Increased Levels of BAMBI Inhibit Canonical TGF-β Signaling in Chronic Wound Tissues
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. In Vitro Scabs
2.3. Tube Formation Assay
2.4. Cytokine Arrays
2.5. Gene Expression Analyses
2.6. Immunostainings of Phosphorylated Smad3
2.6.1. Immunofluorescence Staining with Confocal-Microscopy
2.6.2. Immunohistological Staining
2.7. Luciferase Reporter Assay
2.8. Western Blot
2.9. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Patients with Chronic Wounds Show Altered ANGPT:TIE2 Ratios in the Serum and Increased Levels of Matrix Metalloproteinase 9 in the Wound Tissue
3.3. Increased Expression of FN1, VEGFR2, and Pro-Inflammatory Interleukins in Chronic Wound Tissue
3.4. Increased Expression of TGF-β1 and TGF-β3 in Chronic Wound Tissues
3.5. Expressed TGF-β Is Active but Does Not Reach the Chronic Wound Tissues
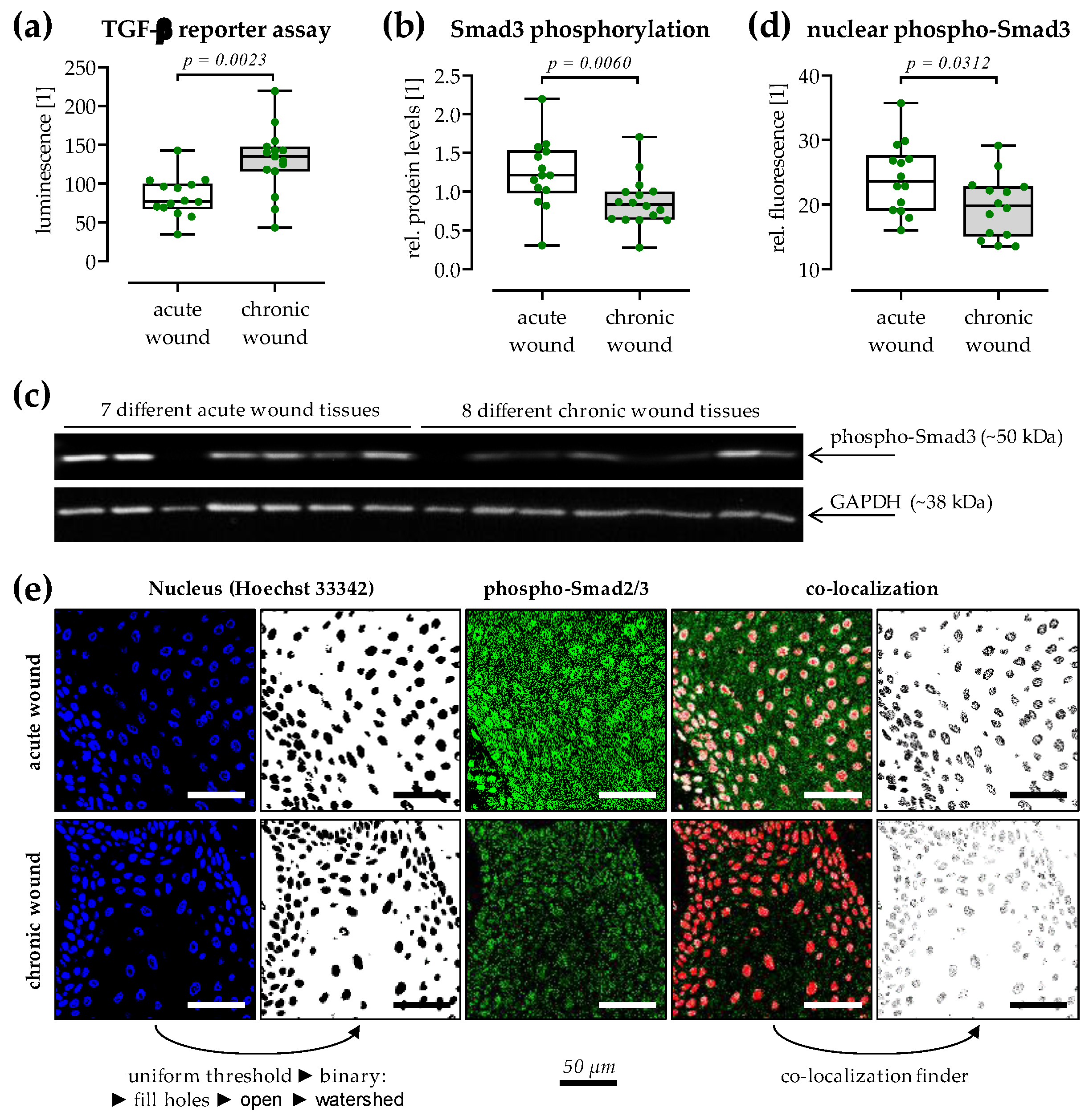
3.6. Reduced TGF-β Signaling in Chronic Wound Tissues
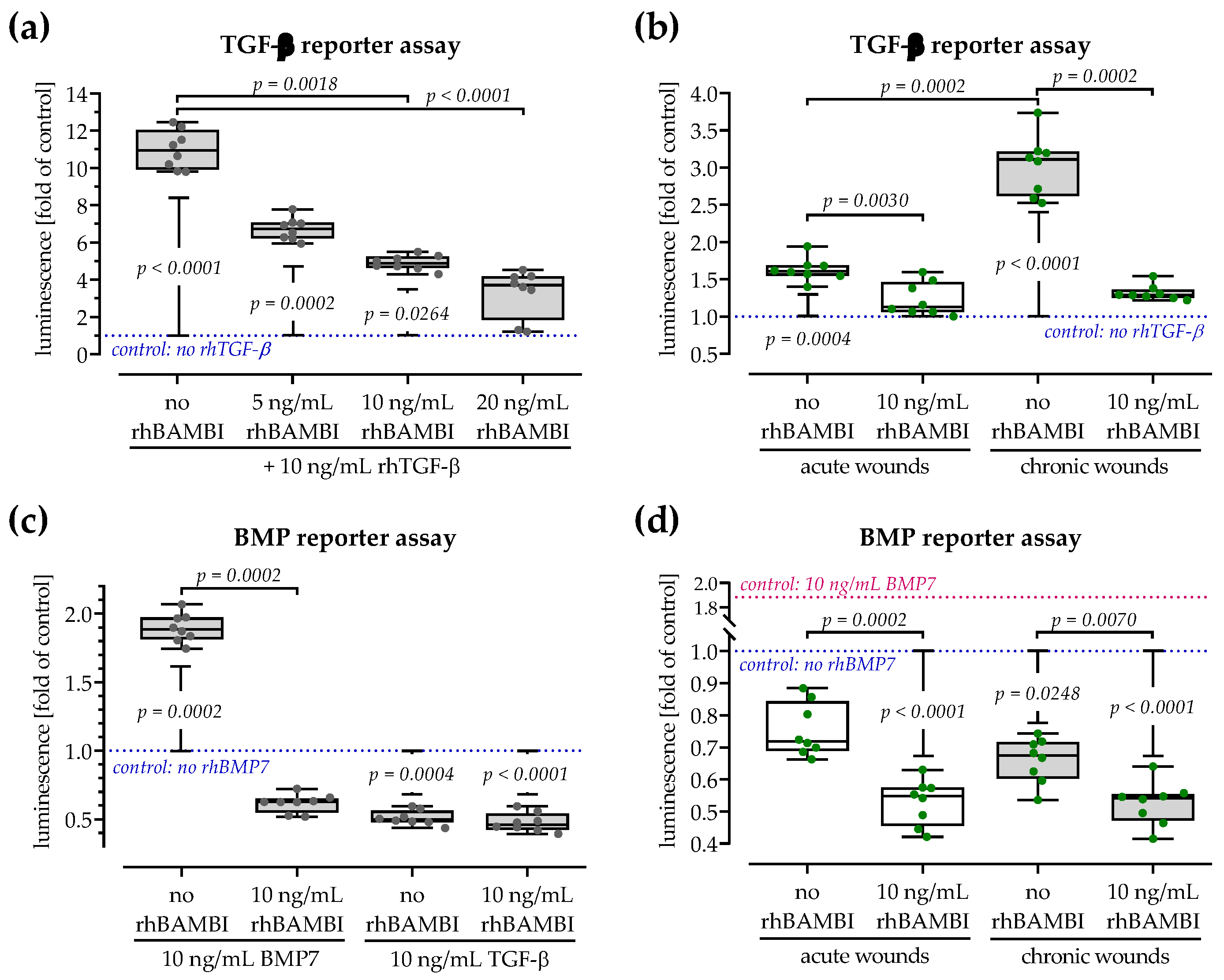
3.7. Expression of the TGF-β Pseudo-Receptor BAMBI Is Increased in Chronic Wound Tissues
3.8. Both Smad-Dependent Signaling Pathways Are Inhibited in the Presence of BAMBI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Jarbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef]
- Chan, B.; Cadarette, S.; Wodchis, W.; Wong, J.; Mittmann, N.; Krahn, M. Cost-of-illness studies in chronic ulcers: A systematic review. J. Wound Care 2017, 26, S4–S14. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Karolczak, K.; Watala, C. Blood Platelets as an Important but Underrated Circulating Source of TGFβ. Int. J. Mol. Sci. 2021, 22, 4492. [Google Scholar] [CrossRef]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.W.D.; Vickaryous, M.K.; Viloria-Petit, A.M. Signalling by Transforming Growth Factor Beta Isoforms in Wound Healing and Tissue Regeneration. J. Dev. Biol. 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Pentinmikko, N.; Ilmonen, M.; Salven, P. Dual action of TGF-β induces vascular growth in vivo through recruitment of angiogenic VEGF-producing hematopoietic effector cells. Angiogenesis 2012, 15, 511–519. [Google Scholar] [CrossRef]
