Adenosinergic System and Neuroendocrine Syncope: What Is the Link?
Abstract
1. Introduction
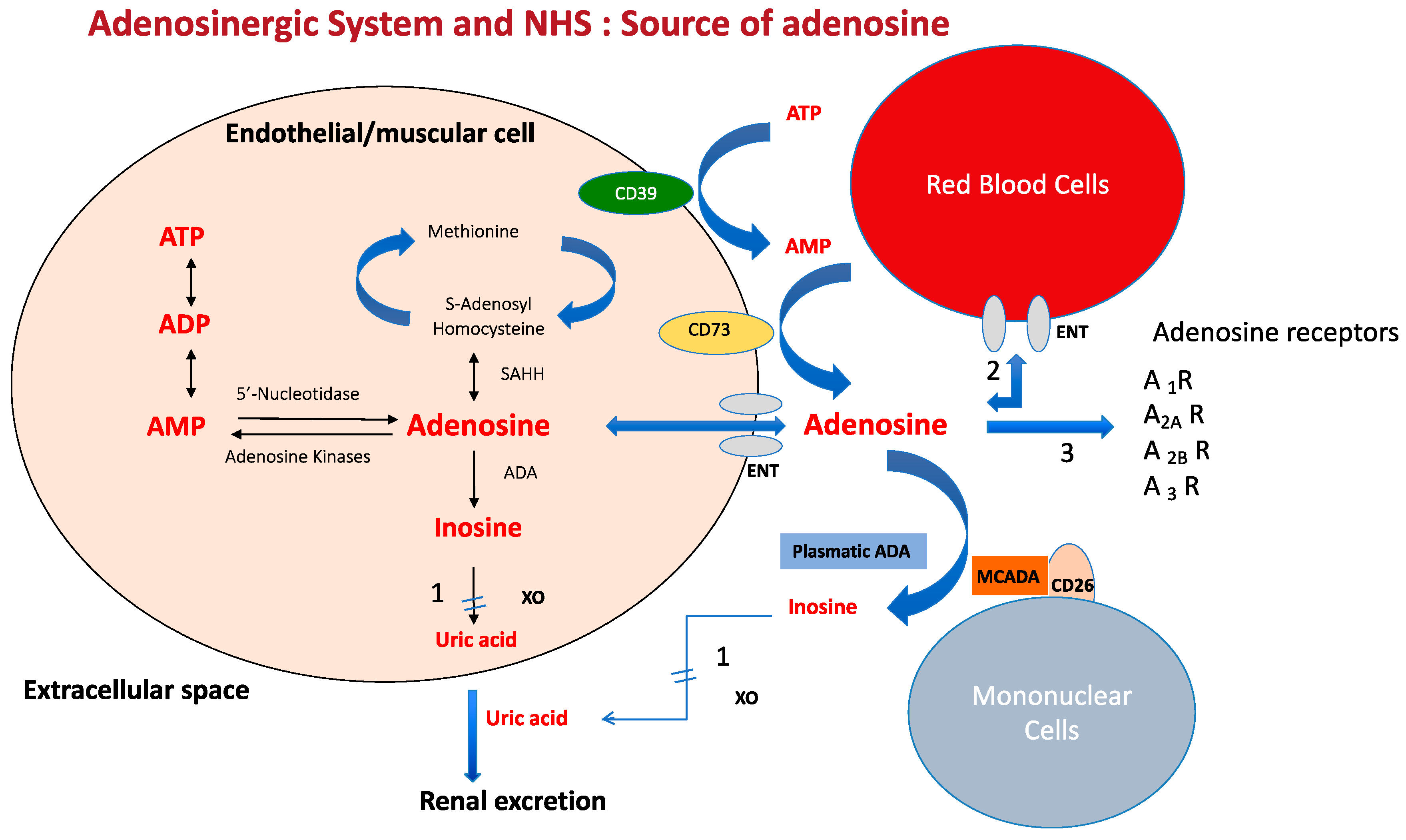
2. Source and Metabolism of Adenosine
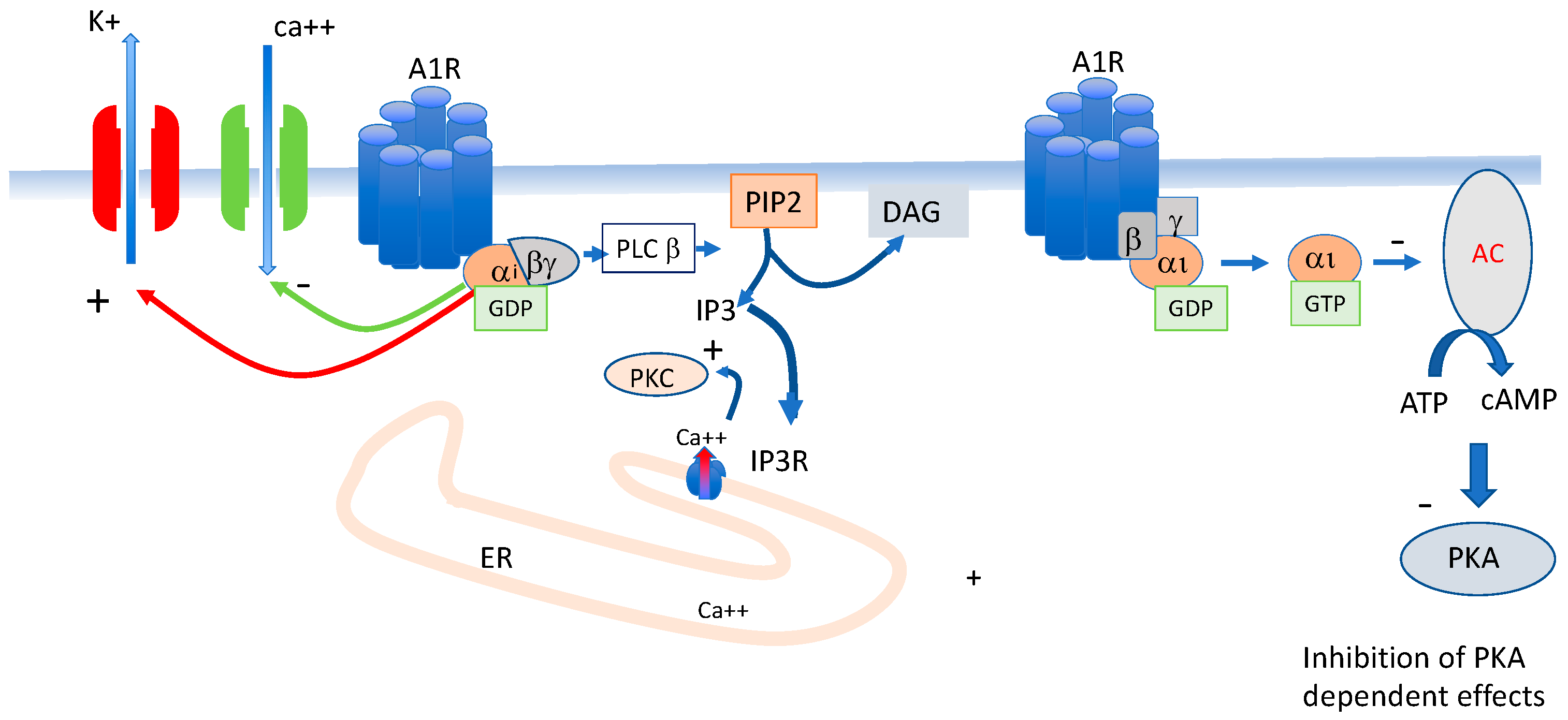
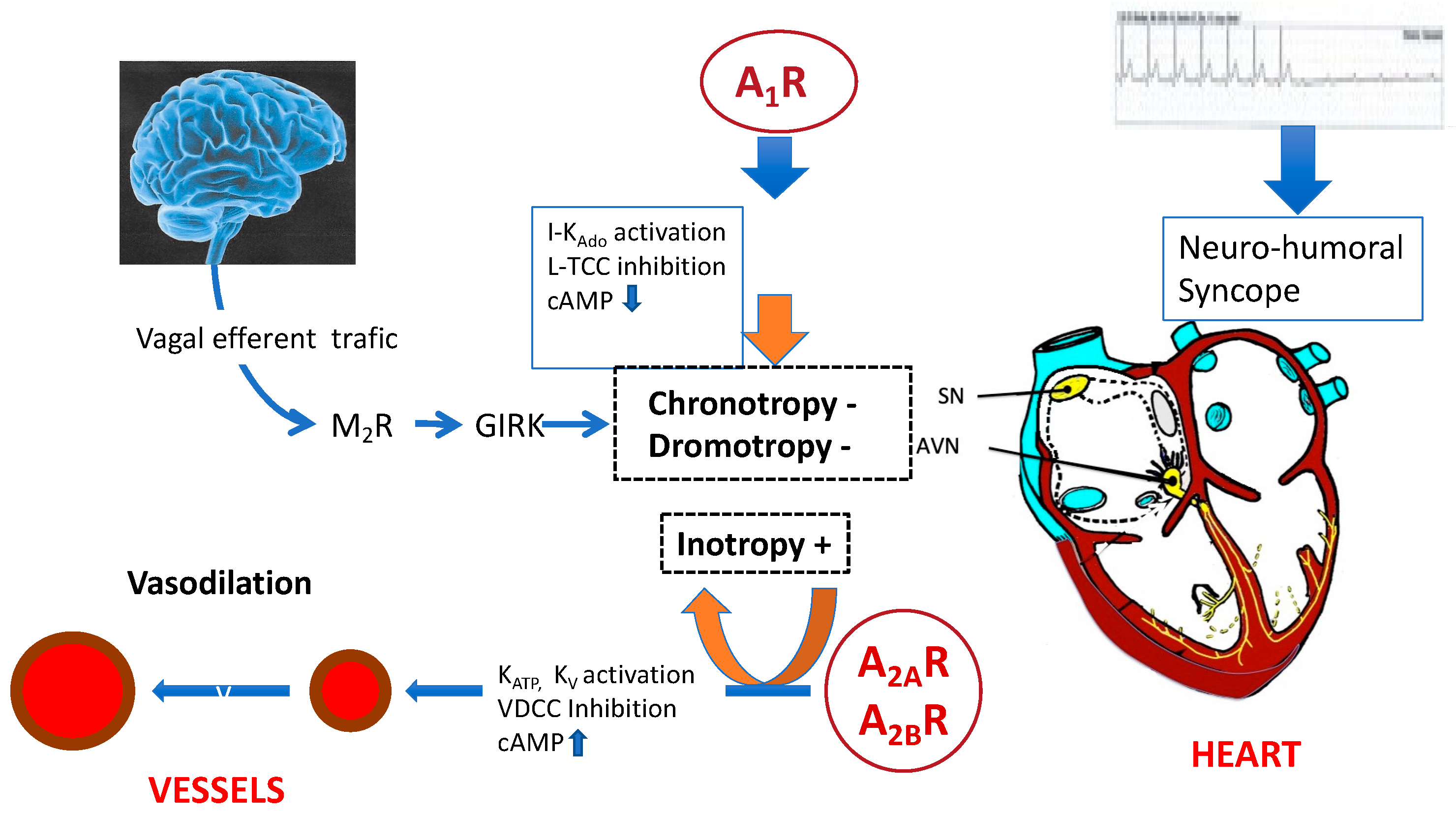
3. Adenosine Receptors Implicated in Blood Pressure and Heart Rate
4. Role of the Adenosinergic System in NES
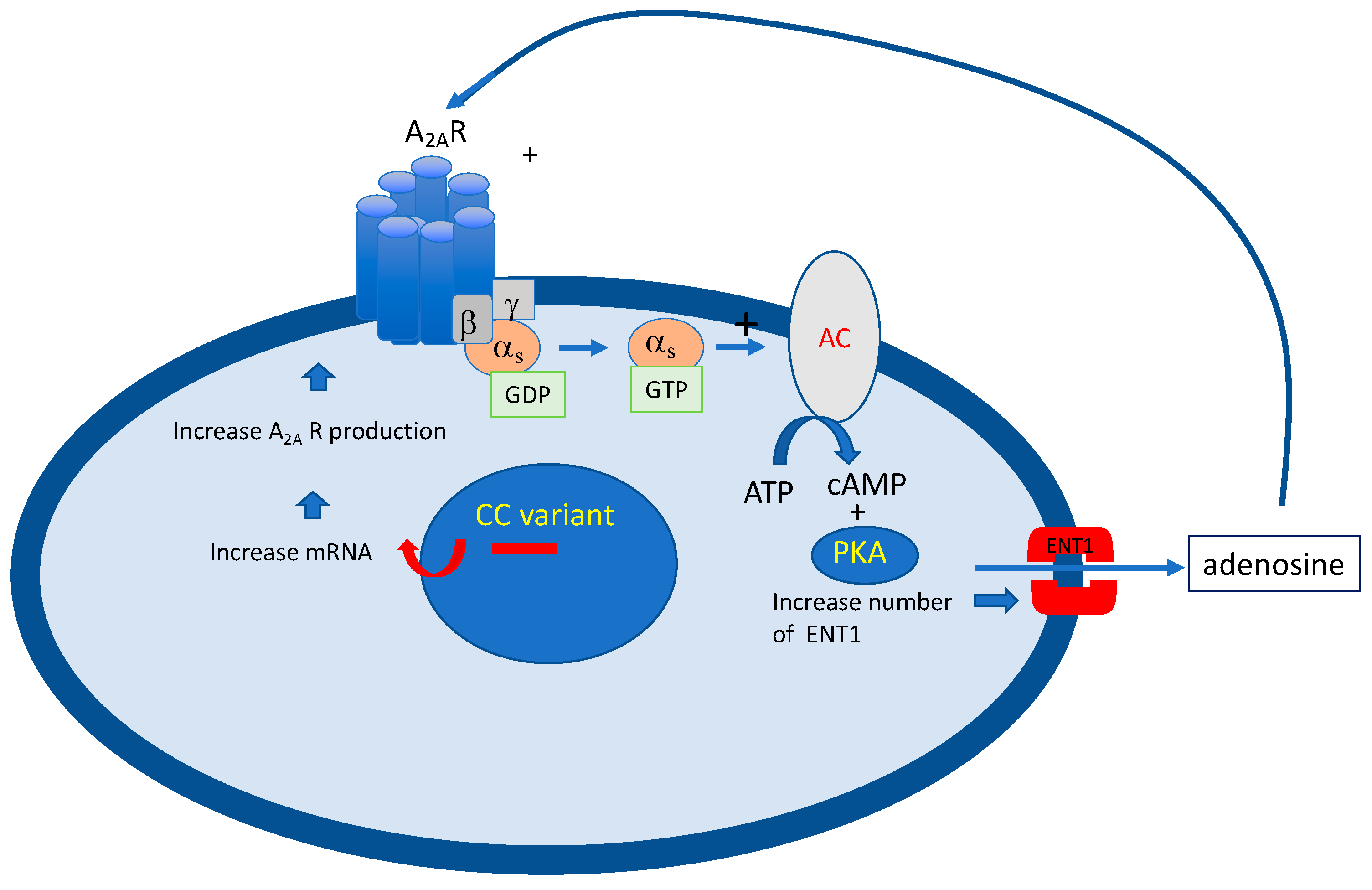
4.1. Adenosinergic Profile in NHS Patients
4.2. Effects of Exogenous Adenosine
4.3. The Question of Adenosine Measurement
4.4. Effects of Dioxygene on the Adenosinergic System
5. Peripheral Blood Mononuclear Cells (PBMCs) Are a Useful Tool for the Exploration of the Adenosinergic System
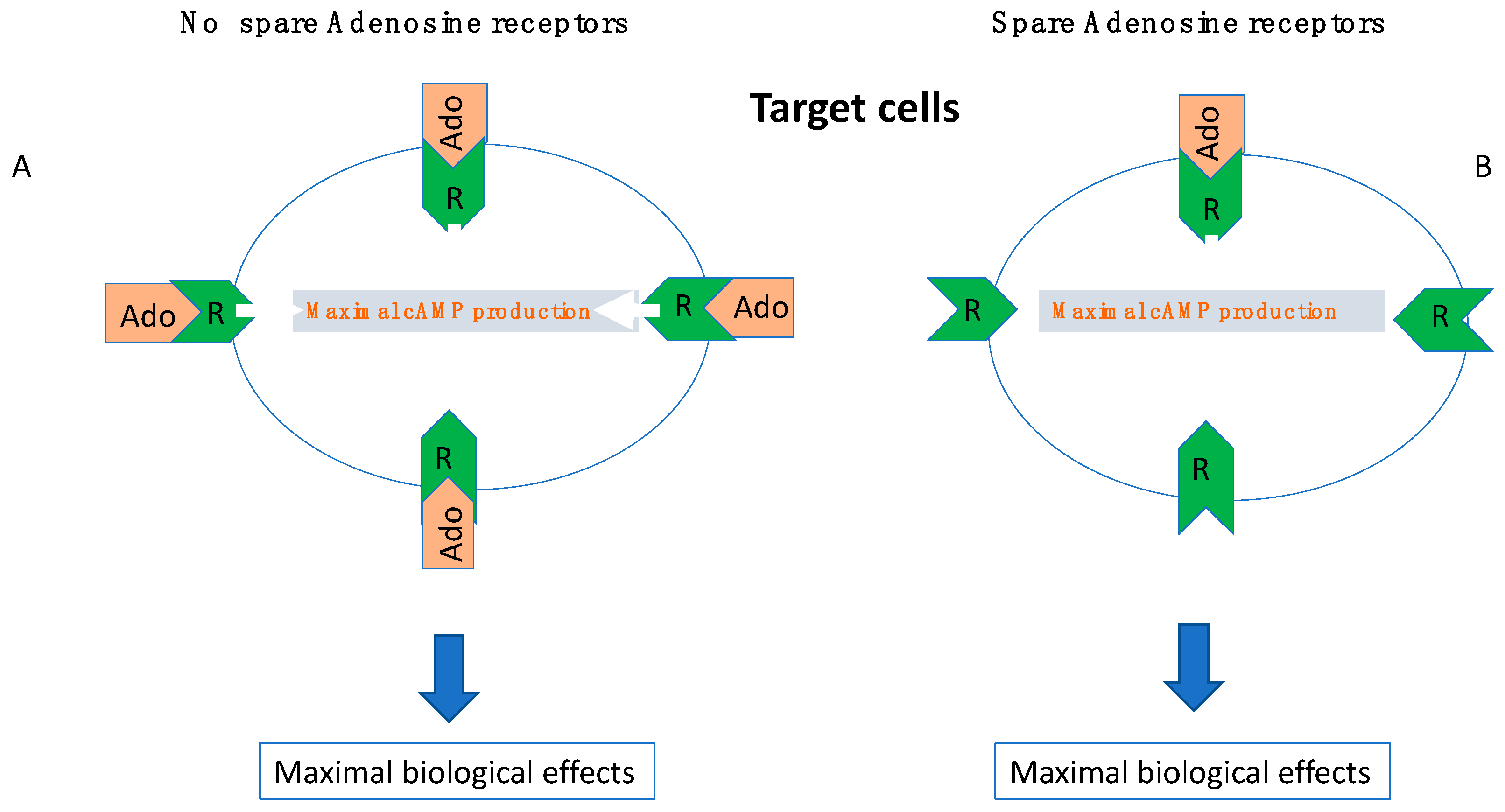
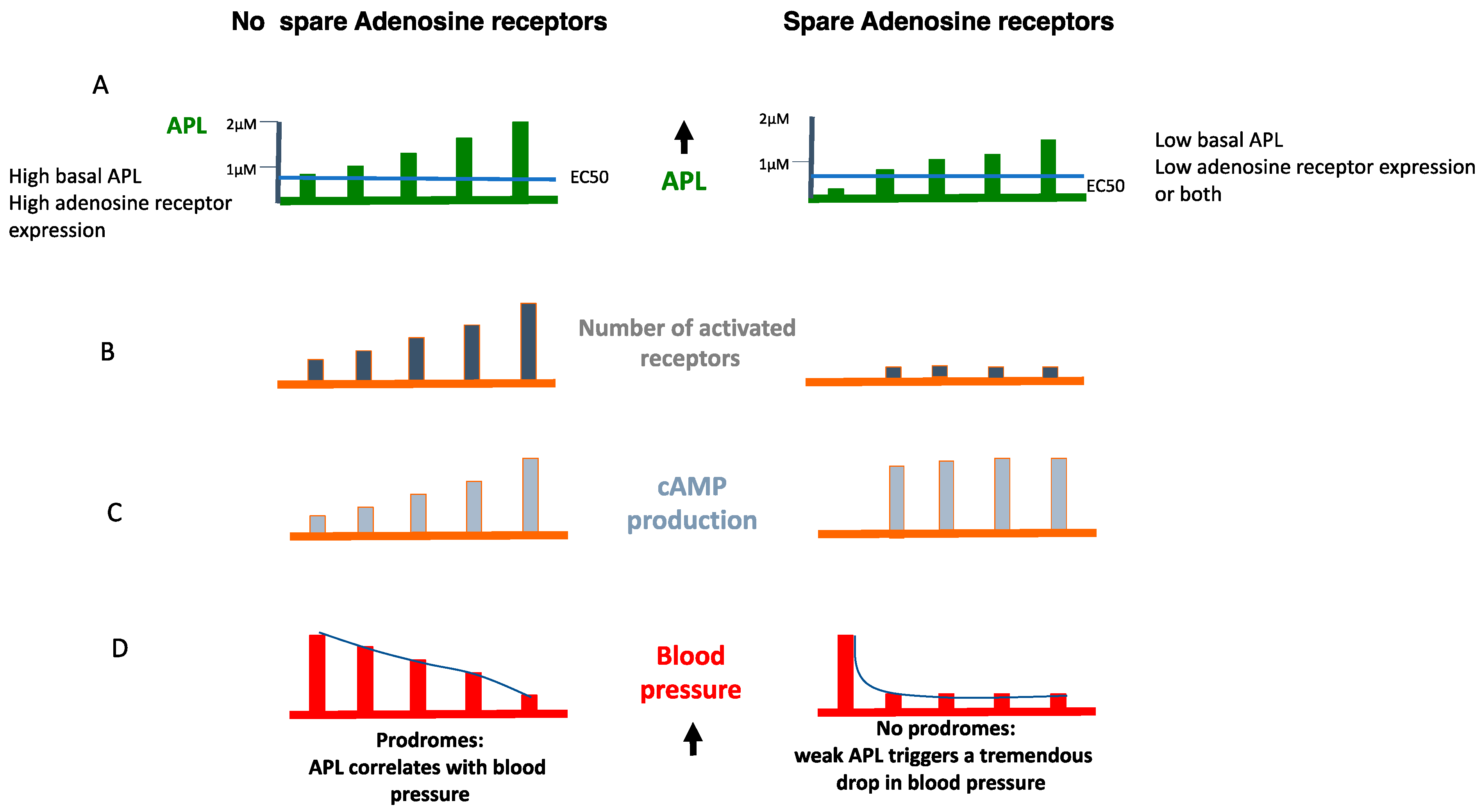
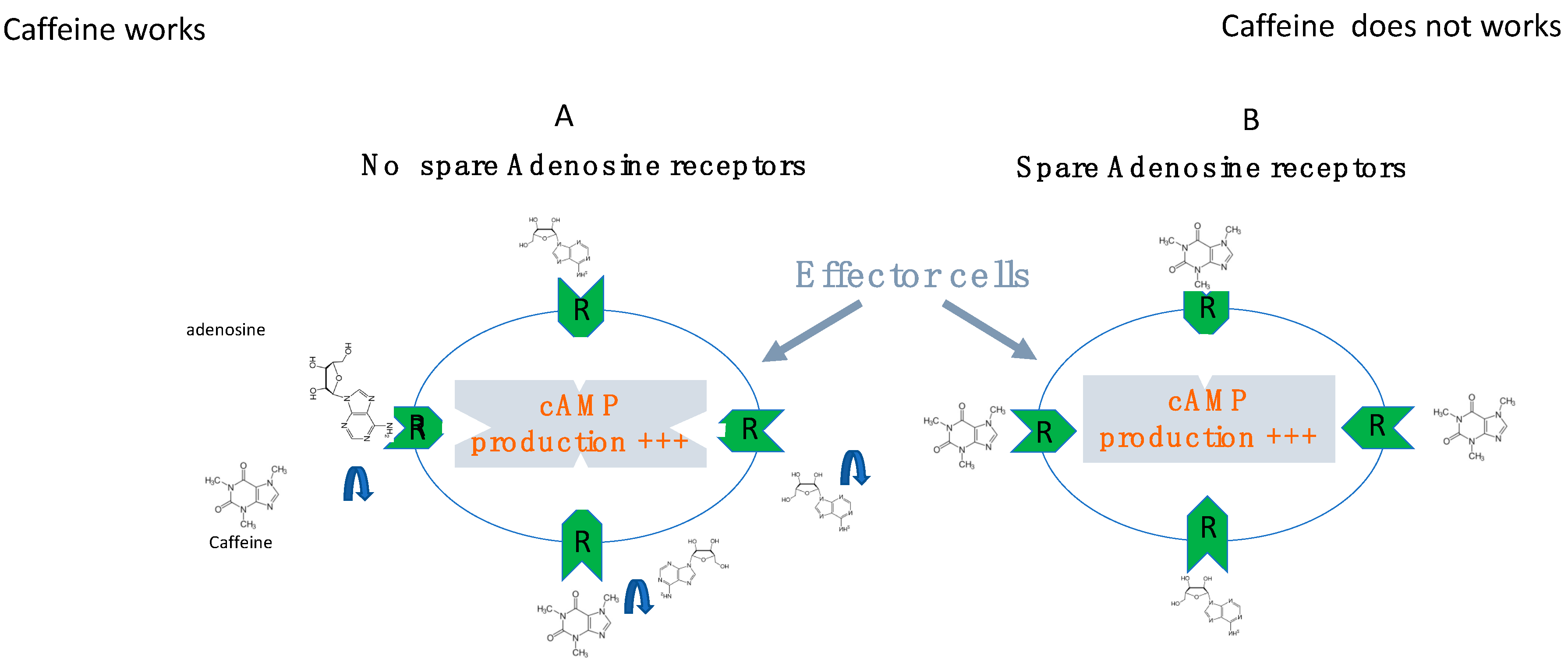
6. The Role of Receptor Reserve (Spare Receptors)
7. Effects of Adenosine Receptor Antagonists
8. Effects of Pacing
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goldberger, Z.D.; Petek, B.J.; Brignole, M.; Shen, W.-K.; Sheldon, R.S.; Solbiati, M.; Deharo, J.-C.; Moya, A.; Hamdan, M.H. ACC/AHA/HRS Versus ESC Guidelines for the Diagnosis and Management of Syncope. J. Am. Coll. Cardiol. 2019, 74, 2410–2423. [Google Scholar] [CrossRef]
- Brignole, M.; Deharo, J.-C.; Guieu, R. Syncope and Idiopathic (Paroxysmal) AV Block. Cardiol. Clin. 2015, 33, 441–447. [Google Scholar] [CrossRef]
- Brignole, M.; Guieu, R.; Tomaino, M.; Iori, M.; Ungar, A.; Bertolone, C.; Unterhuber, M.; Bottoni, N.; Tesi, F.; Deharo, J.C. Mechanism of syncope without prodromes with normal heart and normal electrocardiogram. Heart Rhythm. 2017, 14, 234–239. [Google Scholar] [CrossRef]
- Deharo, J.-C.; Brignole, M.; Guieu, R. Adenosine and neurohumoral syncope. Minerva. Med. 2022, 113, 243–250. [Google Scholar] [CrossRef]
- Sun, B.C. Risk prediction for patients with syncope. Ann. Emerg. Med. 2004, 44, 422–423. [Google Scholar] [CrossRef]
