First-in-Class Colchicine-Based Visible Light Photoswitchable Microtubule Dynamics Disrupting Agent
Abstract
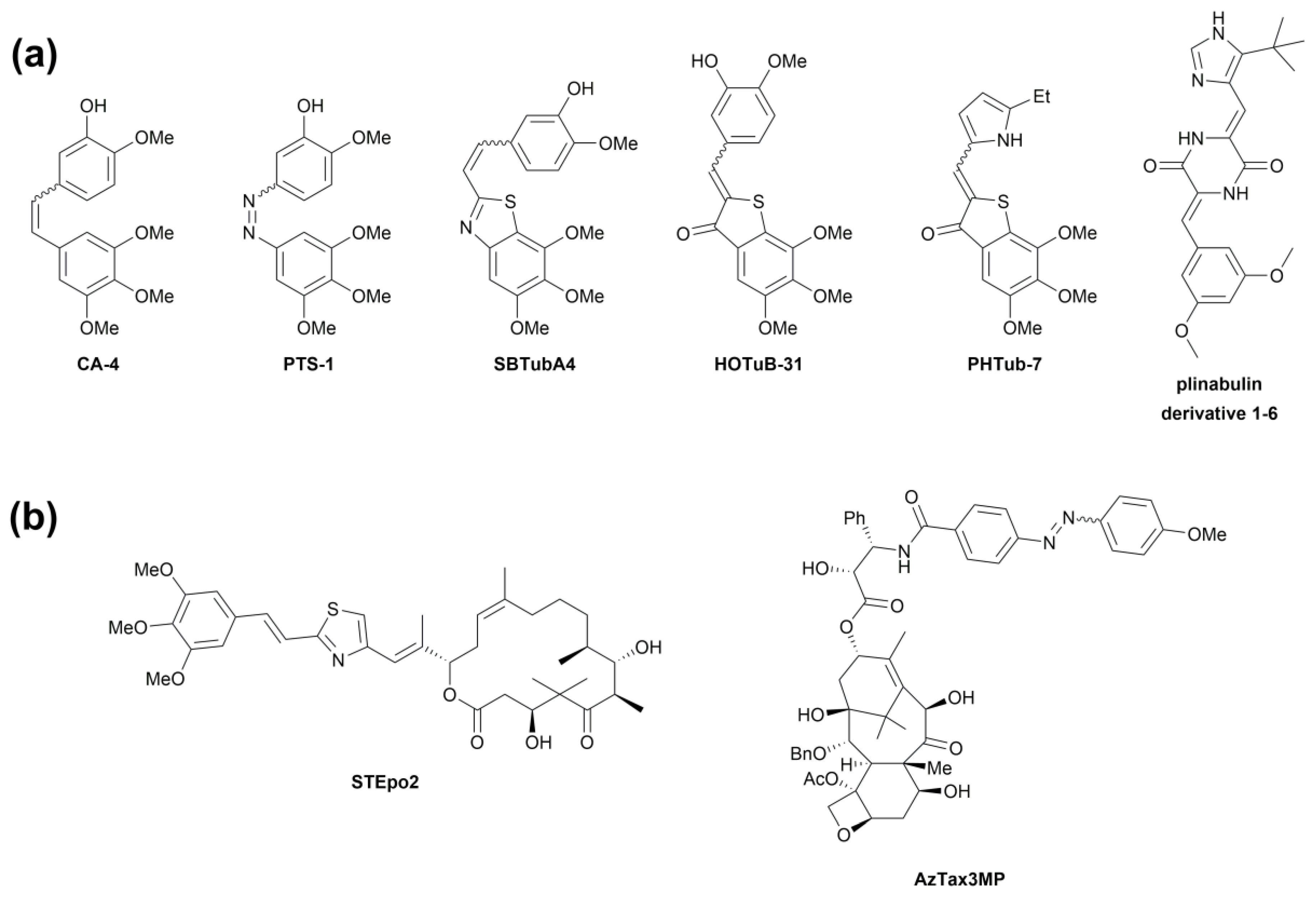
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Tubulin Polymerisation Assay
2.3. Cell Culturing
2.4. MTT Cytotoxicity Assay with Green and Blue Light Irradiation
2.5. Immunofluorescence
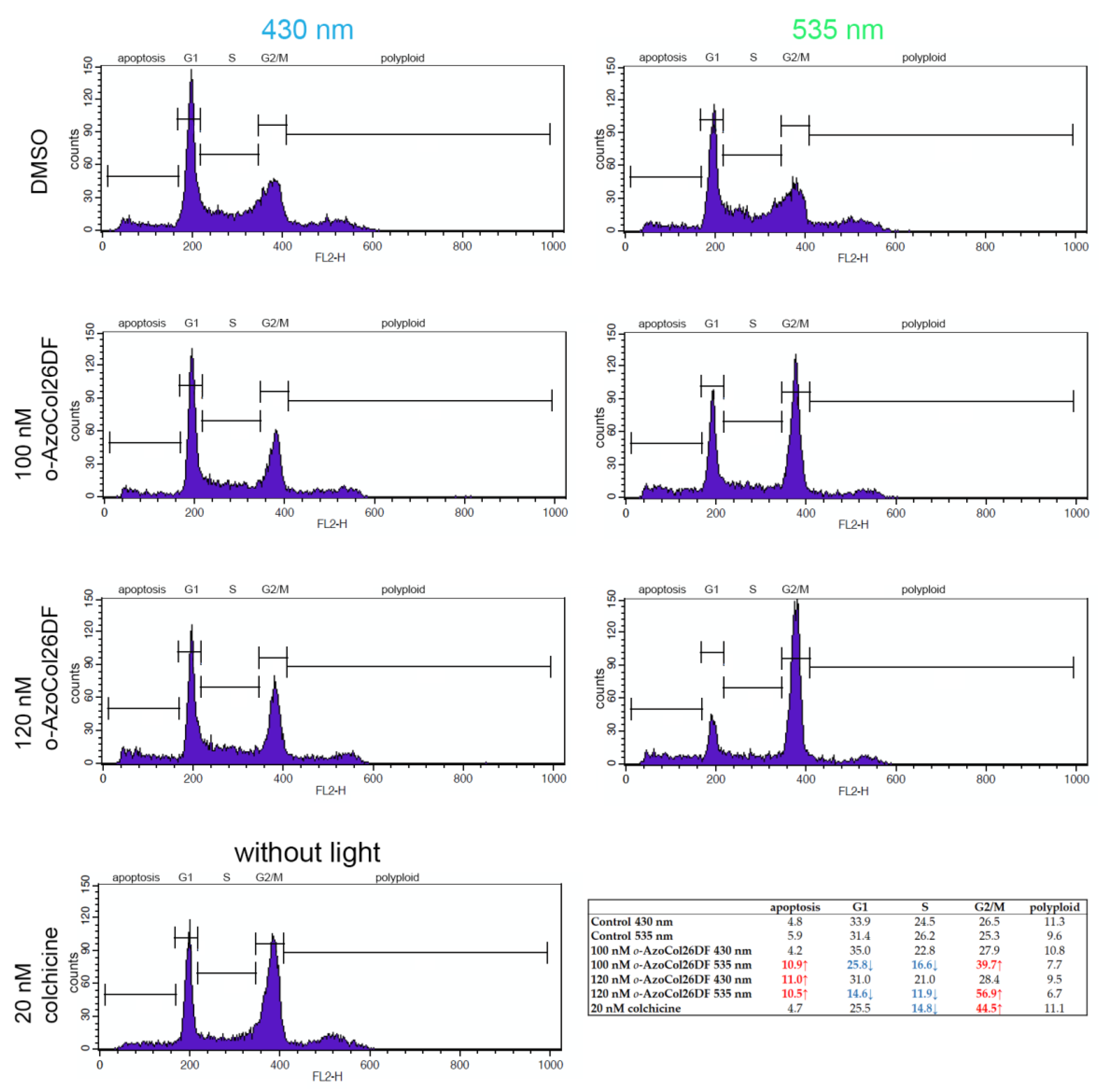
2.6. Cell Cycle Stages Analysis
3. Results and Discussion
3.1. The Computational Study
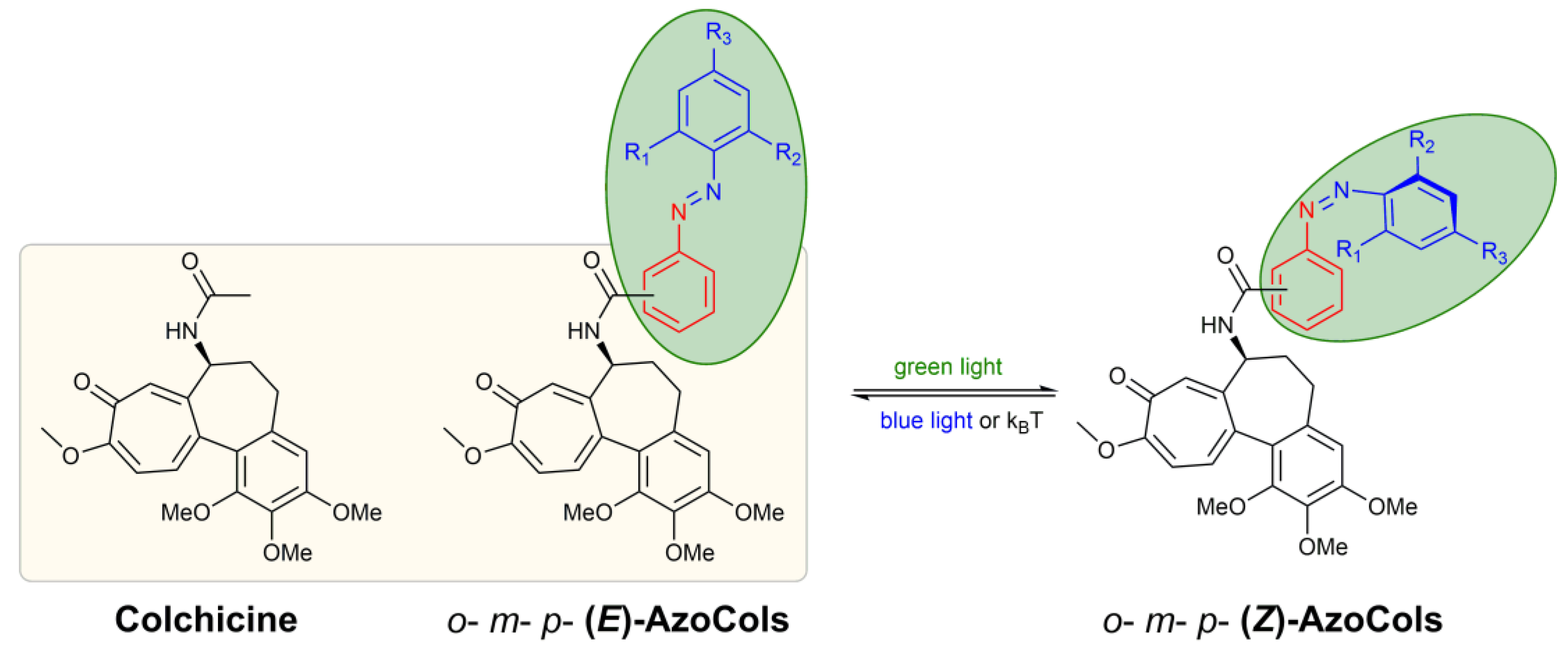
3.2. Design and Synthesis of Azocolchicines
3.3. Photochemical Characterisation
3.4. Photocontrollable in Cellulo Studies
3.5. o-AzoCol26DF Disrupt Tubulin Polymerisation and Cellular Microtubule Organisation in Light-Dependent Manner
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lerch, M.M.; Hansen, M.J.; van Dam, G.M.; Szymanski, W.; Szymanski, W.; Feringa, B.L.; Feringa, B.L. Emerging Targets in Photopharmacology. Angew. Chem. Int. Ed. 2016, 55, 10978–10999. [Google Scholar] [CrossRef] [PubMed]
- Velema, W.A.; Szymanski, W.; Feringa, B. Photopharmacology: Beyond Proof of Principle. J. Am. Chem. Soc. 2014, 136, 2178–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchter, M.J. On the Promise of Photopharmacology Using Photoswitches: A Medicinal Chemist’s Perspective. J. Med. Chem. 2020, 63, 11436–11447. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.H.; Holt, A.; Broichhagen, J.; Donthamsetti, P.; Flannery, J.G.; Isacoff, E.Y. Photopharmacology for vision restoration. Curr. Opin. Pharmacol. 2022, 65, 102259. [Google Scholar] [CrossRef] [PubMed]
- Hauwert, N.J.; Mocking, T.A.M.; Da Costa Pereira, D.; Lion, K.; Huppelschoten, Y.; Vischer, H.F.; de Esch, I.J.P.; Wijtmans, M.; Leurs, R. A Photoswitchable Agonist for the Histamine H3 Receptor, a Prototypic Family A G-protein-coupled Receptor. Angew. Chem. 2019, 58, 4579–4583. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Vaidehi, N. Computational Mapping of the Conformational Transitions in Agonist Selective Pathways of a G-protein Coupled Receptor. J. Am. Chem. Soc. 2010, 132, 8951–8956. [Google Scholar] [CrossRef]
- Agnetta, L.; Kauk, M.; Canizal, M.C.A.; Messerer, R.; Holzgrabe, U.; Hoffmann, C.; Hoffmann, C.; Decker, M. A Photoswitchable Dualsteric Ligand Controlling Receptor Efficacy. Angew. Chem. Int. Ed. 2017, 56, 7282–7287. [Google Scholar] [CrossRef]
