Porcine Macrophage Markers and Populations: An Update
Abstract
:1. Introduction
2. Markers for Studying Monocytes/Macrophages and Their Differentiation in Swine
2.1. CD172a
2.2. CD14
2.3. Fc-Gamma Receptors
2.4. CD163
2.5. Siglecs
2.5.1. CD169
2.5.2. Siglec-3/CD33 and CD33-Related Siglecs
2.6. Beta 2-Integrins
2.7. Toll-Like Receptors (TLRs)
2.8. C-Type Lectin-Like Receptors
2.8.1. CD205
2.8.2. CD206/Mannose Receptor
2.8.3. CD209/DC-SIGN
2.8.4. Dectin-1/CD369
2.8.5. CLEC12A/CD371
2.8.6. CLEC12B
2.9. CD107a/Lamp1
2.10. CD68
2.11. CD115/CSF1R
2.12. CD200R Family
2.13. CD203a
2.14. F4/80 or ADGRE1
3. Origin and Plasticity of Tissue-Resident Macrophages
3.1. Macrophage Polarization
3.2. Macrophage Populations in Different Organs
3.2.1. Spleen
3.2.2. Lymph Nodes
3.2.3. Tonsil
3.2.4. Liver
3.2.5. Lung
3.2.6. Nasal Mucosa
3.2.7. Skin
3.2.8. Placenta
4. Porcine Macrophage Cell Lines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The pig: A model for human infectious diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Pollard, J.W. Trophic macrophages in development and disease. Nat. Rev. Immunol. 2009, 9, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef]
- Davies, L.C.; Taylor, P.R. Tissue-resident macrophages: Then and now. Immunology 2015, 144, 541–548. [Google Scholar] [CrossRef] [PubMed]
- VanderWaal, K.; Deen, J. Global trends in infectious diseases of swine. Proc. Natl. Acad. Sci. USA 2018, 115, 11495–11500. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Alcrudo, D.; Falco, J.R.; Raizman, E.; Dietze, K. Transboundary spread of pig diseases: The role of international trade and travel. BMC Vet. Res. 2019, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Ezquerra, A.; Revilla, C.; Alvarez, B.; Perez, C.; Alonso, F.; Dominguez, J. Porcine myelomonocytic markers and cell populations. Dev. Comp. Immunol. 2009, 33, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Dawson, H.D.; Lunney, J.K. Porcine cluster of differentiation (CD) markers 2018 update. Res. Vet. Sci. 2018, 118, 199–246. [Google Scholar] [CrossRef] [PubMed]
- Haverson, K.; Bailey, M.; Higgins, V.R.; Bland, P.W.; Stokes, C.R. Characterization of monoclonal antibodies specific for monocytes, macrophages and granulocytes from porcine peripheral blood and mucosal tissues. J. Immunol. Methods 1994, 170, 233–245. [Google Scholar] [CrossRef]
- Dominguez, J.; Alvarez, B.; Alonso, F.; Thacker, E.; Haverson, K.; McCullough, K.; Summerfield, A.; Ezquerra, A. Workshop studies on monoclonal antibodies in the myeloid panel with CD11 specificity. Vet. Immunol. Immunopathol. 2001, 80, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Deloizy, C.; Bouguyon, E.; Fossum, E.; Sebo, P.; Osicka, R.; Bole, A.; Pierres, M.; Biacchesi, S.; Dalod, M.; Bogen, B.; et al. Expanding the tools for identifying mononuclear phagocyte subsets in swine: Reagents to porcine CD11c and XCR1. Dev. Comp. Immunol. 2016, 65, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Halloran, P.J.; Sweeney, S.E.; Strohmeier, C.M.; Kim, Y.B. Molecular cloning and identification of the porcine cytolytic trigger molecule G7 as a Fc gamma RIII alpha (CD16) homologue. J. Immunol. 1994, 153, 2631–2641. [Google Scholar] [CrossRef] [PubMed]
- Egli, J.; Schmucki, R.; Loos, B.; Reichl, S.; Grabole, N.; Roller, A.; Ebeling, M.; Odermatt, A.; Iglesias, A. The genomic organization and expression pattern of the low-affinity Fc gamma receptors (FcgammaR) in the Gottingen minipig. Immunogenetics 2019, 71, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Balmelli, C.; Vincent, I.E.; Rau, H.; Guzylack-Piriou, L.; McCullough, K.; Summerfield, A. Fc gamma RII-dependent sensitisation of natural interferon-producing cells for viral infection and interferon-alpha responses. Eur. J. Immunol. 2005, 35, 2406–2415. [Google Scholar] [CrossRef] [PubMed]
- Saalmuller, A.; Lunney, J.K.; Daubenberger, C.; Davis, W.; Fischer, U.; Gobel, T.W.; Griebel, P.; Hollemweguer, E.; Lasco, T.; Meister, R.; et al. Summary of the animal homologue section of HLDA8. Cell Immunol. 2005, 236, 51–58. [Google Scholar] [CrossRef]
- Bullido, R.; Gomez del Moral, M.; Alonso, F.; Ezquerra, A.; Zapata, A.; Sanchez, C.; Ortuno, E.; Alvarez, B.; Dominguez, J. Monoclonal antibodies specific for porcine monocytes/macrophages: Macrophage heterogeneity in the pig evidenced by the expression of surface antigens. Tissue Antigens 1997, 49, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Moffat, L.; Rothwell, L.; Garcia-Morales, C.; Sauter, K.A.; Kapetanovic, R.; Gow, D.J.; Hume, D.A. Development and characterisation of monoclonal antibodies reactive with porcine CSF1R (CD115). Dev. Comp. Immunol. 2014, 47, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Domenech, N.; Vazquez, J.; Alonso, F.; Ezquerra, A.; Dominguez, J. The porcine 2A10 antigen is homologous to human CD163 and related to macrophage differentiation. J. Immunol. 1999, 162, 5230–5237. [Google Scholar] [CrossRef] [PubMed]
- Pescovitz, M.D.; Lunney, J.K.; Sachs, D.H. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J. Immunol. 1984, 133, 368–375. [Google Scholar] [CrossRef]
- Alvarez, B.; Sanchez, C.; Bullido, R.; Marina, A.; Lunney, J.; Alonso, F.; Ezquerra, A.; Dominguez, J. A porcine cell surface receptor identified by monoclonal antibodies to SWC3 is a member of the signal regulatory protein family and associates with protein-tyrosine phosphatase SHP-1. Tissue Antigens 2000, 55, 342–351. [Google Scholar] [CrossRef]
- Poderoso, T.; Martinez de la Riva, P.; Uenishi, H.; Alvarez, B.; Toki, D.; Nieto-Pelegrin, E.; Alonso, F.; Dominguez, J.; Ezquerra, A.; Revilla, C. Analysis of the expression of porcine CD200R1 and CD200R1L by using newly developed monoclonal antibodies. Dev. Comp. Immunol. 2019, 100, 103417. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, S.E.; Jung, S.H.; Kim, Y.K.; Kim, Y.B.; Lee, H.T. Characterization of monoclonal antibodies against porcine pulmonary alveolar macrophages of gnotobiotic miniature swine. Biochem. Biophys. Res. Commun. 2015, 461, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Parra-Sanchez, H.; Puebla-Clark, L.; Resendiz, M.; Valenzuela, O.; Hernandez, J. Characterization and expression of DEC205 in the cDC1 and cDC2 subsets of porcine dendritic cells from spleen, tonsil, and submaxillary and mesenteric lymph nodes. Mol. Immunol. 2018, 96, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Marquet, F.; Bonneau, M.; Pascale, F.; Urien, C.; Kang, C.; Schwartz-Cornil, I.; Bertho, N. Characterization of dendritic cells subpopulations in skin and afferent lymph in the swine model. PLoS ONE 2011, 6, e16320. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Pineyro, P.; Tian, D.; Overend, C.; Yugo, D.M.; Matzinger, S.R.; Rogers, A.J.; Haac, M.E.; Cao, Q.; Heffron, C.L.; et al. In vivo targeting of porcine reproductive and respiratory syndrome virus antigen through porcine DC-SIGN to dendritic cells elicits antigen-specific CD4T cell immunity in pigs. Vaccine 2014, 32, 6768–6775. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, B.; Nieto-Pelegrin, E.; Martinez de la Riva, P.; Toki, D.; Poderoso, T.; Revilla, C.; Uenishi, H.; Ezquerra, A.; Dominguez, J. Characterization of the Porcine CLEC12A and Analysis of Its Expression on Blood Dendritic Cell Subsets. Front. Immunol. 2020, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Pelegrin, E.; Alvarez, B.; Martinez de la Riva, P.; Toki, D.; Poderoso, T.; Revilla, C.; Uenishi, H.; Ezquerra, A.; Dominguez, J. Porcine CLEC12B is expressed on alveolar macrophages and blood dendritic cells. Dev. Comp. Immunol. 2020, 111, 103767. [Google Scholar] [CrossRef] [PubMed]
- Waddell, L.A.; Lefevre, L.; Bush, S.J.; Raper, A.; Young, R.; Lisowski, Z.M.; McCulloch, M.E.B.; Muriuki, C.; Sauter, K.A.; Clark, E.L.; et al. ADGRE1 (EMR1, F4/80) Is a Rapidly-Evolving Gene Expressed in Mammalian Monocyte-Macrophages. Front. Immunol. 2018, 9, 2246. [Google Scholar] [CrossRef] [PubMed]
- Revilla, C.; Poderoso, T.; Martinez, P.; Alvarez, B.; Lopez-Fuertes, L.; Alonso, F.; Ezquerra, A.; Dominguez, J. Targeting to porcine sialoadhesin receptor improves antigen presentation to T cells. Vet. Res. 2009, 40, 14. [Google Scholar] [CrossRef]
- Alvarez, B.; Escalona, Z.; Uenishi, H.; Toki, D.; Revilla, C.; Yuste, M.; Del Moral, M.G.; Alonso, F.; Ezquerra, A.; Dominguez, J. Molecular and functional characterization of porcine Siglec-3/CD33 and analysis of its expression in blood and tissues. Dev. Comp. Immunol. 2015, 51, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Escalona, Z.; Alvarez, B.; Uenishi, H.; Toki, D.; Yuste, M.; Revilla, C.; Gomez del Moral, M.; Alonso, F.; Ezquerra, A.; Dominguez, J. Molecular characterization and expression of porcine Siglec-5. Dev. Comp. Immunol. 2014, 44, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Escalona, Z.; Alvarez, B.; Uenishi, H.; Toki, D.; Yuste, M.; Revilla, C.; del Moral, M.G.; Alonso, F.; Ezquerra, A.; Dominguez, J. Molecular characterization of porcine Siglec-10 and analysis of its expression in blood and tissues. Dev. Comp. Immunol. 2015, 48, 116–123. [Google Scholar] [CrossRef]
