The Influence of Adipocyte Secretome on Selected Metabolic Fingerprints of Breast Cancer Cell Lines Representing the Four Major Breast Cancer Subtypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
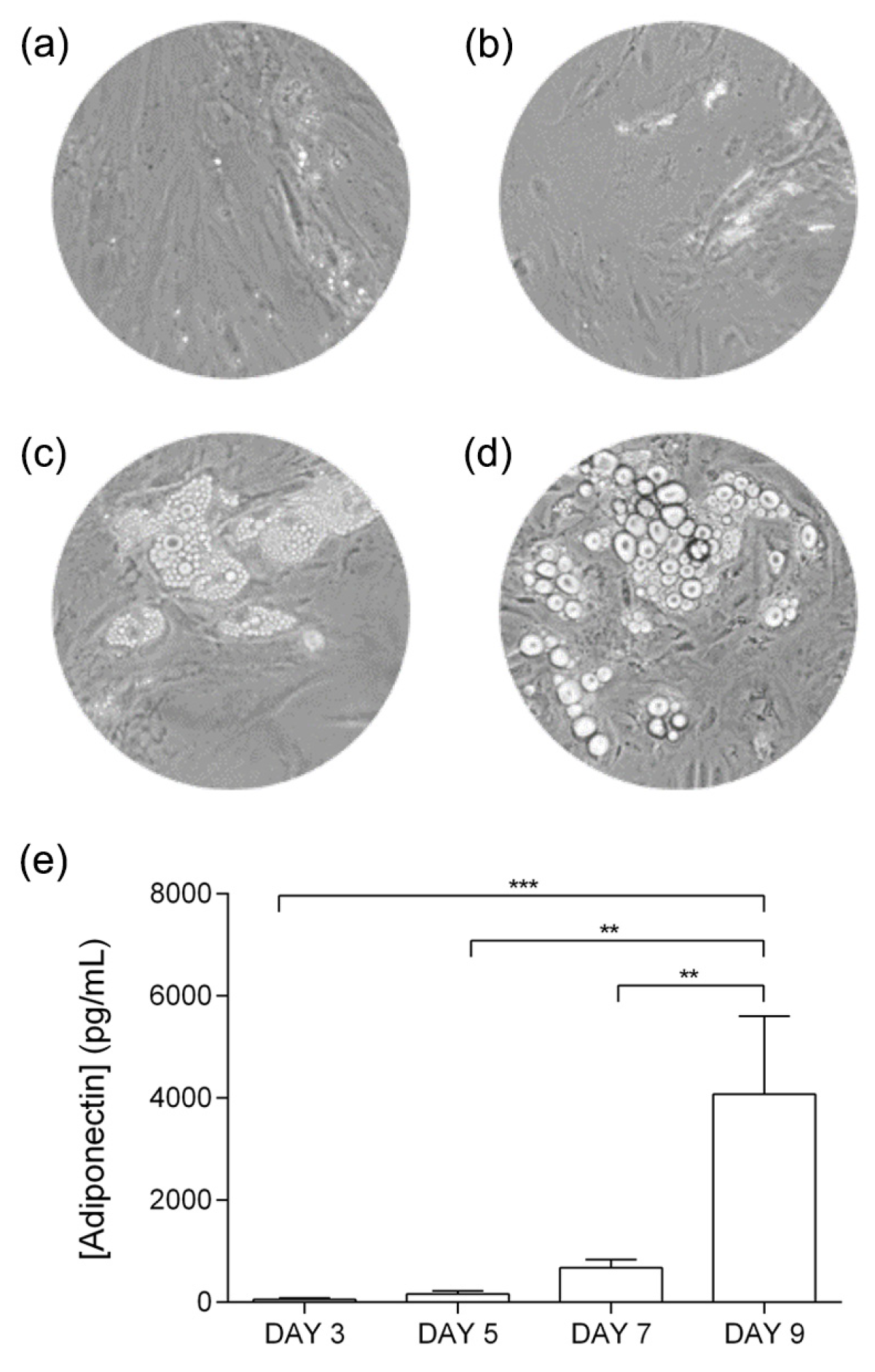
2.2. Adipocyte Differentiation
2.3. Adiponectin Quantification
2.4. Cell Culture
2.5. NMR (Nuclear Magnetic Resonance)-Based Metabolomics
2.6. Triglycerides and Cholesterol Quantification
2.7. RNA Extraction
2.8. RT-PCR
2.9. Statistical Analysis
3. Results
3.1. Adipocyte Differentiation
3.2. Carbohydrate Metabolic Profile
3.3. Amino Acid Metabolic Pattern
3.4. Lipid Characterization
3.5. Glycolytic and Gluconeogenic Enzymatic Fingerprints
3.6. TIGAR Expression Pattern
4. Discussion
4.1. Effect of Obesity in Luminal A Representative MCF-7 Cell Line
4.2. Effect of Obesity in Luminal B Representative BT-474 Cell Line
4.3. Effect of Obesity in HER2+ Representative SK-BR-3 Cell Line
4.4. Effect of Obesity in Triple Negative Representative MDA-MB-231 Cell Line
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perou, C.M.; Sørile, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Ress, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular Portraits of Human Breast Tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Ades, F.; Zardavas, D.; Bozovic-Spasojevic, I.; Pugliano, L.; Fumagalli, D.; De Azambuja, E.; Viale, G.; Sotiriou, C.; Piccart, M. Luminal B Breast Cancer: Molecular Characterization, Clinical Management, and Future Perspectives. J. Clin. Oncol. 2014, 32, 2794–2803. [Google Scholar] [CrossRef] [PubMed]
- Rubin, I.; Yarden, Y. The Basic Biology of HER2. Ann. Oncol. 2001, 12, S3–S8. [Google Scholar] [CrossRef]
- Schneider, B.P.; Winer, E.P.; Foulkes, W.D.; Garber, J.; Perou, C.M.; Richardson, A.; Sledge, G.W.; Carey, L.A. Triple-Negative Breast Cancer: Risk Factors to Potential Targets. Clin. Cancer Res. 2008, 14, 8010–8018. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Au, C.M.C.; Benito-Martin, A.; Ladumor, H.; Oshchepkova, S.; Moges, R.; Brown, K.A. Estrogens and Breast Cancer: Mechanisms Involved in Obesity-Related Development, Growth and Progression. J. Steroid. Biochem. Mol. Biol. 2019, 189, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Pedersen, L.; Halberg, N. Cellular Mechanisms Linking Cancers to Obesity. Cell Stress 2021, 5, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Chen, D.; Moy, I.; Brooks, D.C.; Zhao, H. Aromatase, Breast Cancer and Obesity: A Complex Interaction. Trends Endocrinol. Metab. 2012, 23, 83–89. [Google Scholar] [CrossRef]