- Schönberger, M.; Althaus, M.; Fronius, M.; Fronius, M.; Clauss, W.; Trauner, D. Controlling Epithelial Sodium Channels with Light Using Photoswitchable Amilorides. Nat. Chem. 2014, 6, 712–719. [Google Scholar] [CrossRef]
- Schoenberger, M.; Damijonaitis, A.; Zhang, Z.; Nagel, D.M.; Trauner, D. Development of a New Photochromic Ion Channel Blocker via Azologization of Fomocaine. ACS Chem. Neurosci. 2014, 5, 514–518. [Google Scholar] [CrossRef]
- Fortin, D.L.; Banghart, M.R.; Dunn, T.W.; Borges, K.; Wagenaar, D.A.; Gaudry, Q.; Karakossian, M.H.; Otis, T.S.; Kristan, W.B.; Trauner, D. Photochemical Control of Endogenous Ion Channels and Cellular Excitability. Nat. Methods 2008, 5, 331–338. [Google Scholar] [CrossRef]
- Babii, O.; Afonin, S.; Diel, C.; Huhn, M.; Dommermuth, L.; Schober, T.; Koniev, S.; Hrebonkin, A.; Nesterov-Mueller, A.; Komarov, I.V.; et al. Diarylethene-Based Photoswitchable Inhibitors of Serine Proteases. Angew. Chem. Int. Ed. 2021, 60, 21789–21794. [Google Scholar] [CrossRef]
- Wang, H.; Bisoyi, H.K.; Zhang, X.; Hassan, F.; Li, Q. Visible Light-Driven Molecular Switches and Motors: Recent Developments and Applications. Chem. Eur. J. 2022, 28, e202103906. [Google Scholar] [CrossRef] [PubMed]
- Vetráková, Ľ.; Ladányi, V.; Al Anshori, J.; Dvořák, P.; Wirz, J.; Heger, D. The Absorption Spectrum of Cis-azobenzene. Photochem. Photobiol. Sci. 2017, 16, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Merino, E. Synthesis of Azobenzenes: The Coloured Pieces of Molecular Materials. Chem. Soc. Rev. 2011, 40, 3835–3853. [Google Scholar] [CrossRef] [PubMed]
- Crespi, S.; Simeth, N.A.; König, B. Heteroaryl Azo Dyes as Molecular Photoswitches. Nat. Rev. Chem. 2019, 3, 133–146. [Google Scholar] [CrossRef]
- Griffiths, J., II. Photochemistry of Azobenzene and Its Derivatives. Chem. Soc. Rev. 1972, 1, 481–493. [Google Scholar] [CrossRef]
- Hüll, K.; Morstein, J.; Trauner, D. In Vivo Photopharmacology. Chem. Rev. 2018, 118, 10710–10747. [Google Scholar] [CrossRef]
- Morstein, J.; Trauner, D. New Players in Phototherapy: Photopharmacology and Bio-integrated Optoelectronics. Curr. Opin. Chem. Biol. 2019, 50, 145–151. [Google Scholar] [CrossRef]
- Kirchner, S.; Pianowski, Z. Photopharmacology of antimitotic agents. Int. J. Mol. Sci. 2022, 23, 5657. [Google Scholar] [CrossRef]
- Volarić, J.; Szymanski, W.; Simeth, N.A.; Feringa, B.L. Molecular photoswitches in aqueous environments. Chem. Soc. Rev. 2021, 50, 12377–12449. [Google Scholar] [CrossRef]