- Mercado-Pimentel, M.E.; Runyan, R.B. Multiple transforming growth factor-β isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs 2007, 185, 146–156. [Google Scholar] [CrossRef]
- Roberts, A.B.; Mccune, B.K.; Sporn, M.B. Tgf-β—Regulation of Extracellular-Matrix. Kidney Int. 1992, 41, 557–559. [Google Scholar] [CrossRef]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Lichtman, M.K.; Otero-Vinas, M.; Falanga, V. Transforming growth factor β (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016, 24, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Goumans, M.J.; Liu, Z.; ten Dijke, P. TGF-β signaling in vascular biology and dysfunction. Cell Res. 2009, 19, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.G.; Meng, A.M. Negative regulation of TGF-β signaling in development. Cell Res. 2004, 14, 441–449. [Google Scholar] [CrossRef][Green Version]
- Onichtchouk, D.; Chen, Y.G.; Dosch, R.; Gawantka, V.; Delius, H.; Massague, J.; Niehrs, C. Silencing of TGF-β signalling by the pseudoreceptor BAMBI. Nature 1999, 401, 480–485. [Google Scholar] [CrossRef]
- Fan, Y.; Li, X.; Xiao, W.; Fu, J.; Harris, R.C.; Lindenmeyer, M.; Cohen, C.D.; Guillot, N.; Baron, M.H.; Wang, N.; et al. BAMBI elimination enhances alternative TGF-β signaling and glomerular dysfunction in diabetic mice. Diabetes 2015, 64, 2220–2233. [Google Scholar] [CrossRef]
- Liu, C.; Rinderknecht, H.; Histing, T.; Kolbenschlag, J.; Nussler, A.K.; Ehnert, S. Establishment of an In Vitro Scab Model for Investigating Different Phases of Wound Healing. Bioengineering 2022, 9, 191. [Google Scholar] [CrossRef]
- Faulkner, A.; Purcell, R.; Hibbert, A.; Latham, S.; Thomson, S.; Hall, W.L.; Wheeler-Jones, C.; Bishop-Bailey, D. A thin layer angiogenesis assay: A modified basement matrix assay for assessment of endothelial cell differentiation. BMC Cell Biol. 2014, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Ehnert, S.; Baur, J.; Schmitt, A.; Neumaier, M.; Lucke, M.; Dooley, S.; Vester, H.; Wildemann, B.; Stockle, U.; Nussler, A.K. TGF-β1 as Possible Link between Loss of Bone Mineral Density and Chronic Inflammation. PLoS ONE 2010, 5, e14073. [Google Scholar] [CrossRef]