- Silverstein, M.D.; Singer, D.; Mulley, A.G.; Thibault, G.; Barnett, G. Patients with syncope admitted to medical intensive care units. JAMA 1982, 248, 1185–1189. [Google Scholar] [CrossRef]
- Day, S.C.; Cook, E.; Funkenstein, H.; Goldman, L. Evaluation and outcome of emergency room patients with transient loss of consciousness. Am. J. Med. 1982, 73, 15–23. [Google Scholar] [CrossRef]
- Da Silva, R.M.F.L. Syncope: Epidemiology, etiology, and prognosis. Front. Physiol. 2014, 5, 471. [Google Scholar] [CrossRef]
- Flammang, D.; Pelleg, A.; Benditt, D.G. The Adenosine Triphospate (ATP) Test for Evaluation of Syncope of Unknown Origin. J. Cardiovasc. Electrophysiol. 2005, 16, 1388–1389. [Google Scholar] [CrossRef]
- Flammang, D.; Church, T.; Waynberger, M.; Chassing, A.; Antiel, M. Can Adenosine 5′-Triphosphate Be Used to Select Treatment in Severe Vasovagal Syndrome? Circulation 1997, 96, 1201–1208. [Google Scholar] [CrossRef]
- Saadjian, A.Y.; Lévy, S.; Franceschi, F.D.; Zouher, I.; Paganelli, F.; Guieu, R.G.P. Role of Endogenous Adenosine as a Modulator of Syncope Induced During Tilt Testing. Circulation 2002, 106, 569–574. [Google Scholar] [CrossRef]
- Brignole, M.; Iori, M.; Solari, D.; Bottoni, N.; Rivasi, G.; Ungar, A.; Deharo, J.C.; Guieu, R. Efficacy of theophylline in patients with syncope without prodromes with normal heart and normal ECG. Int. J. Cardiol. 2019, 289, 70–73. [Google Scholar] [CrossRef]
- Brignole, M.; Solari, D.; Iori, M.; Bottoni, N.; Guieu, R.; Deharo, J.C. Efficacy of theophylline in patients affected by low adenosine syncope. Heart Rhythm. 2016, 13, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Belardinelli, L.; Shryock, J.C.; Song, Y.; Wang, D.; Srinivas, M.; Mozzicato, S.; Joshi, B.V.; Jacobson, K.A.; Liang, B.T.; Dougherty, C.; et al. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J. 1995, 9, 359–365. [Google Scholar] [CrossRef]