- Bléger, D.; Hecht, S. Visible-light-activated Molecular Switches. Angew. Chem. Int. Ed. 2015, 54, 11338–11349. [Google Scholar] [CrossRef]
- Leistner, A.L.; Pianowski, Z.L. Smart photochromic materials triggered with visible light. Eur. J. Org. Chem. 2022, 19, e202101271. [Google Scholar] [CrossRef]
- Lameijer, L.N.; Lameijer, L.N.; Budzák, Š.; Simeth, N.A.; Hansen, M.J.; Feringa, B.L.; Jacquemin, D.; Szymanski, W.; Szymanski, W. General Principles for the Design of Visible-light-responsive Photoswitches: Tetra-ortho-chloro-azobenzenes. Angew. Chem. Int. Ed. 2020, 59, 21663–21670. [Google Scholar] [CrossRef] [PubMed]
- Bléger, D.; Schwarz, J.; Brouwer, A.M.; Hecht, S. O-fluoroazobenzenes as Readily Synthesized Photoswitches Offering Nearly Quantitative Two-way Isomerization with Visible Light. J. Am. Chem. Soc. 2012, 134, 20597–20600. [Google Scholar] [CrossRef] [PubMed]
- Agnetta, L.; Bermudez, M.; Riefolo, F.; Matera, C.; Claro, E.; Messerer, R.; Littmann, T.; Wolber, G.; Holzgrabe, U.; Decker, M. Fluorination of Photoswitchable Muscarinic Agonists Tunes Receptor Pharmacology and Photochromic Properties. J. Med. Chem. 2019, 62, 3009–3020. [Google Scholar] [CrossRef] [PubMed]
- Knie, C.; Utecht, M.; Zhao, F.; Kulla, H.; Kovalenko, S.A.; Brouwer, A.M.; Saalfrank, P.; Hecht, S.; Bléger, D. Ortho-fluoroazobenzenes: Visible Light Switches with Very Long-lived Z Isomers. Chem. Eur. J. 2014, 20, 16492–16501. [Google Scholar] [CrossRef] [PubMed]
- Kuntze, K.; Viljakka, J.; Titov, E.; Ahmed, Z.; Kalenius, E.; Saalfrank, P.; Priimagi, A. Towards low-energy-light-driven bistable photoswitches: Ortho-fluoroaminoazobenzenes. Photochem. Photobiol. Sci. 2022, 21, 159–173. [Google Scholar] [CrossRef]
- Steinmetz, M.O.; Steinmetz, M.O.; Prota, A.E. Microtubule-targeting Agents: Strategies to Hijack the Cytoskeleton. Trends Cell Biol. 2018, 28, 776–792. [Google Scholar] [CrossRef]
- Borys, F.; Borys, F.; Joachimiak, E.; Krawczyk, H.; Fabczak, H. Intrinsic and Extrinsic Factors Affecting Microtubule Dynamics in Normal and Cancer Cells. Molecules 2020, 25, 3705. [Google Scholar] [CrossRef]
- Dumontet, C.; Jordan, M.A. Microtubule-binding Agents: A Dynamic Field of Cancer Therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [Green Version]
- Borys, F.; Tobiasz, P.; Poterała, M.; Krawczyk, H. Development of novel derivatives of stilbene and macrocyclic compounds as potent of anti-microtubule factors. Biomed. Pharmacother. 2021, 133, 110973. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.R.; Mitchison, T.J. Small Molecules, Big Impact: A History of Chemical Inhibitors and the Cytoskeleton. Chem. Biol. 2002, 9, 1275–1285. [Google Scholar] [CrossRef] [Green Version]