- Warburg, O. The Metabolism of Carcinoma Cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Luis, C.; Duarte, F.; Faria, I.; Jarak, I.; Oliveira, P.F.; Alves, M.G.; Soares, R.; Fernandes, R. Warburg Effect Inversion: Adiposity Shifts Central Primary Metabolism in MCF-7 Breast Cancer Cells. Life Sci. 2019, 223, 38–46. [Google Scholar] [CrossRef]
- Kishida, K.; Funahashi, T.; Shimomura, I. Adiponectin as a Routine Clinical Biomarker. In Proceedings of the Best Practice and Research: Clinical Endocrinology and Metabolism. Clin. Endocrinol. Metab. 2014, 28, 119–130. [Google Scholar]
- Holliday, D.L.; Speirs, V. Choosing the Right Cell Line for Breast Cancer Research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Sander, C.; Stuart, J.M.; Chang, K.; Creighton, C.J.; et al. The Cancer Genome Atlas Pan-Cancer Analysis Project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.; Das, G.M. Metabolic Reprogramming in Breast Cancer and Its Therapeutic Implications. Cells 2019, 8, 89. [Google Scholar] [CrossRef]
- Pavlides, S.; Whitaker-Menezes, D.; Castello-Cros, R.; Flomenberg, N.; Witkiewicz, A.K.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; et al. The Reverse Warburg Effect: Aerobic Glycolysis in Cancer Associated Fibroblasts and the Tumor Stroma. Cell Cycle 2009, 8, 3984–4001. [Google Scholar] [CrossRef]
- Willmann, L.; Schlimpert, M.; Halbach, S.; Erbes, T.; Stickeler, E.; Kammerer, B. Metabolic Profiling of Breast Cancer: Differences in Central Metabolism between Subtypes of Breast Cancer Cell Lines. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 1000, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Hue, L.; Taegtmeyer, H. The Randle Cycle Revisited: A New Head for an Old Hat. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E578–E591. [Google Scholar] [CrossRef]
- Matus-Ortega, G.; Romero-Aguilar, L.; González, J.; Guerra Sánchez, G.; Matus-Ortega, M.; Del Castillo-Falconi, V.; Pardo, J.P. The Randle Cycle, the Precarious Link between Sugars and Fats. TIP Rev. Espec. En Cienc. Químico-Biológicas 2020, 23, 1–10. [Google Scholar] [CrossRef]