- De Waard, M.; Hering, J.; Weiss, N.; Feltz, A. How do G proteins directly control neuronal Ca2+ channel function? Trends Pharmacol. Sci. 2005, 26, 427–436. [Google Scholar] [CrossRef]
- Guieu, R.; Degioanni, C.; Fromonot, J.; De Maria, L.; Ruf, J.; Deharo, J.C.; Brignole, M. Adenosine, Adenosine Receptors and Neurohumoral Syncope: From Molecular Basis to Personalized Treatment. Biomedicines 2022, 10, 1127. [Google Scholar] [CrossRef]
- Beukers, M.W.; Dulk, H.D.; van Tilburg, E.W.; Brouwer, J.; Ijzerman, A.P. Why Are A2B Receptors Low-Affinity Adenosine Receptors? Mutation of Asn273 to Tyr Increases Affinity of Human A2B Receptor for 2-(1-Hexynyl)adenosine. Mol. Pharmacol. 2000, 58, 1349–1356. [Google Scholar] [CrossRef]
- Sutton, R. The role of adenosine in syncope. Int. J. Cardiol. 2022, 365, 47–48. [Google Scholar] [CrossRef]
- Lazurova, Z.; Mitro, P.; Popovnakova, M. The Role of Adenosine and Its Degradation Enzymes—Adenosinedeaminase and Adenosinekinase in Pathogenesis of Vasovagal Syncope. Eur. J. Intern. Med. 2022, 105, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Fragakis, N.; Antoniadis, A.P.; Sotiriadou, M.; Virgiliou, C.; Ballauri, I.; Gika, H.G.; Bougiouklis, D.; Gerou, S.; Lazaridis, C.; Vergopoulos, S.; et al. Syncope without prodromes is associated with excessive plasma release of adenosine at the time of syncope during head-up tilt table test. Int. J. Cardiol. 2022, 363, 43–48. [Google Scholar] [CrossRef]
- Shryock, J.C.; Snowdy, S.; Baraldi, P.G.; Cacciari, B.; Spalluto, G.; Monopoli, A.; Ongini, E.; Baker, S.P.; Belardinelli, L. A2A—Adenosine Receptor Reserve for Coronary Vasodilation. Circulation 1998, 98, 711–718. [Google Scholar] [CrossRef]
- Cohen, F.R.; Lazareno, S.; Birdsall, N.J. The affinity of adenosine for the high- and low-affinity states of the human adenosine A1 receptor. Eur. J. Pharmacol. 1996, 309, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Groppelli, A.; Brambilla, R.; Caldara, G.L.; Torresani, E.; Parati, G.; Solari, D.; Ungar, A.; Rafanelli, M.; Deharo, J.C.; et al. Plasma adenosine and neurally mediated syncope: Ready for clinical use. Eur. 2020, 22, 847–853. [Google Scholar] [CrossRef]