- Jordan, M.A. Mechanism of Action of Antitumor Drugs That Interact with Microtubules and Tubulin. Anticancer Agents Med. Chem. 2012, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Kaku, K.; Robles, A.J.; Hamel, E.; Mooberry, S.L.; Gangjee, A. Simple monocyclic pyrimidine analogs as microtubule targeting agents binding to the colchicine site. Bioorg. Med. Chem. 2023, 82, 117217. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Xu, R.M.; Zheng, G.H.; Jin, Y.Z.; Li, Y.; Chen, X.Y.; Tian, Y.S. Development of Combretastatin A-4 Analogues as Potential Anticancer Agents with Improved Aqueous Solubility. Molecules 2023, 28, 1717. [Google Scholar] [CrossRef]
- Grillone, K.; Riillo, C.; Rocca, R.; Ascrizzi, S.; Spanò, V.; Scionti, F.; Polerà, N.; Maruca, A.; Barreca, M.; Juli, G.; et al. The new microtubule-targeting agent SIX2G induces immunogenic cell death in multiple myeloma. Int. J. Mol. Sci. 2022, 23, 10222. [Google Scholar] [CrossRef]
- Borowiak, M.; Borowiak, M.; Nahaboo, W.; Reynders, M.; Nekolla, K.; Jalinot, P.; Hasserodt, J.; Rehberg, M.; Delattre, M.; Zahler, S. Photoswitchable Inhibitors of Microtubule Dynamics Optically Control Mitosis and Cell Death. Cell 2015, 162, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Engdahl, A.J.; Torres, E.A.; Lock, S.E.; Engdahl, T.B.; Mertz, P.S.; Streu, C. Synthesis, Characterization, and Bioactivity of the Photoisomerizable Tubulin Polymerization Inhibitor Azo-combretastatin A4. Org. Lett. 2015, 17, 4546–4549. [Google Scholar] [CrossRef]
- Sheldon, J.E.; Dcona, M.M.; Lyons, C.E.; Hackett, J.C.; Hartman, M.C.T. Photoswitchable Anticancer Activity via Trans–cis Isomerization of a Combretastatin A-4 Analog. Org. Biomol. Chem. 2016, 14, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Sailer, A.; Ermer, F.; Kraus, Y.; Lutter, F.H.; Donau, C.A.; Bremerich, M.; Ahlfeld, J.; Thorn-Seshold, O. Hemithioindigos for Cellular Photopharmacology: Desymmetrised Molecular Switch Scaffolds Enabling Design Control over the Isomer-dependency of Potent Antimitotic Bioactivity. ChemBioChem 2019, 20, 1305–1314. [Google Scholar] [CrossRef]
