Bile Acids, Intestinal Barrier Dysfunction, and Related Diseases
Abstract
1. Introduction
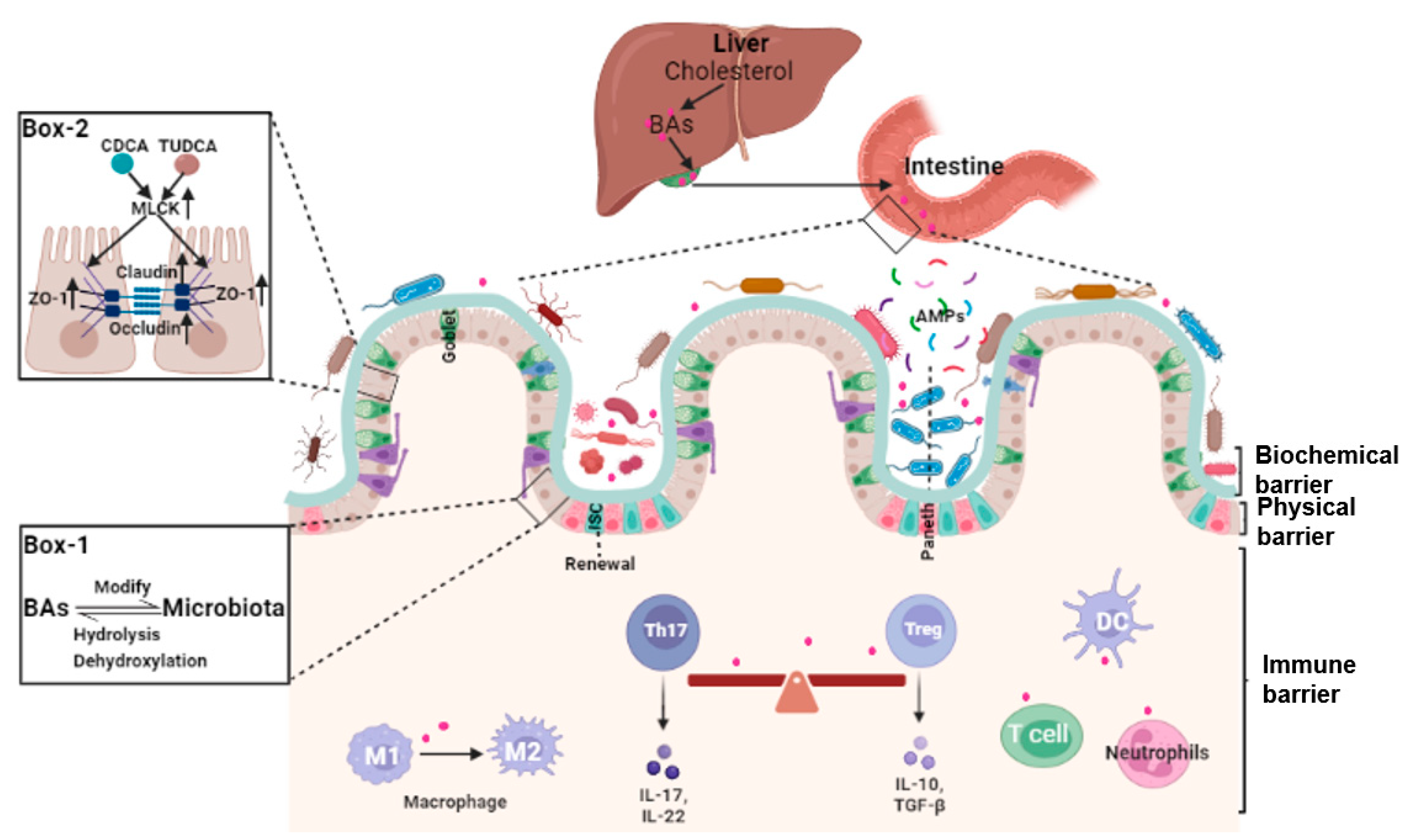
2. Bile Acids and Intestinal Barrier
2.1. Bile Acids and Intestinal Epithelial Cells Tight Junctions
2.2. Bile Acids and Gut Microbiota
2.3. Bile Acids, Intestinal Stem Cells (ISCs), and Epithelial Injury Repair
2.4. Bile Acids and Intestinal Local Immune Homeostasis
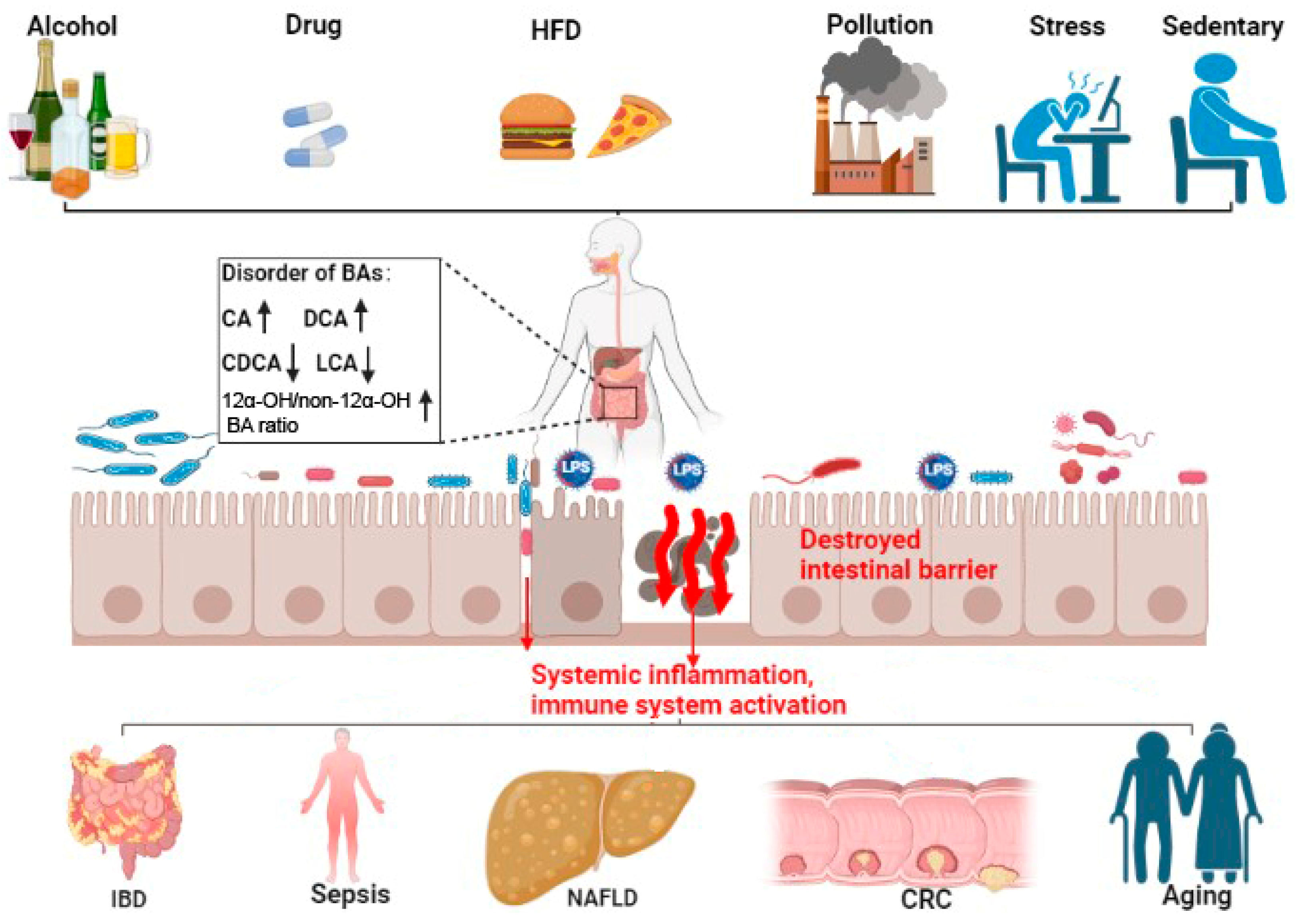
3. Bile Acid and Intestinal-Barrier-Dysfunction-Related Diseases
3.1. Inflammatory Bowel Diseases (IBDs)
3.2. Gut Origin of Sepsis
3.3. Non-Alcoholic Fatty Liver Disease (NAFLD)
3.4. Colorectal Cancer (CRC)
3.5. Aging
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breugelmans, T.; Oosterlinck, B.; Arras, W.; Ceuleers, H.; De Man, J.; Hold, G.L.; De Winter, B.Y.; Smet, A. The role of mucins in gastrointestinal barrier function during health and disease. Lancet Gastroenterol. Hepatol. 2022, 7, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.W.; Rimal, B.; Jiang, C.T.; Chiang, J.Y.L.; Patterson, A.D. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef]
- Bhargava, P.; Smith, M.D.; Mische, L.; Harrington, E.; Fitzgerald, K.C.; Martin, K.; Kim, S.; Reyes, A.A.; Gonzalez-Cardona, J.; Volsko, C.; et al. Bile acid metabolism is altered in multiple sclerosis and supplementation ameliorates neuroinflammation. J. Clin. Investig. 2020, 130, 3467–3482. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.C.; Xu, C.J.; Feng, B.S.; Gao, X.; Liu, Z.J. Critical roles of bile acids in regulating intestinal mucosal immune responses. Ther. Adv. Gastroenterol. 2021, 14, 17562848211018098. [Google Scholar] [CrossRef]
- Li, C.G.; Wang, Y.; Liu, D.B.; Wong, C.C.; Coker, O.O.; Zhang, X.; Liu, C.A.; Zhou, Y.F.; Liu, Y.L.; Kang, W.; et al. Squalene epoxidase drives cancer cell proliferation and promotes gut dysbiosis to accelerate colorectal carcinogenesis. Gut 2022, 71, 2253–2265. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Atarashi, K.; Plichta, D.R.; Arai, Y.; Sasajima, S.; Kearney, S.M.; Suda, W.; Takeshita, K.; Sasaki, T.; Okamoto, S.; et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 2021, 599, 458–464. [Google Scholar] [CrossRef]
- Ding, L.; Yang, L.; Wang, Z.T.; Huang, W.D. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm. Sin. B 2015, 5, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Chen, W.D.; Huang, W.D. FXR, a target for different diseases. Histol. Histopathol. 2008, 23, 621–627. [Google Scholar] [PubMed]
- Arifuzzaman, M.; Won, T.H.; Li, T.T.; Yano, H.; Digumarthi, S.; Heras, A.F.; Zhang, W.; Parkhurst, C.N.; Kashyap, S.; Jin, W.B.; et al. Inulin fibre promotes microbiota-derived bile acids and type 2 inflammation. Nature 2022, 611, 578–584. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.F.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.Y.; Wang, Y.J.; Liu, C.; Wang, W.Z.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.J.; Zhu, M.; Wang, K.; Zhao, X.Y.; Hu, L.L.; Jing, W.H.; Lu, H.T.; Wang, S.C. Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism. Pharmacol. Res. 2021, 171, 105767. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut Microbiota and Intestinal Trans-Epithelial Permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Dong, W.X.; Wang, S.N.; Zhang, Y.J.; Liu, T.Y.; Xie, R.X.; Wang, B.M.; Cao, H.L. Deoxycholic acid disrupts the intestinal mucosal barrier and promotes intestinal tumorigenesis. Food Funct. 2018, 9, 5588–5597. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.Y.; He, J.N.; Yin, X.; Shi, Y.; Wan, J.; Tian, Z. The protective effect of lithocholic acid on the intestinal epithelial barrier is mediated by the vitamin D receptor via a SIRT1/Nrf2 and NF-kappa B dependent mechanism in Caco-2 cells. Toxicol. Lett. 2019, 316, 109–118. [Google Scholar] [CrossRef]
- Ruan, D.; Wu, S.W.; Fouad, A.M.; Zhu, Y.W.; Huang, W.J.; Chen, Z.L.; Gou, Z.Y.; Wang, Y.B.; Han, Y.Q.; Yan, S.J.; et al. Curcumin alleviates LPS-induced intestinal homeostatic imbalance through reshaping gut microbiota structure and regulating group 3 innate lymphoid cells in chickens. Food Funct. 2022, 13, 11811–11824. [Google Scholar] [CrossRef]
- Buckley, A.; Turner, J.R. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029314. [Google Scholar] [CrossRef]
- Song, M.; Ye, J.Y.; Zhang, F.L.; Su, H.; Yang, X.H.; He, H.W.; Liu, F.F.; Zhu, X.T.; Wang, L.N.; Gao, P.; et al. Chenodeoxycholic Acid (CDCA) Protects against the Lipopolysaccharide-Induced Impairment of the Intestinal Epithelial Barrier Function via the FXR-MLCK Pathway. J. Agric. Food Chem. 2019, 67, 8868–8874. [Google Scholar] [CrossRef]
- Song, M.; Zhang, F.L.; Fu, Y.M.; Yi, X.; Feng, S.C.; Liu, Z.C.; Deng, D.; Yang, Q.; Yu, M.; Zhu, C.J.; et al. Tauroursodeoxycholic acid (TUDCA) improves intestinal barrier function associated with TGR5-MLCK pathway and the alteration of serum metabolites and gut bacteria in weaned piglets. J. Anim. Sci. Biotechnol. 2022, 13, 73. [Google Scholar] [CrossRef]