- Walia, A.; Yang, J.F.; Huang, Y.H.; Rosenblatt, M.I.; Chang, J.H.; Azar, D.T. Endostatin’s emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim. Biophys. Acta 2015, 1850, 2422–2438. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.E.; Parks, W.C. Metalloproteinases and their inhibitors: Regulators of wound healing. Int. J. Biochem. Cell Biol. 2008, 40, 1334–1347. [Google Scholar] [CrossRef]
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; De Winther, M.P.; Donners, M.M. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014, 17, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zajac, E.; Schweighofer, B.; Kupriyanova, T.A.; Juncker-Jensen, A.; Minder, P.; Quigley, J.P.; Deryugina, E.I. Angiogenic capacity of M1- and M2-polarized macrophages is determined by the levels of TIMP-1 complexed with their secreted proMMP-9. Blood 2013, 122, 4054–4067. [Google Scholar] [CrossRef]
- Vaalamo, M.; Leivo, T.; Saarialho-Kere, U. Differential expression of tissue inhibitors of metalloproteinases (TIMP-1, -2, -3, and -4) in normal and aberrant wound healing. Hum. Pathol. 1999, 30, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.J.; Butler, G.S.; Rodriguez, D.; Overall, C.M. Matrix metalloproteinase proteomics: Substrates, targets, and therapy. Curr. Opin. Cell Biol. 2009, 21, 645–653. [Google Scholar] [CrossRef]
- Purohit, T.; Qin, Z.; Quan, C.; Lin, Z.; Quan, T. Smad3-dependent CCN2 mediates fibronectin expression in human skin dermal fibroblasts. PLoS ONE 2017, 12, e0173191. [Google Scholar] [CrossRef] [PubMed]
- Bielefeld, K.A.; Amini-Nik, S.; Whetstone, H.; Poon, R.; Youn, A.; Wang, J.; Alman, B.A. Fibronectin and beta-catenin act in a regulatory loop in dermal fibroblasts to modulate cutaneous healing. J. Biol. Chem. 2011, 286, 27687–27697. [Google Scholar] [CrossRef]