- Cai, Q.; Lin, T.; Kamarajugadda, S.; Lu, J. Regulation of Glycolysis and the Warburg Effect by Estrogen-Related Receptors. Oncogene 2013, 32, 2079–2086. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg Effect: Historical Dogma versus Current Understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef]
- Edwards, D.N.; Ngwa, V.M.; Raybuck, A.L.; Wang, S.; Hwang, Y.; Kim, L.C.; Cho, S.H.; Paik, Y.; Wang, Q.; Zhang, S.; et al. Selective Glutamine Metabolism Inhibition in Tumor Cells Improves Antitumor T Lymphocyte Activity in Triple-Negative Breast Cancer. J. Clin. Investig. 2021, 131, 1–16. [Google Scholar] [CrossRef]
- Silvente-Poirot, S.; Poirot, M. Cholesterol Metabolism and Cancer: The Good, the Bad and the Ugly. Curr. Opin. Pharmacol. 2012, 12, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhang, W.; Li, S.; Yang, H. The Role of Cholesterol Metabolism in Cancer. Am. J. Cancer Res. 2019, 9, 219–227. [Google Scholar] [PubMed]
- El-Bacha, T.; De Freitas, M.S.; Sola-Penna, M. Cellular Distribution of Phosphofructokinase Activity and Implications to Metabolic Regulation in Human Breast Cancer. Mol. Genet. Metab. 2003, 79, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Lehotzky, A.; Telegdi, M.; Liliom, K.; Ovadi, J. Interaction of Phosphofructokinase with Tubulin and Microtubules. Quantitative Evaluation of the Mutual Effects. J. Biol. Chem. 1993, 268, 10888–10894. [Google Scholar] [CrossRef]
- Schug, Z.T.; Vande Voorde, J.; Gottlieb, E. The Metabolic Fate of Acetate in Cancer. Nat. Rev. Cancer 2016, 16, 708–717. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, C. Gluconeogenesis in Cancer: Function and Regulation of PEPCK, FBPase, and G6Pase. Trends Cancer 2019, 5, 30–45. [Google Scholar] [CrossRef]
- Dornier, E.; Rabas, N.; Mitchell, L.; Novo, D.; Dhayade, S.; Marco, S.; Mackay, G.; Sumpton, D.; Pallares, M.; Nixon, C. Glutaminolysis Drives Membrane Trafficking to Promote Invasiveness of Breast Cancer Cells. Nat. Commun. 2017, 8, 2255. [Google Scholar] [CrossRef]
- Bertelsen, V.; Stang, E. The Mysterious Ways of ErbB2/HER2 Trafficking. Membranes 2014, 4, 424–446. [Google Scholar] [CrossRef]
- Zeleznik, O.A.; Balasubramanian, R.; Ren, Y.; Tobias, D.K.; Rosner, B.A.; Peng, C.; Bever, A.M.; Frueh, L.; Jeanfavre, S.; Avila-Pacheco, J.; et al. Branched-Chain Amino Acids and Risk of Breast Cancer. JNCI Cancer Spectr. 2021, 5, pkab059. [Google Scholar] [CrossRef]
