1. Introduction
The mechanical properties of living cells, including their shape, rigidity, and internal dynamics, play a crucial role in cell physiology and pathology [
1,
2]. The mechanical response of the cell generally depends on its three main mechanical components: the cytoskeleton, the nucleus, and the cytoplasm. The cell cytoskeleton largely determines its shape and rigidity [
2,
3]. An important component of the cell cytoskeleton is the dense cortical actin mesh at the outer borders of the cytoskeleton. That structure is mechanically connected to the inner surface of the cell membrane [
4]. This actin mesh elasticity and cross-linking control the overall cell stiffness [
4].
More specifically, interactions of molecular motors such as kinesin, dynein, and myosin II with the cytoskeleton produce forces and mechanical work that significantly affect the motion and diffusion of intracellular constituents [
5]. These forces cause a direct motion of cell’s constituents such as organelles (the power of motion in this case is around 2; i.e., <∆r
2>~C∆t
α, where α = 2).
A dissipative part of that intracellular mechanical work is generated by the incoherent component of the action of those molecular motors [
5]. That dissipative part contributes to significant vibrations of the cytoskeleton elastic mesh. In turn, those vibrations have a major impact on the active intracellular diffusivity [
6] (the power of motion/diffusion in this case is less than 2; i.e., α < 2) and intracellular organization [
7].
The balance between cellular elasticity and intracellular mechanical work influences the motion of intracellular content, and thus significantly affects the dynamics and organization of the cell’s constituents [
6,
7]. While intracellular elasticity increases cellular stiffness, dis-homogeneity of intra-organelles content and reduces intracellular constituent motion, the intracellular dissipative part of mechanical work can overcome those dynamical effects and restore intracellular indirect motion and intra-organelle homogeneity [
7].
Intracellular liquid–liquid phase separation (LLPS), which has a profound impact on cell physiology [
8], has been found to be negatively correlated with intracellular mechanical work [
9], particularly cortical actin mechanical work [
10]. Consequently, the control and balance of intracellular elasticity and the dissipation of mechanical work are expected to significantly influence cell physiology by impacting the dynamics and organization of the cell’s constituents.
Mechanical vibrations occurring in the inner part of the cytoskeleton due to intracellular dissipation of mechanical work are likely to induce related vibrations in the mechanically coupled cortical actin and cell membrane [
11]. These vibrations of the cell membrane can transfer mechanical energy outside the cell, effectively acting as another dissipation process that disperses energy to the surrounding environment. This leakage of mechanical work to the cell surroundings may serve a physiological purpose by balancing and reducing intracellular mechanical work, while also spreading mechanical work to adjacent cells. In this manner, a specific physiological state characterized by a high degree of intracellular dissipated mechanical work in a particular cell could be promoted in other adjacent and mechanically coupled cells through the transfer of mechanical energy.
The cell can actively adjust the tension or stiffness of its cortical actin through ATP-dependent actin-myosin reactions [
4]. By adjusting the cortical actin tension the cell may control its surface vibrations and the leakage of mechanical energy to the cell surroundings. Influx of calcium across the cell membrane is crucial for those ATP-dependent actin-myosin reactions (that in turn generate active cortical actin tension). Reducing such calcium influx may diminish the ability of cortical actin to generate tension and thus, control the cell surface vibrations and leakage of mechanical energy. Mibefradil, a specific inhibitor of voltage-gated T-type calcium channels, is expected to inhibit the calcium influx across the cell membrane, thereby impeding actin-myosin reactions and cortical actin tension.
Malignant cells have reduced stiffness [
12,
13], while the indirect dynamics of intracellular constituents are heightened [
5,
14,
15,
16]. As mentioned, these dynamics would be reflected in pronounced vibrations of the cytoskeleton, its cortical part and the cell membrane.