- Sailer, A.; Ermer, F.; Kraus, Y.; Bingham, R.; Lutter, F.H.; Ahlfeld, J.; Thorn-Seshold, O. Potent Hemithioindigo-based Antimitotics Photocontrol the Microtubule Cytoskeleton in Cellulo. Beilstein J. Org. Chem. 2020, 16, 125–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sailer, A.; Meiring, J.C.M.; Heise, C.; Pettersson, L.N.; Akhmanova, A.; Thorn-Seshold, J.; Thorn-Seshold, O. Pyrrole Hemithioindigo Antimitotics with Near-quantitative Bidirectional Photoswitching Photocontrol Cellular Microtubule Dynamics with Single-cell Precision. Angew. Chem. Int. Ed. 2021, 60, 23695–23704. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.K.; Zhao, Z.; Gildner, M.B.; Shoulders, B.A.; Velasquez, T.L.; Blumenthal, M.O.; Wang, L.; Li, X.; Hudnall, T.W.; Betancourt, T. Synthesis, Optical Properties and in Vitro Cell Viability of Novel Spiropyrans and Their Photostationary States. Tetrahedron 2021, 80, 131854. [Google Scholar] [CrossRef]
- Nilsson, J.R.; Li, S.; Önfelt, B.; Önfelt, B.; Andréasson, J. Light-induced Cytotoxicity of a Photochromic Spiropyran. Chem. Commun. 2011, 47, 11020–11022. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Meiring, J.C.M.; Kraus, Y.; Wranik, M.; Weinert, T.; Pritzl, S.D.; Bingham, R.; Ntouliou, E.; Jansen, K.I.; Olieric, N. A Robust, Gfp-orthogonal Photoswitchable Inhibitor Scaffold Extends Optical Control over the Microtubule Cytoskeleton. Cell Chem. Biol. 2021, 28, 228–241. [Google Scholar] [CrossRef]
- Gao, L.; Meiring, J.C.; Varady, A.; Ruider, I.E.; Heise, C.; Wranik, M.; Velasco, C.D.; Taylor, J.A.; Terni, B.; Weinert, T.; et al. In vivo photocontrol of microtubule dynamics and integrity, migration and mitosis, by the potent GFP-imaging-compatible photoswitchable reagents SBTubA4P and SBTub2M. J. Am. Chem. Soc. 2022, 144, 5614–5628. [Google Scholar] [CrossRef]
- Kirchner, S.; Leistner, A.L.; Gödtel, P.; Seliwjorstow, A.; Weber, S.; Karcher, J.; Niger, M.; Pianowski, Z. Hemipiperazines as peptide-derived molecular photoswitches with low-nanomolar cytotoxicity. Nat. Commun. 2022, 13, 6066. [Google Scholar] [CrossRef]
- Müller-Deku, A.; Meiring, J.C.M.; Loy, K.; Kraus, Y.; Heise, C.; Bingham, R.; Jansen, K.I.; Qu, X.; Bartolini, F.; Kapitein, L.C. Photoswitchable Paclitaxel-based Microtubule Stabilisers Allow Optical Control over the Microtubule Cytoskeleton. Nat. Commun. 2020, 11, 4640. [Google Scholar] [CrossRef]
- Gao, L.; Meiring, J.C.; Heise, C.; Rai, A.; Müller-Deku, A.; Akhmanova, A.; Thorn-Seshold, J.; Thorn-Seshold, O. Photoswitchable Epothilone-Based Microtubule Stabilisers Allow GFP-Imaging-Compatible, Optical Control over the Microtubule Cytoskeleton. Angew. Chem. Int. Ed. 2022, 61, e202114614. [Google Scholar] [CrossRef]