- Alvarez, B.; Revilla, C.; Domenech, N.; Perez, C.; Martinez, P.; Alonso, F.; Ezquerra, A.; Domiguez, J. Expression of toll-like receptor 2 (TLR2) in porcine leukocyte subsets and tissues. Vet. Res. 2008, 39, 13. [Google Scholar] [CrossRef]
- Wen, K.; Azevedo, M.S.; Gonzalez, A.; Zhang, W.; Saif, L.J.; Li, G.; Yousef, A.; Yuan, L. Toll-like receptor and innate cytokine responses induced by lactobacilli colonization and human rotavirus infection in gnotobiotic pigs. Vet. Immunol. Immunopathol. 2009, 127, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, B.; de la Riva, P.M.; Handler, A.; Revilla, C.; Alonso, F.; Ezquerra, A.; Dominguez, J. Expression of TLR4 in swine as assessed by a newly developed monoclonal antibody. Vet. Immunol. Immunopathol. 2013, 153, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Schneberger, D.; Lewis, D.; Caldwell, S.; Singh, B. Expression of toll-like receptor 9 in lungs of pigs, dogs and cattle. Int. J. Exp. Pathol. 2011, 92, 1–7. [Google Scholar] [CrossRef]
- Blecha, F.; Kielian, T.; McVey, D.S.; Lunney, J.K.; Walker, K.; Stokes, C.R.; Stevens, K.; Kim, Y.B.; Chu, R.M.; Chen, T.S.; et al. Workshop studies on monoclonal antibodies reactive against porcine myeloid cells. Vet. Immunol. Immunopathol. 1994, 43, 269–272. [Google Scholar] [CrossRef]
- Summerfield, A.; Guzylack-Piriou, L.; Schaub, A.; Carrasco, C.P.; Tache, V.; Charley, B.; McCullough, K.C. Porcine peripheral blood dendritic cells and natural interferon-producing cells. Immunology 2003, 110, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, A.; McCullough, K.C. Porcine bone marrow myeloid cells: Phenotype and adhesion molecule expression. J. Leukoc. Biol. 1997, 62, 176–185. [Google Scholar] [CrossRef]
- Summerfield, A.; Auray, G.; Ricklin, M. Comparative dendritic cell biology of veterinary mammals. Annu. Rev. Anim. Biosci. 2015, 3, 533–557. [Google Scholar] [CrossRef] [PubMed]
- Sinkora, M.; Sinkorova, J. B cell lymphogenesis in swine is located in the bone marrow. J. Immunol. 2014, 193, 5023–5032. [Google Scholar] [CrossRef]
- Alvarez, B.; Gomez, N.; Jose Garrido, J.; Yerle, M.; Revilla, C.; Chamorro, S.; Alonso, F.; Dominguez, J.; Ezquerra, A. Molecular cloning characterization and expression of porcine immunoreceptor SIRPalpha. Dev. Comp. Immunol. 2007, 31, 307–318. [Google Scholar] [CrossRef]
- Barclay, A.N.; Van den Berg, T.K. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: Structure, function, and therapeutic target. Annu. Rev. Immunol. 2014, 32, 25–50. [Google Scholar] [CrossRef]
- Boettcher, A.N.; Cunnick, J.E.; Powell, E.J.; Egner, T.K.; Charley, S.E.; Loving, C.L.; Tuggle, C.K. Porcine signal regulatory protein alpha binds to human CD47 to inhibit phagocytosis: Implications for human hematopoietic stem cell transplantation into severe combined immunodeficient pigs. Xenotransplantation 2019, 26, e12466. [Google Scholar] [CrossRef]
- Maeda, A.; Kogata, S.; Toyama, C.; Lo, P.C.; Okamatsu, C.; Yamamoto, R.; Masahata, K.; Kamiyama, M.; Eguchi, H.; Watanabe, M.; et al. The Innate Cellular Immune Response in Xenotransplantation. Front. Immunol. 2022, 13, 858604. [Google Scholar] [CrossRef] [PubMed]
- Janssen, W.J.; McPhillips, K.A.; Dickinson, M.G.; Linderman, D.J.; Morimoto, K.; Xiao, Y.Q.; Oldham, K.M.; Vandivier, R.W.; Henson, P.M.; Gardai, S.J. Surfactant proteins A and D suppress alveolar macrophage phagocytosis via interaction with SIRP alpha. Am. J. Respir. Crit. Care Med. 2008, 178, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, T.K.; van Beek, E.M.; Buhring, H.J.; Colonna, M.; Hamaguchi, M.; Howard, C.J.; Kasuga, M.; Liu, Y.; Matozaki, T.; Neel, B.G.; et al. A nomenclature for signal regulatory protein family members. J. Immunol. 2005, 175, 7788–7789. [Google Scholar] [CrossRef]
- Triantafilou, M.; Triantafilou, K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002, 23, 301–304. [Google Scholar] [CrossRef]
- Shimazu, R.; Akashi, S.; Ogata, H.; Nagai, Y.; Fukudome, K.; Miyake, K.; Kimoto, M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 1999, 189, 1777–1782. [Google Scholar] [CrossRef]
- Hailman, E.; Lichenstein, H.S.; Wurfel, M.M.; Miller, D.S.; Johnson, D.A.; Kelley, M.; Busse, L.A.; Zukowski, M.M.; Wright, S.D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J. Exp. Med. 1994, 179, 269–277. [Google Scholar] [CrossRef]
- Dziarski, R.; Tapping, R.I.; Tobias, P.S. Binding of bacterial peptidoglycan to CD14. J. Biol. Chem. 1998, 273, 8680–8690. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, M.G.; Gorham, J.D.; Murphy, T.L.; Tuomanen, E.; Murphy, K.M. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect. Immun. 1996, 64, 1906–1912. [Google Scholar] [CrossRef]
- Tada, H.; Nemoto, E.; Shimauchi, H.; Watanabe, T.; Mikami, T.; Matsumoto, T.; Ohno, N.; Tamura, H.; Shibata, K.; Akashi, S.; et al. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol. Immunol. 2002, 46, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Savedra, R., Jr.; Delude, R.L.; Ingalls, R.R.; Fenton, M.J.; Golenbock, D.T. Mycobacterial lipoarabinomannan recognition requires a receptor that shares components of the endotoxin signaling system. J. Immunol. 1996, 157, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Haziot, A.; Chen, S.; Ferrero, E.; Low, M.G.; Silber, R.; Goyert, S.M. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J. Immunol. 1988, 141, 547–552. [Google Scholar] [CrossRef]
- Bazil, V.; Strominger, J.L. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J. Immunol. 1991, 147, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Ranoa, D.R.E.; Kelley, S.L.; Tapping, R.I. Human lipopolysaccharide-binding protein (LBP) and CD14 independently deliver triacylated lipoproteins to Toll-like receptor 1 (TLR1) and TLR2 and enhance formation of the ternary signaling complex. J. Biol. Chem. 2013, 288, 9729–9741. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.B.; Nygard, A.B.; Fredholm, M.; Aasted, B.; Salomonsen, J. Cloning, characterization and mapping of porcine CD14 reveals a high conservation of mammalian CD14 structure, expression and locus organization. Dev. Comp. Immunol. 2007, 31, 729–737. [Google Scholar] [CrossRef]
- Sanz, G.; Perez, E.; Jimenez-Marin, A.; Mompart, F.; Morera, L.; Barbancho, M.; Llanes, D.; Garrido, J.J. Molecular cloning, chromosomal location, and expression analysis of porcine CD14. Dev. Comp. Immunol. 2007, 31, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, H.W.; Appl, B.; Kafferlein, E.; Loffler, T.; Jahn-Henninger, H.; Gutensohn, W.; Nores, J.R.; McCullough, K.; Passlick, B.; Labeta, M.O.; et al. The antibody MY4 recognizes CD14 on porcine monocytes and macrophages. Scand. J. Immunol. 1994, 40, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Kielian, T.; McVey, D.S.; Davis, W.C.; Kim, Y.B.; Blecha, F. Competitive binding analysis of monoclonal antibodies reactive with porcine alveolar macrophages using anti-CD14 and anti-CD18. Vet. Immunol. Immunopathol. 1994, 43, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Thacker, E.; Summerfield, A.; McCullough, K.; Ezquerra, A.; Dominguez, J.; Alonso, F.; Lunney, J.; Sinkora, J.; Haverson, K. Summary of workshop findings for porcine myelomonocytic markers. Vet. Immunol. Immunopathol. 2001, 80, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, J.; Ezquerra, A.; Alonso, F.; McCullough, K.; Summerfield, A.; Bianchi, A.; Zwart, R.J.; Kim, Y.B.; Blecha, F.; Eicher, S.; et al. Porcine myelomonocytic markers: Summary of the Second International Swine CD Workshop. Vet. Immunol. Immunopathol. 1998, 60, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Shahriar, F.; Gordon, J.R.; Simko, E. Identification of lipopolysaccharide-binding proteins in porcine milk. Can. J. Vet. Res. 2006, 70, 243–250. [Google Scholar] [PubMed]
- Guilliams, M.; Bruhns, P.; Saeys, Y.; Hammad, H.; Lambrecht, B.N. The function of Fcgamma receptors in dendritic cells and macrophages. Nat. Rev. Immunol. 2014, 14, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Junker, F.; Gordon, J.; Qureshi, O. Fc Gamma Receptors and Their Role in Antigen Uptake, Presentation, and T Cell Activation. Front. Immunol. 2020, 11, 1393. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef]
- Qiao, S.; Zhang, G.; Xia, C.; Zhang, H.; Zhang, Y.; Xi, J.; Song, H.; Li, X. Cloning and characterization of porcine Fc gamma receptor II (FcgammaRII). Vet. Immunol. Immunopathol. 2006, 114, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Qiao, S.; Li, Q.; Wang, X.; Duan, Y.; Wang, L.; Xiao, Z.; Xia, C. Molecular cloning and expression of the porcine high-affinity immunoglobulin G Fc receptor (FcgammaRI). Immunogenetics 2006, 58, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, B.; Mwangi, W.; Rijal, P.; Schwartz, J.C.; Noble, A.; Shaw, A.; Sealy, J.E.; Bonnet-Di Placido, M.; Graham, S.P.; Townsend, A.; et al. Fc-Mediated Functions of Porcine IgG Subclasses. Front. Immunol. 2022, 13, 903755. [Google Scholar] [CrossRef]