- Ramirez-Perez, O.; Cruz-Ramon, V.; Chinchilla-Lopez, P.; Mendez-Sanchez, N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann. Hepatol. 2017, 16, S21–S26. [Google Scholar] [CrossRef]
- Cai, J.; Sun, L.L.; Gonzalez, F.J. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- He, Z.Y.; Ma, Y.L.; Yang, S.R.; Zhang, S.Y.; Liu, S.; Xiao, J.X.; Wang, Y.J.; Wang, W.; Yang, H.J.; Li, S.L.; et al. Gut microbiota-derived ursodeoxycholic acid from neonatal dairy calves improves intestinal homeostasis and colitis to attenuate extended-spectrum beta-lactamase-producing enteroaggregative Escherichia coli infection. Microbiome 2022, 10, 79. [Google Scholar] [CrossRef]
- Gadaleta, R.M.; Garcia-Irigoyen, O.; Cariello, M.; Scialpi, N.; Peres, C.; Vetrano, S.; Fiorino, G.; Danese, S.; Ro, B.; Luo, J.; et al. Fibroblast Growth Factor 19 modulates intestinal microbiota and inflammation in presence of Farnesoid X Receptor. Ebiomedicine 2020, 54, 102719. [Google Scholar] [CrossRef]
- Nakanishi, T.; Fukui, H.; Wang, X.; Nishiumi, S.; Yokota, H.; Makizaki, Y.; Tanaka, Y.; Ohno, H.; Tomita, T.; Oshima, T.; et al. Effect of a High-Fat Diet on the Small-Intestinal Environment and Mucosal Integrity in the Gut-Liver Axis. Cells 2021, 10, 3168. [Google Scholar] [CrossRef]
- Anhe, F.F.; Nachbar, R.T.; Varin, T.V.; Trottier, J.; Dudonne, S.; Le Barz, M.; Feutry, P.; Pilon, G.; Barbier, O.; Desjardins, Y.; et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut 2019, 68, 453–464. [Google Scholar] [CrossRef]
- He, Y.; Song, Z.; Ji, Y.; Tso, P.; Wu, Z.L. Preventive Effects of L-Glutamine on High-Fat Diet-Induced Metabolic Disorders Linking with Regulation of Intestinal Barrier Integrity, Hepatic Lipid Metabolism, and Gut Microbiota in Rats. J. Agric. Food Chem. 2022, 70, 11923–11934. [Google Scholar] [CrossRef]
- Yang, Y.H.; Zhang, Y.H.; Xu, Y.C.; Luo, T.Y.; Ge, Y.T.; Jiang, Y.G.; Shi, Y.H.; Sun, J.; Le, G.W. Dietary methionine restriction improves the gut microbiota and reduces intestinal permeability and inflammation in high-fat-fed mice. Food Funct. 2019, 10, 5952–5968. [Google Scholar] [CrossRef]
- Li, D.K.; Chaudhari, S.N.; Lee, Y.; Sojoodi, M.; Adhikari, A.A.; Zukerberg, L.; Shroff, S.; Barrett, S.C.; Tanabe, K.; Chung, R.T.; et al. Inhibition of microbial deconjugation of micellar bile acids protects against intestinal permeability and liver injury. Sci. Adv. 2022, 8, eabo2794. [Google Scholar] [CrossRef]
- Williams, J.M.; Duckworth, C.A.; Burkitt, M.D.; Watson, A.J.M.; Campbell, B.J.; Pritchard, D.M. Epithelial Cell Shedding and Barrier Function: A Matter of Life and Death at the Small Intestinal Villus Tip. Vet. Pathol. 2015, 52, 445–455. [Google Scholar] [CrossRef]
- Liu, C.Y.; Cham, C.M.; Chang, E.B. Epithelial wound healing in inflammatory bowel diseases: The next therapeutic frontier. Transl. Res. 2021, 236, 35–51. [Google Scholar] [CrossRef]
- Chen, L.; Jiao, T.Y.; Liu, W.W.; Luo, Y.H.; Wang, J.; Guo, X.Z.; Tong, X.; Lin, Z.M.; Sun, C.Y.; Wang, K.L.; et al. Hepatic cytochrome P450 8B1 and cholic acid potentiate intestinal epithelial injury in colitis by suppressing intestinal stem cell renewal. Cell Stem Cell 2022, 29, 1366–1381. [Google Scholar] [CrossRef]
- Huang, D.; Xiong, M.L.; Xu, X.J.; Wu, X.W.; Xu, J.X.; Cai, X.B.; Lu, L.G.; Zhou, H. Bile acids elevated by high-fat feeding induce endoplasmic reticulum stress in intestinal stem cells and contribute to mucosal barrier damage. Biochem. Biophys. Res. Commun. 2020, 529, 289–295. [Google Scholar] [CrossRef]
- Sorrentino, G.; Perino, A.; Yildiz, E.; El Alam, G.; Sleiman, M.B.; Gioiello, A.; Pellicciari, R.; Schoonjans, K. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology 2020, 159, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Mroz, M.S.; Lajczak, N.K.; Goggins, B.J.; Keely, S.; Keely, S.J. The bile acids, deoxycholic acid and ursodeoxycholic acid, regulate colonic epithelial wound healing. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 314, G378–G387. [Google Scholar] [CrossRef]
- Yamada, S.; Masuno, H.; Kagechika, H.; Tanatani, A.; Kanda, Y. A Novel Lithocholic Acid Derivative Upregulates Detoxification-Related Genes in Human Induced Pluripotent Stem Cell-Derived Intestinal Organoids. Biol. Pharm. Bull. 2022, 45, 1720–1724. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Morbe, U.M.; Jorgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef]
- Tanoue, T.; Atarashi, K.; Honda, K. Development and maintenance of intestinal regulatory T cells. Nat. Rev. Immunol. 2016, 16, 295–309. [Google Scholar] [CrossRef]
- Paik, D.; Yao, L.N.; Zhang, Y.C.; Bae, S.; D’Agostino, G.D.; Zhang, M.H.; Kim, E.; Franzosa, E.A.; Avila-Pacheco, J.; Bisanz, J.E.; et al. Human gut bacteria produce T(H)17-modulating bile acid metabolites. Nature 2022, 603, 907–912. [Google Scholar] [CrossRef]
