 232, 1069–1078. [Google Scholar] [CrossRef]
- Shelton, M.; Ritso, M.; Liu, J.; O’Neil, D.; Kocharyan, A.; Rudnicki, M.A.; Stanford, W.L.; Skerjanc, I.S.; Blais, A. Gene expression profiling of skeletal myogenesis in human embryonic stem cells reveals a potential cascade of transcription factors regulating stages of myogenesis, including quiescent/activated satellite cell-like gene expression. PLoS ONE 2019, 14, e0222946. [Google Scholar] [CrossRef]
- Rosen, G.D.; Sanes, J.R.; LaChance, R.; Cunningham, J.M.; Roman, J.; Dean, D.C. Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell 1992, 69, 1107–1119. [Google Scholar] [CrossRef]
- Fujita, M.; Takada, Y.K.; Takada, Y. The chemokine fractalkine can activate integrins without CX3CR1 through direct binding to a ligand-binding site distinct from the classical RGD-binding site. PLoS ONE 2014, 9, e96372. [Google Scholar] [CrossRef] [Green Version]
- Choo, H.J.; Canner, J.P.; Vest, K.E.; Thompson, Z.; Pavlath, G.K. A tale of two niches: Differential functions for VCAM-1 in satellite cells under basal and injured conditions. Am. J. Physiol. Cell Physiol. 2017, 313, C392–C404. [Google Scholar] [CrossRef] [Green Version]
- Fujita, M.; Takada, Y.K.; Takada, Y. Integrins alphavbeta3 and alpha4beta1 act as coreceptors for fractalkine, and the integrin-binding defective mutant of fractalkine is an antagonist of CX3CR1. J. Immunol. 2012, 189, 5809–5819. [Google Scholar] [CrossRef] [Green Version]
- Imaizumi, T.; Yoshida, H.; Satoh, K. Regulation of CX3CL1/fractalkine expression in endothelial cells. J. Atheroscler. Thromb. 2004, 11, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal. 2011, 15, 1607–1638. [Google Scholar] [CrossRef] [Green Version]
- Xuan, W.; Liao, Y.; Chen, B.; Huang, Q.; Xu, D.; Liu, Y.; Bin, J.; Kitakaze, M. Detrimental effect of fractalkine on myocardial ischaemia and heart failure. Cardiovasc. Res. 2011, 92, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Budai, Z.; Balogh, L.; Sarang, Z. Altered Gene Expression of Muscle Satellite Cells Contributes to Agerelated Sarcopenia in Mice. Curr. Aging Sci. 2018, 11, 165–172. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, M.; Tipparaju, S.M.; Kim, J.W.; Xuan, W. Chemokine/ITGA4 Interaction Directs iPSC-Derived Myogenic Progenitor Migration to Injury Sites in Aging Muscle for Regeneration. Cells 2023, 12, 1837. https://doi.org/10.3390/cells12141837
Ashraf M, Tipparaju SM, Kim JW, Xuan W. Chemokine/ITGA4 Interaction Directs iPSC-Derived Myogenic Progenitor Migration to Injury Sites in Aging Muscle for Regeneration. Cells. 2023; 12(14):1837. https://doi.org/10.3390/cells12141837
Chicago/Turabian StyleAshraf, Muhammad, Srinivas M. Tipparaju, Joung Woul Kim, and Wanling Xuan. 2023. "Chemokine/ITGA4 Interaction Directs iPSC-Derived Myogenic Progenitor Migration to Injury Sites in Aging Muscle for Regeneration" Cells 12, no. 14: 1837. https://doi.org/10.3390/cells12141837
APA StyleAshraf, M., Tipparaju, S. M., Kim, J. W., & Xuan, W. (2023). Chemokine/ITGA4 Interaction Directs iPSC-Derived Myogenic Progenitor Migration to Injury Sites in Aging Muscle for Regeneration. Cells, 12(14), 1837. https://doi.org/10.3390/cells12141837