- Cheong, W.F.; Prahl, S.A.; Welch, A.J. A Review of the Optical Properties of Biological Tissues. IEEE J. Quantum Electron. 1990, 26, 2166–2185. [Google Scholar] [CrossRef] [Green Version]
- Nery, A.L.P.; Quina, F.H.; Moreira, P.F.; Medeiros, C.E.R.; Baader, W.J.; Shimizu, K.; Catalani, L.H.; Bechara, E.J.H. Does the Photochemical Conversion of Colchicine into Lumicolchicines Involve Triplet Transients? A Solvent Dependence Study. Photochem. Photobiol. 2001, 73, 213–218. [Google Scholar] [CrossRef]
- Ghanem, R.; Baker, H.M.; Abu Seif, M.; Al-Qawasmeh, R.A.; Mataneh, A.-A.; Al-Gharabli, S. Photochemical Transformation of Colchicine: A Kinetic Study. J. Solution Chem. 2010, 39, 441–456. [Google Scholar] [CrossRef]
- Bagnato, J.D.; Eilers, A.L.; Horton, R.A.; Grissom, C.B. Synthesis and Characterization of a Cobalamin-colchicine Conjugate as a Novel Tumor-targeted Cytotoxin. J. Org. Chem. 2004, 69, 8987–8996. [Google Scholar] [CrossRef]
- Gell, C.; Friel, C.T.; Borgonovo, B.; Drechsel, D.N.; Hyman, A.A.; Howard, J. Purification of Tubulin from Porcine Brain. In Microtubule Dynamics; Humana: Totowa, NJ, USA, 2011; Volume 777, pp. 15–28. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision E.01; Gaussian Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Siewertsen, R.; Schönborn, J.B.; Hartke, B.; Renth, F.; Temps, F. Superior Z → E and E → Z Photoswitching Dynamics of Dihydrodibenzodiazocine, a Bridged Azobenzene, by S1(nπ*) Excitation at λ = 387 and 490 nm. Phys. Chem. Chem. Phys. 2011, 13, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yuan, S.; Fang, W.-H.; Zhang, Y. Probing Highly Efficient Photoisomerization of a Bridged Azobenzene by a Combination of CASPT2//CASSCF Calculation with Semiclassical Dynamics Simulation. J. Phys. Chem. A 2011, 115, 10027–10034. [Google Scholar] [CrossRef] [PubMed]
- Konrad, D.B.; Savasci, G.; Allmendinger, L.; Trauner, D.; Ochsenfeld, C.; Ali, A.M. Computational Design and Synthesis of a Deeply Red-shifted and Bistable Azobenzene. J. Am. Chem. Soc. 2020, 142, 6538–6547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosentino, L.; Redondo-Horcajo, M.; Zhao, Y.; Santos, A.R.; Chowdury, K.F.; Vinader, V.; Abdallah, Q.M.A.; Abdel-Rahman, H.M.; Fournier-Dit-Chabert, J.; Shnyder, S.D. Synthesis and Biological Evaluation of Colchicine B-ring Analogues Tethered with Halogenated Benzyl Moieties. J. Med. Chem. 2012, 55, 11062–11066. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Kumar, A.; Kumar, A.; Joshi, P.; Joshi, P.; Guru, S.K.; Kumar, S.; Kumar, S.; Wani, Z.A.; Mahajan, G. Colchicine Derivatives with Potent Anticancer Activity and Reduced P-glycoprotein Induction Liability. Org. Biomol. Chem. 2015, 13, 5674–5689. [Google Scholar] [CrossRef]
- Tobiasz, P.; Borys, F.; Borecka, M.; Krawczyk, H. Synthesis and Investigations of Building Blocks with Dibenzo[b,f]oxepine for Use in Photopharmacology. Int. J. Mol. Sci. 2021, 22, 11033. [Google Scholar] [CrossRef]
- Wegener, M.; Hansen, M.J.; Driessen, A.J.M.; Szymanski, W.; Feringa, B.L. Photocontrol of Antibacterial Activity: Shifting from UV to Red Light Activation. J. Am. Chem. Soc. 2017, 139, 17979–17986. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, W.; Szymanski, W.; Ourailidou, M.E.; Velema, W.A.; Dekker, F.J.; Feringa, B.L. Light-controlled Histone Deacetylase (HDAC) Inhibitors: Towards Photopharmacological Chemotherapy. Chem. Eur. J. 2015, 21, 16517–16524. [Google Scholar] [CrossRef] [Green Version]
- Wutz, D.-F.; Gluhacevic, D.; Chakrabarti, A.; Schmidtkunz, K.; Robaa, D.; Erdmann, F.; Romier, C.; Sippl, W.; Jung, M.; König, B. Photochromic Histone Deacetylase Inhibitors Based on Dithienylethenes and Fulgimides. Org. Biomol. Chem. 2017, 15, 4882–4896. [Google Scholar] [CrossRef] [PubMed]
- Slobodnick, A.; Shah, B.; Pillinger, M.H.; Krasnokutsky, S. Colchicine: Old and New. Am. J. Med. 2015, 128, 461–470. [Google Scholar] [CrossRef]
- Vilanova, C.; Díaz-Oltra, S.; Murga, J.; Falomir, E.; Carda, M.; Redondo-Horcajo, M.; Díaz, J.F.; Barasoain, I.; Marco, J.A. Design and Synthesis of Pironetin Analogue/colchicine Hybrids and Study of Their Cytotoxic Activity and Mechanisms of Interaction with Tubulin. J. Med. Chem. 2014, 57, 10391–10403. [Google Scholar] [CrossRef] [Green Version]
- Yasobu, N.; Kitajima, M.; Kogure, N.; Shishido, Y.; Matsuzaki, T.; Nagaoka, M.; Takayama, H. Design, Synthesis, and Antitumor Activity of 4-halocolchicines and Their Pro-drugs Activated by Cathepsin B. ACS Med. Chem. Lett. 2011, 2, 348–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| (E)-isomer a | (Z)-isomer b | ||||||
|---|---|---|---|---|---|---|---|
| Compound | λmax (π−π*) [nm] | λmax (n−π*) [nm] | λmax (n−π*) [nm] | Δλ (n−π*) [nm] | t1/2[h] | PSS(E) c (%) | PSS(Z) d (%) |
| o-3a | 324 | 448 | 440 | 8 | 6 | 95 | 46 |
