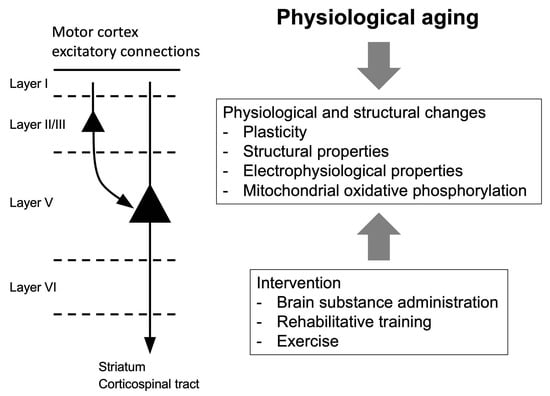
Neuronal Plasticity and Age-Related Functional Decline in the Motor Cortex
Abstract
1. Introduction
2. Motor Cortex
2.1. Pathway
2.2. Plasticity in the Motor Cortex
2.2.1. Synaptic Plasticity and Motor Learning
2.2.2. Cortical Plasticity Induced by Non-Invasive Stimulation
2.2.3. Structural Plasticity and Motor Learning
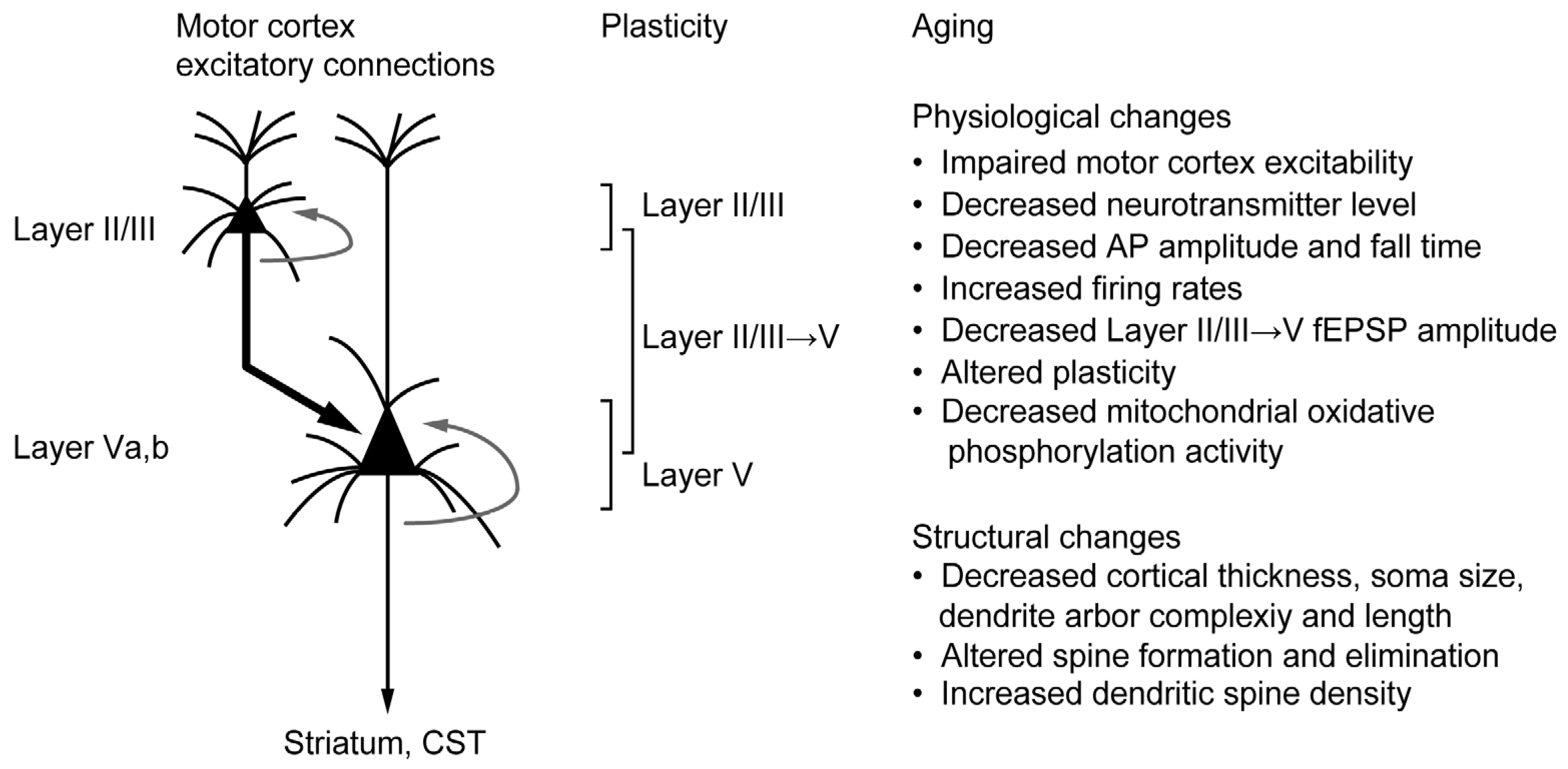
3. Aging in the Motor Cortex
3.1. Structural and Functional Alteration in the Motor Cortex
3.2. Aging and Cortical Plasticity in the Motor Cortex
3.3. Aging and Mitochondria in the Motor Cortex
4. Interventions for Age-Related Declines of Motor Function
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, B.C.; Taylor, J.L. Age-related changes in motor cortical properties and voluntary activation of skeletal muscle. Curr. Aging Sci. 2011, 4, 192–199. [Google Scholar] [CrossRef]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef]
- Clark, B.C. Neuromuscular Changes with Aging and Sarcopenia. J. Frailty Aging 2019, 8, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Freire, M.; de Cabo, R.; Studenski, S.A.; Ferrucci, L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front. Aging Neurosci. 2014, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Manini, T.M.; Hong, S.L.; Clark, B.C. Aging and muscle: A neuron’s perspective. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R. Sarcopenia and its implications for the elderly. Eur. J. Clin. Nutr. 2000, 54 (Suppl. S3), S40–S47. [Google Scholar] [CrossRef] [PubMed]
- Willadt, S.; Nash, M.; Slater, C. Age-related changes in the structure and function of mammalian neuromuscular junctions. Ann. N. Y. Acad. Sci. 2018, 1412, 41–53. [Google Scholar] [CrossRef]
- Jin, L.; Hahn, M.E. Comparison of lower extremity joint mechanics between healthy active young and middle age people in walking and running gait. Sci. Rep. 2019, 9, 5568. [Google Scholar] [CrossRef]
- Yurek, D.M.; Hipkens, S.B.; Hebert, M.A.; Gash, D.M.; Gerhardt, G.A. Age-related decline in striatal dopamine release and motoric function in brown Norway/Fischer 344 hybrid rats. Brain Res. 1998, 791, 246–256. [Google Scholar] [CrossRef]
- Takahashi, K.; Ohsawa, I.; Shirasawa, T.; Takahashi, M. Early-onset motor impairment and increased accumulation of phosphorylated alpha-synuclein in the motor cortex of normal aging mice are ameliorated by coenzyme Q. Exp. Gerontol. 2016, 81, 65–75. [Google Scholar] [CrossRef]
- Broom, L.; Stephen, J.; Nayar, V.; VanderHorst, V.G. Shifts in Gait Signatures Mark the End of Lifespan in Mice, with Sex Differences in Timing. Front. Aging Neurosci. 2021, 13, 716993. [Google Scholar] [CrossRef] [PubMed]
- Valdez, G.; Tapia, J.C.; Kang, H.; Clemenson, G.D., Jr.; Gage, F.H.; Lichtman, J.W.; Sanes, J.R. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc. Natl. Acad. Sci. USA 2010, 107, 14863–14868. [Google Scholar] [CrossRef]
- Sheth, K.A.; Iyer, C.C.; Wier, C.G.; Crum, A.E.; Bratasz, A.; Kolb, S.J.; Clark, B.C.; Burghes, A.H.M.; Arnold, W.D. Muscle strength and size are associated with motor unit connectivity in aged mice. Neurobiol. Aging 2018, 67, 128–136. [Google Scholar] [CrossRef]
- Chugh, D.; Iyer, C.C.; Wang, X.; Bobbili, P.; Rich, M.M.; Arnold, W.D. Neuromuscular junction transmission failure is a late phenotype in aging mice. Neurobiol. Aging 2020, 86, 182–190. [Google Scholar] [CrossRef]
- Seidler, R.D.; Bernard, J.A.; Burutolu, T.B.; Fling, B.W.; Gordon, M.T.; Gwin, J.T.; Kwak, Y.; Lipps, D.B. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010, 34, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Cassady, K.; Gagnon, H.; Lalwani, P.; Simmonite, M.; Foerster, B.; Park, D.; Peltier, S.J.; Petrou, M.; Taylor, S.F.; Weissman, D.H.; et al. Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. Neuroimage 2019, 186, 234–244. [Google Scholar] [CrossRef]
- Kossev, A.R.; Schrader, C.; Dauper, J.; Dengler, R.; Rollnik, J.D. Increased intracortical inhibition in middle-aged humans; a study using paired-pulse transcranial magnetic stimulation. Neurosci. Lett. 2002, 333, 83–86. [Google Scholar] [CrossRef] [PubMed]
- McGinley, M.; Hoffman, R.L.; Russ, D.W.; Thomas, J.S.; Clark, B.C. Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Exp. Gerontol. 2010, 45, 671–678. [Google Scholar] [CrossRef]
- Alexandre, F.; Heraud, N.; Tremey, E.; Oliver, N.; Bourgouin, D.; Varray, A. Specific motor cortex hypoexcitability and hypoactivation in COPD patients with peripheral muscle weakness. BMC Pulm. Med. 2020, 20, 1. [Google Scholar] [CrossRef]
- Khedr, E.M.; Ahmed, M.A.; Hamdy, A.; Shawky, O.A. Cortical excitability of amyotrophic lateral sclerosis: Transcranial magnetic stimulation study. Neurophysiol. Clin. 2011, 41, 73–79. [Google Scholar] [CrossRef]
