Repetitive Transcranial Magnetic Stimulation Reduces Depressive-like Behaviors, Modifies Dendritic Plasticity, and Generates Global Epigenetic Changes in the Frontal Cortex and Hippocampus in a Rodent Model of Chronic Stress
Abstract
:1. Introduction
2. Materials and Methods
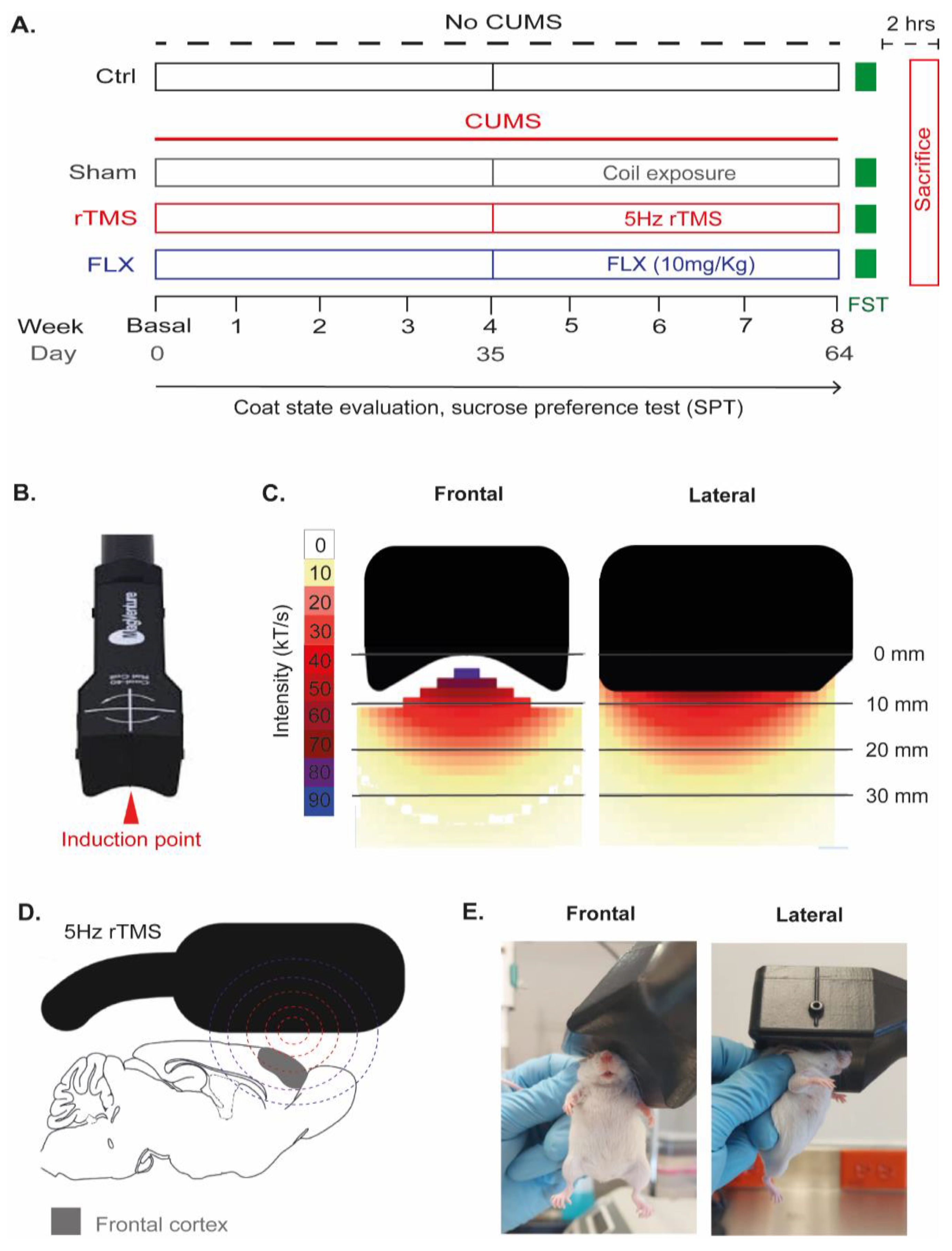
2.1. Experimental Design
2.2. Animals
2.3. Chronic Unpredictable Mild Stress Protocol
2.4. Repetitive Transcranial Magnetic Stimulation (rTMS)
2.5. Drugs
2.6. Behavioral Tests
2.6.1. Evaluation of the Coat State
2.6.2. Sucrose Preference Test
2.6.3. Forced-Swim Test
2.7. Brain Processing for Golgi Cox and DNA Isolation
2.8. Golgi–Cox Staining
Morphological Analysis of the Dendritic Spines and Dendritic Tree Complexity
2.9. Immunohistochemistry
2.9.1. Tissue Processing for Immunohistochemistry
2.9.2. Antibodies and Immunodetection
2.9.3. Measurement of Immunoreactivity
2.10. Immunofluorescence and Image Analysis
2.11. DNA Extraction and Methylation Analysis
2.12. Statistics
3. Results
3.1. Repetitive Transcranial Magnetic Stimulation and Fluoxetine Reverse Depressive-like Behaviors Generated by Chronic Unpredictable Mild Stress
3.2. rTMS Modifies the Density of the Dendritic Spines and the Complexity of the Dendritic Trees in the Pyramidal Neurons of the Frontal Cortex and the Granule Cells of the Hippocampal Dentate Gyrus
3.3. rTMS Increased the Immunoreactivity of Synaptophysin without Influencing the Immunoreactivity for Neurogranin
3.4. Repetitive Transcranial Magnetic Stimulation or Fluoxetine Induce Differential Effects on the Immunoreactivity of the Histone H2B (K16) Acetylation and the Histone H3 Trimethylation
3.5. rTMS Increased DNA Methylation Globally and at the Level of Mature Neurons in the Hippocampal Dentate Gyrus
3.6. Stress-Exposure Increased 5hmC in the Frontal Cortex
3.7. Principal Component Analysis of the Most Representative Parameters at the Level of Dendritic Remodeling, Synapse Proteins, Histone Modification, and DNA De/Methylation
4. Discussion
4.1. Findings
4.2. rTMS at 5 Hz Reverses the Behavioral Alterations Generated by Chronic Stress
4.3. 5 Hz rTMS Reverses Dendritic Atrophy at the Level of the Dendritic Trees and Spines Generated by Chronic Stress in the Frontal Cortex and Dentate Gyrus of the Hippocampus
4.4. rTMS May Promote the Increase in the Immunoreactivity of Presynaptic Proteins in the Pyramidal Neurons of the Frontal Cortex and the Granular Cells of the Dentate Gyrus
4.5. FLX Favors Histone Acetylation Whereas 5 Hz rTMS Increases Histone Trimethylation in the Frontal Cortex
4.6. DNA Methylation in the DG Is Favored by rTMS and May Be the Mechanism by Which Dendritic Remodeling Is Promoted in the Mature Neurons
4.7. Chronic Stress Induces Demethylation in the Frontal Cortex Independently of rTMS and FLX Application
5. Conclusions