- Saadjian, A.Y.; Gerolami, V.; Giorgi, R.; Mercier, L.; Berge-Lefranc, J.-L.; Paganelli, F.; Ibrahim, Z.; By, Y.; Guéant, J.L.; Lévy, S.; et al. Head-up tilt induced syncope and adenosine A2A receptor gene polymorphism. Eur. Heart J. 2009, 30, 1510–1515. [Google Scholar] [CrossRef]
- Mitro, P.; Habalova, V.; Evin, L.; Muller, E.; Simurda, M.; Slaba, E.; Murin, P.; Valocik, G. Gene Polymorphism of the Adenosine A2a Receptor in Patients with Vasovagal Syncope. Pacing Clin. Electrophysiol. 2016, 39, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Deharo, J.-C.; Mechulan, A.; Giorgi, R.; Franceschi, F.; Prevot, S.; Peyrouse, E.; Condo, J.; By, Y.; Ruf, J.; Brignole, M.; et al. Adenosine plasma level and A2A adenosine receptor expression: Correlation with laboratory tests in patients with neurally mediated syncope. Heart 2012, 98, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Guieu, R.; Deharo, J.-C.; Ruf, J.; Mottola, G.; Kipson, N.; Bruzzese, L.; Gerolami, V.; Franceschi, F.; Ungar, A.; Tomaino, M.; et al. Adenosine and Clinical Forms of Neurally-Mediated Syncope. J. Am. Coll. Cardiol. 2015, 66, 204–205. [Google Scholar] [CrossRef]
- Mitro, P.; Lazúrová, Z.; Kerekanič, M.; Farkaš, J.; Popovňáková, M. Adenosine, adenosine-deaminase and implantable recorder outcome in syncopal patients. Pacing Clin. Electrophysiol. 2022, 45, 768–772. [Google Scholar] [CrossRef]
- Fragakis, N.; Antoniadis, A.P.; Saviano, M.; Vassilikos, V.; Pappone, C. The use of adenosine and adenosine triphosphate testing in the diagnosis, risk stratification and management of patients with syncope: Current evidence and future perspectives. Int. J. Cardiol. 