- Stoffels, J.M.; Zhao, C.; Baron, W. Fibronectin in tissue regeneration: Timely disassembly of the scaffold is necessary to complete the build. Cell. Mol. Life Sci. 2013, 70, 4243–4253. [Google Scholar] [CrossRef]
- Fuchs, P.O.; Calitz, C.; Pavlovic, N.; Binet, F.; Solbak, S.M.O.; Danielson, U.H.; Kreuger, J.; Heindryckx, F.; Gerwins, P. Fibrin fragment E potentiates TGF-beta-induced myofibroblast activation and recruitment. Cell Signal 2020, 72, 109661. [Google Scholar] [CrossRef]
- Youn, M.; Huang, H.; Chen, C.; Kam, S.; Wilkes, M.C.; Chae, H.D.; Sridhar, K.J.; Greenberg, P.L.; Glader, B.; Narla, A.; et al. MMP9 inhibition increases erythropoiesis in RPS14-deficient del(5q) MDS models through suppression of TGF-β pathways. Blood Adv. 2019, 3, 2751–2763. [Google Scholar] [CrossRef] [PubMed]
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, X.F.; Wang, Z.C.; Lou, D.; Fang, Q.Q.; Hu, Y.Y.; Zhao, W.Y.; Zhang, L.Y.; Wu, L.H.; Tan, W.Q. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed. Pharmacother. 2020, 129, 110287. [Google Scholar] [CrossRef] [PubMed]
- Secker, G.A.; Shortt, A.J.; Sampson, E.; Schwarz, Q.P.; Schultz, G.S.; Daniels, J.T. TGF β stimulated re-epithelialisation is regulated by CTGF and Ras/MEK/ERK signalling. Exp. Cell Res. 2008, 314, 131–142. [Google Scholar] [CrossRef]
- Frazier, K.; Williams, S.; Kothapalli, D.; Klapper, H.; Grotendorst, G.R. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J. Investig. Dermatol. 1996, 107, 404–411. [Google Scholar] [CrossRef]
- Sun, S.W.; Chen, L.; Zhou, M.; Wu, J.H.; Meng, Z.J.; Han, H.L.; Miao, S.Y.; Zhu, C.C.; Xiong, X.Z. BAMBI regulates macrophages inducing the differentiation of Treg through the TGF-beta pathway in chronic obstructive pulmonary disease. Respir. Res. 2019, 20, 26. [Google Scholar] [CrossRef]
- Ehnert, S.; Zhao, J.; Pscherer, S.; Freude, T.; Dooley, S.; Kolk, A.; Stockle, U.; Nussler, A.K.; Hube, R. Transforming growth factor beta1 inhibits bone morphogenic protein (BMP)-2 and BMP-7 signaling via upregulation of Ski-related novel protein N (SnoN): Possible mechanism for the failure of BMP therapy? BMC Med. 2012, 10, 101. [Google Scholar] [CrossRef]