6. Limitations and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eid, R.S.; Gobinath, A.R.; Galea, L.A.M. Sex Differences in Depression: Insights from Clinical and Preclinical Studies. Prog. Neurobiol. 2019, 176, 86–102. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Pandemic Triggers 25% Increase in Prevalence of Anxiety and Depression Worldwide. 2023. Available online: https://www.who.int/news/item/02-03-2022-covid-19-pandemic-triggers-25-increase-in-prevalence-of-anxiety-and-depression-worldwide (accessed on 7 April 2023).
- Malhi, G.S.; Moore, J.; McGuffin, P. The Genetics of Major Depressive Disorder. Curr. Psychiatry Rep. 2000, 2, 165–169. [Google Scholar] [CrossRef]
- Nestler, E.J.; Barrot, M.; DiLeone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of Depression. Neuron 2002, 34, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.-C.; Cao, X.; Das, M.; Zhu, X.H.; Gao, T.M. Behavioral Animal Models of Depression. Neurosci. Bull. 2010, 26, 327–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flint, J.; Kendler, K.S. Review the Genetics of Major Depression. Neuron 2014, 81, 484–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Asis, J.M.; Stern, E.; Alexopoulos, G.S.; Pan, H.; van Gorp, W.; Blumberg, H.; Kalayam, B.; Eidelberg, D.; Kiosses, D.; Silbersweig, D.A. Hippocampal and Anterior Cingulate Activation Deficits in Patients with Geriatric Depression. Am. J. Psychiatry 2001, 158, 1321–1323. [Google Scholar] [CrossRef]
- Weissman, D.G.; Lambert, H.K.; Rodman, A.M.; Peverill, M.; Sheridan, M.A.; McLaughlin, K.A. Reduced Hippocampal and Amygdala Volume as a Mechanism Underlying Stress Sensitization to Depression Following Childhood Trauma. Depress. Anxiety 2020, 37, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, T.J.; McCausland, H.C.; Morris, H.D.; Padmanaban, V.; Cameron, H.A. Stress and Loss of Adult Neurogenesis Differentially Reduce Hippocampal Volume. Biol. Psychiatry 2017, 82, 914–923. [Google Scholar] [CrossRef]
- Qiao, H.; Li, M.-X.; Xu, C.; Chen, H.-B.; An, S.-C.; Ma, X.-M. Dendritic Spines in Depression: What We Learned from Animal Models. Neural Plast. 2016, 2016, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Hering, H.; Sheng, M. Dentritic spines: Structure, dynamics and regulation. Nat. Rev. Neurosci. 2001, 2, 880–888. [Google Scholar] [CrossRef]
- Dioli, C.; Patrício, P.; Sousa, N.; Kokras, N.; Dalla, C.; Guerreiro, S.; Santos-Silva, M.A.; Rego, A.C.; Pinto, L.; Ferreiro, E.; et al. Chronic Stress Triggers Divergent Dendritic Alterations in Immature Neurons of the Adult Hippocampus, Depending on Their Ultimate Terminal Fields. Transl. Psychiatry 2019, 9, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallés, A.S.; Barrantes, F.J. Nanoscale Sub-Compartmentalization of the Dendritic Spine Compartment. Biomolecules 2021, 11, 1697. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.A.; Christoffel, D.J.; Heshmati, M.; Hodes, G.E.; Magida, J.; Davis, K.; Cahill, M.E.; Dias, C.; Ribeiro, E.; Ables, J.L.; et al. Epigenetic Regulation of RAC1 Induces Synaptic Remodeling in Stress Disorders and Depression. Nat. Med. 2013, 19, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smrt, R.D.; Zhao, X. Epigenetic regulation of neuronal dendrite and dendritic spine development. Front. Biol. 2010, 4, 304–323. [Google Scholar] [CrossRef] [Green Version]