- Boric, K.; Munoz, P.; Gallagher, M.; Kirkwood, A. Potential adaptive function for altered long-term potentiation mechanisms in aging hippocampus. J. Neurosci. 2008, 28, 8034–8039. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.C. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca2+ channels in senescent synaptic plasticity. Prog. Neurobiol. 2012, 96, 283–303. [Google Scholar] [CrossRef]
- Abe, H.; Jitsuki, S.; Nakajima, W.; Murata, Y.; Jitsuki-Takahashi, A.; Katsuno, Y.; Tada, H.; Sano, A.; Suyama, K.; Mochizuki, N.; et al. CRMP2-binding compound, edonerpic maleate, accelerates motor function recovery from brain damage. Science 2018, 360, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T. Novel synaptic plasticity enhancer drug to augment functional recovery with rehabilitation. Curr. Opin. Neurol. 2019, 32, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Arber, S.; Costa, R.M. Connecting neuronal circuits for movement. Science 2018, 360, 1403–1404. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.G. The discovery of motor cortex and its background. J. Hist. Neurosci. 2007, 16, 320–331. [Google Scholar] [CrossRef]
- Nudo, R.J.; Masterton, R.B. Descending pathways to the spinal cord, III: Sites of origin of the corticospinal tract. J. Comp. Neurol. 1990, 296, 559–583. [Google Scholar] [CrossRef]
- Tennant, K.A.; Adkins, D.L.; Donlan, N.A.; Asay, A.L.; Thomas, N.; Kleim, J.A.; Jones, T.A. The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cereb. Cortex 2011, 21, 865–876. [Google Scholar] [CrossRef]
- Weiler, N.; Wood, L.; Yu, J.; Solla, S.A.; Shepherd, G.M. Top-down laminar organization of the excitatory network in motor cortex. Nat. Neurosci. 2008, 11, 360–366. [Google Scholar] [CrossRef]
- Petreanu, L.; Mao, T.; Sternson, S.M.; Svoboda, K. The subcellular organization of neocortical excitatory connections. Nature 2009, 457, 1142–1145. [Google Scholar] [CrossRef]
- Sanders, T.H.; Jaeger, D. Optogenetic stimulation of cortico-subthalamic projections is sufficient to ameliorate bradykinesia in 6-ohda lesioned mice. Neurobiol. Dis. 2016, 95, 225–237. [Google Scholar] [CrossRef]
- Anderson, C.T.; Sheets, P.L.; Kiritani, T.; Shepherd, G.M. Sublayer-specific microcircuits of corticospinal and corticostriatal neurons in motor cortex. Nat. Neurosci. 2010, 13, 739–744. [Google Scholar] [CrossRef]
- Yu, J.; Anderson, C.T.; Kiritani, T.; Sheets, P.L.; Wokosin, D.L.; Wood, L.; Shepherd, G.M. Local-Circuit Phenotypes of Layer 5 Neurons in Motor-Frontal Cortex of YFP-H Mice. Front. Neural Circuits 2008, 2, 6. [Google Scholar] [CrossRef]
- Hooks, B.M.; Hires, S.A.; Zhang, Y.X.; Huber, D.; Petreanu, L.; Svoboda, K.; Shepherd, G.M. Laminar analysis of excitatory local circuits in vibrissal motor and sensory cortical areas. PLoS Biol. 2011, 9, e1000572. [Google Scholar] [CrossRef] [PubMed]
- Davidoff, R.A. The pyramidal tract. Neurology 1990, 40, 332–339. [Google Scholar] [CrossRef]
- Jane, J.A.; Yashon, D.; DeMyer, W.; Bucy, P.C. The contribution of the precentral gyrus to the pyramidal tract of man. J. Neurosurg. 1967, 26, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Kawai, R.; Markman, T.; Poddar, R.; Ko, R.; Fantana, A.L.; Dhawale, A.K.; Kampff, A.R.; Olveczky, B.P. Motor cortex is required for learning but not for executing a motor skill. Neuron 2015, 86, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Cantarero, G.; Lloyd, A.; Celnik, P. Reversal of long-term potentiation-like plasticity processes after motor learning disrupts skill retention. J. Neurosci. 2013, 33, 12862–12869. [Google Scholar] [CrossRef]
- Papale, A.E.; Hooks, B.M. Circuit changes in motor cortex during motor skill learning. Neuroscience 2018, 368, 283–297. [Google Scholar] [CrossRef]
- Kida, H.; Tsuda, Y.; Ito, N.; Yamamoto, Y.; Owada, Y.; Kamiya, Y.; Mitsushima, D. Motor Training Promotes Both Synaptic and Intrinsic Plasticity of Layer II/III Pyramidal Neurons in the Primary Motor Cortex. Cereb. Cortex 2016, 26, 3494–3507. [Google Scholar] [CrossRef]
- Kida, H.; Kawakami, R.; Sakai, K.; Otaku, H.; Imamura, K.; Han, T.Z.; Sakimoto, Y.; Mitsushima, D. Motor training promotes both synaptic and intrinsic plasticity of layer V pyramidal neurons in the primary motor cortex. J. Physiol. 2023, 601, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Rioult-Pedotti, M.S.; Friedman, D.; Donoghue, J.P. Learning-induced LTP in neocortex. Science 2000, 290, 533–536. [Google Scholar] [CrossRef]
- Rioult-Pedotti, M.S.; Donoghue, J.P.; Dunaevsky, A. Plasticity of the synaptic modification range. J. Neurophysiol. 2007, 98, 3688–3695. [Google Scholar] [CrossRef] [PubMed]