- Song, X.Y.; Sun, X.M.; Oh, S.F.; Wu, M.; Zhang, Y.B.; Zheng, W.; Geva-Zatorsky, N.; Jupp, R.; Mathis, D.; Benoist, C.; et al. Microbial bile acid metabolites modulate gut ROR gamma(+) regulatory T cell homeostasis. Nature 2020, 577, 410–415. [Google Scholar] [CrossRef]
- Campbell, C.; McKenney, P.T.; Konstantinovsky, D.; Isaeva, O.I.; Schizas, M.; Verter, J.; Mai, C.; Jin, W.B.; Guo, C.J.; Violante, S.; et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 2020, 581, 475–479. [Google Scholar] [CrossRef]
- Wang, L.Y.; Gong, Z.Z.; Zhang, X.Y.; Zhu, F.X.X.; Liu, Y.C.; Jin, C.Z.; Du, X.X.; Xu, C.F.; Chen, Y.W.; Cai, W.; et al. Gut microbial bile acid metabolite skews macrophage polarization and contributes to high-fat diet-induced colonic inflammation. Gut Microbes 2020, 12, 1819155. [Google Scholar] [CrossRef]
- Pi, Y.; Wu, Y.J.; Zhang, X.Y.; Lu, D.D.; Han, D.D.; Zhao, J.C.; Zheng, X.J.; Zhang, S.Y.; Ye, H.; Lian, S.; et al. Gut microbiota-derived ursodeoxycholic acid alleviates low birth weight-induced colonic inflammation by enhancing M2 macrophage polarization. Microbiome 2023, 11, 19. [Google Scholar] [CrossRef]
- Shi, T.; Malik, A.; Yang Vom Hofe, A.; Matuschek, L.; Mullen, M.; Lages, C.S.; Kudira, R.; Singh, R.; Zhang, W.; Setchell, K.D.R.; et al. Farnesoid X receptor antagonizes macrophage-dependent licensing of effector T lymphocytes and progression of sclerosing cholangitis. Sci. Transl. Med. 2022, 14, eabi4354. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Author Correction: Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 898. [Google Scholar] [CrossRef]
- Jagt, J.Z.; Verburgt, C.M.; de Vries, R.; de Boer, N.K.H.; Benninga, M.A.; de Jonge, W.J.; van Limbergen, J.E.; de Meij, T.G.J. Faecal Metabolomics in Paediatric Inflammatory Bowel Disease: A Systematic Review. J. Crohns Colitis 2022, 16, 1777–1790. [Google Scholar] [CrossRef]
- Battat, R.; Scherl, E.J.; Lukin, D.; Charilaou, P.; Mahtani, P.; Gerber, J.; Gandara, J.A.; Bank, J.R.I.L.C.; Dundar, F.; Zumbo, P.; et al. Increased Primary Bile Acids with Ileocolonic Resection Impact Ileal Inflammation and Gut Microbiota in Inflammatory Bowel Disease. J. Crohns Colitis 2022, 17, 795–803. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, L.; Duan, Y.; Gao, Y.; Gao, H.; Mao, D.; Luo, Y. Gut microbiota exaggerates triclosan-induced liver injury via gut-liver axis. J. Hazard. Mater. 2022, 421, 126707. [Google Scholar] [CrossRef]
- Liu, T.C.; Kern, J.T.; Jain, U.; Sonnek, N.M.; Xiong, S.; Simpson, K.F.; VanDussen, K.L.; Winkler, E.S.; Haritunians, T.; Malique, A.; et al. Western diet induces Paneth cell defects through microbiome alterations and farnesoid X receptor and type I interferon activation. Cell Host Microbe 2021, 29, 988–1001.e6. [Google Scholar] [CrossRef]
- Baptista, L.; Pollard, D.; Di Bella, A. Evaluation of Resting Serum Bile Acid Concentrations in Dogs with Sepsis. Vet. Sci. 2022, 9, 627. [Google Scholar] [CrossRef]
- Zohrer, E.; Meinel, K.; Fauler, G.; Moser, V.A.; Greimel, T.; Zobl, J.; Schlagenhauf, A.; Jahnel, J. Neonatal sepsis leads to early rise of rare serum bile acid tauro-omega-muricholic acid (TOMCA). Pediatr. Res. 2018, 84, 66–70. [Google Scholar] [CrossRef]
- Ainosah, R.H.; Hagras, M.M.; Alharthi, S.E.; Saadah, O.I. The effects of ursodeoxycholic acid on sepsis-induced cholestasis management in an animal model. J. Taibah Univ. Med. Sci. 2020, 15, 312–320. [Google Scholar] [CrossRef]
- Golden, J.M.; Escobar, O.H.; Nguyen, M.V.L.; Mallicote, M.U.; Kavarian, P.; Frey, M.R.; Gayer, C.P. Ursodeoxycholic acid protects against intestinal barrier breakdown by promoting enterocyte migration via EGFR- and COX-2-dependent mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G259–G271. [Google Scholar] [CrossRef]
- Yuan, S.; Fang, Y.; Tang, M.; Hu, Z.; Rao, C.; Chen, J.; Xia, Y.; Zhang, M.; Yan, J.; Tang, B.; et al. Tauroursodeoxycholic acid prevents Burkholderia pseudomallei-induced endoplasmic reticulum stress and is protective during melioidosis in mice. BMC Microbiol. 2021, 21, 137. [Google Scholar] [CrossRef]
- Wong, W.Y.; Chan, B.D.; Sham, T.T.; Lee, M.M.L.; Chan, C.O.; Chau, C.T.; Mok, D.K.W.; Kwan, Y.W.; Tai, W.C.S. Lactobacillus casei Strain Shirota Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice by Increasing Taurine-Conjugated Bile Acids and Inhibiting NF-kappa B Signaling via Stabilization of I kappa B alpha. Front. Nutr. 2022, 9, 816836. [Google Scholar] [CrossRef]
- Smirnova, E.; Muthiah, M.D.; Narayan, N.; Siddiqui, M.S.; Puri, P.; Luketic, V.A.; Contos, M.J.; Idowu, M.; Chuang, J.C.; Billin, A.N.; et al. Metabolic reprogramming of the intestinal microbiome with functional bile acid changes underlie the development of NAFLD. Hepatology 2022, 76, 1811–1824. [Google Scholar] [CrossRef]