- McKinney, B.; Ding, Y.; Lewis, D.A.; Sweet, R.A. DNA Methylation as a Putative Mechanism for Reduced Dendritic Spine Density in the Superior Temporal Gyrus of Subjects with Schizophrenia. Transl. Psychiatry 2017, 7, 1032–1038. [Google Scholar] [CrossRef]
- Russo-neustadt, A.; Ha, T.; Ramirez, R.; Kesslak, J.P. Physical Activity—Antidepressant Treatment Combination: Impact on Brain-Derived Neurotrophic Factor and Behavior in an Animal Model. Behav. Brain Res. 2001, 120, 87–95. [Google Scholar] [CrossRef] [PubMed]
- CovIngtonii, H.E.; Maze, I.; LaPlant, Q.C.; Vialou, V.F.; Ohnishi, Y.N.; Berton, O.; Fass, D.M.; Renthal, W.; Rush, A.; Wu, E.Y.; et al. Antidepressant Actions of Histone Deacetylase Inhibitors. J. Neurosci. 2009, 29, 11451–11460. [Google Scholar] [CrossRef] [Green Version]
- Covington, H.E.; Vialou, V.F.; LaPlant, Q.; Ohnishi, Y.N.; Nestler, E.J. Hippocampal-Dependent Antidepressant-like Activity of Histone Deacetylase Inhibition. Neurosci. Lett. 2011, 493, 122–126. [Google Scholar] [CrossRef] [Green Version]
- Uchida, S.; Hara, K.; Kobayashi, A.; Otsuki, K.; Yamagata, H.; Hobara, T.; Suzuki, T.; Miyata, N.; Watanabe, Y. Epigenetic Status of Gdnf in the Ventral Striatum Determines Susceptibility and Adaptation to Daily Stressful Events. Neuron 2011, 69, 359–372. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, F.A.; Lin, C.L.; Crusio, W.E.; Akbarian, S. Antidepressant-Like Effects of the Histone Deacetylase Inhibitor, Sodium Butyrate, in the Mouse. Biol. Psychiatry 2007, 62, 55–64. [Google Scholar] [CrossRef]
- Covington, H.E.; Maze, I.; Sun, H.; Bomze, H.M.; DeMaio, K.D.; Wu, E.Y.; Dietz, D.M.; Lobo, M.K.; Ghose, S.; Mouzon, E.; et al. A Role for Repressive Histone Methylation in Cocaine-Induced Vulnerability to Stress. Neuron 2011, 71, 656–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.; Kim, J.; Lee, J.; Kim, S.; Bae, K.; Kim, S.; Shin, I.; Kim, H.; Shin, M.; Yoon, J. BDNF Promoter Methylation and Suicidal Behavior in Depressive Patients. J. Affect. Disord. 2013, 151, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, H.; Iwamoto, K.; Bundo, M.; Ueda, J.; Miyauchi, T.; Komori, A.; Kazuno, A.; Adati, N.; Kusumi, I.; Okazaki, Y.; et al. Hypermethylation of Serotonin Transporter Gene in Bipolar Disorder Detected by Epigenome Analysis of Discordant Monozygotic Twins. Transl. Psychiatry 2011, 1, e24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reszka, E.; Jabłońska, E.; Lesicka, M.; Wieczorek, E.; Kapelski, P.; Szczepankiewicz, A.; Pawlak, J.; Dmitrzak-Węglarz, M. An Altered Global DNA Methylation Status in Women with Depression. J. Psychiatr. Res. 2021, 137, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Food and Drugs Administration. Guidance for Industry and Food and Drug Administration Staff Class II Special Controls Guidance Document: Electrocardiograph Electrodes; FDA: Silver Spring, MD, USA, 2011; pp. 1–15.