- Rioult-Pedotti, M.S.; Friedman, D.; Hess, G.; Donoghue, J.P. Strengthening of horizontal cortical connections following skill learning. Nat. Neurosci. 1998, 1, 230–234. [Google Scholar] [CrossRef]
- Hodgson, R.A.; Ji, Z.; Standish, S.; Boyd-Hodgson, T.E.; Henderson, A.K.; Racine, R.J. Training-induced and electrically induced potentiation in the neocortex. Neurobiol. Learn. Mem. 2005, 83, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Monfils, M.H.; Teskey, G.C. Skilled-learning-induced potentiation in rat sensorimotor cortex: A transient form of behavioural long-term potentiation. Neuroscience 2004, 125, 329–336. [Google Scholar] [CrossRef]
- Biane, J.S.; Takashima, Y.; Scanziani, M.; Conner, J.M.; Tuszynski, M.H. Thalamocortical Projections onto Behaviorally Relevant Neurons Exhibit Plasticity during Adult Motor Learning. Neuron 2016, 89, 1173–1179. [Google Scholar] [CrossRef]
- Kida, H.; Mitsushima, D. Mechanisms of motor learning mediated by synaptic plasticity in rat primary motor cortex. Neurosci. Res. 2018, 128, 14–18. [Google Scholar] [CrossRef]
- Barbati, S.A.; Cocco, S.; Longo, V.; Spinelli, M.; Gironi, K.; Mattera, A.; Paciello, F.; Colussi, C.; Podda, M.V.; Grassi, C. Enhancing Plasticity Mechanisms in the Mouse Motor Cortex by Anodal Transcranial Direct-Current Stimulation: The Contribution of Nitric Oxide Signaling. Cereb. Cortex 2020, 30, 2972–2985. [Google Scholar] [CrossRef]
- Inoue, R.; Miura, M.; Yanai, S.; Nishimune, H. Coenzyme Q(10) supplementation improves the motor function of middle-aged mice by restoring the neuronal activity of the motor cortex. Sci. Rep. 2023, 13, 4323. [Google Scholar] [CrossRef]
- Ziemann, U.; Ilic, T.V.; Pauli, C.; Meintzschel, F.; Ruge, D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J. Neurosci. 2004, 24, 1666–1672. [Google Scholar] [CrossRef]
- Rosenkranz, K.; Kacar, A.; Rothwell, J.C. Differential modulation of motor cortical plasticity and excitability in early and late phases of human motor learning. J. Neurosci. 2007, 27, 12058–12066. [Google Scholar] [CrossRef] [PubMed]
- Cantarero, G.; Tang, B.; O’Malley, R.; Salas, R.; Celnik, P. Motor learning interference is proportional to occlusion of LTP-like plasticity. J. Neurosci. 2013, 33, 4634–4641. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Boyd, T.E.; Froc, D.J.; Racine, R.J. Laminar differences in field potential morphology and long-term potentiation in motor cortex coronal slices from both unstimulated and previously potentiated rats. Eur. J. Neurosci. 2005, 22, 1455–1462. [Google Scholar] [CrossRef]
- Molina-Luna, K.; Pekanovic, A.; Rohrich, S.; Hertler, B.; Schubring-Giese, M.; Rioult-Pedotti, M.S.; Luft, A.R. Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS ONE 2009, 4, e7082. [Google Scholar] [CrossRef]
- Yang, G.; Pan, F.; Gan, W.B. Stably maintained dendritic spines are associated with lifelong memories. Nature 2009, 462, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Yu, X.; Perlik, A.J.; Tobin, W.F.; Zweig, J.A.; Tennant, K.; Jones, T.; Zuo, Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 2009, 462, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Zemmar, A.; Weinmann, O.; Kellner, Y.; Yu, X.; Vicente, R.; Gullo, M.; Kasper, H.; Lussi, K.; Ristic, Z.; Luft, A.R.; et al. Neutralization of Nogo-A enhances synaptic plasticity in the rodent motor cortex and improves motor learning in vivo. J. Neurosci. 2014, 34, 8685–8698. [Google Scholar] [CrossRef]
- Guo, L.; Xiong, H.; Kim, J.I.; Wu, Y.W.; Lalchandani, R.R.; Cui, Y.; Shu, Y.; Xu, T.; Ding, J.B. Dynamic rewiring of neural circuits in the motor cortex in mouse models of Parkinson’s disease. Nat. Neurosci. 2015, 18, 1299–1309. [Google Scholar] [CrossRef]
- Gloor, C.; Luft, A.R.; Hosp, J.A. Biphasic plasticity of dendritic fields in layer V motor neurons in response to motor learning. Neurobiol. Learn. Mem. 2015, 125, 189–194. [Google Scholar] [CrossRef]
- Tjia, M.; Yu, X.; Jammu, L.S.; Lu, J.; Zuo, Y. Pyramidal Neurons in Different Cortical Layers Exhibit Distinct Dynamics and Plasticity of Apical Dendritic Spines. Front. Neural Circuits 2017, 11, 43. [Google Scholar] [CrossRef]
- Donoghue, J.P.; Wise, S.P. The motor cortex of the rat: Cytoarchitecture and microstimulation mapping. J. Comp. Neurol. 1982, 212, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Hosp, J.A.; Pekanovic, A.; Rioult-Pedotti, M.S.; Luft, A.R. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J. Neurosci. 2011, 31, 2481–2487. [Google Scholar] [CrossRef] [PubMed]
- Ridding, M.C.; Ziemann, U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. Physiol. 2010, 588, 2291–2304. [Google Scholar] [CrossRef]