- Nimer, N.; Choucair, I.; Wang, Z.N.; Nemet, I.; Li, L.; Gukasyan, J.; Weeks, T.L.; Alkhouri, N.; Zein, N.; Tang, W.H.W.; et al. Bile acids profile, histopathological indices and genetic variants for non-alcoholic fatty liver disease progression. Metabolism 2021, 116, 154457. [Google Scholar] [CrossRef]
- Caussy, C.; Hsu, C.; Singh, S.; Bassirian, S.; Kolar, J.; Faulkner, C.; Sinha, N.; Bettencourt, R.; Gara, N.; Valasek, M.A.; et al. Serum bile acid patterns are associated with the presence of NAFLD in twins, and dose-dependent changes with increase in fibrosis stage in patients with biopsy-proven NAFLD. Aliment. Pharm. Ther. 2019, 49, 183–193. [Google Scholar] [CrossRef]
- Tawfiq, R.A.; Nassar, N.N.; Hammam, O.A.; Allam, R.M.; Elmazar, M.M.; Abdallah, D.M.; Attia, Y.M. Obeticholic acid orchestrates the crosstalk between ileal autophagy and tight junctions in non-alcoholic steatohepatitis: Role of TLR4/TGF-beta 1 axis. Chem.-Biol. Interact. 2022, 361, 109953. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Zhu, L.; Liu, H.N.; Tseng, Y.J.; Weng, S.Q.; Liu, T.T.; Dong, L.; Shen, X.Z. The protective effect and mechanism of the FXR agonist obeticholic acid via targeting gut microbiota in non-alcoholic fatty liver disease. Drug Des. Dev. Ther. 2019, 13, 2249–2270. [Google Scholar] [CrossRef]
- Xiao, Y.T.; Wang, Y.; Liu, Y.; Wang, W.P.; Tian, X.B.; Chen, S.S.; Lu, Y.; Du, J.; Cai, W. A nonbile acid farnesoid X receptor agonist tropifexor potently inhibits cholestatic liver injury and fibrosis by modulating the gut-liver axis. Liver Int. 2021, 41, 2117–2131. [Google Scholar] [CrossRef]
- Wang, W.J.; Zhao, J.F.; Gui, W.F.; Sun, D.; Dai, H.J.; Xiao, L.; Chu, H.K.; Du, F.; Zhu, Q.J.; Schnabl, B.; et al. Tauroursodeoxycholic acid inhibits intestinal inflammation and barrier disruption in mice with non-alcoholic fatty liver disease. Br. J. Pharmacol. 2018, 175, 469–484. [Google Scholar] [CrossRef]
- Wan, Y.; Yuan, J.H.; Li, J.; Li, H.; Zhang, J.J.; Tang, J.; Ni, Y.; Huang, T.; Wang, F.L.; Zhao, F.; et al. Unconjugated and secondary bile acid profiles in response to higher-fat, lower-carbohydrate diet and associated with related gut microbiota: A 6-month randomized controlled-feeding trial. Clin. Nutr. 2020, 39, 395–404. [Google Scholar] [CrossRef]
- Gao, J.; Mao, K.M.; Wang, X.H.; Mi, S.; Fu, M.Q.; Li, X.Y.; Xiao, J.B.; Simal-Gandara, J.; Sang, Y.X. Tibet Kefir Milk Regulated Metabolic Changes Induced by High-Fat Diet via Amino Acids, Bile Acids, and Equol Metabolism in Human-Microbiota-Associated Rats. J. Agric. Food Chem. 2021, 69, 6720–6732. [Google Scholar] [CrossRef]
- Niekamp, P.; Kim, C.H. Microbial Metabolite Dysbiosis and Colorectal Cancer. Gut Liver 2023, 17, 190. [Google Scholar] [CrossRef]
- Liu, L.; Yang, M.; Dong, W.X.; Liu, T.Y.; Song, X.L.; Gu, Y.; Wang, S.N.; Liu, Y.; Abla, Z.; Qiao, X.M.; et al. Gut Dysbiosis and Abnormal Bile Acid Metabolism in Colitis-Associated Cancer. Gastroenterol. Res. Pract. 2021, 2021, 6645970. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Goldberg, A.A.; Richard, V.R.; Kyryakov, P.; Bourque, S.D.; Beach, A.; Burstein, M.T.; Glebov, A.; Koupaki, O.; Boukh-Viner, T.; Gregg, C.; et al. Chemical genetic screen identifies lithocholic acid as an anti-aging compound that extends yeast chronological life span in a TOR-independent manner, by modulating housekeeping longevity assurance processes. Aging 2010, 2, 393–414. [Google Scholar] [CrossRef]
- Frommherz, L.; Bub, A.; Hummel, E.; Rist, M.J.; Roth, A.; Watzl, B.; Kulling, S.E. Age-Related Changes of Plasma Bile Acid Concentrations in Healthy Adults-Results from the Cross-Sectional KarMeN Study. PLoS ONE 2016, 11, e0153959. [Google Scholar] [CrossRef]
- Lee, G.; Lee, H.; Hong, J.; Lee, S.H.; Jung, B.H. Quantitative profiling of bile acids in rat bile using ultrahigh-performance liquid chromatography-orbitrap mass spectrometry: Alteration of the bile acid composition with aging. J. Chromatogr. B 2016, 1031, 37–49. [Google Scholar] [CrossRef]
- Shao, Y.P.; Ouyang, Y.; Li, T.B.; Liu, X.Y.; Xu, X.J.; Li, S.; Xu, G.W.; Le, W.D. Alteration of Metabolic Profile and Potential Biomarkers in the Plasma of Alzheimer’s Disease. Aging Dis. 2020, 11, 1459–1470. [Google Scholar] [CrossRef]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef]
- Keita, A.V.; Lindqvist, C.M.; Ost, A.; Magana, C.D.L.; Schoultz, I.; Halfvarson, J. Gut Barrier Dysfunction-A Primary Defect in Twins with Crohn’s Disease Predominantly Caused by Genetic Predisposition. J. Crohns Colitis 2018, 12, 1200–1209. [Google Scholar] [CrossRef]
- Pi, Y.; Zhang, X.Y.; Wu, Y.J.; Wang, Z.Y.; Bai, Y.; Liu, X.Y.; Han, D.D.; Zhao, J.B.; Tobin, I.; Zhao, J.C.; et al. Alginate Alleviates Dextran Sulfate Sodium-Induced Colitis by Promoting Bifidobacterium animalis and Intestinal Hyodeoxycholic Acid Synthesis in Mice. Microbiol. Spectr. 2022, 10, e02979-22. [Google Scholar] [CrossRef]