- Liang, D.; Song, Z.; Liang, W.; Li, Y.; Liu, S. Metformin inhibits TGF-β 1-induced MCP-1 expression through BAMBI-mediated suppression of MEK/ERK1/2 signalling. Nephrology 2019, 24, 481–488. [Google Scholar] [CrossRef]
- Lin, L.; Wang, Y.; Liu, W.; Huang, Y. BAMBI inhibits skin fibrosis in keloid through suppressing TGF-β 1-induced hypernomic fibroblast cell proliferation and excessive accumulation of collagen I. Int. J. Clin. Exp. Med. 2015, 8, 13227–13234. [Google Scholar]
- Yang, L.; Gao, Q.; Lv, F.; Guo, M.; Zhao, D. miR-519d-3p promotes TGFbeta/Smad mediated postoperative epidural scar formation via suppression of BAMBI. Mol. Med. Rep. 2019, 20, 3901–3909. [Google Scholar]
- Guillot, N.; Kollins, D.; Gilbert, V.; Xavier, S.; Chen, J.; Gentle, M.; Reddy, A.; Bottinger, E.; Jiang, R.; Rastaldi, M.P.; et al. BAMBI regulates angiogenesis and endothelial homeostasis through modulation of alternative TGFbeta signaling. PLoS ONE 2012, 7, e39406. [Google Scholar] [CrossRef] [PubMed]
- Guillot, N.; Kollins, D.; Badimon, J.J.; Schlondorff, D.; Hutter, R. Accelerated reendothelialization, increased neovascularization and erythrocyte extravasation after arterial injury in BAMBI−/− mice. PLoS ONE 2013, 8, e58550. [Google Scholar] [CrossRef] [PubMed]





| Primer | NM_ | Forward Sequence | Reverse Sequence | TA | Size |
|---|---|---|---|---|---|
| 18s | 003286 | GGACAGGATTGACAGATTGAT | AGTCTCGTTCGTTATCGGAAT | 56 °C | 111 bp |
| ANGPT1 | 001146.5 | CGATGGCAACTGTCGTGAGA | CATGGTAGCCGTGTGGTTCT | 60 °C | 232 bp |
| ANGPT2 | 001147.3 | CTTGGAACACTCCCTCTCGAC | GCTTGTCTTCCATAGCTAGCAC | 60 °C | 125 bp |
| BAMBI | 012342.2 | TGCACGATGTTCTCTCTCCT | GAAGTCAGCTCCTGCACCTT | 59 °C | 106 bp |
| COL1A1 | 000088.3 | CAGCCGCTTCACCTACAGC | TTTTGTATTCAATCACTGTCTTGCC | 60 °C | 83 bp |
| CTGF | 001901.3 | CCAATGACAACGCCTCCTG | TGGTGCAGCCAGAAAGCTC | 62 °C | 159 bp |
| FN1 | 002026.2 | CCCCATTCCAGGACACTTCTG | GCCCACGGTAACAACCTCTT | 60 °C | 203 bp |