- Nudo, R.J.; Milliken, G.W.; Jenkins, W.M.; Merzenich, M.M. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J. Neurosci. 1996, 16, 785–807. [Google Scholar] [CrossRef]
- Wu, A.D.; Fregni, F.; Simon, D.K.; Deblieck, C.; Pascual-Leone, A. Noninvasive brain stimulation for Parkinson’s disease and dystonia. Neurotherapeutics 2008, 5, 345–361. [Google Scholar] [CrossRef]
- Godeiro, C.; Franca, C.; Carra, R.B.; Saba, F.; Saba, R.; Maia, D.; Brandao, P.; Allam, N.; Rieder, C.R.M.; Freitas, F.C.; et al. Use of non-invasive stimulation in movement disorders: A critical review. Arq. Neuropsiquiatr. 2021, 79, 630–646. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.L.; Santarnecchi, E.; Buch, E.R.; Cohen, L.G. Non-invasive brain stimulation in neurorehabilitation: Local and distant effects for motor recovery. Front. Hum. Neurosci. 2014, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Liebetanz, D.; Nitsche, M.A.; Tergau, F.; Paulus, W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 2002, 125, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, B.; Reis, J.; Martinowich, K.; Schambra, H.M.; Ji, Y.; Cohen, L.G.; Lu, B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron 2010, 66, 198–204. [Google Scholar] [CrossRef]
- Rahman, A.; Reato, D.; Arlotti, M.; Gasca, F.; Datta, A.; Parra, L.C.; Bikson, M. Cellular effects of acute direct current stimulation: Somatic and synaptic terminal effects. J. Physiol. 2013, 591, 2563–2578. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.C.; Zito, K. LTP-induced long-term stabilization of individual nascent dendritic spines. J. Neurosci. 2013, 33, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, A.; Profice, P.; Tonali, P.A.; Pilato, F.; Saturno, E.; Dileone, M.; Ranieri, F.; Di Lazzaro, V. Effects of aging on motor cortex excitability. Neurosci. Res. 2006, 55, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Petitet, P.; Spitz, G.; Emir, U.E.; Johansen-Berg, H.; O’Shea, J. Age-related decline in cortical inhibitory tone strengthens motor memory. Neuroimage 2021, 245, 118681. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, D.L.; Kabaso, D.; Rocher, A.B.; Luebke, J.I.; Wearne, S.L.; Hof, P.R. Changes in the structural complexity of the aged brain. Aging Cell 2007, 6, 275–284. [Google Scholar] [CrossRef]
- Chang, Y.M.; Rosene, D.L.; Killiany, R.J.; Mangiamele, L.A.; Luebke, J.I. Increased action potential firing rates of layer 2/3 pyramidal cells in the prefrontal cortex are significantly related to cognitive performance in aged monkeys. Cereb. Cortex 2005, 15, 409–418. [Google Scholar] [CrossRef]
- Luebke, J.I.; Chang, Y.M. Effects of aging on the electrophysiological properties of layer 5 pyramidal cells in the monkey prefrontal cortex. Neuroscience 2007, 150, 556–562. [Google Scholar] [CrossRef]
- Coskren, P.J.; Luebke, J.I.; Kabaso, D.; Wearne, S.L.; Yadav, A.; Rumbell, T.; Hof, P.R.; Weaver, C.M. Functional consequences of age-related morphologic changes to pyramidal neurons of the rhesus monkey prefrontal cortex. J. Comput. Neurosci. 2015, 38, 263–283. [Google Scholar] [CrossRef]
- Luebke, J.I.; Chang, Y.M.; Moore, T.L.; Rosene, D.L. Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience 2004, 125, 277–288. [Google Scholar] [CrossRef]
- Rizzo, V.; Richman, J.; Puthanveettil, S.V. Dissecting mechanisms of brain aging by studying the intrinsic excitability of neurons. Front. Aging Neurosci. 2014, 6, 337. [Google Scholar] [CrossRef]
- Davidson, A.M.; Mejia-Gomez, H.; Jacobowitz, M.; Mostany, R. Dendritic Spine Density and Dynamics of Layer 5 Pyramidal Neurons of the Primary Motor Cortex Are Elevated With Aging. Cereb. Cortex 2020, 30, 767–777. [Google Scholar] [CrossRef]
- Brecht, M.; Schneider, M.; Sakmann, B.; Margrie, T.W. Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature 2004, 427, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Kishore, A.; Popa, T.; James, P.; Yahia-Cherif, L.; Backer, F.; Varughese Chacko, L.; Govind, P.; Pradeep, S.; Meunier, S. Age-related decline in the responsiveness of motor cortex to plastic forces reverses with levodopa or cerebellar stimulation. Neurobiol. Aging 2014, 35, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Tennant, K.A.; Adkins, D.L.; Scalco, M.D.; Donlan, N.A.; Asay, A.L.; Thomas, N.; Kleim, J.A.; Jones, T.A. Skill learning induced plasticity of motor cortical representations is time and age-dependent. Neurobiol. Learn. Mem. 2012, 98, 291–302. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burianova, H.; Marstaller, L.; Rich, A.N.; Williams, M.A.; Savage, G.; Ryan, M.; Sowman, P.F. Motor neuroplasticity: A MEG-fMRI study of motor imagery and execution in healthy ageing. Neuropsychologia 2020, 146, 107539. [Google Scholar] [CrossRef]