- Lee, J.W.J.; Plichta, D.; Hogstrom, L.; Borren, N.Z.; Lau, H.; Gregory, S.M.; Tan, W.; Khalili, H.; Clish, C.; Vlamakis, H.; et al. Multi-omics reveal microbial determinants impacting responses to biologic therapies in inflammatory bowel disease. Cell Host Microbe 2021, 29, 1294–1304.e4. [Google Scholar] [CrossRef]
- Vantrappen, G.; Ghoos, Y.; Rutgeerts, P.; Janssens, J. Bile acid studies in uncomplicated Crohn’s disease. Gut 1977, 18, 730–735. [Google Scholar] [CrossRef]
- Hernandez-Rocha, C.; Borowski, K.; Turpin, W.; Filice, M.; Nayeri, S.; Raygoza Garay, J.A.; Stempak, J.M.; Silverberg, M.S. Integrative Analysis of Colonic Biopsies from Inflammatory Bowel Disease Patients Identifies an Interaction Between Microbial Bile Acid-inducible Gene Abundance and Human Angiopoietin-like 4 Gene Expression. J. Crohns Colitis 2021, 15, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Huang, X.S.; Wang, H.T.; Hegner, C.; Liu, Y.J.; Shang, J.S.; Eliason, A.; Diao, H.T.; Park, H.; Frey, B.; et al. CAR directs T cell adaptation to bile acids in the small intestine. Nature 2021, 593, 147–151. [Google Scholar] [CrossRef]
- Foley, S.E.; Tuohy, C.; Dunford, M.; Grey, M.J.; De Luca, H.; Cawley, C.; Szabady, R.L.; Maldonado-Contreras, A.; Houghton, J.M.; Ward, D.V.; et al. Gut microbiota regulation of P-glycoprotein in the intestinal epithelium in maintenance of homeostasis. Microbiome 2021, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, Y.; Li, C.; Xie, Z.; Dai, L. Amelioration of Colitis by a Gut Bacterial Consortium Producing Anti-Inflammatory Secondary Bile Acids. Microbiol. Spectr. 2023, 11, e0333022. [Google Scholar] [CrossRef]
- Gao, R.Y.; Shearn, C.T.; Orlicky, D.J.; Battista, K.D.; Alexeev, E.E.; Cartwright, I.M.; Lanis, J.M.; Kostelecky, R.E.; Ju, C.; Colgan, S.P.; et al. Bile acids modulate colonic MAdCAM-1 expression in a murine model of combined cholestasis and colitis. Mucosal Immunol. 2021, 14, 479–490. [Google Scholar] [CrossRef]
- Huo, X.K.; Li, D.W.; Wu, F.; Li, S.H.; Qiao, Y.L.; Wang, C.; Wang, Y.; Zhou, C.J.; Sun, L.Q.; Luan, Z.L.; et al. Cultivated human intestinal fungus Candida metapsilosis M2006B attenuates colitis by secreting acyclic sesquiterpenoids as FXR agonists. Gut 2022, 71, 2205–2217. [Google Scholar] [CrossRef]
- Xu, M.Q.; Shen, Y.Q.; Cen, M.S.; Zhu, Y.B.; Cheng, F.L.; Tang, L.L.; Zheng, X.; Kim, J.J.; Dai, N.; Hu, W.L. Modulation of the Gut Microbiota-farnesoid X Receptor Axis Improves Deoxycholic Acid-induced Intestinal Inflammation in Mice. J. Crohns Colitis 2021, 15, 1197–1210. [Google Scholar] [CrossRef]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Marshall, J.C.; Christou, N.V.; Meakins, J.L. The gastrointestinal tract. The “undrained abscess” of multiple organ failure. Ann. Surg. 1993, 218, 111–119. [Google Scholar] [CrossRef]
- Adiliaghdam, F.; Cavallaro, P.; Mohad, V.; Almpani, M.; Kuhn, F.; Gharedaghi, M.H.; Najibi, M.; Rahme, L.G.; Hodin, R.A. Targeting the gut to prevent sepsis from a cutaneous burn. JCI Insight 2020, 5, e137128. [Google Scholar] [CrossRef]
- Potruch, A.; Schwartz, A.; Ilan, Y. The role of bacterial translocation in sepsis: A new target for therapy. Ther. Adv. Gastroenterol. 2022, 15, 17562848221094214. [Google Scholar] [CrossRef]
- Horvatits, T.; Drolz, A.; Rutter, K.; Roedl, K.; Langouche, L.; Van den Berghe, G.; Fauler, G.; Meyer, B.; Hulsmann, M.; Heinz, G.; et al. Circulating bile acids predict outcome in critically ill patients. Ann. Intensive Care 2017, 7, 48. [Google Scholar] [CrossRef]
- Kosyakovsky, L.B.; Somerset, E.; Rogers, A.J.; Sklar, M.; Mayers, J.R.; Toma, A.; Szekely, Y.; Soussi, S.; Wang, B.; Fan, C.S.; et al. Machine learning approaches to the human metabolome in sepsis identify metabolic links with survival. Intensive Care Med. Exp. 2022, 10, 24. [Google Scholar] [CrossRef]
- Lorenzo-Zuniga, V.; Bartoli, R.; Planas, R.; Hofmann, A.F.; Vinado, B.; Hagey, L.R.; Hernandez, J.M.; Mane, J.; Alvarez, M.A.; Ausina, V.; et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology 2003, 37, 551–557. [Google Scholar] [CrossRef]
- Xiao, L.X.; Qi, L.; Zhang, X.L.; Zhou, Y.Q.; Yue, H.L.; Yu, E.D.; Li, Q.Y. Liver injury in septic mice were suppressed by a camptothecin-bile acid conjugate via inhibiting NF-kappa B signaling pathway. Life Sci. 2020, 257, 118130. [Google Scholar] [CrossRef]
- Chen, L.F.; Li, H.Y.; Chen, Y.; Yang, Y.M. Probiotic Lactobacillus rhamnosus GG reduces mortality of septic mice by modulating gut microbiota composition and metabolic profiles. Nutrition 2020, 78, 110863. [Google Scholar] [CrossRef]
- Li, Y.F.; Sheng, H.D.; Qian, J.; Wang, Y. The Chinese medicine babaodan suppresses LPS-induced sepsis by inhibiting NLRP3-mediated inflammasome activation. J. Ethnopharmacol. 2022, 292, 115205. [Google Scholar] [CrossRef]