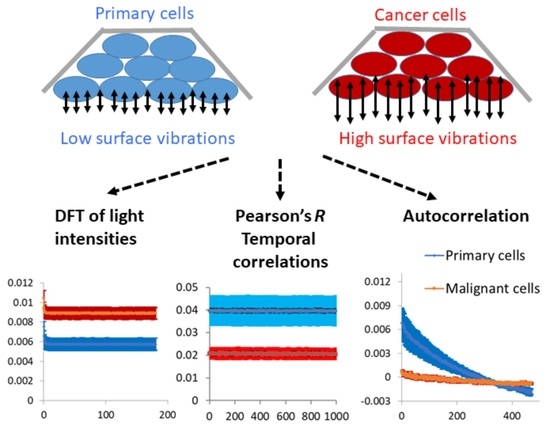
Solid malignant tumors typically consist of a dense conglomerate of malignant cells, whereas healthy tissues usually exhibit more spacious cell arrangements with significant amounts of connective tissue between them. The dense cellular architecture observed in malignant tumors, coupled with the anticipated high leakage of mechanical work from the cells, could facilitate the propagation of their distinctive physiological condition, characterized by elevated levels of intracellular indirect dynamics and mechanical work, among neighboring malignant cells. Consequently, this leakage of mechanical work may enable malignant cells to induce their malignant-related physiological state in adjacent cells. As such, sensitive evaluation of surface vibrations and dynamics of cells may assist in detecting or monitoring their malignancy.
To test these hypotheses, we examined cell membrane vibrations in primary lymphocytes and compared them to malignant CD8 and Jurkat cells. This analysis was conducted using TIRF microscopy within an immune synapse model. Additionally, we explored the response of primary lymphocytes, CD8 cells, and Jurkat cells to Mibefradil, an inhibitor of voltage-gated T-type calcium channels that should affect cortical actin tension generation [
17]. Furthermore, we evaluated the dynamics of cell surface movement in an in vitro tissue model by employing a cell plaque and reflected laser speckle analysis. This study encompassed four pairs of malignant and matched benign primary cells. We then explored the potential of cell membrane vibrations as a biomarker for the detection and monitoring of malignant cells and tissues.
2. Materials and Methods
2.1. Materials
Complete Medium (medium): RPMI-1640, DMEM medium, heat-inactivated fetal calf serum (FCS), penicillin, streptomycin, glutamine, sodium pyruvate, and HEPES obtained from Biological Industries (Kibbutz Beit Haemek, Israel). Mibefradil from Sigma-Aldrich (St. Louis, MO, USA). CD45 proteins were purchased from BioLegend (San Diego, CA, USA). Anti-human CD3 from eBioscience Inc. (ThermoFisher Scientific, Waltham, MA, USA).
2.2. Cell Lines
Jurkat (human leukemic) E6.1 (CD4+) T cells were a kind gift from the Samelson lab at the NIH. Jurkat cells were maintained in RPMI-1640 medium supplemented with 10% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, 2% glutamine, 2% sodium pyruvate, and 2% HEPES. Cells were maintained in completely humidified air with 5% CO2 at 37 °C.
Primary lymphocyte cells were provided by the Immunology and advanced CAR-T cell therapy Laboratory, Research & Development Department, Tel-Aviv Sourasky Medical Center, Tel Aviv, Israel.
The following cells were purchased from ATCC, delivered frozen, thawed, and maintained according to the vendor instructions.
NCI-H1573 cell line (lung adenocarcinoma) (ATCC CRL-5877)
Primary small airway epithelial cells (ATCC PCS-301-010)
PC-3 cell line (prostate adenocarcinoma) (ATCC CRL-1435)
Primary prostate epithelial cells (ATCC PCS-440-010)
TCCSUP cell line (transitional cell carcinoma) (ATCC HTB-5)
Primary bladder epithelial cells (ATCC PCS-420-010)
SK-MEL-24 cell line (melanoma) (ATCC HTB-71)
Primary epidermal melanocytes (ATCC PCS-200-013)
Immunostaining
CD45 proteins were labelled using mouse anti-human primary antibodies conjugated to alexa647 fluorophore (BioLegend, 304056). The labeling procedure followed the manufacturers’ protocols. Briefly, 0.5 µg of mouse anti human anti-CD45 monoclonal antibody conjugated to alexa647 was added to 500 × 103 cells suspended in FACS buffer for 45 min on ice. Cells were then washed in phosphate-buffered saline (PBS) three times and suspended in imaging buffer (RPMI without phenol red, 10% FBS, 25 mM HEPES).