- Fitzgerald, P.B. An Update on the Clinical Use of Repetitive Transcranial Magnetic Stimulation in the Treatment of Depression. J. Affect. Disord. 2020, 276, 90–103. [Google Scholar] [CrossRef]
- Philip, N.S.; Carpenter, S.L.; Ridout, S.J.; Sanchez, G.; Albright, S.E.; Tyrka, A.R.; Price, L.H.; Carpenter, L.L. 5 Hz Repetitive Transcranial Magnetic Stimulation to Left Prefrontal Cortex for Major Depression. J. Affect. Disord. 2015, 186, 13–17. [Google Scholar] [CrossRef] [Green Version]
- González-Olvera, J.J.; Ricardo-Garcell, J.; García Anaya, M.L. Análisis de fuentes del EEG en Pacientes Tratados Con Estimulación Magnética Transcraneal a 5Hz Como Tratamiento Antidepresivo. Salud Ment. 2013, 36, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, K.E.M.; Muthukumaraswamy, S.D.; Stinear, C.M.; Hoeh, N. Neuroimaging Effect of RTMS on GABA and Glutamate Levels in Treatment-Resistant Depression: An MR Spectroscopy Study. Psychiatry Res. Neuroimaging 2021, 317, 111377. [Google Scholar] [CrossRef]
- Chen, Q.-M.; Yao, F.-R.; Sun, H.-W.; Chen, Z.-G.; Ke, J.; Liao, J.; Cai, X.-Y.; Yu, L.-Q.; Wu, Z.-Y.; Wang, Z.; et al. Combining Inhibitory and Facilitatory Repetitive Transcranial Magnetic Stimulation (RTMS) Treatment Improves Motor Function by Modulating GABA in Acute Ischemic Stroke Patients. Restor. Neurol. Neurosci. 2021, 39, 419–434. [Google Scholar] [CrossRef]
- Poh, E.Z.; Hahne, D.; Moretti, J.; Harvey, A.R.; Clarke, M.W.; Rodger, J. Simultaneous Quantification of Dopamine, Serotonin, Their Metabolites and Amino Acids by LC-MS/MS in Mouse Brain Following Repetitive Transcranial Magnetic Stimulation. Neurochem. Int. 2019, 131, 104546. [Google Scholar] [CrossRef]
- Wang, H.Y.; Crupi, D.; Liu, J.; Stucky, A.; Cruciata, G.; di Rocco, A.; Friedman, E.; Quartarone, A.; Ghilardi, M.F. Repetitive Transcranial Magnetic Stimulation Enhances BDNF-TrkB Signaling in Both Brain and Lymphocyte. J. Neurosci. 2011, 31, 11044–11054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueyama, E.; Ukai, S.; Ogawa, A.; Yamamoto, M.; Kawaguchi, S.; Ishii, R.; Shinosaki, K. Chronic Repetitive Transcranial Magnetic Stimulation Increases Hippocampal Neurogenesis in Rats. Psychiatry Clin. Neurosci. 2011, 65, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Cambiaghi, M.; Crupi, R.; Bautista, E.L.; Elsamadisi, A.; Malik, W.; Pozdniakova, H.; Han, Z.; Bu, M.; Battaglia, F. The Effects of 1-Hz rTMS on Emotional Behavior and Dendritic Complexity of Mature and Newly Generated Dentate Gyrus Neurons in Male Mice. Int. J. Environ. 2020, 17, 4074. [Google Scholar] [CrossRef] [PubMed]
- Etiévant, A.; Manta, S.; Latapy, C.; Magno, L.A.; Fecteau, S.; Beaulieu, J.M. Repetitive Transcranial Magnetic Stimulation Induces Long-Lasting Changes in Protein Expression and Histone Acetylation. Sci. Rep. 2015, 5, 16873. [Google Scholar] [CrossRef] [Green Version]
- Armas-Castañeda, G.; Ricardo-Garcell, J.; Romo-Nava, F.; Heinze-Martin, G.; González-Olvera, J.J. Transcranial Magnetic Stimulation: Antidepressant Efficacy and Three-Month Follow-Up. Brain Stimul. 2015, 8, 343. [Google Scholar] [CrossRef]
- Willner, P.; Muscat, R.; Papp, M. Chronic Mild Stress-Induced Anhedonia: A Realistic Animal Model of Depression. Neurosci. Biobehav. Rev. 1992, 16, 525–534. [Google Scholar] [CrossRef]
- Tanti, A.; Rainer, Q.; Minier, F.; Surget, A.; Belzung, C. Differential Environmental Regulation of Neurogenesis along the Septo-Temporal Axis of the Hippocampus. Neuropharmacology 2012, 63, 374–384. [Google Scholar] [CrossRef]
- Yalcin, I.; Belzung, C.; Surget, A. Mouse Strain Differences in the Unpredictable Chronic Mild Stress: A Four-Antidepressant Survey. Behav. Brain Res. 2008, 193, 140–143. [Google Scholar] [CrossRef]
- Liu, M.; Yin, C.; Zhu, L.; Zhu, X.; Xu, C.; Luo, C.; Chen, H.; Zhu, D.; Zhou, Q. Sucrose Preference Test for Measurement of Stress-Induced Anhedonia in Mice. Nat. Protoc. 2008, 193, 140–143. [Google Scholar] [CrossRef]
- Gorman-Sandler, E.; Hollis, F. The Forced Swim Test: Giving up on Behavioral Despair (Commentary on Molendijk & de Kloet, 2021). Eur. J. Neurosci. 2022, 55, 2832–2835. [Google Scholar] [CrossRef]
- Ano, Y.; Ohya, R.; Kita, M.; Taniguchi, Y.; Kondo, K. Theaflavins Improve Memory Impairment and Depression-like Behavior by Regulating Microglial Activation. Molecules 2019, 24, 467. [Google Scholar] [CrossRef] [Green Version]
- Allen Institute for Brain Science. Allen Brain Reference Atlases. Available online: https://atlas.brain-map.org/atlas?atlas=2 (accessed on 12 April 2023).