- Liu, T.T.; Tang, X.M.; Cui, Y.; Xiong, X.; Xu, Y.Y.; Hu, S.H.; Feng, S.Y.; Shao, L.J.; Ren, Y.Q.; Miao, H.J.; et al. Fibroblast Growth Factor 19 Improves LPS-Induced Lipid Disorder and Organ Injury by Regulating Metabolomic Characteristics in Mice. Oxid. Med. Cell Longev. 2022, 2022, 9673512. [Google Scholar] [CrossRef]
- Jin, P.; Deng, S.X.; Tian, M.; Lenahan, C.; Wei, P.J.; Wang, Y.; Tan, J.Y.; Wen, H.M.; Zhao, F.; Gao, Y.Q.; et al. INT-777 prevents cognitive impairment by activating Takeda G protein-coupled receptor 5 (TGR5) and attenuating neuroinflammation via cAMP/PKA/ CREB signaling axis in a rat model of sepsis. Exp. Neurol. 2021, 335, 113504. [Google Scholar] [CrossRef]
- Hao, H.; Cao, L.; Jiang, C.; Che, Y.; Zhang, S.; Takahashi, S.; Wang, G.; Gonzalez, F.J. Farnesoid X Receptor Regulation of the NLRP3 Inflammasome Underlies Cholestasis-Associated Sepsis. Cell Metab. 2017, 25, 856–867.e5. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Yilmaz, Y.; Yu, M.L.; Wong, V.W.S.; Fernandez, M.C.; Isakov, V.A.; Duseja, A.K.; Mendez-Sanchez, N.; Eguchi, Y.; Bugianesi, E.; et al. Clinical and Patient-Reported Outcomes From Patients With Nonalcoholic Fatty Liver Disease Across the World: Data From the Global Non-Alcoholic Steatohepatitis (NASH)/Non-Alcoholic Fatty Liver Disease (NAFLD) Registry. Clin. Gastroenterol. Hepatol. 2022, 20, 2296–2306. [Google Scholar] [CrossRef]
- Wu, S.S.; Yuan, C.Z.; Yang, Z.R.; Liu, S.; Zhang, Q.; Zhang, S.T.; Zhu, S.T. Non-alcoholic fatty liver is associated with increased risk of irritable bowel syndrome: A prospective cohort study. BMC Med. 2022, 20, 262. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Adolph, T.E.; Trauner, M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef]
- He, S.Y.; Cui, S.D.; Song, W.; Jiang, Y.H.; Chen, H.S.; Liao, D.J.; Lu, X.P.; Li, J.; Chen, X.Q.; Peng, L. Interleukin-17 Weakens the NAFLD/NASH Process by Facilitating Intestinal Barrier Restoration Depending on the Gut Microbiota. Mbio 2022, 13, e03688-21. [Google Scholar] [CrossRef]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef]
- Sun, J.Y.; Fan, J.M.; Li, T.T.; Yan, X.X.; Jiang, Y.H. Nuciferine Protects Against High-Fat Diet-Induced Hepatic Steatosis via Modulation of Gut Microbiota and Bile Acid Metabolism in Rats. J. Agric. Food Chem. 2022, 70, 12014–12028. [Google Scholar] [CrossRef] [PubMed]
- Fiaschini, N.; Mancuso, M.; Tanori, M.; Colantoni, E.; Vitali, R.; Diretto, G.; Rebenaque, L.L.; Stronati, L.; Negroni, A. Liver Steatosis and Steatohepatitis Alter Bile Acid Receptors in Brain and Induce Neuroinflammation: A Contribution of Circulating Bile Acids and Blood-Brain Barrier. Int. J. Mol. Sci. 2022, 23, 14254. [Google Scholar] [CrossRef]
- Rubio, C.; Puerto, M.; Garcia-Rodriquez, J.J.; Lu, V.B.; Garcia-Martinez, I.; Alen, R.; Sanmartin-Salinas, P.; Toledo-Lobo, M.V.; Saiz, J.; Ruperez, J.; et al. Impact of global PTP1B deficiency on the gut barrier permeability during NASH in mice. Mol. Metab. 2020, 35, 100954. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Ijaz, M.U.; Hussain, M.; ul Haq, I.; Zhao, D.; Li, C.B. High-Fat Proteins Drive Dynamic Changes in Gut Microbiota, Hepatic Metabolome, and Endotoxemia-TLR-4-NF kappa B-Mediated Inflammation in Mice. J. Agric. Food Chem. 2020, 68, 11710–11725. [Google Scholar] [CrossRef]
- Finn, P.D.; Rodriguez, D.; Kohler, J.; Jiang, Z.; Wan, S.; Blanco, E.; King, A.J.; Chen, T.; Bell, N.; Dragoli, D.; et al. Intestinal TGR5 agonism improves hepatic steatosis and insulin sensitivity in Western diet-fed mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 316, G412–G424. [Google Scholar] [CrossRef]
- Zhai, Y.Y.; Zhou, W.L.; Yan, X.; Qiao, Y.; Guan, L.L.; Zhang, Z.C.; Liu, H.; Jiang, J.Z.; Liu, J.; Peng, L. Astragaloside IV ameliorates diet-induced hepatic steatosis in obese mice by inhibiting intestinal FXR via intestinal flora remodeling. Phytomedicine 2022, 107, 154444. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Baker, S.S.; Chapa-Rodriguez, A.; Liu, W.S.; Nugent, C.A.; Tsompana, M.; Mastrandrea, L.; Buck, M.J.; Baker, R.D.; Genco, R.J.; et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018, 67, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; You, H.J.; Bajaj, J.S.; Joo, S.K.; Yu, J.; Park, S.; Kang, H.; Park, J.H.; Kim, J.H.; Lee, D.H.; et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat. Commun. 2020, 11, 4982. [Google Scholar] [CrossRef]
- Duan, R.Q.; Huang, K.; Guan, X.; Li, S.; Xia, J.; Shen, M.; Sun, Z.; Yu, Z.Q. Tectorigenin ameliorated high-fat diet-induced nonalcoholic fatty liver disease through anti-inflammation and modulating gut microbiota in mice. Food Chem. Toxicol. 2022, 164, 112948. [Google Scholar] [CrossRef] [PubMed]
- Carbajo-Pescador, S.; Porras, D.; Garcia-Mediavilla, M.V.; Martinez-Florez, S.; Juarez-Fernandez, M.; Cuevas, M.J.; Mauriz, J.L.; Gonzalez-Gallego, J.; Nistal, E.; Sanchez-Campos, S. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. Dis. Model. Mech. 2019, 12, dmm039206. [Google Scholar] [CrossRef]