| o-3b | 326 | 445 | 437 | 8 | 5 | 99 | 48 |
| o-3c | 330 | 444 | 433 | 11 | 23 | 96 | 66 |
| o-3d | 315 | 450 | 420 | 30 | >48 | 97 | 88 |
| m-3a | 321 | 437 | 429 | 8 | >48 | 94 | 35 |
| m-3b | 322 | 431 | 423 | 8 | >48 | 95 | 32 |
| m-3c | 326 | 440 | 423 | 17 | >48 | 96 | 46 |
| m-3d | 315 | 448 | 417 | 31 | >48 | 96 | 77 |
| p-3a | 330 | 447 | 440 | 7 | 17 | 91 | 35 |
| p-3b | 330 | 446 | 440 | 6 | 16 | 99 | 47 |
| p-3c | 334 | 448 | 437 | 11 | 23 | 93 | 47 |
| p-3d | 322 | 454 | 421 | 33 | >48 | 91 | 71 |
| Compound | Cell Line | IC50 Blue Light (nM) | IC50 Green Light (nM) | IC Ratio |
|---|---|---|---|---|
| p-AzoCol | MCF-7 (N = 1) | 48 ± 2 | 44 ± 2 | 1.1 |
| p-AzoCol4F | MCF-7 (N = 1) | 44 ± 1 | 43 ± 1 | 1.0 |
| p-AzoCol24DF | MCF-7 (N = 3) | 50 ± 1 | 36 ± 1 | 1.4 |
| p-AzoCol26DF | MCF-7 (N = 1) | 55 ± 1 | 54 ± 1 | 1.0 |
| m-AzoCol | MCF-7 (N = 1) | 31 ± 2 | 27 ± 1 | 1.1 |
| m-AzoCol4F | MCF-7 (N = 1) | 42 ± 1 | 40 ± 1 | 1.1 |
| m-AzoCol24DF | MCF-7 (N = 1) | 49 ± 1 | 47 ± 1 | 1.0 |
| m-AzoCol26DF | MCF-7 (N = 1) | 45 ± 2 | 46 ± 4 | 1.0 |
| o-AzoCol | MCF-7 (N = 1) | nc | nc | nc |
| o-AzoCol4F | MCF-7 (N = 1) | nc | nc | nc |
| o-AzoCol24DF | MCF-7 (N = 1) | 183 | 174 | 1.1 |
| o-AzoCol26DF | MCF-7 (N = 3) | 184 ± 4 | 126 ± 2 | 1.5 |
| o-AzoCol26DF | HCT116 (N = 2) | 187 ± 9 | 97 ± 2 | 1.9 |
| o-AzoCol26DF | HEK293 (N = 3) | >250 | >250 | - |
| Colchicine | MCF-7 | 12 a | - | - |
| Colchicine | HCT116 | 11 b | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borys, F.; Tobiasz, P.; Fabczak, H.; Joachimiak, E.; Krawczyk, H. First-in-Class Colchicine-Based Visible Light Photoswitchable Microtubule Dynamics Disrupting Agent. Cells 2023, 12, 1866. https://doi.org/10.3390/cells12141866
Borys F, Tobiasz P, Fabczak H, Joachimiak E, Krawczyk H. First-in-Class Colchicine-Based Visible Light Photoswitchable Microtubule Dynamics Disrupting Agent. Cells. 2023; 12(14):1866. https://doi.org/10.3390/cells12141866
Chicago/Turabian StyleBorys, Filip, Piotr Tobiasz, Hanna Fabczak, Ewa Joachimiak, and Hanna Krawczyk. 2023. "First-in-Class Colchicine-Based Visible Light Photoswitchable Microtubule Dynamics Disrupting Agent" Cells 12, no. 14: 1866. https://doi.org/10.3390/cells12141866
APA StyleBorys, F., Tobiasz, P., Fabczak, H., Joachimiak, E., & Krawczyk, H. (2023). First-in-Class Colchicine-Based Visible Light Photoswitchable Microtubule Dynamics Disrupting Agent. Cells, 12(14), 1866. https://doi.org/10.3390/cells12141866