2015, 183, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Sutton, R.; Menozzi, C.; Garcia-Civera, R.; Moya, A.; Wieling, W.; Andresen, D.; Benditt, D.G.; Grovale, N.; De Santo, T.; et al. Lack of correlation between the responses to tilt testing and adenosine triphosphate test and the mechanism of spontaneous neurally mediated syncope. Eur. Heart J. 2006, 27, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Guieu, R.; Sampiéri, F.; Bechis, G.; Rochat, H. Use of HPLC to measure circulating adenosine levels in migrainous patients. Clin. Chim. Acta 1994, 227, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Marlinge, M.; Vairo, D.; Marolda, V.; Bruzzese, L.; Adjriou, N.; Guiol, C.; Kipson, N.; Bonnardel, A.; Gastaldi, M.; Kerbaul, F.; et al. Rapid measurment of adenosine concentration in human blood using fixed potential amperometry: Comparison with mass spectrometry and high performance liquid chroma-tography. J. Anal. Bioanal. Tech. 2017, 4, 1–4. [Google Scholar]
- Groppelli, A.; Brignole, M.; Chefrour, M.; Gastaldi, M.; El Oufir, F.; Deharo, J.C.; Parati, G.; Guieu, R. Adenosine Concentration in Patients with Neurally Mediated Syncope. Front. Cardiovasc. Med. 2022, 9, 900023. [Google Scholar] [CrossRef]
- Bruzzese, L.; Fromonot, J.; By, Y.; Durand-Gorde, J.-M.; Condo, J.; Kipson, N.; Guieu, R.; Fenouillet, E.; Ruf, J. NF-κB enhances hypoxia-driven T-cell immunosuppression via upregulation of adenosine A2A receptors. Cell Signal. 2014, 26, 1060–1067. [Google Scholar] [CrossRef]
- Bruzzese, L.; Rostain, J.-C.; Née, L.; Condo, J.; Mottola, G.; Adjriou, N.; Mercier, L.; Berge-Lefranc, J.-L.; Fromonot, J.; Kipson, N.; et al. Effect of hyperoxic and hyperbaric conditions on the adenosinergic pathway and CD26 expression in rat. J. Appl. Physiol. 2015, 119, 140–147. [Google Scholar] [CrossRef]
- Fromonot, J.; Chaumet, G.; Gavarry, O.; Rostain, J.-C.; Lucciano, M.; Joulia, F.; Brignole, M.; Deharo, J.-C.; Guieu, R.; Boussuges, A. Hyperoxia Improves Hemodynamic Status During Head-up Tilt Testing in Healthy Volunteers: A Randomized Study. Medicine 2016, 95, e2876. [Google Scholar] [CrossRef]