2.3. Sample Preparation
Coverslip preparation was as follows: coverslips (#1.5 glass chambers, iBidi, Gräfelfing, Germany) were washed with acidic ethanol at room temperature (RT) for 10 min and dried at 37 °C for 1 h. Coverslips were then incubated at RT for 15 min with 0.01% poly-L-lysine (Sigma) diluted in water. This was followed by washing and drying of the coverslips at 37 °C for 1 h. For the immune synapse model experiment, the poly-L-lysine covered coverslips were incubated for 2 h at 37 °C with 10 μg/mL anti CD3 antibodies diluted in PBS. Then, the chambers were washed 3 times with PBS and left with PBS until the application of cells. Finally, cells were suspended in imaging buffer at a concentration of 1 million and 100,000–500,000 cells and were applied onto coverslips.
2.4. Treatment of Jurkat, CD8 Cells and Primary Lymphocytes with Mibefradil
Upon completion of measurements in all the cells, Mibefradil 10 µM was added to the cells medium. The samples were then incubated for 30 min on the microscope stage. At the end of incubation, cells were measured again.
2.5. AFM Measurements
Measurements were carried out using commercial AFM MFP-3D-Bio of Asylum Research (Oxford Instruments) combined with an inverted microscope. We used a relatively large and spherical probe with the following specifications: silicon Nitride (Si3N4) AFM cantilevers with colloidal SiO tips (CP-PNPL-SiO-C, Ø = 6.62 μm, sQUBE). The AFM probes were cleaned and oxidized using O2 Plasma (Atto, Diener Electronic) for 5 min prior to use. Next, the AFM probe was applied on the surface of live Jurkat cells that were suspended in imaging buffer on Falcon Petri Dish 50 × 9 mm (Corning, NY, USA). We conducted a single measurement procedure for each cell. Cantilever force constants ranged from 0.04–0.08 N/m as determined via individual calibration of the cantilever using the thermal noise method. During measurements, the AFM probe indented the cells at a constant speed of 2 μm/s with 2048 Hz frequency of measurement. Indentation and measurements were seized after the applied force on the probe reached a maximal value of 3 nN.
The power spectra were calculated by applying Discrete Fourier Transform (DFT) analysis on the time-dependent results using Matlab (version R2021b, MathWorks Inc., Natick, MA, USA).
2.6. Microscopy
TIRF microscopy: Cells were imaged using a TiE Nikon microscope. The cells were excited using a 647 mn pulsed laser (90 ps) at 2 mW/cm2 (20% power). Samples were imaged using a (CFI-SR-HP) Apochromat TIRF X100, NA of 1.49, oil-immersion objective (Nikon Instruments, Melville, NY, USA). Image stacks were generated by taking 1000 serial images with an acquisition time of 4.8 ms per individual frames of 128 × 128 pixels (160 nm pixel size). The reflection light was detected using an avalanche photodiode (APD) with a band-pass filter of 650–720 nm.
Sedimented cell pairs (primary and malignant) microscopy: the plaques of sedimented cells were imaged using a TiE Nikon microscope. The cells were excited using a 647 mn pulsed laser (90 ps) at 10 mW/cm2 (100% power). Samples were imaged using a (CFI-SR-HP) Apochromat X10, NA of 0.3, objective (Nikon Instruments). Image stacks were generated by taking 1000 serial images with an acquisition time of 2.6 ms per individual frames of 50 × 50 pixels (1.6 µm pixel size). The reflection light was detected using an avalanche photodiode (APD) with a band-pass filter of 650–720 nm.
2.7. Images Analysis
TIRF images analysis: In each cell, a squared region of interest (ROI) of 121 pixels was chosen at the cell interface with the coverslip. The fluorescence intensity of each pixel in each image was normalized by dividing its intensity with the average intensity of that time-dependent image. The temporal fluctuations of the normalized fluorescence intensities were analyzed via DFT for each pixel in a ROI. The amplitudes of the DFT analyzes were then averaged for each frequency for all the pixels of an ROI to obtain the averaged DFT results of each ROI (or cell) in each condition.