- Risher, W.C.; Ustunkaya, T.; Alvarado, J.S.; Eroglu, C. Rapid Golgi Analysis Method for Efficient and Unbiased Classification of Dendritic Spines. PLoS ONE 2014, 9, e107591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Rodríguez, G.B.; Olvera-Hernández, S.; Vega-Rivera, N.M.; Ortiz-López, L. Melatonin Influences Structural Plasticity in the Axons of Granule Cells in the Dentate Gyrus of BALB/c Mice. Int. J. Mol. Sci. 2019, 20, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zocher, S.; Overall, R.W.; Berdugo-Vega, G.; Rund, N.; Karasinsky, A.; Adusumilli, V.S.; Steinhauer, C.; Scheibenstock, S.; Händler, K.; Schultze, J.L.; et al. De Novo DNA Methylation Controls Neuronal Maturation during Adult Hippocampal Neurogenesis. EMBO J. 2021, 40, e107100. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; He, J.; Richardson, J.S.; Li, X.M. The Response of Synaptophysin and Microtubule-Associated Protein 1 to Restraint Stress in Rat Hippocampus and Its Modulation by Venlafaxine. J. Neurochem. 2004, 91, 1380–1388. [Google Scholar] [CrossRef]
- Li, H.; Lin, W.; Li, J.; Wang, W. Altered Neurogranin Phosphorylation and Protein Levels Are Associated with Anxiety- and Depression-like Behaviors in Rats Following Forced Swim Stress. J. Behav. Brain Sci. 2014, 04, 506–522. [Google Scholar] [CrossRef] [Green Version]
- Zilles, K.; Palomero-Gallagher, N.; Amunts, K. Cytoarchitecture and Maps of the Human Cerebral Cortex; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 3, ISBN 9780123970251. [Google Scholar]
- Palomero-Gallagher, N.; Kedo, O.; Mohlberg, H.; Zilles, K.; Amunts, K. Multimodal Mapping and Analysis of the Cyto- and Receptorarchitecture of the Human Hippocampus. Brain Struct. Funct. 2020, 225, 881–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, D.N.; Stowell, M.H.B. Room for Two: The Synaptophysin/Synaptobrevin Complex. Front. Synaptic Neurosci. 2021, 13, 740318. [Google Scholar] [CrossRef] [PubMed]
- Auclair, G.; Weber, M. Mechanisms of DNA Methylation and Demethylation in Mammals. Biochimie 2012, 94, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Rosenblat, J.D.; Brietzke, E.; Pan, Z.; Lee, Y.; Cao, B.; Zuckerman, H.; Kalantarova, A.; McIntyre, R.S. Stress, Epigenetics and Depression: A Systematic Review. Neurosci. Biobehav. Rev. 2019, 102, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Nollet, M.; le Guisquet, A.-M.; Belzung, C. Models of Depression: Unpredictable Chronic Mild Stress in Mice. Curr. Protoc. Pharmacol. 2013, 61, 5–65. [Google Scholar] [CrossRef]
- Ibarguen-Vargas, Y.; Surget, A.; Touma, C.; Palme, R.; Belzung, C. Multifaceted strainspecific effects in a mouse model of depression and of antidepressant reversal. Psychoneuroendocrinology 2018, 33, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of Hippocampal Neurogenesis for the Behavioral Effects of Antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef] [Green Version]
- David, D.J.; Samuels, B.A.; Rainer, Q.; Wang, J.-W.; Marsteller, D.; Mendez, I.; Drew, M.; Craig, D.A.; Guiard, B.P.; Guilloux, J.-P.; et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 2009, 4, 479–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isingrini, E.; Camus, V.; le Guisquet, A.M.; Pingaud, M.; Devers, S.; Belzung, C. Association between Repeated Unpredictable Chronic Mild Stress (UCMS) Procedures with a High Fat Diet: A Model of Fluoxetine Resistance in Mice. PLoS ONE 2010, 5, e10404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Rodríguez, G.B.; Meneses-San Juan, D.; Rico-Becerra, A.I.; González-Olvera, J.J.; Reyes-Galindo, V. Repetitive Transcranial Magnetic Stimulation and Fluoxetine Reverse Depressive-like Behavior but with Differential Effects on Olig2-Positive Cells in Chronically Stressed Mice. Neuropharmacology 2023, 236, 109567. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.F.; Shi, T.Y.; Fan-Yang; Wang, W.N.; Chen, Y.C.; Tan, Q.R. Long-Lasting Effects of Chronic RTMS to Treat Chronic Rodent Model of Depression. Behav. Brain Res. 2012, 232, 245–251. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhang, R.G.; Xue, F.; Wang, H.N.; Chen, Y.C.; Hu, G.T.; Peng, Y.; Peng, Z.W.; Tan, Q.R. Quetiapine and Repetitive Transcranial Magnetic Stimulation Ameliorate Depression-like Behaviors and up-Regulate the Proliferation of Hippocampal-Derived Neural Stem Cells in a Rat Model of Depression: The Involvement of the BDNF/ERK Signal Pathway. Pharmacol. Biochem. Behav. 2015, 136, 39–46. [Google Scholar] [CrossRef]
- Peng, Z.-W.; Xue, F.; Zhou, C.-H.; Zhang, R.-G.; Wang, Y.; Liu, L.; Sang, H.-F.; Wang, H.-N.; Tan, Q.-R. Repetitive Transcranial Magnetic Stimulation Inhibits Sirt1/MAO-A Signaling in the Prefrontal Cortex in a Rat Model of Depression and Cortex-Derived Astrocytes. Mol. Cell. Biochem. 2017, 442, 59–72. [Google Scholar] [CrossRef]
- Garcia-Anaya, M.; Gonzalez-Olvera, J.; Ricardo-Garcell, J.; Armas, G.; Miranda, E.; Reyes, E.; Adelina Otero, G. Clinical and Electrophysiological Effect of Right and Left Repetitive Transcranial Magnetic Stimulation in Patients with Major Depressive Disorder. Salud Ment. 2011, 34, 291–299. [Google Scholar]
- Duman, C.H.; Duman, R.S. Spine Synapse Remodeling in the Pathophysiology and Treatment of Depression. Neurosci. Lett. 2014, 601, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Radley, J.J.; Rocher, A.B.; Miller, M.; Janssen, W.G.M.; Liston, C.; Hof, P.R.; McEwen, B.S.; Morrison, J.H. Repeated Stress Induces Dendritic Spine Loss in the Rat Medial Prefrontal Cortex. Cereb. Cortex 2006, 16, 313–320. [Google Scholar] [CrossRef]
- Chen, Y.; Rex, C.S.; Rice, C.J.; Dubé, C.M.; Gall, C.M.; Lynch, G. Correlated Memory Defects and Hippocampal Dendritic Spine Loss after Acute Stress Involve Corticotropin-Releasing Hormone Signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 13123–13128. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, Z.; Su, Y.; Kang, L.; Geng, D.; Wang, Y.; Luan, F.; Wang, M.; Cui, H. Magnetic Stimulation Modulates Structural Synaptic Plasticity and Regulates BDNF-TrkB Signal Pathway in Cultured Hippocampal Neurons. Neurochem. Int. 2013, 62, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, A.; Mu, F.; Rosskopp, J.; Lenz, M.; Ziemann, U.; Deller, T. Repetitive Magnetic Stimulation Induces Functional and Structural Plasticity of Excitatory Postsynapses in Mouse Organotypic Hippocampal Slice Cultures. J. Neurosci. 2012, 32, 17514–17523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czéh, B.; Welt, T.; Fischer, A.K.; Erhardt, A.; Schmitt, W.; Müller, M.B.; Toschi, N.; Fuchs, E.; Keck, M.E. Chronic Psychosocial Stress and Concomitant Repetitive Transcranial Magnetic Stimulation: Effects on Stress Hormone Levels and Adult Hippocampal Neurogenesis. Biol. Psychiatry 2002, 52, 1057–1065. [Google Scholar] [CrossRef]
- Cajigas, I.J.; Will, T.; Schuman, E.M. Protein Homeostasis and Synaptic Plasticity. EMBO J. 2010, 29, 2746–2752. [Google Scholar] [CrossRef] [Green Version]
- Tarsa, L.; Goda, Y. Synaptophysin Regulates Activity-Dependent Synapse Formation in Cultured Hippocampal Neurons. Proc. Natl. Acad. Sci. USA 2002, 99, 1012–1016. [Google Scholar] [CrossRef]
- Bianchi, S.; Stimpson, C.D.; Dukaa, T.; Larsen, M.D.; Janssen, W.G.M.; Collins, Z.; Bauernfeind, A.L.; Schapirod, S.J.; Bazed, W.B.; Mcarthurd, M.J.; et al. Synaptogenesis and Development of Pyramidal Neuron Dendritic Morphology in the Chimpanzee Neocortex Resembles Humans. Proc. Natl. Acad. Sci. USA 2013, 110, 10395–10401. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Z.; Kang, L.; Geng, D.; Wang, Y.; Wang, M.; Cui, H. Repetitive Transcranial Magnetic Stimulation (RTMS) Influences Spatial Cognition and Modulates Hippocampal Structural Synaptic Plasticity in Aging Mice. Exp. Gerontol. 2014, 58, 256–268. [Google Scholar] [CrossRef]