- Varani, K.; Laghi-Pasini, F.; Camurri, A.; Capecchi, P.L.; Maccherini, M.; Diciolla, F.; Ceccatelli, L.; Lazzerini, P.E.; Ulouglu, C.; Cattabeni, F.; et al. Changes of peripheral A2A adenosine receptors in chronic heart failure and cardiac transplantation. FASEB J. 2003, 17, 280–282. [Google Scholar] [CrossRef]
- Gariboldi, V.; Vairo, D.; Guieu, R.; Marlingue, M.; Ravis, E.; Lagier, D.; Mari, A.; Thery, E.; Collart, F.; Gaudry, M.; et al. Expressions of adenosine A2A receptors in coronary arteries and peripheral blood mononuclear cells are correlated in coronary artery disease patients. Int. J. Cardiol. 2017, 230, 427–431. [Google Scholar] [CrossRef]
- Gaudry, M.; Marlinge, M.; Deharo, P.; Vairo, D.; Bottone, S.; Mottola, G.; Kipson, N.; Criado, C.; Mace, P.; Chefrour, M.; et al. Pharmacological profile of adenosine A2A receptors in patients with lower extremity peripheral artery disease and associated coronary artery disease: A pilot study. Int. J. Cardiol. 2019, 285, 121–127. [Google Scholar] [CrossRef]
- By, Y.; Durand-Gorde, J.-M.; Condo, J.; Lejeune, P.-J.; Mallet, B.; Carayon, P.; Guieu, R.; Ruf, J. Production of an agonist-like monoclonal antibody to the human A2A receptor of adenosine for clinical use. Mol. Immunol. 2009, 46, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, F.; By, Y.; Peyrouse, E.; Fromonot, J.; Gerolami, V.; Kipson, N.; Boussuges, A.; Brignole, M.; Fenouillet, E.; Deharo, J.C.; et al. A2A adenosine receptor function in patients with vasovagal syncope. Europace 2013, 15, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Jacquin, L.; Franceschi, F.; By, Y.; Durand-Gorde, J.-M.; Condo, J.; Deharo, J.-C.; Michelet, P.; Fenouillet, E.; Guieu, R.; Ruf, J. Search for adenosine A2A spare receptors on peripheral human lymphocytes. FEBS Open Bio 2012, 3, 1–5. [Google Scholar] [CrossRef]
- Clark, A.J. Mode of Action of Drugs on Cells; Edward Arnold and Co.: London, UK, 1933. [Google Scholar]