- Qiao, S.; Jiang, Z.; Tian, X.; Wang, R.; Xing, G.; Wan, B.; Bao, D.; Liu, Y.; Hao, H.; Guo, J.; et al. Porcine FcgammaRIIb mediates enhancement of porcine reproductive and respiratory syndrome virus (PRRSV) infection. PLoS ONE 2011, 6, e28721. [Google Scholar] [CrossRef]
- Farber, D.L.; Sears, D.W. Rat CD16 is defined by a family of class III Fc gamma receptors requiring co-expression of heteroprotein subunits. J. Immunol. 1991, 146, 4352–4361. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, S.E.; Halloran, P.J.; Kim, Y.B. Identification of a unique porcine Fc gamma RIIIA alpha molecular complex. Cell Immunol. 1996, 172, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Dato, M.E.; Wierda, W.G.; Kim, Y.B. A triggering structure recognized by G7 monoclonal antibody on porcine lymphocytes and granulocytes. Cell Immunol. 1992, 140, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L. Monocyte subsets in man and other species. Cell Immunol. 2014, 289, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Liu, X.; Zhang, Y.; Duan, E.; Zhang, Z.; Chen, J.; Mu, C.; Cui, B. Porcine Fc gamma RIIb sub-isoforms are generated by alternative splicing. Vet. Immunol. Immunopathol. 2012, 145, 386–394. [Google Scholar] [CrossRef]
- Xia, P.; Liu, Y.; Liu, X.; Zhang, Z.; Duan, E.; Lu, X.; Zhao, J.; Cui, B. Molecular cloning and characterization of a porcine Fc gamma RIIb sub-isoform(FcgammaRIIb1). Vet. Immunol. Immunopathol. 2011, 141, 144–150. [Google Scholar] [CrossRef]
- Jie, H.B.; Yim, D.; Kim, Y.B. Porcine Fc gammaRIII isoforms are generated by alternative splicing. Mol. Immunol. 2009, 46, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Law, S.K.; Micklem, K.J.; Shaw, J.M.; Zhang, X.P.; Dong, Y.; Willis, A.C.; Mason, D.Y. A new macrophage differentiation antigen which is a member of the scavenger receptor superfamily. Eur. J. Immunol. 1993, 23, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.; Ortuño, E.; Gómez, N.; García-Briones, M.; Alvarez, B.; Martínez de la Riva, P.; Alonso, F.; Revilla, C.; Domínguez, J.; Ezquerra, Á. Cloning and expression of porcine CD163: Its use for characterization of monoclonal antibodies to porcine CD163 and development of an ELISA to measure soluble CD163 in biological fluids. Span. J. Agric. Res. 2008, 6, 59–72. [Google Scholar] [CrossRef]
- Calvert, J.G.; Slade, D.E.; Shields, S.L.; Jolie, R.; Mannan, R.M.; Ankenbauer, R.G.; Welch, S.K. CD163 expression confers susceptibility to porcine reproductive and respiratory syndrome viruses. J. Virol. 2007, 81, 7371–7379. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.J.; Law, S.K.; Moestrup, S.K. Identification of the haemoglobin scavenger receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Schaer, C.A.; Schoedon, G.; Imhof, A.; Kurrer, M.O.; Schaer, D.J. Constitutive endocytosis of CD163 mediates hemoglobin-heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circ. Res. 2006, 99, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Levine, Y.A.; Gunasekaran, M.K.; Wang, Y.; Addorisio, M.; Zhu, S.; Li, W.; Li, J.; de Kleijn, D.P.; et al. Identification of CD163 as an antiinflammatory receptor for HMGB1-haptoglobin complexes. JCI Insight 2016, 1, e85375. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Ritter, M.; Orso, E.; Langmann, T.; Klucken, J.; Schmitz, G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J. Leukoc. Biol. 2000, 67, 97–103. [Google Scholar] [CrossRef]
- Sulahian, T.H.; Hogger, P.; Wahner, A.E.; Wardwell, K.; Goulding, N.J.; Sorg, C.; Droste, A.; Stehling, M.; Wallace, P.K.; Morganelli, P.M.; et al. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine 2000, 12, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Schaer, D.J.; Boretti, F.S.; Schoedon, G.; Schaffner, A. Induction of the CD163-dependent haemoglobin uptake by macrophages as a novel anti-inflammatory action of glucocorticoids. Br. J. Haematol. 2002, 119, 239–243. [Google Scholar] [CrossRef]
- Singleton, H.; Graham, S.P.; Bodman-Smith, K.B.; Frossard, J.P.; Steinbach, F. Establishing Porcine Monocyte-Derived Macrophage and Dendritic Cell Systems for Studying the Interaction with PRRSV-1. Front. Microbiol. 2016, 7, 832. [Google Scholar] [CrossRef] [PubMed]
- Fabriek, B.O.; van Bruggen, R.; Deng, D.M.; Ligtenberg, A.J.; Nazmi, K.; Schornagel, K.; Vloet, R.P.; Dijkstra, C.D.; van den Berg, T.K. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood 2009, 113, 887–892. [Google Scholar] [CrossRef]
- Van Gorp, H.; Van Breedam, W.; Delputte, P.L.; Nauwynck, H.J. Sialoadhesin and CD163 join forces during entry of the porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2008, 89, 2943–2953. [Google Scholar] [CrossRef]
- Burkard, C.; Lillico, S.G.; Reid, E.; Jackson, B.; Mileham, A.J.; Ait-Ali, T.; Whitelaw, C.B.; Archibald, A.L. Precision engineering for PRRSV resistance in pigs: Macrophages from genome edited pigs lacking CD163 SRCR5 domain are fully resistant to both PRRSV genotypes while maintaining biological function. PLoS Pathog. 2017, 13, e1006206. [Google Scholar] [CrossRef]
- Whitworth, K.M.; Rowland, R.R.; Ewen, C.L.; Trible, B.R.; Kerrigan, M.A.; Cino-Ozuna, A.G.; Samuel, M.S.; Lightner, J.E.; McLaren, D.G.; Mileham, A.J.; et al. Gene-edited pigs are protected from porcine reproductive and respiratory syndrome virus. Nat. Biotechnol. 2016, 34, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, Y.; Luo, Y.; Zheng, J.; Gong, L.; Wang, H.; Feng, Y.; Gong, T.; Wu, D.; Wu, R.; et al. Adaptation of African swine fever virus to porcine kidney cells stably expressing CD163 and Siglec1. Front. Immunol. 2022, 13, 1015224. [Google Scholar] [CrossRef]
- Sanchez-Torres, C.; Gomez-Puertas, P.; Gomez-del-Moral, M.; Alonso, F.; Escribano, J.M.; Ezquerra, A.; Dominguez, J. Expression of porcine CD163 on monocytes/macrophages correlates with permissiveness to African swine fever infection. Arch. Virol. 2003, 148, 2307–2323. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.; Gaudreault, N.N.; Whitworth, K.M.; Murgia, M.V.; Nietfeld, J.C.; Mileham, A.; Samuel, M.; Wells, K.D.; Prather, R.S.; Rowland, R.R.R. Genetically edited pigs lacking CD163 show no resistance following infection with the African swine fever virus isolate, Georgia 2007/1. Virology 2017, 501, 102–106. [Google Scholar] [CrossRef]
- Etzerodt, A.; Maniecki, M.B.; Moller, K.; Moller, H.J.; Moestrup, S.K. Tumor necrosis factor alpha-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163. J. Leukoc. Biol. 2010, 88, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Niu, J.; Yu, H.; Gu, W.; Li, R.; Luo, X.; Huang, M.; Tian, Z.; Feng, L.; Wang, Y. Modulation of CD163 expression by metalloprotease ADAM17 regulates porcine reproductive and respiratory syndrome virus entry. J. Virol. 2014, 88, 10448–10458. [Google Scholar] [CrossRef] [PubMed]
- Etzerodt, A.; Moestrup, S.K. CD163 and inflammation: Biological, diagnostic, and therapeutic aspects. Antioxid. Redox Signal. 2013, 18, 2352–2363. [Google Scholar] [CrossRef] [PubMed]
- Hogger, P.; Sorg, C. Soluble CD163 inhibits phorbol ester-induced lymphocyte proliferation. Biochem. Biophys. Res. Commun. 2001, 288, 841–843. [Google Scholar] [CrossRef]
- Akahori, H.; Karmali, V.; Polavarapu, R.; Lyle, A.N.; Weiss, D.; Shin, E.; Husain, A.; Naqvi, N.; Van Dam, R.; Habib, A.; et al. CD163 interacts with TWEAK to regulate tissue regeneration after ischaemic injury. Nat. Commun. 2015, 6, 7792. [Google Scholar] [CrossRef] [PubMed]
- Cabezon, O.; Munoz-Gonzalez, S.; Colom-Cadena, A.; Perez-Simo, M.; Rosell, R.; Lavin, S.; Marco, I.; Fraile, L.; de la Riva, P.M.; Rodriguez, F.; et al. African swine fever virus infection in Classical swine fever subclinically infected wild boars. BMC Vet. Res. 2017, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, J.A.; MacPhee, D.J.; Harding, J.C.S. Development and application of a porcine specific ELISA for the quantification of soluble CD163. Vet. Immunol. Immunopathol. 2019, 210, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Costa-Hurtado, M.; Olvera, A.; Martinez-Moliner, V.; Galofre-Mila, N.; Martinez, P.; Dominguez, J.; Aragon, V. Changes in macrophage phenotype after infection of pigs with Haemophilus parasuis strains with different levels of virulence. Infect. Immun. 2013, 81, 2327–2333. [Google Scholar] [CrossRef]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef]
- Pillai, S.; Netravali, I.A.; Cariappa, A.; Mattoo, H. Siglecs and immune regulation. Annu. Rev. Immunol. 2012, 30, 357–392. [Google Scholar] [CrossRef]
- Angata, T. Molecular diversity and evolution of the Siglec family of cell-surface lectins. Mol. Divers. 2006, 10, 555–566. [Google Scholar] [CrossRef]
- Varki, A.; Angata, T. Siglecs—The major subfamily of I-type lectins. Glycobiology 2006, 16, 1R–27R. [Google Scholar] [CrossRef]