Sedimented cells microscopy: In each field of view, multiple squared ROI’s of 2500 pixels were chosen. The light intensity of each pixel in a ROI was normalized by dividing its intensity with the average intensity of that time-dependent image. The temporal fluctuations of the normalized intensities were analyzed via DFT and autocorrelation for each pixel in a ROI. The amplitudes of the DFT analyzes or autocorrelation values were then averaged for each frequency or lag for all the pixels of an ROI to obtain the averaged DFT and autocorrelation results of each ROI in each condition. The Pearson correlation coefficients (R) were calculated for the series of images of each ROI for different time lags. Next, the average R values for each lag in each type of cell werecalculated. The Pearson correlation coefficient formula is , where cov stands for the covariance of intensities in the two images, i is the light intensity in pixelx,y,t, and σ stands for the standard deviation.
2.8. Statistical Analyses
The acquired data were exported to Excel spreadsheets (Microsoft Office Professional plus 2021, Microsoft Inc., Redmond, Washington, DC, USA) for graph and table presentation and for statistical analysis with Real Statistic Resource pack. Significance of differences between groups were calculated using Analysis of Variance (ANOVA) single factor function or t-test for paired samples, with statistical significance set at p < 0.05.
4. Discussion
Several studies have extensively described the role of cortical actin in determining cell shape and rigidity [
4,
18]. However, the impact of cortical actin mechanics on cell physiology requires further clarification. To investigate the function of cortical actin as the primary generator of active cellular stiffness, we manipulated T cells using the T-type calcium channel blocker, Mibefradil [
17]. This drug reduces the calcium flux through the cell membrane, while this flux is needed for the cortical actin active tension generation by the actin myosin ATP reactions with Ca
++ as their obligatory co-factor. We then measured the cells stiffness and surface vibrations using AFM and found that Mibefradil reduced cell stiffness while increasing cell membrane fluctuations.
To clarify the negative correlation between both high membrane fluctuations and the dissipation of mechanical energy to the cell surroundings with cortical actin tension, we estimated the extent of dissipated energy and related it to stiffness values (
Figure 2a). As expected, in non-manipulated cells, dissipated mechanical work was negatively correlated with the stiffness of the cell or cortical actin. Conversely, in cells treated with Mibefradil, the dissipated mechanical work values were higher, as depicted in
Figure 2b. These findings suggest that the active tension regulation of the cortical actin structure plays a crucial role in the regulation and balance of mechanical energy leakage or flow to the outer surroundings of the cell.
Malignant cells are softer [
12,
13] with higher levels of intracellular mechanical work [
5,
14,
15,
16] in comparison to their benign precursors. Accordingly, it is expected that their cell membrane vibration will be higher. Following that, we utilized TIRF microscopy to investigate surface vibrations in primary lymphocytes and leukemic CD8 and Jurkat cells. The vibrations were analyzed within two frequency ranges: <3 Hz, associated with active, ATP-dependent intracellular vibrations, and 10–25 Hz, which corresponds to thermal motion of the cortical actin and adjacent cell membrane. We observed heightened vibrations in the leukemic CD8 and Jurkat cells, in both frequency ranges. The increased active vibrations in the malignant cells under study likely result from their higher intracellular indirect mechanical work, a characteristic of malignant cell physiology [
5]. Moreover, the elevated passive thermal vibrations in these malignant cells are likely due to their lower cortical actin stiffness, which exhibits a negative correlation with cell membrane fluctuations (
Figure 2a). The augmentation of both active and thermal vibrations following Mibefradil treatment in the malignant cell types indicates that their underlying lower cortical actin tension could be further reduced. This range of potential tension conditions for the cortical actin may enable these cells to regulate the dissipation of intracellular mechanical work to the surroundings by maintaining a preferred state of cortical actin tension. Our results demonstrate that all cell types, benign and malignant, responded to Mibefradil treatment by increasing cell membrane vibrations at both frequency ranges (
Figure 3). These results highlight the role of cortical actin in controlling cell surface vibrations in both benign and malignant cells (
Figure 3c,d). Consequently, we propose that cortical actin may function as an inhibitor or regulator of cell surface vibrations, and dissipative leakage of mechanical energy. This is predominant in both benign and malignant cells.