- Stephenson, R.P. A modification of receptor theory. Br. J. Pharmacol. Chemother. 1956, 11, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Fenouillet, E.; Mottola, G.; Kipson, N.; Paganelli, F.; Guieu, R.; Ruf, J. Adenosine Receptor Profiling Reveals an Association between the Presence of Spare Receptors and Cardiovascular Disorders. Int. J. Mol. Sci. 2019, 20, 5964. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, M.; Shryock, J.C.; Dennis, D.M.; Baker, S.P.; Belardinelli, L. Differential A1 Adenosine Receptor Reserve for Two Actions of Adenosine on Guinea Pig Atrial Myocytes. Mol. Pharmacol. 1997, 52, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Kiss, Z.; Pak, K.; Zsuga, J.; Juhasz, B.; Varga, B.; Szentmiklosi, A.J.; Haines, D.D.; Tosaki, A.; Gesztelyi, R. The guinea pig atrial A1 adenosine receptor reserve for the direct negative inotropic effect of adenosine. Gen. Physiol. Biophys. 2013, 32, 325–335. [Google Scholar] [CrossRef]
- Daly, J.W.; Shi, D.; Nikodijevic, O.; Jacobson, K.A. The role of adenosine receptors in the central action of caffeine. Pharmacopsychoecologia 2015, 7, 201–213. [Google Scholar]
- Nagatomo, K.; Kubo, Y. Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc. Natl. Acad. Sci. USA 2008, 105, 17373–17378. [Google Scholar] [CrossRef]
- Robertson, D.; Frölich, J.C.; Carr, R.K.; Watson, J.T.; Hollifield, J.W.; Shand, D.G.; Oates, J.A. Effects of Caffeine on Plasma Renin Activity, Catecholamines and Blood Pressure. N. Engl. J. Med. 1978, 298, 181–186. [Google Scholar] [CrossRef]
- Tofovic, S.P.; Kusaka, H.; Rominski, B.; Jackson, E.K. Caffeine Increases Renal Renin Secretion in a Rat Model of Genetic Heart Failure. J. Cardiovasc. Pharmacol. 1999, 33, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Sra, J.S.; Jazayeri, M.R.; Avitall, B.; Dhala, A.; Deshpande, S.; Blanck, Z.; Akhtar, M. Comparison of Cardiac Pacing with Drug Therapy in the Treatment of Neurocardiogenic (Vasovagal) Syncope with Bradycardia or Asystole. N. Engl. J. Med. 1993, 328, 1085–1090. [Google Scholar] [CrossRef]