- Qin, T.; Gao, X.; Lei, L.; Feng, J.; Zhang, W.; Hu, Y.; Shen, Z.; Liu, Z.; Huan, Y.; Wu, S.; et al. Machine learning- and structure-based discovery of a novel chemotype as FXR agonists for potential treatment of nonalcoholic fatty liver disease. Eur. J. Med. Chem. 2023, 252, 115307. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, W.W.; Ma, Y.M.; Zhao, X.H.; He, L.M.; Sun, P.; Wang, H.Y. High-fat diet aggravates colitis-associated carcinogenesis by evading ferroptosis in the ER stress-mediated pathway. Free. Radic. Biol. Med. 2021, 177, 156–166. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Nguyen, L.H.; Mehta, R.S.; Song, M.Y.; Huttenhower, C.; Chan, A.T. Association Between the Sulfur Microbial Diet and Risk of Colorectal Cancer. JAMA Netw. Open 2021, 4, e2134308. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Ou, F.S.; Bainter, T.M.; Fuchs, C.S.; Niedzwiecki, D.; Zhang, S.; Saltz, L.B.; Mayer, R.J.; Hantel, A.; Benson, A.; et al. Associations Between Unprocessed Red Meat and Processed Meat With Risk of Recurrence and Mortality in Patients With Stage III Colon Cancer. JAMA Netw. Open 2022, 5, e220145. [Google Scholar] [CrossRef]
- Zeng, H.W.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef]
- Song, X.L.; An, Y.P.; Chen, D.F.; Zhang, W.R.; Wu, X.M.; Li, C.Q.; Wang, S.N.; Dong, W.X.; Wang, B.M.; Liu, T.Y.; et al. Microbial metabolite deoxycholic acid promotes vasculogenic mimicry formation in intestinal carcinogenesis. Cancer Sci. 2022, 113, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Ocvirk, S.; Wilson, A.S.; Posma, J.M.; Li, J.V.; Koller, K.R.; Day, G.M.; Flanagan, C.A.; Otto, J.E.; Sacco, P.E.; Sacco, F.D.; et al. A prospective cohort analysis of gut microbial co-metabolism in Alaska Native and rural African people at high and low risk of colorectal cancer. Am. J. Clin. Nutr. 2020, 111, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.X.; Liu, L.; Dou, Y.; Xu, M.Q.; Liu, T.Y.; Wang, S.N.; Zhang, Y.J.; Deng, B.R.; Wang, B.M.; Cao, H.L. Deoxycholic acid activates epidermal growth factor receptor and promotes intestinal carcinogenesis by ADAM17-dependent ligand release. J. Cell Mol. Med. 2018, 22, 4263–4273. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Gillilland, M.; Lee, A.A.; Wu, X.Y.; Zhou, S.Y.; Owyang, C. Secondary bile acids mediate high-fat diet-induced upregulation of R-spondin 3 and intestinal epithelial proliferation. JCI Insight 2022, 7, e148309. [Google Scholar] [CrossRef]
- Wang, S.A.; Dong, W.X.; Liu, L.; Xu, M.Q.; Wang, Y.; Liu, T.Y.; Zhang, Y.J.; Wang, B.M.; Cao, H.L. Interplay between bile acids and the gut microbiota promotes intestinal carcinogenesis. Mol. Carcinog. 2019, 58, 1155–1167. [Google Scholar] [CrossRef]
- Zhou, M.J.; Wang, D.F.; Li, X.; Cao, Y.; Yi, C.X.; Ocansey, D.K.W.; Zhou, Y.L.; Mao, F. Farnesoid-X receptor as a therapeutic target for inflammatory bowel disease and colorectal cancer. Front. Pharmacol. 2022, 13, 1016836. [Google Scholar] [CrossRef]
- Fu, T.; Coulter, S.; Yoshihara, E.; Oh, T.G.; Fang, S.; Cayabyab, F.; Zhu, Q.Y.; Zhang, T.; Leblanc, M.; Liu, S.H.; et al. FXR Regulates Intestinal Cancer Stem Cell Proliferation. Cell 2019, 176, 1098–1112. [Google Scholar] [CrossRef]
- Li, X.Y.; Khan, I.; Huang, G.X.; Lu, Y.Y.; Wang, L.P.; Liu, Y.Y.; Lu, L.L.; Hsiao, W.; Liu, Z.Q. Kaempferol acts on bile acid signaling and gut microbiota to attenuate the tumor burden in ApcMin/plus mice. Eur. J. Pharmacol. 2022, 918, 174773. [Google Scholar] [CrossRef]
- Li, S.; Xu, Z.S.; Guo, J.; Zheng, J.B.; Sun, X.J.; Yu, J.H. Farnesoid X receptor activation induces antitumour activity in colorectal cancer by suppressing JAK2/STAT3 signalling via transactivation of SOCS3 gene. J. Cell Mol. Med. 2020, 24, 14549–14560. [Google Scholar] [CrossRef]
- Yu, J.H.; Yang, K.; Zheng, J.B.; Zhao, P.W.; Xia, J.; Sun, X.J.; Zhao, W. Activation of FXR and inhibition of EZH2 synergistically inhibit colorectal cancer through cooperatively accelerating FXR nuclear location and upregulating CDX2 expression. Cell Death Dis. 2022, 13, 388. [Google Scholar] [CrossRef]
- Perino, A.; Demagny, H.; Velazquez-Villegas, L.; Schoonjans, K. Molecular Physiology of Bile Acid Signaling in Health, Disease, and Aging. Physiol. Rev. 2021, 101, 683–731. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Rimal, B.; Patterson, A.D. Role of bile acids and gut bacteria in healthy ageing of centenarians. Nature 2021, 599, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Hofer, U. Unique bile acid metabolism in centenarians. Nat. Rev. Microbiol. 2021, 19, 618. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Green, C.L.; Soltow, Q.A.; Mitchell, S.E.; Derous, D.; Wang, Y.C.; Chen, L.N.; Han, J.D.J.; Promislow, D.E.L.; Lusseau, D.; Douglas, A.; et al. The Effects of Graded Levels of Calorie Restriction: XIII. Global Metabolomics Screen Reveals Graded Changes in Circulating Amino Acids, Vitamins, and Bile Acids in the Plasma of C57BL/6 Mice. J. Gerontol. A-Biol. 2019, 74, 16–26. [Google Scholar] [CrossRef]