- Qian, F.F.; He, Y.H.; Du, X.H.; Lu, H.X.; He, R.H.; Fan, J.Z. Repetitive Transcranial Magnetic Stimulation Promotes Neurological Functional Recovery in Rats with Traumatic Brain Injury by Upregulating Synaptic Plasticity-Related Proteins. Neural Regen. Res. 2023, 18, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, Y.; Tian, Q.; Zhu, B.; Zhao, Z. Repetitive Transcranial Magnetic Stimulation Increases Serum Brain-Derived Neurotrophic Factor and Decreases Interleukin-1β and Tumor Necrosis Factor-α in Elderly Patients with Refractory Depression. J. Int. Med. Res. 2019, 47, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Pan, W. Repetitive Transcranial Magnetic Stimulation (RTMS) Modulates Hippocampal Structural Synaptic Plasticity in Rats. Physiol. Res. 2019, 68, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Amunts, K.; Schleicher, A.; Zilles, K. Cytoarchitecture of the Cerebral Cortex-More than Localization. Neuroimage 2007, 37, 1061–1065. [Google Scholar] [CrossRef]
- Molyneaux, B.J.; Arlotta, P.; Menezes, R.L.; Macklis, J.D. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 2007, 8, 427–437. [Google Scholar] [CrossRef]
- Opris, I.; Hampson, R.E.; Stanford, T.R.; Gerhardt, G.A.; Deadwyler, S.A. Neural Activity in Frontal Cortical Cell Layers: Evidence for Columnar Sensorimotor Processing. J. Cogn. Neurosci. 2011, 23, 1507–1521. [Google Scholar] [CrossRef] [Green Version]
- Ezama, L.; Hernández-Cabrera, J.A.; Seoane, S.; Pereda, E.; Janssen, N. Functional Connectivity of the Hippocampus and Its Subfields in Resting-State Networks. Eur. J. Neurosci. 2021, 53, 3378–3393. [Google Scholar] [CrossRef]
- Talpalar, A.E.; Giugliano, M.; Grossman, Y. Enduring Medial Perforant Path Short-Term Synaptic Depression at High Pressure. Front. Cell. Neurosci. 2010, 4, 128. [Google Scholar] [CrossRef] [Green Version]
- De Bruyckere, E.; Simon, R.; Nestel, S.; Heimrich, B.; Kätzel, D.; Egorov, A.; Liu, P.; Jenkins, N.A.; Copeland, N.G.; Schwegler, H.; et al. Stability and Function of Hippocampal Mossy Fiber Synapses Depend on Bcl11b/Ctip2. Front. Mol. Neurosci. 2018, 11, 103. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Juan, D.M.S.; González-Olvera, J.J. 5 Hz of Repetitive Transcranial Magnetic Stimulation Improves cognition and Induces Modifications in Hippocampal Neurogenesis in Adult Female Swiss Webster Mice. Brain Res. Bull. 2022, 186, 91–105. [Google Scholar] [CrossRef]
- Yang, H.Y.; Liu, Y.; Xie, J.C.; Liu, N.N.; Tian, X. Effects of Repetitive Transcranial Magnetic Stimulation on Synaptic Plasticity and Apoptosis in Vascular Dementia Rats. Behav. Brain Res. 2015, 281, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Rock, P.L.; Roiser, J.P.; Riedel, W.J.; Blackwell, A.D. Cognitive Impairment in Depression: A Systematic Review and Meta-Analysis. Psychol. Med. 2014, 44, 2029–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, H.; Chen, H.; Wang, H.; Wang, L. The Histone Modifications of Neuronal Plasticity. Neural Plast. 2021, 2021, 6690523. [Google Scholar] [CrossRef]
- Wang, H.T.; Huang, F.L.; Hu, Z.L.; Zhang, W.J.; Qiao, X.Q.; Huang, Y.Q.; Dai, R.P.; Li, F.; Li, C.Q. Early-Life Social Isolation-Induced Depressive-Like Behavior in Rats Results in Microglial Activation and Neuronal Histone Methylation That Are Mitigated by Minocycline. Neurotox. Res. 2017, 31, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.S.; Maitra, S.; Chakravarty, S.; Kumar, A. Histone Lysine Demethylases of JMJD2 or KDM4 Family Are Important Epigenetic Regulators in Reward Circuitry in the Etiopathology of Depression. Neuropsychopharmacology 2017, 42, 854–863. [Google Scholar] [CrossRef] [Green Version]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Patchev, A.V.; Daniel, G.; Almeida, O.F.X.; Spengler, D. Early-Life Stress Reduces DNA Methylation of the Pomc Gene in Male Mice. Endocrinology 2014, 155, 1751–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murgatroyd, C.; Patchev, A.V.; Wu, Y.; Micale, V.; Bockmühl, Y.; Fischer, D.; Holsboer, F.; Wotjak, C.T.; Almeida, O.F.X.; Spengler, D. Dynamic DNA Methylation Programs Persistent Adverse Effects of Early-Life Stress. Nat. Neurosci. 2009, 12, 1559–1566. [Google Scholar] [CrossRef]