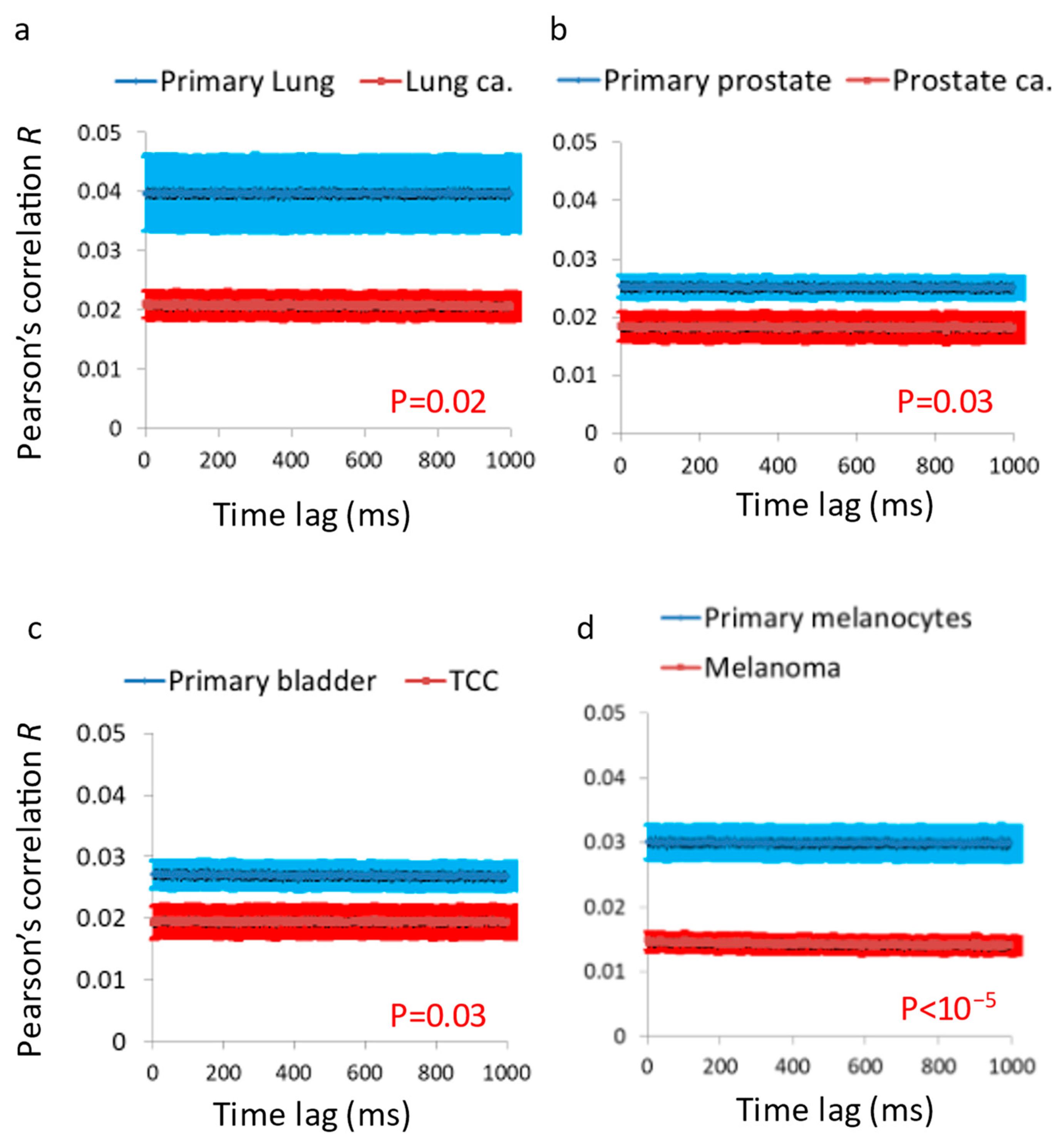
After investigating the mechanical vibrations of the cell membrane in single-cell measurements and confirming that these vibrations are indeed higher in malignant cells compared to benign cells, the next step was to extend these findings to four additional pairs of primary benign and matched malignant cell lines using a simplified in vitro model of multicellular tissues. In this model, a cluster of sedimented cells was subject to imaging via a reflected laser beam, and the resulting time-dependent speckle patterns were analyzed to assess