- Cao, H.; Crocker, P.R. Evolution of CD33-related siglecs: Regulating host immune functions and escaping pathogen exploitation? Immunology 2011, 132, 18–26. [Google Scholar] [CrossRef]
- Vanderheijden, N.; Delputte, P.L.; Favoreel, H.W.; Vandekerckhove, J.; Van Damme, J.; van Woensel, P.A.; Nauwynck, H.J. Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. J. Virol. 2003, 77, 8207–8215. [Google Scholar] [CrossRef]
- Xie, J.; Christiaens, I.; Yang, B.; Breedam, W.V.; Cui, T.; Nauwynck, H.J. Molecular cloning of porcine Siglec-3, Siglec-5 and Siglec-10, and identification of Siglec-10 as an alternative receptor for porcine reproductive and respiratory syndrome virus (PRRSV). J. Gen. Virol. 2017, 98, 2030–2042. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R.; Gordon, S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J. Exp. Med. 1989, 169, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Hartnell, A.; Steel, J.; Turley, H.; Jones, M.; Jackson, D.G.; Crocker, P.R. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood 2001, 97, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Delputte, P.L.; Van Breedam, W.; Barbe, F.; Van Reeth, K.; Nauwynck, H.J. IFN-alpha treatment enhances porcine Arterivirus infection of monocytes via upregulation of the porcine Arterivirus receptor sialoadhesin. J. Interferon Cytokine Res. 2007, 27, 757–766. [Google Scholar] [CrossRef]
- Sautter, C.A.; Auray, G.; Python, S.; Liniger, M.; Summerfield, A. Phenotypic and functional modulations of porcine macrophages by interferons and interleukin-4. Dev. Comp. Immunol. 2018, 84, 181–192. [Google Scholar] [CrossRef]
- Munday, J.; Floyd, H.; Crocker, P.R. Sialic acid binding receptors (siglecs) expressed by macrophages. J. Leukoc. Biol. 1999, 66, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Virji, M.; Crocker, P.R. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol. Microbiol. 2003, 49, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Kawasaki, N.; Nycholat, C.M.; Han, S.; Pilotte, J.; Crocker, P.R.; Paulson, J.C. Antigen delivery to macrophages using liposomal nanoparticles targeting sialoadhesin/CD169. PLoS ONE 2012, 7, e39039. [Google Scholar] [CrossRef]
- Van Dinther, D.; Veninga, H.; Revet, M.; Hoogterp, L.; Olesek, K.; Grabowska, J.; Borg, E.G.F.; Kalay, H.; van Kooyk, Y.; den Haan, J.M.M. Comparison of Protein and Peptide Targeting for the Development of a CD169-Based Vaccination Strategy against Melanoma. Front. Immunol. 2018, 9, 1997. [Google Scholar] [CrossRef]
- Delputte, P.L.; Van Gorp, H.; Favoreel, H.W.; Hoebeke, I.; Delrue, I.; Dewerchin, H.; Verdonck, F.; Verhasselt, B.; Cox, E.; Nauwynck, H.J. Porcine sialoadhesin (CD169/Siglec-1) is an endocytic receptor that allows targeted delivery of toxins and antigens to macrophages. PLoS ONE 2011, 6, e16827. [Google Scholar] [CrossRef]
- Poderoso, T.; Martinez, P.; Alvarez, B.; Handler, A.; Moreno, S.; Alonso, F.; Ezquerra, A.; Dominguez, J.; Revilla, C. Delivery of antigen to sialoadhesin or CD163 improves the specific immune response in pigs. Vaccine 2011, 29, 4813–4820. [Google Scholar] [CrossRef] [PubMed]
- Delputte, P.L.; Van Breedam, W.; Delrue, I.; Oetke, C.; Crocker, P.R.; Nauwynck, H.J. Porcine arterivirus attachment to the macrophage-specific receptor sialoadhesin is dependent on the sialic acid-binding activity of the N-terminal immunoglobulin domain of sialoadhesin. J. Virol. 2007, 81, 9546–9550. [Google Scholar] [CrossRef] [PubMed]
- Delputte, P.L.; Costers, S.; Nauwynck, H.J. Analysis of porcine reproductive and respiratory syndrome virus attachment and internalization: Distinctive roles for heparan sulphate and sialoadhesin. J. Gen. Virol. 2005, 86, 1441–1445. [Google Scholar] [CrossRef] [PubMed]
- Prather, R.S.; Rowland, R.R.; Ewen, C.; Trible, B.; Kerrigan, M.; Bawa, B.; Teson, J.M.; Mao, J.; Lee, K.; Samuel, M.S.; et al. An intact sialoadhesin (Sn/SIGLEC1/CD169) is not required for attachment/internalization of the porcine reproductive and respiratory syndrome virus. J. Virol. 2013, 87, 9538–9546. [Google Scholar] [CrossRef]
- De Baere, M.I.; Van Gorp, H.; Nauwynck, H.J.; Delputte, P.L. Antibody binding to porcine sialoadhesin reduces phagocytic capacity without affecting other macrophage effector functions. Cell Immunol. 2011, 271, 462–473. [Google Scholar] [CrossRef]
- De Baere, M.I.; Van Gorp, H.; Delputte, P.L.; Nauwynck, H.J. Interaction of the European genotype porcine reproductive and respiratory syndrome virus (PRRSV) with sialoadhesin (CD169/Siglec-1) inhibits alveolar macrophage phagocytosis. Vet. Res. 2012, 43, 47. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, R.; Qiao, S.; Chen, X.X.; Deng, R.; Zhang, G. Porcine sialoadhesin suppresses type I interferon production to support porcine reproductive and respiratory syndrome virus infection. Vet. Res. 2020, 51, 18. [Google Scholar] [CrossRef] [PubMed]
- Poderoso, T.; De la Riva, P.M.; Alvarez, B.; Nieto-Pelegrin, E.; Ezquerra, A.; Dominguez, J.; Revilla, C. Expression of Siglec-1, -3, -5 and -10 in porcine cDC1 and cDC2 subsets from blood, spleen and lymph nodes and functional capabilities of these cells. Dev. Comp. Immunol. 2020, 109, 103692. [Google Scholar] [CrossRef]
- Ando, M.; Tu, W.; Nishijima, K.; Iijima, S. Siglec-9 enhances IL-10 production in macrophages via tyrosine-based motifs. Biochem. Biophys. Res. Commun. 2008, 369, 878–883. [Google Scholar] [CrossRef]
- Carlin, A.F.; Lewis, A.L.; Varki, A.; Nizet, V. Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. J. Bacteriol. 2007, 189, 1231–1237. [Google Scholar] [CrossRef]
- Zou, Z.; Chastain, A.; Moir, S.; Ford, J.; Trandem, K.; Martinelli, E.; Cicala, C.; Crocker, P.; Arthos, J.; Sun, P.D. Siglecs facilitate HIV-1 infection of macrophages through adhesion with viral sialic acids. PLoS ONE 2011, 6, e24559. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Pan, Q.; Xiao, J.; Li, W.; Ma, H.; Chen, H.; Cai, X.; Xu, X. Sialidase of Glaesserella parasuis Augments Inflammatory Response via Desialylation and Abrogation of Negative Regulation of Siglec-5. Infect. Immun. 2021, 89, e00696-20. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.S.; Springer, T.A. Structure and function of leukocyte integrins. Immunol. Rev. 1990, 114, 181–217. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Madrid, F.; Corbi, A.L. Leukocyte integrins: Structure, function and regulation of their activity. Semin. Cell Biol. 1992, 3, 199–210. [Google Scholar] [CrossRef]
- Van der Vieren, M.; Le Trong, H.; Wood, C.L.; Moore, P.F.; St John, T.; Staunton, D.E.; Gallatin, W.M. A novel leukointegrin, alpha d beta 2, binds preferentially to ICAM-3. Immunity 1995, 3, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Sanz, G.; Jimenez-Marin, A.; Barbancho, M.; Garrido, J.J. Molecular cloning, characterization and gene expression of the full length cDNA encoding the porcine CD11b(alphaM) and chromosomal localization of the porcine CD11a(alphaL)-CD11b(alphaM)-CD11b(alphaD) gene cluster. Vet. Immunol. Immunopathol. 2012, 145, 505–510. [Google Scholar] [CrossRef]
- Bullido, R.; Alonso, F.; Gomez del Moral, M.; Ezquerra, A.; Alvarez, B.; Ortuno; Dominguez, J. Monoclonal antibody 2F4/11 recognizes the alpha chain of a porcine beta 2 integrin involved in adhesion and complement mediated phagocytosis. J. Immunol. Methods 1996, 195, 125–134. [Google Scholar] [CrossRef]
- Whittall, J.T.; Parkhouse, R.M. Monoclonal antibodies defining differentiation antigens of swine lymphoid and myeloid cells. Vet. Immunol. Immunopathol. 1997, 60, 149–160. [Google Scholar] [CrossRef]
- Baert, K.; Sonck, E.; Goddeeris, B.M.; Devriendt, B.; Cox, E. Cell type-specific differences in beta-glucan recognition and signalling in porcine innate immune cells. Dev. Comp. Immunol. 2015, 48, 192–203. [Google Scholar] [CrossRef]
- Uenishi, H.; Shinkai, H. Porcine Toll-like receptors: The front line of pathogen monitoring and possible implications for disease resistance. Dev. Comp. Immunol. 2009, 33, 353–361. [Google Scholar] [CrossRef]
- Jungi, T.W.; Farhat, K.; Burgener, I.A.; Werling, D. Toll-like receptors in domestic animals. Cell Tissue Res. 2011, 343, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Annu. Rev. Immunol. 2003, 21, 335–376. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Innate immunity to virus infection. Immunol. Rev. 2009, 227, 75–86. [Google Scholar] [CrossRef]
- Underhill, D.M.; Ozinsky, A. Toll-like receptors: Key mediators of microbe detection. Curr. Opin. Immunol. 2002, 14, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef]
- Lin, Y.T.; Chen, Y.P.; Fang, C.H.; Huang, P.Y.; Liang, S.M. Capsid proteins of foot-and-mouth disease virus interact with TLR2 and CD14 to induce cytokine production. Immunol. Lett. 2020, 223, 10–16. [Google Scholar] [CrossRef]
- Cao, Z.; Zheng, M.; Lv, H.; Guo, K.; Zhang, Y. Tissue expression of Toll-like receptors 2, 3, 4 and 7 in swine in response to the Shimen strain of classical swine fever virus. Mol. Med. Rep. 2018, 17, 7122–7130. [Google Scholar] [CrossRef]