- Faits, T.; Walker, M.E.; Rodriguez-Morato, J.; Meng, H.C.; Gervis, J.E.; Galluccio, J.M.; Lichtenstein, A.H.; Johnson, W.E.; Matthan, N.R. Exploring changes in the human gut microbiota and microbial-derived metabolites in response to diets enriched in simple, refined, or unrefined carbohydrate-containing foods: A post hoc analysis of a randomized clinical trial. Am. J. Clin. Nutr. 2020, 112, 1631–1641. [Google Scholar] [CrossRef]
- Barcena, C.; Quiros, P.M.; Durand, S.; Mayoral, P.; Rodriguez, F.; Caravia, X.M.; Marino, G.; Garabaya, C.; Fernandez-Garcia, M.T.; Kroemer, G.; et al. Methionine Restriction Extends Lifespan in Progeroid Mice and Alters Lipid and Bile Acid Metabolism. Cell Rep. 2018, 24, 2392–2403. [Google Scholar] [CrossRef]
- Barcena, C.; Lopez-Otin, C.; Kroemer, G. Methionine restriction for improving progeria: Another autophagy-inducing anti-aging strategy? Autophagy 2019, 15, 558–559. [Google Scholar] [CrossRef]
- Barcena, C.; Valdes-Mas, R.; Mayoral, P.; Garabaya, C.; Durand, S.; Rodriguez, F.; Fernandez-Garcia, M.T.; Salazar, N.; Nogacka, A.M.; Garatachea, N.; et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 2019, 25, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.Q.; Li, Y.Z.; Che, M.; Li, C.W.; Wu, Q.; Sun, C. Melatonin relieves hepatic lipid dysmetabolism caused by aging via modifying the secondary bile acid pattern of gut microbes. Cell. Mol. Life Sci. 2022, 79, 527. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Davis, J.E.; Rappai, T.; McDonald, K.G.; Kulkarni, D.H.; Knoop, K.A.; Hogan, S.P.; Fitzpatrick, J.A.J.; Lencer, W.I.; Newberry, R.D. Intestinal goblet cells sample and deliver lumenal antigens by regulated endocytic uptake and transcytosis. eLife 2021, 10, e67292. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Gohir, W.; Kennedy, K.M.; Wallace, J.G.; Saoi, M.; Bellissimo, C.J.; Britz-McKibbin, P.; Petrik, J.J.; Surette, M.G.; Sloboda, D.M. High-fat diet intake modulates maternal intestinal adaptations to pregnancy and results in placental hypoxia, as well as altered fetal gut barrier proteins and immune markers. J. Physiol. 2019, 597, 3029–3051. [Google Scholar] [CrossRef]
- Hasan, M.N.; Chen, J.; Wang, H.; Du, Y.; Clayton, Y.D.; Gu, L.; Li, T. Glycine-β-Muricholic Acid Improves Liver Fibrosis and Gut Barrier Function by Reducing Bile Acid Pool Size and Hydrophobicity in Male Cyp2c70 Knockout Mice. Cells 2023, 12, 1371. [Google Scholar] [CrossRef]
- Jian, Y.P.; Yang, G.; Zhang, L.H.; Liang, J.Y.; Zhou, H.L.; Wang, Y.S.; Xu, Z.X. Lactobacillus plantarum alleviates irradiation-induced intestinal injury by activation of FXR-FGF15 signaling in intestinal epithelia. J. Cell. Physiol. 2022, 237, 1845–1856. [Google Scholar] [CrossRef]
| Diseases | BAs | BARs | Mechanism | Reference |
|---|---|---|---|---|
| IBD | PBAs↑ SBAs↓ | Inhibit FXR and TGR5 | Alter the expression of tight junction proteins and the renewal of intestinal stem cells; inhibit Paneth cell function and type I interferon signaling. | [32,47,48,49,50,51] |
| Sepsis | TBAs↑ TωMCA↑ | Inhibit FXR and TGR5 | TUDCA prevents sepsis through inhibiting TGR5-NF-κB and endoplasmic reticulum stress. TBAs increase the gut barrier integrity. | [52,53,54,55,56,57] |
| NAFLD | DCA↑ Conjugated PBAs↑ Unconjugated PBAs↓ | Inhibit FXR | Interfere with TLR4/TGF-β1 signaling pathway, activate autophagy and intestinal integrity, decrease intestinal barrier function, and induce changes in microbiota composition. | [58,59,60,61,62,63,64] |
| CRC | CA↑ DCA↑ CDCA↓ LCA↓ | Inhibit FXR-FGF15 Enhance TGR5 | Promotes dysregulated immunity, loss of the intestinal barrier, invasion of microbial and pathogenic metabolites, and increases inflammation. | [65,66,67,68] |
| Aging | LCA↓ iso-LCA↓ 3-oxo-LCA↓ | Inhibit RORγt | Encourage age-related immune dysregulation and promote chronic inflammation. | [42,69,70,71,72,73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Jin, L.; Huang, W. Bile Acids, Intestinal Barrier Dysfunction, and Related Diseases. Cells 2023, 12, 1888. https://doi.org/10.3390/cells12141888
Shi L, Jin L, Huang W. Bile Acids, Intestinal Barrier Dysfunction, and Related Diseases. Cells. 2023; 12(14):1888. https://doi.org/10.3390/cells12141888
Chicago/Turabian StyleShi, Linsen, Lihua Jin, and Wendong Huang. 2023. "Bile Acids, Intestinal Barrier Dysfunction, and Related Diseases" Cells 12, no. 14: 1888. https://doi.org/10.3390/cells12141888
APA StyleShi, L., Jin, L., & Huang, W. (2023). Bile Acids, Intestinal Barrier Dysfunction, and Related Diseases. Cells, 12(14), 1888. https://doi.org/10.3390/cells12141888