- Davey, G.P.; Clark, J.B. Threshold effects and control of oxidative phosphorylation in nonsynaptic rat brain mitochondria. J. Neurochem. 1996, 66, 1617–1624. [Google Scholar] [CrossRef]
- Davey, G.P.; Peuchen, S.; Clark, J.B. Energy thresholds in brain mitochondria. Potential involvement in neurodegeneration. J. Biol. Chem. 1998, 273, 12753–12757. [Google Scholar] [CrossRef]
- Lores-Arnaiz, S.; Bustamante, J. Age-related alterations in mitochondrial physiological parameters and nitric oxide production in synaptic and non-synaptic brain cortex mitochondria. Neuroscience 2011, 188, 117–124. [Google Scholar] [CrossRef]
- Lores-Arnaiz, S.; Lombardi, P.; Karadayian, A.G.; Orgambide, F.; Cicerchia, D.; Bustamante, J. Brain cortex mitochondrial bioenergetics in synaptosomes and non-synaptic mitochondria during aging. Neurochem. Res. 2016, 41, 353–363. [Google Scholar] [CrossRef]
- Todorova, V.; Blokland, A. Mitochondria and Synaptic Plasticity in the Mature and Aging Nervous System. Curr. Neuropharmacol. 2017, 15, 166–173. [Google Scholar] [CrossRef]
- Mattson, M.P. Mitochondrial regulation of neuronal plasticity. Neurochem. Res. 2007, 32, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Takahashi, M. Exogenous administration of coenzyme Q10 restores mitochondrial oxygen consumption in the aged mouse brain. Mech. Ageing Dev. 2013, 134, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Eckert, A. Brain aging and neurodegeneration: From a mitochondrial point of view. J. Neurochem. 2017, 143, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.M.; Johannsen, D.L.; Ravussin, E. Skeletal muscle mitochondria and aging: A review. J. Aging Res. 2012, 2012, 194821. [Google Scholar] [CrossRef]
- Short, K.R.; Bigelow, M.L.; Kahl, J.; Singh, R.; Coenen-Schimke, J.; Raghavakaimal, S.; Nair, K.S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 5618–5623. [Google Scholar] [CrossRef]
- Garcia, M.L.; Fernandez, A.; Solas, M.T. Mitochondria, motor neurons and aging. J. Neurol. Sci. 2013, 330, 18–26. [Google Scholar] [CrossRef]
- Anagnostou, M.E.; Hepple, R.T. Mitochondrial Mechanisms of Neuromuscular Junction Degeneration with Aging. Cells 2020, 9, 197. [Google Scholar] [CrossRef]
- Carter, H.N.; Chen, C.C.; Hood, D.A. Mitochondria, muscle health, and exercise with advancing age. Physiology 2015, 30, 208–223. [Google Scholar] [CrossRef]
- Pandya, J.D.; Grondin, R.; Yonutas, H.M.; Haghnazar, H.; Gash, D.M.; Zhang, Z.; Sullivan, P.G. Decreased mitochondrial bioenergetics and calcium buffering capacity in the basal ganglia correlates with motor deficits in a nonhuman primate model of aging. Neurobiol. Aging 2015, 36, 1903–1913. [Google Scholar] [CrossRef]
- Niklowitz, P.; Onur, S.; Fischer, A.; Laudes, M.; Palussen, M.; Menke, T.; Doring, F. Coenzyme Q10 serum concentration and redox status in European adults: Influence of age, sex, and lipoprotein concentration. J. Clin. Biochem. Nutr. 2016, 58, 240–245. [Google Scholar] [CrossRef]
- Kalen, A.; Appelkvist, E.L.; Dallner, G. Age-related changes in the lipid compositions of rat and human tissues. Lipids 1989, 24, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Soderberg, M.; Edlund, C.; Kristensson, K.; Dallner, G. Lipid compositions of different regions of the human brain during aging. J. Neurochem. 1990, 54, 415–423. [Google Scholar] [CrossRef]
- Friedrich, T.; van Heek, P.; Leif, H.; Ohnishi, T.; Forche, E.; Kunze, B.; Jansen, R.; Trowitzsch-Kienast, W.; Hofle, G.; Reichenbach, H.; et al. Two binding sites of inhibitors in NADH: Ubiquinone oxidoreductase (complex I). Relationship of one site with the ubiquinone-binding site of bacterial glucose:ubiquinone oxidoreductase. Eur. J. Biochem. 1994, 219, 691–698. [Google Scholar] [CrossRef]
- Brandt, U. Proton-translocation by membrane-bound NADH:ubiquinone-oxidoreductase (complex I) through redox-gated ligand conduction. Biochim. Biophys. Acta 1997, 1318, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, I.E. Molecular genetics of succinate:quinone oxidoreductase in eukaryotes. Prog. Nucleic Acid. Res. Mol. Biol. 1998, 60, 267–315. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Camacho, J.D.; Bernier, M.; Lopez-Lluch, G.; Navas, P. Coenzyme Q10 Supplementation in Aging and Disease. Front. Physiol. 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Emmanuele, V.; Lopez, L.C.; Berardo, A.; Naini, A.; Tadesse, S.; Wen, B.; D’Agostino, E.; Solomon, M.; DiMauro, S.; Quinzii, C.; et al. Heterogeneity of coenzyme Q10 deficiency: Patient study and literature review. Arch. Neurol. 2012, 69, 978–983. [Google Scholar] [CrossRef]