- Kuzemtseva, L.; de la Torre, E.; Martin, G.; Soldevila, F.; Ait-Ali, T.; Mateu, E.; Darwich, L. Regulation of toll-like receptors 3, 7 and 9 in porcine alveolar macrophages by different genotype 1 strains of porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 2014, 158, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Kuzemtseva, L.; Perez, M.; Mateu, E.; Segales, J.; Darwich, L. Expression of Toll-like receptor 9 (TLR9) in the lungs and lymphoid tissue of pigs. Vet. J. 2015, 203, 259–261. [Google Scholar] [CrossRef]
- Muneta, Y.; Uenishi, H.; Kikuma, R.; Yoshihara, K.; Shimoji, Y.; Yamamoto, R.; Hamashima, N.; Yokomizo, Y.; Mori, Y. Porcine TLR2 and TLR6: Identification and their involvement in Mycoplasma hyopneumoniae infection. J. Interferon Cytokine Res. 2003, 23, 583–590. [Google Scholar] [CrossRef]
- Alvarez, B.; Martinez, P.; Yuste, M.; Poderoso, T.; Alonso, F.; Dominguez, J.; Ezquerra, A.; Revilla, C. Phenotypic and functional heterogeneity of CD169(+) and CD163(+) macrophages from porcine lymph nodes and spleen. Dev. Comp. Immunol. 2014, 44, 44–49. [Google Scholar] [CrossRef]
- Sancho, D.; Reis e Sousa, C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu. Rev. Immunol. 2012, 30, 491–529. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.; Gringhuis, S.I. C-type lectin receptors in the control of T helper cell differentiation. Nat. Rev. Immunol. 2016, 16, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Thalhammer, T.; Tzakos, A.G.; Stojanovska, L. Targeting antigens to dendritic cell receptors for vaccine development. J. Drug Deliv. 2013, 2013, 869718. [Google Scholar] [CrossRef] [PubMed]
- Zelensky, A.N.; Gready, J.E. The C-type lectin-like domain superfamily. FEBS J. 2005, 272, 6179–6217. [Google Scholar] [CrossRef]
- Brown, G.D.; Willment, J.A.; Whitehead, L. C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 2018, 18, 374–389. [Google Scholar] [CrossRef]
- Kerrigan, A.M.; Brown, G.D. Syk-coupled C-type lectins in immunity. Trends Immunol. 2011, 32, 151–156. [Google Scholar] [CrossRef]
- Redelinghuys, P.; Brown, G.D. Inhibitory C-type lectin receptors in myeloid cells. Immunol. Lett. 2011, 136, 1–12. [Google Scholar] [CrossRef]
- Del Fresno, C.; Iborra, S.; Saz-Leal, P.; Martinez-Lopez, M.; Sancho, D. Flexible Signaling of Myeloid C-Type Lectin Receptors in Immunity and Inflammation. Front. Immunol. 2018, 9, 804. [Google Scholar] [CrossRef]
- Huang, Y.W.; Dryman, B.A.; Li, W.; Meng, X.J. Porcine DC-SIGN: Molecular cloning, gene structure, tissue distribution and binding characteristics. Dev. Comp. Immunol. 2009, 33, 464–480. [Google Scholar] [CrossRef] [PubMed]
- Sonck, E.; Stuyven, E.; Goddeeris, B.; Cox, E. Identification of the porcine C-type lectin dectin-1. Vet. Immunol. Immunopathol. 2009, 130, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Tacken, P.J.; de Vries, I.J.; Torensma, R.; Figdor, C.G. Dendritic-cell immunotherapy: From ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 2007, 7, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mendoza, L.; Sotelo-Mundo, R.R.; Dawson, H.; Mwangi, W.; Hernandez, J. Characterization of porcine CD205. Dev. Comp. Immunol. 2010, 34, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mendoza, L.; Velazquez, C.; Bray, J.; Njongmeta, L.; Mwangi, W.; Hernandez, J. Development and characterization of a monoclonal antibody against porcine CD205. Vet. Immunol. Immunopathol. 2012, 146, 74–80. [Google Scholar] [CrossRef]
- Auray, G.; Keller, I.; Python, S.; Gerber, M.; Bruggmann, R.; Ruggli, N.; Summerfield, A. Characterization and Transcriptomic Analysis of Porcine Blood Conventional and Plasmacytoid Dendritic Cells Reveals Striking Species-Specific Differences. J. Immunol. 2016, 197, 4791–4806. [Google Scholar] [CrossRef]
- Kato, M.; McDonald, K.J.; Khan, S.; Ross, I.L.; Vuckovic, S.; Chen, K.; Munster, D.; MacDonald, K.P.; Hart, D.N. Expression of human DEC-205 (CD205) multilectin receptor on leukocytes. Int. Immunol. 2006, 18, 857–869. [Google Scholar] [CrossRef]
- Gazi, U.; Martinez-Pomares, L. Influence of the mannose receptor in host immune responses. Immunobiology 2009, 214, 554–561. [Google Scholar] [CrossRef]
- Marquet, F.; Vu Manh, T.P.; Maisonnasse, P.; Elhmouzi-Younes, J.; Urien, C.; Bouguyon, E.; Jouneau, L.; Bourge, M.; Simon, G.; Ezquerra, A.; et al. Pig skin includes dendritic cell subsets transcriptomically related to human CD1a and CD14 dendritic cells presenting different migrating behaviors and T cell activation capacities. J. Immunol. 2014, 193, 5883–5893. [Google Scholar] [CrossRef]
- Maisonnasse, P.; Bouguyon, E.; Piton, G.; Ezquerra, A.; Urien, C.; Deloizy, C.; Bourge, M.; Leplat, J.J.; Simon, G.; Chevalier, C.; et al. The respiratory DC/macrophage network at steady-state and upon influenza infection in the swine biomedical model. Mucosal Immunol. 2016, 9, 835–849. [Google Scholar] [CrossRef]
- Brown, G.D. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.R.; Tsoni, S.V.; Willment, J.A.; Dennehy, K.M.; Rosas, M.; Findon, H.; Haynes, K.; Steele, C.; Botto, M.; Gordon, S.; et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 2007, 8, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Lahoud, M.H.; Proietto, A.I.; Ahmet, F.; Kitsoulis, S.; Eidsmo, L.; Wu, L.; Sathe, P.; Pietersz, S.; Chang, H.W.; Walker, I.D.; et al. The C-type lectin Clec12A present on mouse and human dendritic cells can serve as a target for antigen delivery and enhancement of antibody responses. J. Immunol. 2009, 182, 7587–7594. [Google Scholar] [CrossRef] [PubMed]
- Hutten, T.J.; Thordardottir, S.; Fredrix, H.; Janssen, L.; Woestenenk, R.; Tel, J.; Joosten, B.; Cambi, A.; Heemskerk, M.H.; Franssen, G.M.; et al. CLEC12A-Mediated Antigen Uptake and Cross-Presentation by Human Dendritic Cell Subsets Efficiently Boost Tumor-Reactive T Cell Responses. J. Immunol. 2016, 197, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Huysamen, C.; Brown, G.D. The fungal pattern recognition receptor, Dectin-1, and the associated cluster of C-type lectin-like receptors. FEMS Microbiol. Lett. 2009, 290, 121–128. [Google Scholar] [CrossRef]
- Fukuda, M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J. Biol. Chem. 1991, 266, 21327–21330. [Google Scholar] [CrossRef]
- Febbraio, M.; Silverstein, R.L. Identification and characterization of LAMP-1 as an activation-dependent platelet surface glycoprotein. J. Biol. Chem. 1990, 265, 18531–18537. [Google Scholar] [CrossRef]
- Zhang, L.; Lun, Y.; Yan, D.; Yu, L.; Ma, W.; Du, B.; Zhu, X. Proteomic analysis of macrophages: A new way to identify novel cell-surface antigens. J. Immunol. Methods 2007, 321, 80–85. [Google Scholar] [CrossRef]
- Betts, M.R.; Brenchley, J.M.; Price, D.A.; De Rosa, S.C.; Douek, D.C.; Roederer, M.; Koup, R.A. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 2003, 281, 65–78. [Google Scholar] [CrossRef]
- Alter, G.; Malenfant, J.M.; Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 2004, 294, 15–22. [Google Scholar] [CrossRef]
- Eskelinen, E.L.; Tanaka, Y.; Saftig, P. At the acidic edge: Emerging functions for lysosomal membrane proteins. Trends Cell Biol. 2003, 13, 137–145. [Google Scholar] [CrossRef]
- Chen, J.W.; Cha, Y.; Yuksel, K.U.; Gracy, R.W.; August, J.T. Isolation and sequencing of a cDNA clone encoding lysosomal membrane glycoprotein mouse LAMP-1. Sequence similarity to proteins bearing onco-differentiation antigens. J. Biol. Chem. 1988, 263, 8754–8758. [Google Scholar] [CrossRef] [PubMed]
- Domenech, N.; Rodriguez-Carreno, M.P.; Filgueira, P.; Alvarez, B.; Chamorro, S.; Dominguez, J. Identification of porcine macrophages with monoclonal antibodies in formalin-fixed, paraffin-embedded tissues. Vet. Immunol. Immunopathol. 2003, 94, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. Colony-stimulating factor-1 receptor. Blood 1990, 75, 1–12. [Google Scholar] [CrossRef]
- Hume, D.A.; MacDonald, K.P. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 2012, 119, 1810–1820. [Google Scholar] [CrossRef]
- Sasmono, R.T.; Ehrnsperger, A.; Cronau, S.L.; Ravasi, T.; Kandane, R.; Hickey, M.J.; Cook, A.D.; Himes, S.R.; Hamilton, J.A.; Hume, D.A. Mouse neutrophilic granulocytes express mRNA encoding the macrophage colony-stimulating factor receptor (CSF-1R) as well as many other macrophage-specific transcripts and can transdifferentiate into macrophages in vitro in response to CSF-1. J. Leukoc. Biol. 2007, 82, 111–123. [Google Scholar] [CrossRef]
- Gow, D.J.; Garceau, V.; Kapetanovic, R.; Sester, D.P.; Fici, G.J.; Shelly, J.A.; Wilson, T.L.; Hume, D.A. Cloning and expression of porcine Colony Stimulating Factor-1 (CSF-1) and Colony Stimulating Factor-1 Receptor (CSF-1R) and analysis of the species specificity of stimulation by CSF-1 and Interleukin 34. Cytokine 2012, 60, 793–805. [Google Scholar] [CrossRef]
- Wright, G.J.; Cherwinski, H.; Foster-Cuevas, M.; Brooke, G.; Puklavec, M.J.; Bigler, M.; Song, Y.; Jenmalm, M.; Gorman, D.; McClanahan, T.; et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J. Immunol. 2003, 171, 3034–3046. [Google Scholar] [CrossRef] [PubMed]