the dynamics of the cell surface. In all of these malignant cell lines, we observed higher surface vibrations compared to their respective matched benign cell lines (
Figure 5 and
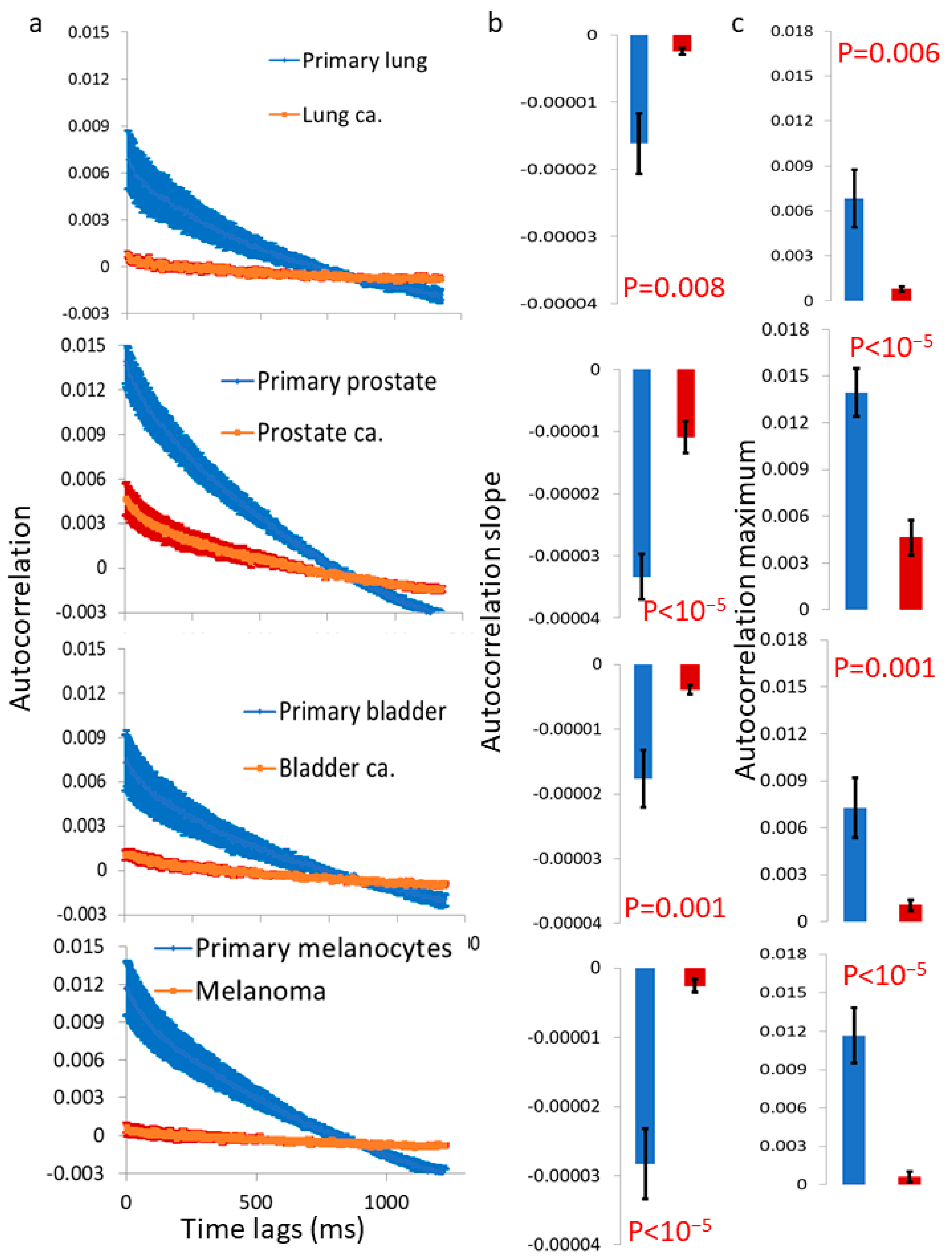
Figure 6). The autocorrelation values of these dynamics were consistently lower in the malignant cell lines compared to their matched benign counterparts (
Figure 7). Therefore, we can conclude that surface vibrations in malignant cells have a higher amplitude, but the motion is less correlated and more chaotic compared to the surface of matched benign cells.