| IL-1β | 000576.2 | CTCGCCAGTGAAATGATGGCT | GTCGGAGATTCGTAGCTGGAT | 60 °C | 144 bp |
| IL-6 | 000600.4 | AACCTGAACCTTCCAAAGATGG | TCTGGCTTGTTCCTCACTACT | 58 °C | 159 bp |
| IL-8 (CXCL8) | 000584.3 | TAGCAAAATTGAGGCCAAGG | AAACCAAGGCACAGTGGAAC | 60 °C | 227 bp |
| MMP1 | 002421.4 | CCCAGCGACTCTAGAAACACA | TCTTGGCAAATCTGGCGTGT | 60 °C | 322 bp |
| MMP9 | 004994.3 | TCTATGGTCCTCGCCCTGAA | CATCGTCCACCGGACTCAAA | 64 °C | 219 bp |
| RPL13a | 012423.3 | AAGTACCAGGCAGTGACAG | CCTGTTTCCGTAGCCTCATG | 56 °C | 100 bp |
| TGF-β1 | 000660.6 | TCCGGACCAGCCCTCGGGAG | CGGTCGCGGGTGCTGTTGTA | 58 °C | 680 bp |
| TGF-β2 | 001135599.1 | GCAGGTATTGATGGCACCTC | AGGCAGCAATTATCCTGCAC | 58 °C | 206 bp |
| TGF-β3 | 003239.2 | CAGCTGCCTTGCCACCCCTC | TGCAGCCTTCCTCCCTCTCCC | 58 °C | 601 bp |
| Tie2 (TEK) | 000459.5 | GGTCAAGCAACCCAGCCTTTTC | CAGGTCATTCCAGCAGAGCCAA | 64 °C | 121 bp |
| TIMP1 | 003254.3 | AGTTTTGTGGCTCCCTGGAA | AAGCCCTTTTCAGAG-CCTTG | 60 °C | 179 bp |
| TIMP2 | 003255.5 | ATGCAGATGTAGTGATCAGGGC | GTGATGTGCATCTTGCCGTC | 60 °C | 256 bp |
| VEGFa | 001204384.1 | CTACCTCCACCATGCCAAGT | GCAGTAGCTGCGCTGATAGA | 59 °C | 109 bp |
| VEGFRI (FLT1) | 001160030.1 | TCTCACACATCGACAAACCAATACA | GGTAGCAGTACAATTGAGGACAAGA | 60 °C | 106 bp |
| VEGFRI (KDR) | 002253.2 | CAGGGGACAGAGGGACTTG | GAGGCCATCGCTGCACTCA | 60 °C | 91 bp |
| Acute Wounds | Chronic Wounds | p-Value * | ||
|---|---|---|---|---|
| Healing | Delayed Healing | |||
| # of patients | N = 15 | N = 5 | N = 18 | - |
| males | N = 11 | N = 2 | N = 11 | >0.9999 |
| females | N = 4 | N = 3 | N = 7 | |
| age (years) | 52.9 ± 17.1 (18–82) | 64.2 ± 11.8 (53–82) | 58.3 ± 18.4 (20–81) | 0.6182 |
| wound duration at sampling (days) | 22.4 ± 11.9 (0–37) | 18.8 ± 9.2 (8–30) | 203.8 ± 226.2 (64–730) | <0.0001 |
| wound size at sampling (cm2) | 33.3 ± 24.9 (8.3–96.0) | 38.6 ± 29.8 (12.0–88.0) | 32.7 ± 22.3 (2.3–80.0) | >0.9999 |
| # of traumatic wounds | N = 6 (40.0%) | N = 3 (75.0%) | N = 8 (44.4%) | >0.9999 |
| # of iatrogenic wounds | N = 2 (13.3%) | N = 0 (0.0%) | N = 3 (16.7%) | >0.9999 |