- Mihrshahi, R.; Barclay, A.N.; Brown, M.H. Essential roles for Dok2 and RasGAP in CD200 receptor-mediated regulation of human myeloid cells. J. Immunol. 2009, 183, 4879–4886. [Google Scholar] [CrossRef] [PubMed]
- Mihrshahi, R.; Brown, M.H. Downstream of tyrosine kinase 1 and 2 play opposing roles in CD200 receptor signaling. J. Immunol. 2010, 185, 7216–7222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cherwinski, H.; Sedgwick, J.D.; Phillips, J.H. Molecular mechanisms of CD200 inhibition of mast cell activation. J. Immunol. 2004, 173, 6786–6793. [Google Scholar] [CrossRef] [PubMed]
- Voehringer, D.; Rosen, D.B.; Lanier, L.L.; Locksley, R.M. CD200 receptor family members represent novel DAP12-associated activating receptors on basophils and mast cells. J. Biol. Chem. 2004, 279, 54117–54123. [Google Scholar] [CrossRef] [PubMed]
- Gorczynski, R.; Chen, Z.; Kai, Y.; Lee, L.; Wong, S.; Marsden, P.A. CD200 is a ligand for all members of the CD200R family of immunoregulatory molecules. J. Immunol. 2004, 172, 7744–7749. [Google Scholar] [CrossRef] [PubMed]
- Hatherley, D.; Cherwinski, H.M.; Moshref, M.; Barclay, A.N. Recombinant CD200 protein does not bind activating proteins closely related to CD200 receptor. J. Immunol. 2005, 175, 2469–2474. [Google Scholar] [CrossRef] [PubMed]
- Rijkers, E.S.; de Ruiter, T.; Baridi, A.; Veninga, H.; Hoek, R.M.; Meyaard, L. The inhibitory CD200R is differentially expressed on human and mouse T and B lymphocytes. Mol. Immunol. 2008, 45, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Poderoso, T.; De la Riva, P.M.; Alvarez, B.; Dominguez, J.; Ezquerra, A.; Revilla, C. CD200R family receptors are expressed on porcine monocytes and modulate the production of IL-8 and TNF-alpha triggered by TLR4 or TLR7 in these cells. Mol. Immunol. 2022, 144, 166–177. [Google Scholar] [CrossRef]
- Copland, D.A.; Calder, C.J.; Raveney, B.J.; Nicholson, L.B.; Phillips, J.; Cherwinski, H.; Jenmalm, M.; Sedgwick, J.D.; Dick, A.D. Monoclonal antibody-mediated CD200 receptor signaling suppresses macrophage activation and tissue damage in experimental autoimmune uveoretinitis. Am. J. Pathol. 2007, 171, 580–588. [Google Scholar] [CrossRef]
- Jenmalm, M.C.; Cherwinski, H.; Bowman, E.P.; Phillips, J.H.; Sedgwick, J.D. Regulation of myeloid cell function through the CD200 receptor. J. Immunol. 2006, 176, 191–199. [Google Scholar] [CrossRef]
- Snelgrove, R.J.; Goulding, J.; Didierlaurent, A.M.; Lyonga, D.; Vekaria, S.; Edwards, L.; Gwyer, E.; Sedgwick, J.D.; Barclay, A.N.; Hussell, T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat. Immunol. 2008, 9, 1074–1083. [Google Scholar] [CrossRef]
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef]
- Koning, N.; van Eijk, M.; Pouwels, W.; Brouwer, M.S.; Voehringer, D.; Huitinga, I.; Hoek, R.M.; Raes, G.; Hamann, J. Expression of the inhibitory CD200 receptor is associated with alternative macrophage activation. J. Innate Immun. 2010, 2, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ambarus, C.A.; Krausz, S.; van Eijk, M.; Hamann, J.; Radstake, T.R.; Reedquist, K.A.; Tak, P.P.; Baeten, D.L. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J. Immunol. Methods 2012, 375, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, J.; Ezquerra, A.; Alonso, F.; Bullido, R.; McCullough, K.; Summerfield, A.; Bianchi, A.; Zwart, R.J.; Kim, Y.B.; Blecha, F.; et al. Workshop studies with monoclonal antibodies identifying a novel porcine differentiation antigen, SWC9. Vet. Immunol. Immunopathol. 1998, 60, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.B.; Nygard, A.B.; Viuff, B.; Fredholm, M.; Aasted, B.; Salomonsen, J. Porcine ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1/CD203a): Cloning, transcription, expression, mapping, and identification of an NPP1/CD203a epitope for swine workshop cluster 9 (SWC9) monoclonal antibodies. Dev. Comp. Immunol. 2007, 31, 618–631. [Google Scholar] [CrossRef] [PubMed]
- McCullough, K.C.; Schaffner, R.; Natale, V.; Kim, Y.B.; Summerfield, A. Phenotype of porcine monocytic cells: Modulation of surface molecule expression upon monocyte differentiation into macrophages. Vet. Immunol. Immunopathol. 1997, 58, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Ondrackova, P.; Nechvatalova, K.; Kucerova, Z.; Leva, L.; Dominguez, J.; Faldyna, M. Porcine mononuclear phagocyte subpopulations in the lung, blood and bone marrow: Dynamics during inflammation induced by Actinobacillus pleuropneumoniae. Vet. Res. 2010, 41, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, S.F.; Wan, B.; Ming, S.L.; Li, G.L.; Su, B.Q.; Liu, J.Y.; Wei, Y.S.; Yang, G.Y.; Chu, B.B. Maintenance of cyclic GMP-AMP homeostasis by ENPP1 is involved in pseudorabies virus infection. Mol. Immunol. 2018, 95, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Linden, J.; Koch-Nolte, F.; Dahl, G. Purine Release, Metabolism, and Signaling in the Inflammatory Response. Annu. Rev. Immunol. 2019, 37, 325–347. [Google Scholar] [CrossRef]
- Onyedibe, K.I.; Wang, M.; Sintim, H.O. ENPP1, an Old Enzyme with New Functions, and Small Molecule Inhibitors-A STING in the Tale of ENPP1. Molecules 2019, 24, 4192. [Google Scholar] [CrossRef]
- Trautmann, A. Extracellular ATP in the immune system: More than just a “danger signal”. Sci. Signal. 2009, 2, pe6. [Google Scholar] [CrossRef]
- Gordon, S.; Lawson, L.; Rabinowitz, S.; Crocker, P.R.; Morris, L.; Perry, V.H. Antigen markers of macrophage differentiation in murine tissues. Curr. Top. Microbiol. Immunol. 1992, 181, 1–37. [Google Scholar] [CrossRef]
- Gautier, E.L.; Shay, T.; Miller, J.; Greter, M.; Jakubzick, C.; Ivanov, S.; Helft, J.; Chow, A.; Elpek, K.G.; Gordonov, S.; et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012, 13, 1118–1128. [Google Scholar] [CrossRef]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Hettinger, J.; Richards, D.M.; Hansson, J.; Barra, M.M.; Joschko, A.C.; Krijgsveld, J.; Feuerer, M. Origin of monocytes and macrophages in a committed progenitor. Nat. Immunol. 2013, 14, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Yona, S.; Kim, K.W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and tissue specialization. Annu. Rev. Immunol. 2015, 33, 643–675. [Google Scholar] [CrossRef]
- Rehakova, Z.; Trebichavsky, I.; Sinkora, J.; Splichal, I.; Sinkora, M. Early ontogeny of monocytes and macrophages in the pig. Physiol. Res. 1998, 47, 357–363. [Google Scholar]
- Bordet, E.; Maisonnasse, P.; Renson, P.; Bouguyon, E.; Crisci, E.; Tiret, M.; Descamps, D.; Bernelin-Cottet, C.; Urien, C.; Lefevre, F.; et al. Porcine Alveolar Macrophage-like cells are pro-inflammatory Pulmonary Intravascular Macrophages that produce large titers of Porcine Reproductive and Respiratory Syndrome Virus. Sci. Rep. 2018, 8, 10172. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Gratchev, A.; Kzhyshkowska, J.; Kothe, K.; Muller-Molinet, I.; Kannookadan, S.; Utikal, J.; Goerdt, S. Mphi1 and Mphi2 can be re-polarized by Th2 or Th1 cytokines, respectively, and respond to exogenous danger signals. Immunobiology 2006, 211, 473–486. [Google Scholar] [CrossRef]
- Porcheray, F.; Viaud, S.; Rimaniol, A.C.; Leone, C.; Samah, B.; Dereuddre-Bosquet, N.; Dormont, D.; Gras, G. Macrophage activation switching: An asset for the resolution of inflammation. Clin. Exp. Immunol. 2005, 142, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.D.; Jiang, C.; Matta, B.; Tietzel, I.; Watkins, S.K.; Suttles, J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 2005, 175, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Roszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Locati, M. Macrophage polarization comes of age. Immunity 2005, 23, 344–346. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003, 73, 209–212. [Google Scholar] [CrossRef]
- Edwards, J.P.; Zhang, X.; Frauwirth, K.A.; Mosser, D.M. Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 2006, 80, 1298–1307. [Google Scholar] [CrossRef]
- Yu, S.; Ge, H.; Li, S.; Qiu, H.J. Modulation of Macrophage Polarization by Viruses: Turning Off/On Host Antiviral Responses. Front. Microbiol. 2022, 13, 839585. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef]
- Ghassabeh, G.H.; De Baetselier, P.; Brys, L.; Noel, W.; Van Ginderachter, J.A.; Meerschaut, S.; Beschin, A.; Brombacher, F.; Raes, G. Identification of a common gene signature for type II cytokine-associated myeloid cells elicited in vivo in different pathologic conditions. Blood 2006, 108, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Raes, G.; Van den Bergh, R.; De Baetselier, P.; Ghassabeh, G.H.; Scotton, C.; Locati, M.; Mantovani, A.; Sozzani, S. Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J. Immunol. 2005, 174, 6561–6562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.O.; Laubach, V.E.; Alley, E.W.; Edwards, K.A.; Sherman, P.A.; Russell, S.W.; Murphy, W.J. Transcriptional basis for hyporesponsiveness of the human inducible nitric oxide synthase gene to lipopolysaccharide/interferon-gamma. J. Leukocyte Biol. 1996, 59, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Schneemann, M.; Schoeden, G. Macrophage biology and immunology: Man is not a mouse. J. Leukoc. Biol. 2007, 81, 579; discussion 580. [Google Scholar] [CrossRef] [PubMed]