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.-C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martín, A.; et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 39, 1883–1948. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Europace 2022, 24, 71–164. [Google Scholar] [CrossRef] [PubMed]
- Flammang, D.; Church, T.R.; De Roy, L.; Blanc, J.-J.; Leroy, J.; Mairesse, G.H.; Otmani, A.; Graux, P.J.; Frank, R.; Purnode, P.; et al. Treatment of Unexplained Syncope: A multicenter, randomized trial of cardiac pacing guided by adenosin 5′-triphosphate testing. Circulation 2012, 125, 31–36. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guieu, R.; Fromonot, J.; Mottola, G.; Maille, B.; Marlinge, M.; Groppelli, A.; Conte, S.; Bechah, Y.; Lalevee, N.; Michelet, P.; et al. Adenosinergic System and Neuroendocrine Syncope: What Is the Link? Cells 2023, 12, 2027. https://doi.org/10.3390/cells12162027
Guieu R, Fromonot J, Mottola G, Maille B, Marlinge M, Groppelli A, Conte S, Bechah Y, Lalevee N, Michelet P, et al. Adenosinergic System and Neuroendocrine Syncope: What Is the Link? Cells. 2023; 12(16):2027. https://doi.org/10.3390/cells12162027
Chicago/Turabian StyleGuieu, Régis, Julien Fromonot, Giovanna Mottola, Baptiste Maille, Marion Marlinge, Antonella Groppelli, Samantha Conte, Yassina Bechah, Nathalie Lalevee, Pierre Michelet, and et al. 2023. "Adenosinergic System and Neuroendocrine Syncope: What Is the Link?" Cells 12, no. 16: 2027. https://doi.org/10.3390/cells12162027
APA StyleGuieu, R., Fromonot, J., Mottola, G., Maille, B., Marlinge, M., Groppelli, A., Conte, S., Bechah, Y., Lalevee, N., Michelet, P., Hamdan, M., Brignole, M., & Deharo, J. C. (2023). Adenosinergic System and Neuroendocrine Syncope: What Is the Link? Cells, 12(16), 2027. https://doi.org/10.3390/cells12162027