Previously, active vibrations of the cell membrane were demonstrated in frequencies of around 100 Hz [
10,
11]. Those vibrations are probably related to the mechanically coupled cortical actin and its active tension generation. ATP-depleted cells were found to be softer [
5,
10] and to lack such fast and active vibrations [
10,
11]. Those previous and present results support our assumption that in benign cells, the cell surface, together with its cortical actin cytoskeleton, actively produces (ATP-dependent) work to reduce surface vibrations and minimize the leakage of intracellular mechanical energy. Since the generation process of cortical actin tension in benign cells is dominant and active (rather than passive or random), it is expected that the dynamics of the cortical actin and cell membrane would exhibit higher correlations and less randomness. In malignant cells, the generation process of cortical actin tension is diminished, leading to more thermal and random dynamics in these components. As a result, the measured autocorrelation values are lower, and the leakage of intracellular mechanical work to the surroundings becomes more significant in malignant cells.
Possible inhibition of tension generation by the cortical actin in malignant cells may explain their relative softness. Such softness may promote malignancy in multiple ways. First, it likely assists the migration and penetration abilities of these cells [
19]. Second, it may interfere with engagement and clearance of the cancer cells by immune cells through a proper immune synapse. In that case, the immune synapse may be less effective and compromise the specificity of antigen recognition by the immune cells and their response amplitude. Thus, malignant cells may escape an effective immune response of the host [
20].
In addition, the softness of malignant cells may enhance cell membrane vibrations and leakage of essential intracellular mechanical work to the cell surroundings. As mentioned, this work is crucial for important physiological processes of the cells, such as intracellular motion and active organization. Thus, the malignant cell must find a balance regarding the cortical actin tension that will enable on one hand, a high degree of softness that promotes invasiveness and immune escape, and on the other hand, sufficient intracellular mechanical work to preserve its high level of dynamics and homogeneity.
Interfering with the balance of cortical actin tension in malignant cells by Mibefradil treatment caused apoptosis and inhibits proliferation and autophagia in these cells [
21]. The interruption of the homeostasis of their mechanical work could have caused a major physiological deterioration in some of the cells that resulted in apoptosis.
Figure 8 illustrates the suggested principals of intracellular mechanical work homeostasis. Since living cells can be considered to be in a thermodynamic non-equilibrium steady state, we follow the general principals of thermodynamic non-equilibrium steady state by Oono et al. [
22]. Such a description includes three energetic components: the input driving power to the system (Q
A), the dissipated fraction of Q
A, which is termed ‘the house keeping heat’ (Q
H), and the net incorporated energy to the system, or ‘excess heat’ (Q
ex), where Q
ex = Q
A − Q
H. From our description so far, the basic intracellular indirect mechanical work corresponds to Q
A, while the dissipated mechanical work to the cell surroundings corresponds to Q
H. The indirect mechanical work that remains in the cell corresponds to Q
ex.
Since malignant cells are usually arranged as a dense conglomerate of closely packed cells it may enable more efficient transfer of mechanical energy between the adjacent cells. In addition, the higher leakage of mechanical energy in these cells makes this possible phenomenon more significant. In this situation, each cell physiology could directly affect the physiology of its adjacent cells, and the homeostatic balance may be determined in a relatively wide scale across clusters of cells.
This characteristic phenomenon of malignant tissues exhibiting a cooperative high level of mechanical energy that leaks to the surroundings may offer potential benefits for the detection of malignant tumors. In addition, multiple studies have suggested a positive correlation between the clinical degree of aggressiveness in cancer cells and their softness [
13,
23]. Since softer cells are anticipated to exhibit larger amplitudes of cell surface vibrations, monitoring the vibrations on the surface of malignant cells could potentially introduce a novel parameter for the clinical assessment of malignant tumors.
To conclude, cancer cells vary from their precursor benign cells in their mechanical properties. The cancer cells are softer and have larger amplitudes of less correlated surface vibrations. These mechanical differences may be exploited to detect and follow malignant tumors, utilizing sensitive methods for analyzing vibrations of tissue surfaces such as the analysis of reflective laser speckles.