- Mitsui, J.; Matsukawa, T.; Uemura, Y.; Kawahara, T.; Chikada, A.; Porto, K.J.L.; Naruse, H.; Tanaka, M.; Ishiura, H.; Toda, T.; et al. High-dose ubiquinol supplementation in multiple-system atrophy: A multicentre, randomised, double-blinded, placebo-controlled phase 2 trial. eClinicalMedicine 2023, 101920. [Google Scholar] [CrossRef]
- Lopez-Lluch, G.; Del Pozo-Cruz, J.; Sanchez-Cuesta, A.; Cortes-Rodriguez, A.B.; Navas, P. Bioavailability of coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition 2019, 57, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, N.; Dupuis, F.; Arab-Tehrany, E.; Denis, F.M.; Paris, C.; Lartaud, I.; Linder, M. Formulation, characterization and pharmacokinetic studies of coenzyme Q(1)(0) PUFA’s nanoemulsions. Eur. J. Pharm. Sci. 2012, 47, 305–312. [Google Scholar] [CrossRef]
- Hatanaka, J.; Kimura, Y.; Lai-Fu, Z.; Onoue, S.; Yamada, S. Physicochemical and pharmacokinetic characterization of water-soluble Coenzyme Q(10) formulations. Int. J. Pharm. 2008, 363, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, M.; Lanthier, P.; Miller, H.; Beyers, M.; Sodja, C.; Zurakowski, B.; Gangaraju, S.; Pandey, S.; Sandhu, J.K. Nanomicellar formulation of coenzyme Q10 (Ubisol-Q10) effectively blocks ongoing neurodegeneration in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model: Potential use as an adjuvant treatment in Parkinson’s disease. Neurobiol. Aging 2014, 35, 2329–2346. [Google Scholar] [CrossRef] [PubMed]
- Molina, D.P.; Ariwodola, O.J.; Linville, C.; Sonntag, W.E.; Weiner, J.L.; Brunso-Bechtold, J.K.; Adams, M.M. Growth hormone modulates hippocampal excitatory synaptic transmission and plasticity in old rats. Neurobiol. Aging 2012, 33, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Abuelezz, S.A.; Hendawy, N.; Magdy, Y. The potential benefit of combined versus monotherapy of coenzyme Q10 and fluoxetine on depressive-like behaviors and intermediates coupled to Gsk-3beta in rats. Toxicol. Appl. Pharmacol. 2018, 340, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Alhusaini, A.; Hasan, I.H.; Alrumayyan, B.; Alesikri, M.; Alanazi, K.; Almasoud, R.; Almarshad, S. Neuroprotective efficacy of nano-CoQ against propionic acid toxicity in rats: Role of BDNF and CREB protein expressions. J. Biochem. Mol. Toxicol. 2020, 34, e22449. [Google Scholar] [CrossRef] [PubMed]
- Zagrebelsky, M.; Korte, M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology 2014, 76 Pt C, 628–638. [Google Scholar] [CrossRef]
- Andreska, T.; Rauskolb, S.; Schukraft, N.; Luningschror, P.; Sasi, M.; Signoret-Genest, J.; Behringer, M.; Blum, R.; Sauer, M.; Tovote, P.; et al. Induction of BDNF Expression in Layer II/III and Layer V Neurons of the Motor Cortex Is Essential for Motor Learning. J. Neurosci. 2020, 40, 6289–6308. [Google Scholar] [CrossRef]
- Castren, E.; Antila, H. Neuronal plasticity and neurotrophic factors in drug responses. Mol. Psychiatry 2017, 22, 1085–1095. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, Z.; Zhu, L.; Huang, G.; Li, B.; Chen, C.; Huang, J.; Ma, F.; Liu, T.C. Effects of different physical activities on brain-derived neurotrophic factor: A systematic review and bayesian network meta-analysis. Front. Aging Neurosci. 2022, 14, 981002. [Google Scholar] [CrossRef]
- van der Vliet, R.; Ribbers, G.M.; Vandermeeren, Y.; Frens, M.A.; Selles, R.W. BDNF Val66Met but not transcranial direct current stimulation affects motor learning after stroke. Brain Stimul. 2017, 10, 882–892. [Google Scholar] [CrossRef]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Kuczewski, N.; Porcher, C.; Gaiarsa, J.L. Activity-dependent dendritic secretion of brain-derived neurotrophic factor modulates synaptic plasticity. Eur. J. Neurosci. 2010, 32, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.A.; Chan, S.; Pringle, E.; Schallert, K.; Procaccio, V.; Jimenez, R.; Cramer, S.C. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat. Neurosci. 2006, 9, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.J.; Weickert, C.S.; Herman, M.M.; Kleinman, J.E. BDNF mRNA expression during postnatal development, maturation and aging of the human prefrontal cortex. Brain Res. Dev. Brain Res. 2002, 139, 139–150. [Google Scholar] [CrossRef]
- Hayashi, M.; Yamashita, A.; Shimizu, K. Somatostatin and brain-derived neurotrophic factor mRNA expression in the primate brain: Decreased levels of mRNAs during aging. Brain Res. 1997, 749, 283–289. [Google Scholar] [CrossRef]
- Dluzen, D.E.; McDermott, J.L.; Anderson, L.I.; Kucera, J.; Joyce, J.N.; Osredkar, T.; Walro, J.M. Age-related changes in nigrostriatal dopaminergic function are accentuated in +/- brain-derived neurotrophic factor mice. Neuroscience 2004, 128, 201–208. [Google Scholar] [CrossRef]