- Du, Y.; Meng, Q.; Zhang, Q.; Guo, F. Isoleucine or Valine Deprivation Stimulates Fat Loss via Increasing Energy Expenditure and Regulating Lipid Metabolism in WAT. Amino Acids 2012, 43, 725–734. [Google Scholar] [CrossRef]





| Cell Line | Type | Molecular Subtype | Receptor Status |
|---|---|---|---|
| MCF-7 | Invasive Ductal Carcinoma | Luminal A | ER+ PR+ HER2− |
| BT-474 | Invasive Ductal Carcinoma | Luminal B | ER+ PR+ HER2+ |
| SK-BR-3 | Adenocarcinoma | HER2+ | ER− PR− HER2+ |
| MDA-MB-231 | Adenocarcinoma | Triple Negative | ER− PR− HER2− |
| Gene | Primer Sequence (5′-3′) |
|---|---|
| ACTB * | F—GACGACATGGAGAAAATCTG R—ATGATCTGGGTCATCTTCTC |
| HK-2 | F—CTAAACTAGACGAGAGTTTCC R—CATCATAACCACAGGTCATC |
| PFK-L | F—GAAAGACACTGATTTCGAGC R—CATACTGATGCGGTATTGTG |
| PK-L/R | F—AAAATTGAGAACCACGAAGG R—CAATCATCATCTTCTGAGCC |
| PC | F—TTATGGTGCAGAATGGATTG R—TCAGTACCTTAGAGCGAAAG |
| PCK-2 | F—GGCATTCGAGATTTTGTAGAG R—CAGCAGTTATTGTACTTGGG |
| FBP-1 | F—CCAGATAATTCAGCTCCTTATG R—AGAAATATCCCTCCGTAGAC |
| G6P | F—ACTGTGCATACATGTTCATC R—TGAATGTTTTGACCTAGTGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luís, C.; Guerra-Carvalho, B.; Braga, P.C.; Guedes, C.; Patrício, E.; Alves, M.G.; Fernandes, R.; Soares, R. The Influence of Adipocyte Secretome on Selected Metabolic Fingerprints of Breast Cancer Cell Lines Representing the Four Major Breast Cancer Subtypes. Cells 2023, 12, 2123. https://doi.org/10.3390/cells12172123
Luís C, Guerra-Carvalho B, Braga PC, Guedes C, Patrício E, Alves MG, Fernandes R, Soares R. The Influence of Adipocyte Secretome on Selected Metabolic Fingerprints of Breast Cancer Cell Lines Representing the Four Major Breast Cancer Subtypes. Cells. 2023; 12(17):2123. https://doi.org/10.3390/cells12172123
Chicago/Turabian StyleLuís, Carla, Bárbara Guerra-Carvalho, Patrícia C. Braga, Carla Guedes, Emília Patrício, Marco G. Alves, Ruben Fernandes, and Raquel Soares. 2023. "The Influence of Adipocyte Secretome on Selected Metabolic Fingerprints of Breast Cancer Cell Lines Representing the Four Major Breast Cancer Subtypes" Cells 12, no. 17: 2123. https://doi.org/10.3390/cells12172123
APA StyleLuís, C., Guerra-Carvalho, B., Braga, P. C., Guedes, C., Patrício, E., Alves, M. G., Fernandes, R., & Soares, R. (2023). The Influence of Adipocyte Secretome on Selected Metabolic Fingerprints of Breast Cancer Cell Lines Representing the Four Major Breast Cancer Subtypes. Cells, 12(17), 2123. https://doi.org/10.3390/cells12172123