| # of pressure/sheer wounds | N = 3 (20.0%) | N = 0 (0.0%) | N = 3 (16.7%) | >0.9999 |
| # of wounds with other reasons° | N = 1 (6.7%) | N = 0 (0.0%) | N = 1 (5.6%) | >0.9999 |
| # of wounds positive for germs | N = 7 (46.7%) | N = 3 (60.0%) | N = 12 (66.7%) | 0.4323 |
| # of infectious wounds | N = 3 (20.0%) | N = 1 (25.0%) | N = 3 (16.7%) | >0.9999 |
| CRP (mg/L) | 39.1 ± 70.3 (0.2–256.0) | 4.1 ± 7.4 (0.2–17.3) | 24.5 ± 37.7 (0.2–127.2) | 0.4939 |
| Neutrophil count (103/µL) | 4646 ± 1648 (1700–7130) | 3654 ± 730 (2600–4360) | 4104 ± 1516 (1990–7510) | 0.9461 |
| # of diabetics | N = 3 | N = 2 | N = 5 | >0.9999 |
| # of active smokers | N = 5 | N = 2 | N = 6 | >0.9999 |
| # of former smokers | N = 5 | N = 2 | N = 7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehnert, S.; Rinderknecht, H.; Liu, C.; Voss, M.; Konrad, F.M.; Eisler, W.; Alexander, D.; Ngamsri, K.-C.; Histing, T.; Rollmann, M.F.; et al. Increased Levels of BAMBI Inhibit Canonical TGF-β Signaling in Chronic Wound Tissues. Cells 2023, 12, 2095. https://doi.org/10.3390/cells12162095
Ehnert S, Rinderknecht H, Liu C, Voss M, Konrad FM, Eisler W, Alexander D, Ngamsri K-C, Histing T, Rollmann MF, et al. Increased Levels of BAMBI Inhibit Canonical TGF-β Signaling in Chronic Wound Tissues. Cells. 2023; 12(16):2095. https://doi.org/10.3390/cells12162095
Chicago/Turabian StyleEhnert, Sabrina, Helen Rinderknecht, Chao Liu, Melanie Voss, Franziska M. Konrad, Wiebke Eisler, Dorothea Alexander, Kristian-Christos Ngamsri, Tina Histing, Mika F. Rollmann, and et al. 2023. "Increased Levels of BAMBI Inhibit Canonical TGF-β Signaling in Chronic Wound Tissues" Cells 12, no. 16: 2095. https://doi.org/10.3390/cells12162095
APA StyleEhnert, S., Rinderknecht, H., Liu, C., Voss, M., Konrad, F. M., Eisler, W., Alexander, D., Ngamsri, K.-C., Histing, T., Rollmann, M. F., & Nussler, A. K. (2023). Increased Levels of BAMBI Inhibit Canonical TGF-β Signaling in Chronic Wound Tissues. Cells, 12(16), 2095. https://doi.org/10.3390/cells12162095