- Mueller, K.; Arelin, K.; Moller, H.E.; Sacher, J.; Kratzsch, J.; Luck, T.; Riedel-Heller, S.; Villringer, A.; Schroeter, M.L. Serum BDNF correlates with connectivity in the (pre)motor hub in the aging human brain--a resting-state fMRI pilot study. Neurobiol. Aging 2016, 38, 181–187. [Google Scholar] [CrossRef]
- Lohmann, G.; Margulies, D.S.; Horstmann, A.; Pleger, B.; Lepsien, J.; Goldhahn, D.; Schloegl, H.; Stumvoll, M.; Villringer, A.; Turner, R. Eigenvector centrality mapping for analyzing connectivity patterns in fMRI data of the human brain. PLoS ONE 2010, 5, e10232. [Google Scholar] [CrossRef]
- McHughen, S.A.; Cramer, S.C. The BDNF val(66)met polymorphism is not related to motor function or short-term cortical plasticity in elderly subjects. Brain Res. 2013, 1495, 1–10. [Google Scholar] [CrossRef]
| Species | Age | Motor Learning Task or In Vivo Stimulation | Stimulation Layer/Area | Recording Layer/Neuron | Plasticity Type | Amount of Plasticity | Recording or Measurement Method | References |
|---|---|---|---|---|---|---|---|---|
| Female rats | Adult | Pellet-reaching task | Layer II/III at 500 μm horizontally from the recording electrode in the M1 forelimb area * | Layer II/III at 200–350 μm below the pial surface in the M1 forelimb area * | LTP | FP amplitude ↑ | Field potential recording | [42,43,44] |
| Male rats | Not mentioned | Pellet reaching task | Layer II/III at 3.0 mm lateral to the midline | Layer II/III at 2.0 mm lateral to the midline in the M1 forelimb area * | LTP | fEPSP amplitude ↑ | Field potential recording In vivo recording | [45] |
| Male rats | Not mentioned | In vivo white matter stimulation (previously potentiated rat) | Layer II/III in the primary motor cortex Layer V in the primary motor cortex | Layer II/III in the primary motor cortex Layer V in the primary motor cortex | FP | FP amplitude ↑ FP amplitude ↔ | Field potential recording | [54] |
| Male rats | 8–10 weeks | None | Layer II/III at 2–2.5 mm lateral to the midline | Layer II/III at 500 μm lateral to the stimulation electrode in the M1 forelimb area * | LTP | fEPSP amplitude ↓ (D1 or D2 receptor antagonist after LTP induction vs. control) | Field potential recording | [55] |
| Mice | 1 month >4 months 1 month | Accelerated rotor rod task | Layer V pyramidal neurons in the M1 forelimb area * | Structural plasticity (2-days trained mice) Structural plasticity (After previous 2-days training) | Spine formation ↑ (2-days) Spine formation ↔ (Next 2-days) | In vivo two-photon imaging | [56] | |
| Mice | 1 month | Pellet reaching task | Layer V pyramidal neurons in the motor cortex | Structural plasticity | Spine formation ↑ Spine elimination ↔ | In vivo two-photon imaging | [57] | |
| Male rats Male and female mice | 5–6 weeks 1 month | None None Pellet reaching task | Layer II/III 2–4 mm lateral to the midline | Layer II/III at 500 μm lateral to the stimulating electrode Layer V neurons in the motor cortex Layer II/III pyramidal neurons in the motor cortex | LTP Structural plasticity Structural plasticity | fEPSP amplitude ↑ (anti-Nogo A vs. control) Spine formation ↑ (anti-Nogo A vs. control) Spine density ↑ (sham and anti-Nogo A) | Field potential recording In vivo two-photon imaging | [58] |
| Male and female PD model mice | 1–3 months | Dopamine depletion | Superficial layers of the motor cortex | Layer V pyramidal neurons in the M1 forelimb area * Layer V pyramidal neurons 10–100 μm below the cortical surface in the motor cortex | LTP Structural plasticity | EPSC amplitude ↓ (DA depletion vs. control) Spine turnover in the dendritic spine ↑ (DA depletion vs. control) | Whole-cell recording In vivo two-photon imaging | [59] |
| Male rats | 10–12 weeks | Pellet reaching task | Layer V neurons | Structural plasticity | Dendritic length ↑ (after 1 month) | Histological analysis | [60] | |
| Rats | 55–59 days | Pellet reaching task | Entire cortical slice centered over the recorded neurons in the primary motor cortex | Layer V neurons in the caudal forelimb area | Photo-induced EPSC | EPSC amplitude↑ PPR ↔ | Whole-cell recording | [47] |
| Male Rats | 4 weeks | Accelerated rotor rod task | Layer II/III at 200–300 μm laterally from the recorded neurons in the primary motor cortex | Layer II/III pyramidal neurons in the M1 forelimb area * | mEPSC mIPSC | Amplitude ↑ (1-day and 2-days trained) Frequency ↔ (1-day), ↑ (2-days trained) AMPA/NMDA ↑ (1 day), ↔ (2-days trained) PPR ↔ (1-day), ↓ (2-days trained) Amplitude ↔ (1-day), ↔ (2-days trained) Frequency ↓ (1 day), ↔ (2-days trained) PPR ↑ (1 day), ↔ (2-days trained) | Whole-cell recording | [40,48] |