- Chen, J.; Evans, A.N.; Liu, Y.; Honda, M.; Saavedra, J.M.; Aguilera, G. Maternal Deprivation in Rats Is Associated with Corticotrophin-Releasing Hormone (CRH) Promoter Hypomethylation and Enhances CRH Transcriptional Responses to Stress in Adulthood. J. Neuroendocrinol. 2012, 24, 1055–1064. [Google Scholar] [CrossRef] [Green Version]
- Jobe, E.M.; Zhao, X. DNA Methylation and Adult Neurogenesis. Brain Plast. 2016, 3, 5–26. [Google Scholar] [CrossRef]
- Wolffe, A.P.; Jones, P.L.; Wade, P.A. DNA Demethylation. Proc. Natl. Acad. Sci. USA 1999, 96, 5894–5896. [Google Scholar] [CrossRef] [PubMed]
- Neidhart, L. DNA Methylation and Complex Human Disease; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Xin, Y.; O’Donnell, A.H.; Ge, Y.; Chanrion, B.; Milekic, M.; Rosoklija, G.; Stankov, A.; Arango, V.; Dwork, A.J.; Gingrich, J.A.; et al. Role of CpG Context and Content in Evolutionary Signatures of Brain DNA Methylation. Epigenetics 2011, 6, 1308–1318. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Jiménez, P.; Fernández-Messina, L.; Ovejero-Benito, M.C.; Chicharro, P.; Vera-Tomé, P.; Vara, A.; Cibrian, D.; Martínez-Fleta, P.; Jiménez-Fernández, M.; Sánchez-García, I.; et al. Growth Arrest and DNA Damage-Inducible Proteins (GADD45) in Psoriasis. Sci. Rep. 2021, 11, 14579. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.S.; Apple, C.G.; Kannan, K.B.; Funk, Z.M.; Plazas, J.M.; Efron, P.A.; Mohr, A.M. Chronic Stress Induces Persistent Low-Grade Inflammation. Am. J. Surg. 2019, 218, 677–683. [Google Scholar] [CrossRef]
- Juszczyk, G.; Mikulska, J.; Kasperek, K.; Pietrzak, D.; Mrozek, W.; Herbet, M. Chronic Stress and Oxidative Stress as Common Factors of the Pathogenesis of Depression and Alzheimer’s Disease; the Role of Antioxidants in Prevention and Treatment. Antioxidants 2021, 10, 1439. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jiao, C.; Wang, K.; Yuan, N. DNA Methylation and Psychiatric Disorders. Prog. Mol. Biol. Transl. Sci. 2018, 157, 175–232. [Google Scholar] [CrossRef]








| Stressor | Time |
|---|---|
| Empty box | 4 h |
| Movement restriction | 1 h |
| Rotating box | 1 h |
| Continuous light | 24 h |
| Cold (5 °C) | 20 min |
| White noise | 12 h |
| Strobe light | 4 h |
| Tilted box (45°) | 3 h |
| Wet bed | 12 h |
| Overcrowding | 4 h |
| Predator odor | 3 h |
| Food deprivation | 18 h |
| Wet box | 1 h 30 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meneses-San Juan, D.; Lamas, M.; Ramírez-Rodríguez, G.B. Repetitive Transcranial Magnetic Stimulation Reduces Depressive-like Behaviors, Modifies Dendritic Plasticity, and Generates Global Epigenetic Changes in the Frontal Cortex and Hippocampus in a Rodent Model of Chronic Stress. Cells 2023, 12, 2062. https://doi.org/10.3390/cells12162062
Meneses-San Juan D, Lamas M, Ramírez-Rodríguez GB. Repetitive Transcranial Magnetic Stimulation Reduces Depressive-like Behaviors, Modifies Dendritic Plasticity, and Generates Global Epigenetic Changes in the Frontal Cortex and Hippocampus in a Rodent Model of Chronic Stress. Cells. 2023; 12(16):2062. https://doi.org/10.3390/cells12162062
Chicago/Turabian StyleMeneses-San Juan, David, Mónica Lamas, and Gerardo Bernabé Ramírez-Rodríguez. 2023. "Repetitive Transcranial Magnetic Stimulation Reduces Depressive-like Behaviors, Modifies Dendritic Plasticity, and Generates Global Epigenetic Changes in the Frontal Cortex and Hippocampus in a Rodent Model of Chronic Stress" Cells 12, no. 16: 2062. https://doi.org/10.3390/cells12162062