- Carta, T.; Razzuoli, E.; Fruscione, F.; Zinellu, S.; Meloni, D.; Anfossi, A.; Chessa, B.; Dei Giudici, S.; Graham, S.P.; Oggiano, A.; et al. Comparative Phenotypic and Functional Analyses of the Effects of IL-10 or TGF-beta on Porcine Macrophages. Animals 2021, 11, 98. [Google Scholar] [CrossRef] [PubMed]
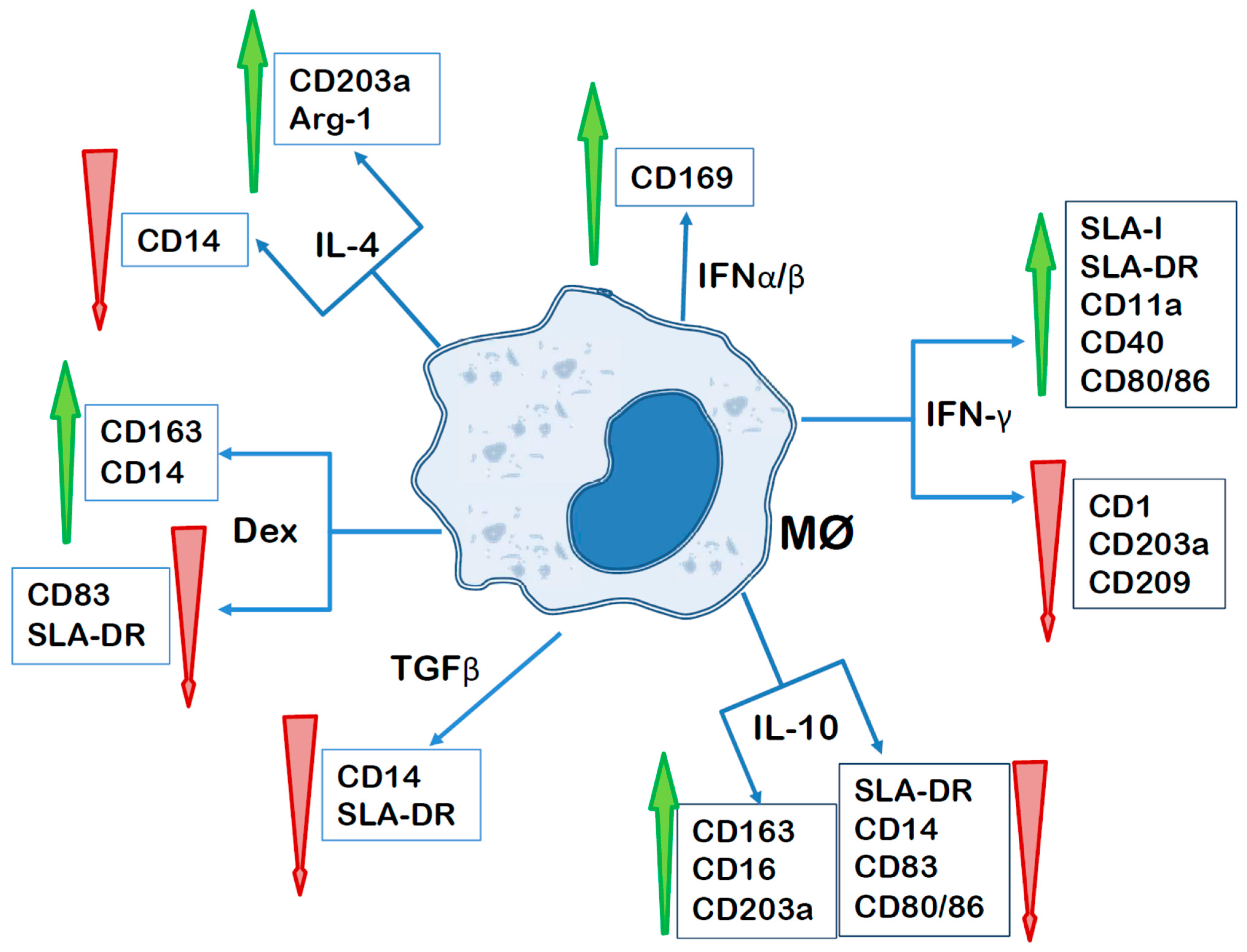
- Franzoni, G.; Mura, L.; Razzuoli, E.; De Ciucis, C.G.; Fruscione, F.; Dell’Anno, F.; Zinellu, S.; Carta, T.; Anfossi, A.G.; Dei Giudici, S.; et al. Heterogeneity of Phenotypic and Functional Changes to Porcine Monocyte-Derived Macrophages Triggered by Diverse Polarizing Factors In Vitro. Int. J. Mol. Sci. 2023, 24, 4671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.N.; Li, Y.N.; Wang, Y.T. Interleukin-4 regulates macrophage polarization via the MAPK signaling pathway to protect against atherosclerosis. Genet. Mol. Res. 2016, 15, 7348. [Google Scholar] [CrossRef]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef]
- Zelnickova, P.; Matiasovic, J.; Pavlova, B.; Kudlackova, H.; Kovaru, F.; Faldyna, M. Quantitative nitric oxide production by rat, bovine and porcine macrophages. Nitric Oxide 2008, 19, 36–41. [Google Scholar] [CrossRef]
- Kapetanovic, R.; Fairbairn, L.; Beraldi, D.; Sester, D.P.; Archibald, A.L.; Tuggle, C.K.; Hume, D.A. Pig bone marrow-derived macrophages resemble human macrophages in their response to bacterial lipopolysaccharide. J. Immunol. 2012, 188, 3382–3394. [Google Scholar] [CrossRef]
- Kim, B.Y.; Son, Y.; Lee, J.; Choi, J.; Kim, C.D.; Bae, S.S.; Eo, S.K.; Kim, K. Dexamethasone inhibits activation of monocytes/macrophages in a milieu rich in 27-oxygenated cholesterol. PLoS ONE 2017, 12, e0189643. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Bordet, E.; Fretaud, M.; Crisci, E.; Bouguyon, E.; Rault, S.; Pezant, J.; Pleau, A.; Renson, P.; Giuffra, E.; Larcher, T.; et al. Macrophage-B Cell Interactions in the Inverted Porcine Lymph Node and Their Response to Porcine Reproductive and Respiratory Syndrome Virus. Front. Immunol. 2019, 10, 953. [Google Scholar] [CrossRef] [PubMed]
- Dubreil, L.; Ledevin, M.; Hervet, C.; Menard, D.; Philippe, C.; Michel, F.J.; Larcher, T.; Meurens, F.; Bertho, N. The Internal Conduit System of the Swine Inverted Lymph Node. Front. Immunol. 2022, 13, 869384. [Google Scholar] [CrossRef] [PubMed]
- Soldevila, F.; Edwards, J.C.; Graham, S.P.; Stevens, L.M.; Crudgington, B.; Crooke, H.R.; Werling, D.; Steinbach, F. Characterization of the Myeloid Cell Populations’ Resident in the Porcine Palatine Tonsil. Front. Immunol. 2018, 9, 1800. [Google Scholar] [CrossRef]
- Lopez-Robles, G.; Silva-Campa, E.; Burgara-Estrella, A.; Hernandez, J. Characterization of antigen-presenting cells from the porcine respiratory system. Res. Vet. Sci. 2015, 100, 80–87. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, Y.W.; Lee, K.J.; Kim, H.S.; Cho, S.W.; van Rooijen, N.; Guan, Y.; Seo, S.H. Alveolar macrophages are indispensable for controlling influenza viruses in lungs of pigs. J. Virol. 2008, 82, 4265–4274. [Google Scholar] [CrossRef] [PubMed]
- Thanawongnuwech, R.; Thacker, E.L.; Halbur, P.G. Effect of porcine reproductive and respiratory syndrome virus (PRRSV) (isolate ATCC VR-2385) infection on bactericidal activity of porcine pulmonary intravascular macrophages (PIMs): In vitro comparisons with pulmonary alveolar macrophages (PAMs). Vet. Immunol. Immunopathol. 1997, 59, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Chitko-McKown, C.G.; Chapes, S.K.; Brown, R.E.; Phillips, R.M.; McKown, R.D.; Blecha, F. Porcine alveolar and pulmonary intravascular macrophages: Comparison of immune functions. J. Leukoc. Biol. 1991, 50, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Frydas, I.S.; Verbeeck, M.; Cao, J.; Nauwynck, H.J. Replication characteristics of porcine reproductive and respiratory syndrome virus (PRRSV) European subtype 1 (Lelystad) and subtype 3 (Lena) strains in nasal mucosa and cells of the monocytic lineage: Indications for the use of new receptors of PRRSV (Lena). Vet. Res. 2013, 44, 73. [Google Scholar] [CrossRef]
- Oh, D.; Xie, J.; Vanderheijden, N.; Nauwynck, H.J. Isolation and characterization of a new population of nasal surface macrophages and their susceptibility to PRRSV-1 subtype 1 (LV) and subtype 3 (Lena). Vet. Res. 2020, 51, 21. [Google Scholar] [CrossRef]
- Karniychuk, U.U.; Nauwynck, H.J. Quantitative changes of sialoadhesin and CD163 positive macrophages in the implantation sites and organs of porcine embryos/fetuses during gestation. Placenta 2009, 30, 497–500. [Google Scholar] [CrossRef]
- Novakovic, P.; Harding, J.C.; Ladinig, A.; Al-Dissi, A.N.; MacPhee, D.J.; Detmer, S.E. Relationships of CD163 and CD169 positive cell numbers in the endometrium and fetal placenta with type 2 PRRSV RNA concentration in fetal thymus. Vet. Res. 2016, 47, 76. [Google Scholar] [CrossRef]
- Noelia, A.-G.; Castrillo, A. Origin and specialization of splenic macrophages. Cell Immunol. 2018, 330, 151–158. [Google Scholar] [CrossRef]
- Binns, R.M. Organisation of the lymphoreticular system and lymphocyte markers in the pig. Vet. Immunol. Immunopathol. 1982, 3, 95–146. [Google Scholar] [CrossRef]
- Pabst, R. The pig as a model for immunology research. Cell Tissue Res. 2020, 380, 287–304. [Google Scholar] [CrossRef]
- Bimczok, D.; Post, A.; Tschernig, T.; Rothkotter, H.J. Phenotype and distribution of dendritic cells in the porcine small intestinal and tracheal mucosa and their spatial relationship to epithelial cells. Cell Tissue Res. 2006, 325, 461–468. [Google Scholar] [CrossRef]
- Berney, C.; Herren, S.; Power, C.A.; Gordon, S.; Martinez-Pomares, L.; Kosco-Vilbois, M.H. A member of the dendritic cell family that enters B cell follicles and stimulates primary antibody responses identified by a mannose receptor fusion protein. J. Exp. Med. 1999, 190, 851–860. [Google Scholar] [CrossRef]
- Mohr, E.; Serre, K.; Manz, R.A.; Cunningham, A.F.; Khan, M.; Hardie, D.L.; Bird, R.; MacLennan, I.C. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J. Immunol. 2009, 182, 2113–2123. [Google Scholar] [CrossRef]
- Gray, E.E.; Cyster, J.G. Lymph node macrophages. J. Innate Immun. 2012, 4, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Carvajal, J.M.; Rodriguez-Gomez, I.M.; Carrasco, L.; Barranco, I.; Alvarez, B.; Dominguez, J.; Salguero, F.J.; Gomez-Laguna, J. Kinetics of the expression of CD163 and CD107a in the lung and tonsil of pigs after infection with PRRSV-1 strains of different virulence. Vet. Res. Commun. 2019, 43, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.J.; Barnes, M.; Tang, H.; Pritchard, M.T.; Nagy, L.E. Kupffer cells in the liver. Compr. Physiol. 2013, 3, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Chihara, R.K.; Paris, L.L.; Reyes, L.M.; Sidner, R.A.; Estrada, J.L.; Downey, S.M.; Wang, Z.Y.; Tector, A.J.; Burlak, C. Primary porcine Kupffer cell phagocytosis of human platelets involves the CD18 receptor. Transplantation 2011, 92, 739–744. [Google Scholar] [CrossRef]
- Cross-Najafi, A.A.; Lopez, K.; Isidan, A.; Park, Y.; Zhang, W.; Li, P.; Yilmaz, S.; Akbulut, S.; Ekser, B. Current Barriers to Clinical Liver Xenotransplantation. Front. Immunol. 2022, 13, 827535. [Google Scholar] [CrossRef] [PubMed]
- Brock, L.G.; Delputte, P.L.; Waldman, J.P.; Nauwynck, H.J.; Rees, M.A. Porcine sialoadhesin: A newly identified xenogeneic innate immune receptor. Am. J. Transplant. 2012, 12, 3272–3282. [Google Scholar] [CrossRef]
- Waldman, J.P.; Vogel, T.; Burlak, C.; Coussios, C.; Dominguez, J.; Friend, P.; Rees, M.A. Blocking porcine sialoadhesin improves extracorporeal porcine liver xenoperfusion with human blood. Xenotransplantation 2013, 20, 239–251. [Google Scholar] [CrossRef]
- Carrascosa, A.L.; Santaren, J.F.; Vinuela, E. Production and titration of African swine fever virus in porcine alveolar macrophages. J. Virol. Methods 1982, 3, 303–310. [Google Scholar] [CrossRef]
- Huang, H.; Potter, A.A.; Campos, M.; Leighton, F.A.; Willson, P.J.; Haines, D.M.; Yates, W.D. Pathogenesis of porcine Actinobacillus pleuropneumonia, part II: Roles of proinflammatory cytokines. Can. J. Vet. Res. 1999, 63, 69–78. [Google Scholar] [PubMed]
- Duan, X.; Nauwynck, H.J.; Pensaert, M.B. Effects of origin and state of differentiation and activation of monocytes/macrophages on their susceptibility to porcine reproductive and respiratory syndrome virus (PRRSV). Arch. Virol. 1997, 142, 2483–2497. [Google Scholar] [CrossRef]
- Maisonnasse, P.; Bordet, E.; Bouguyon, E.; Bertho, N. Broncho Alveolar Dendritic Cells and Macrophages Are Highly Similar to Their Interstitial Counterparts. PLoS ONE 2016, 11, e0167315. [Google Scholar] [CrossRef] [PubMed]