| Male and female mice | 1 month, 4 months | Pellet reaching task | Layer II/III pyramidal neurons Layer V pyramidal neurons | Structural plasticity Structural plasticity | Spine formation and elimination ↔ Spine formation and elimination ↑ | In vivo two-photon imaging | [61] | |
| Male mice | 30–45 days | Repeated tDCS | Layer II/III in the primary motor cortex | Layer II/III at ~200 μm lateral to the stimulation electrode in the primary motor cortex | LTP mEPSC mIPSC Structural plasticity | fEPSP amplitude ↑ PPR (interval: 20 ms) ↓ AMPA/NMDA ratio ↑ Amplitude ↔, Frequency ↑ Amplitude and frequency ↔ Spine density ↑ | Field potential recording Whole-cell recording Histological analysis | [49] |
| Male mice | 15–18 months | CoQ10 suppllementation None | Layer II/III in the radial direction from the recording electrode | Layer V in the primary motor cortex | fEPSP LTP | fEPSP amplitude ↑ (CoQ10 middle-aged vs. age-matched control) fEPSP amplitude ↑ (CoQ10 during LTP induction vs. age-matched control) | Field potential recording | [50] |
| Male rats Male mice | 4 weeks 8–10 weeks | Accelerated rotor rod task Accelerated rotor rod task | Layer II/III at 200–300 μm laterally from the recorded neurons in the primary motor cortex | Layer V pyramidal neurons in the M1 forelimb area * Layer V pyramidal neurons in the motor cortex | mEPSC mIPSC Structural plasticity | Amplitude ↔ (1 day), ↑ (2 days trained) Frequency ↔ (1 day), ↑ (2 days trained) AMPA/NMDA ratio ↔ (1 day), ↑ (2 days trained) PPR ↔ (1 day and 2 days trained) Amplitude ↓ (1 day), ↔ (2 days trained) Frequency ↓ (1 day), ↔ (2 days trained) PPR ↑ (1 day), ↔ (2 days trained) Volume of spines ↑ | Whole-cell recording In vivo two-photon imaging | [41] |
| Intervention Type | Administration Method | Species | Age | Effects on Motor Function | Cell or Brain Region | Target Mechanism | Commercial Availability | References |
|---|---|---|---|---|---|---|---|---|
| Nanomicellar formulation of CoQ10 supplementation | Oral | Male MPTP treated-mice | 8–10 weeks | Decrease of hindlimb faults number during the beam walk test | Substantia nigra, Striatum | Neuroprotection, Astrocytic activation in the midbrain | Yes | [112] |
| Water-soluble nano formula-type CoQ10 (ubiquinone) supplementation | Oral | Male and female mice | 15 months | Improvement of the pole test latency | Motor cortex | Brain mitochondrial oxidative phosphorylation dysfunction | Yes | [10] |
| Oral | Male mice | 15–18 months | Improvement of the pole test latency | Primary motor cortex | Age-related decline of neuronal activity in layer V in the primary motor cortex, Brain mitochondrial oxidative phosphorylation dysfunction | Yes | [50] | |
| High-dose CoQ10 (ubiquinol) supplementation | Oral | Male and female multiple-system atrophy patients | Median age 61.0 years | Improvement of SARA score and time required to walk 10 m | Cerebellum, Motor cortex, Putamen | CoQ10 deficiency (COQ2 mutation) | Yes | [108] |
| Anti-Nogo-A antibodies treatment | Continuous intrathecal infusion | Male rats | 5–6 weeks | Increase in the success rate of the pellet-reaching task | Layer II/III and V neurons in the motor cortex | Spine formation, Spine density modulation upon motor learning in the primary motor cortex | Limited | [58] |
| Combination of edonerpic maleate administration and rehabilitative training | Oral | Male mice after motor cortex cryoinjured Male monkey after motor cortex cryoinjured | 5–13 weeks 5 or 6 years | Facilitation of recovery from injury of the motor cortex (the food-reaching task performance) | Layer V pyramidal neurons in the motor cortex | Experience-dependent synaptic AMPA receptor delivery | Limited | [23] |
| Exercise (Increase in activity-dependent BDNF secretion/TrkB phosphorylation) | Mice/Human | Not mentioned | Improvement of motor learning | Layer II/III neurons in the motor cortex | Activity-dependent BDNF secretion/TrkB phosphorylation, BDNF-mediated synaptic plasticity (LTP) | Not applicable | [70,117,119,120,126,127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, R.; Nishimune, H. Neuronal Plasticity and Age-Related Functional Decline in the Motor Cortex. Cells 2023, 12, 2142. https://doi.org/10.3390/cells12172142
Inoue R, Nishimune H. Neuronal Plasticity and Age-Related Functional Decline in the Motor Cortex. Cells. 2023; 12(17):2142. https://doi.org/10.3390/cells12172142
Chicago/Turabian StyleInoue, Ritsuko, and Hiroshi Nishimune. 2023. "Neuronal Plasticity and Age-Related Functional Decline in the Motor Cortex" Cells 12, no. 17: 2142. https://doi.org/10.3390/cells12172142
APA StyleInoue, R., & Nishimune, H. (2023). Neuronal Plasticity and Age-Related Functional Decline in the Motor Cortex. Cells, 12(17), 2142. https://doi.org/10.3390/cells12172142