- Chitko-McKown, C.G.; Blecha, F. Pulmonary intravascular macrophages: A review of immune properties and functions. Ann. Rech. Vet. 1992, 23, 201–214. [Google Scholar] [PubMed]
- Nunez, A.; Sanchez-Cordon, P.J.; Pedrera, M.; Gomez-Villamandos, J.C.; Carrasco, L. Pulmonary intravascular macrophages regulate the pathogenetic mechanisms of pulmonary lesions during acute courses of classical swine fever. Transbound. Emerg. Dis. 2018, 65, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, L.; Nunez, A.; Salguero, F.J.; Diaz San Segundo, F.; Sanchez-Cordon, P.; Gomez-Villamandos, J.C.; Sierra, M.A. African swine fever: Expression of interleukin-1 alpha and tumour necrosis factor-alpha by pulmonary intravascular macrophages. J. Comp. Pathol. 2002, 126, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Zaba, L.C.; Fuentes-Duculan, J.; Steinman, R.M.; Krueger, J.G.; Lowes, M.A. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J. Clin. Investig. 2007, 117, 2517–2525. [Google Scholar] [CrossRef]
- Karniychuk, U.U.; Nauwynck, H.J. Pathogenesis and prevention of placental and transplacental porcine reproductive and respiratory syndrome virus infection. Vet. Res. 2013, 44, 95. [Google Scholar] [CrossRef]
- Weingartl, H.M.; Sabara, M.; Pasick, J.; van Moorlehem, E.; Babiuk, L. Continuous porcine cell lines developed from alveolar macrophages: Partial characterization and virus susceptibility. J. Virol. Methods 2002, 104, 203–216. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, C.K.; Nam, E.; Kim, S.H.; Lee, O.S.; Lee Du, S.; Lee, C. Generation of a porcine alveolar macrophage cell line for the growth of porcine reproductive and respiratory syndrome virus. J. Virol. Methods 2010, 163, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, G.; Wang, N.; Liu, J.; Cai, Y.; Ren, M.; Li, Z. A simple and efficient method for the generation of a porcine alveolar macrophage cell line for high-efficiency Porcine reproductive and respiratory syndrome virus 2 infection. J. Virol. Methods 2019, 274, 113727. [Google Scholar] [CrossRef] [PubMed]
- de Leon, P.; Bustos, M.J.; Carrascosa, A.L. Laboratory methods to study African swine fever virus. Virus Res. 2013, 173, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Calzada-Nova, G.; Husmann, R.J.; Schnitzlein, W.M.; Zuckermann, F.A. Effect of the host cell line on the vaccine efficacy of an attenuated porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 2012, 148, 116–125. [Google Scholar] [CrossRef]
- Portugal, R.; Goatley, L.C.; Husmann, R.; Zuckermann, F.A.; Dixon, L.K. A porcine macrophage cell line that supports high levels of replication of OURT88/3, an attenuated strain of African swine fever virus. Emerg. Microbes Infect. 2020, 9, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Sagong, M.; Park, C.K.; Kim, S.H.; Lee, K.K.; Lee, O.S.; Lee, D.S.; Cha, S.Y.; Lee, C. Human telomerase reverse transcriptase-immortalized porcine monomyeloid cell lines for the production of porcine reproductive and respiratory syndrome virus. J. Virol. Methods 2012, 179, 26–32. [Google Scholar] [CrossRef]
- Takenouchi, T.; Kitani, H.; Suzuki, S.; Nakai, M.; Fuchimoto, D.I.; Tsukimoto, M.; Shinkai, H.; Sato, M.; Uenishi, H. Immortalization and Characterization of Porcine Macrophages That Had Been Transduced with Lentiviral Vectors Encoding the SV40 Large T Antigen and Porcine Telomerase Reverse Transcriptase. Front. Vet. Sci. 2017, 4, 132. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, T.; Masujin, K.; Miyazaki, A.; Suzuki, S.; Takagi, M.; Kokuho, T.; Uenishi, H. Isolation and immortalization of macrophages derived from fetal porcine small intestine and their susceptibility to porcine viral pathogen infections. Front. Vet. Sci. 2022, 9, 919077. [Google Scholar] [CrossRef]
- Takenouchi, T.; Masujin, K.; Suzuki, S.; Haraguchi, S.; Hiramatsu, K.; Kokuho, T.; Uenishi, H. Establishment and characterization of the immortalized porcine lung-derived mononuclear phagocyte cell line. Front. Vet. Sci. 2022, 9, 1058124. [Google Scholar] [CrossRef] [PubMed]
- Masujin, K.; Kitamura, T.; Kameyama, K.; Okadera, K.; Nishi, T.; Takenouchi, T.; Kitani, H.; Kokuho, T. An immortalized porcine macrophage cell line competent for the isolation of African swine fever virus. Sci. Rep. 2021, 11, 4759. [Google Scholar] [CrossRef]
- Kameyama, K.I.; Kitamura, T.; Okadera, K.; Ikezawa, M.; Masujin, K.; Kokuho, T. Usability of Immortalized Porcine Kidney Macrophage Cultures for the Isolation of ASFV without Affecting Virulence. Viruses 2022, 14, 1794. [Google Scholar] [CrossRef] [PubMed]
- Meek, S.; Watson, T.; Eory, L.; McFarlane, G.; Wynne, F.J.; McCleary, S.; Dunn, L.E.M.; Charlton, E.M.; Craig, C.; Shih, B.; et al. Stem cell-derived porcine macrophages as a new platform for studying host-pathogen interactions. BMC Biol. 2022, 20, 14. [Google Scholar] [CrossRef] [PubMed]
| Specificity | Clone Name | Reference | Supplier |
|---|---|---|---|
| CD11b | MIL4 | [10,11] | Bio-Rad/Serotec |
| CD11c | 3A8 | [12] | Bio-Rad/Serotec |
| CD14 | MIL2 | [10] | Bio-Rad/Serotec |
| CD16 | G7 | [13] | BD Biosciences Bio-Rad/Serotec |
| CD32 | HuCAL32 (CD32a), HuCAL 91 (CD32a/b) | [14] | |
| CD32a/b | AT-10 | [15] | Bio-Rad Thermo Fisher |
| CD68 | EBM11 | [16] | Dako |
| CD107a | 4E9/11 | [8,17] | Bio-Rad/Serotec |
| CD115 | ROS8G11 | [18] | Bio-Rad/Serotec |
| CD163 | 2A10/11 | [19] | Bio-Rad/Serotec |
| CD172a | 74-22-15 BL1H7 | [20] [21] | ATCC/Kingfisher Biotech Bio-Rad/Serotec |
| CD200R1 | PCT1 and PCT3 | [22] | |
| CD200R1L | PCT1 | [22] | |
| CD203a (ENPP1) | PM18-7 | [23] | Bio-Rad/Serotec |
| CD205 | ZH9F7 | [24] | Bio-Rad/Serotec |
| CD206 (MR) | 122D2.08 | [25] | Dendritics |
| CD209 | DC428 | [26] | |
| CLEC12A (CD371) | FA2B10 | [27] | |
| CLEC12B | PELE6 | [28] | |
| F4/80 (ADGRE1) | ROS-4E12-3E6 | [29] | |
| Siglec-1 (CD169) | 3B11 | [30] | Bio-Rad/Serotec |
| Siglec-3 (CD33) | 5D5 | [31] | Bio-Rad/Serotec |
| Siglec-5 (CD170) | 4F7 | [32] | Bio-Rad/Serotec |
| Siglec-10 | 2E9 | [33] | Bio-Rad/Serotec |
| TLR2 (CD282) | 1H11 | [34] | Bio-Rad/Serotec |
| TLR3 (CD283) | TLR3.7 | [9,35] | eBioscience/Thermofisher |
| TLR4 (CD284) | 3H3 | [36] | Bio-Rad/Serotec |
| TLR9 (CD289) | 26C593.2 eB72-1665 | [37] [35] | Novus Biologicals eBioscience |
| Tissue | Location | Phenotype | Comments | Ref. |
|---|---|---|---|---|
| Spleen | ||||
| red pulp | CD163+ CD169−/lo | APC in vitro | [30,121,152] | |
| marginal zone and ellipsoids | CD163− CD169+ | APC in vitro | ||
| follicles white pulp | CD107ahi | Tingible body MØ | [17] | |
| Lymph nodes | ||||
| subcapsular area/periphery of LN | CD163+ CD169+ | Equivalent to mouse medullary sinus macrophages | [30,121,247,248] | |
| perifollicular zone and inside B cell follicles | CD163− CD169+ | Equivalent to murine subcapsular sinus macrophages | ||
| medullary cords | CD163+ CD169− | Equivalent to murine medullary cord | ||
| follicles | CD107ahi | Tingible body MØ | [17] | |
| Tonsil | ||||
| crypt, epithelium, connective tissue and follicles and interfollicular region | CD172ahi CD14− (Tuk4) CD163+ MHC-IIhi | [249] | ||
| Liver | ||||
| hepatic sinusoids | CD107a+ CD163+ CD169+ | Kupffer cells | [17] | |
| Lung | ||||
| alveolar macrophages (AM) | CD163+ CD169+ CD172a+ CD203a+ CD206+ | Suggested embryonic-derived macrophages. Role in protection against influenza virus infection Maintaining airway immune homeostasis. | [170,206,219,250,251] | |
| lung parenchyma (PIM) | Like AM | Suggested embryonic-derived macrophages. | [219,252,253] | |
| lung parenchyma moMacro | CD172aintCD163int | Monocyte-derived cell characteristics, | [170] | |
| Nasal mucosa | ||||
| upper lamina propria and epithelium | CD163+ CD169− | [254,255] | ||
| deep lamina propria, close to cartilage | CD163+ CD169+ | |||
| Skin | ||||
| Dermis | CD172a+CD163+SLA-DR−/lo CD14+CD16+ | Poor T cell stimulatory capacity | [25,169] | |
| Placenta | ||||
| fetal placenta and endometrium, | CD163+ CD169+ | Absent during mid gestation in fetal placenta | [256,257] | |
| fetal placental and endometrium, | CD163+ CD169− |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, B.; Revilla, C.; Poderoso, T.; Ezquerra, A.; Domínguez, J. Porcine Macrophage Markers and Populations: An Update. Cells 2023, 12, 2103. https://doi.org/10.3390/cells12162103
Álvarez B, Revilla C, Poderoso T, Ezquerra A, Domínguez J. Porcine Macrophage Markers and Populations: An Update. Cells. 2023; 12(16):2103. https://doi.org/10.3390/cells12162103
Chicago/Turabian StyleÁlvarez, Belén, Concepción Revilla, Teresa Poderoso, Angel Ezquerra, and Javier Domínguez. 2023. "Porcine Macrophage Markers and Populations: An Update" Cells 12, no. 16: 2103. https://doi.org/10.3390/cells12162103
APA StyleÁlvarez, B., Revilla, C., Poderoso, T., Ezquerra, A., & Domínguez, J. (2023). Porcine Macrophage Markers and Populations: An Update. Cells, 12(16), 2103. https://doi.org/10.3390/cells12162103






