Pretreatment of Mesenchymal Stem Cells with Electrical Stimulation as a Strategy to Improve Bone Tissue Engineering Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics and Animal Care
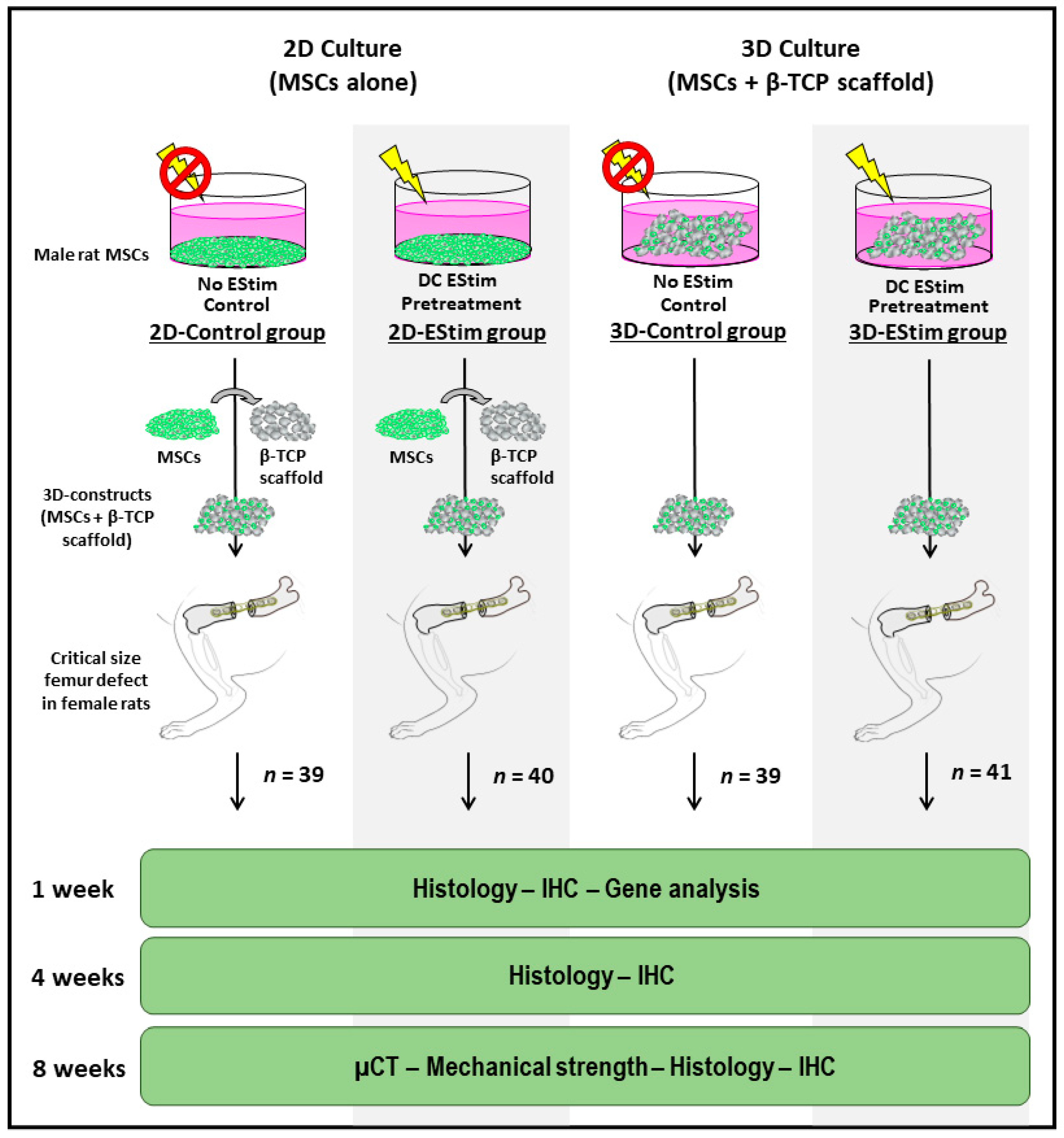
2.2. Treatment Groups
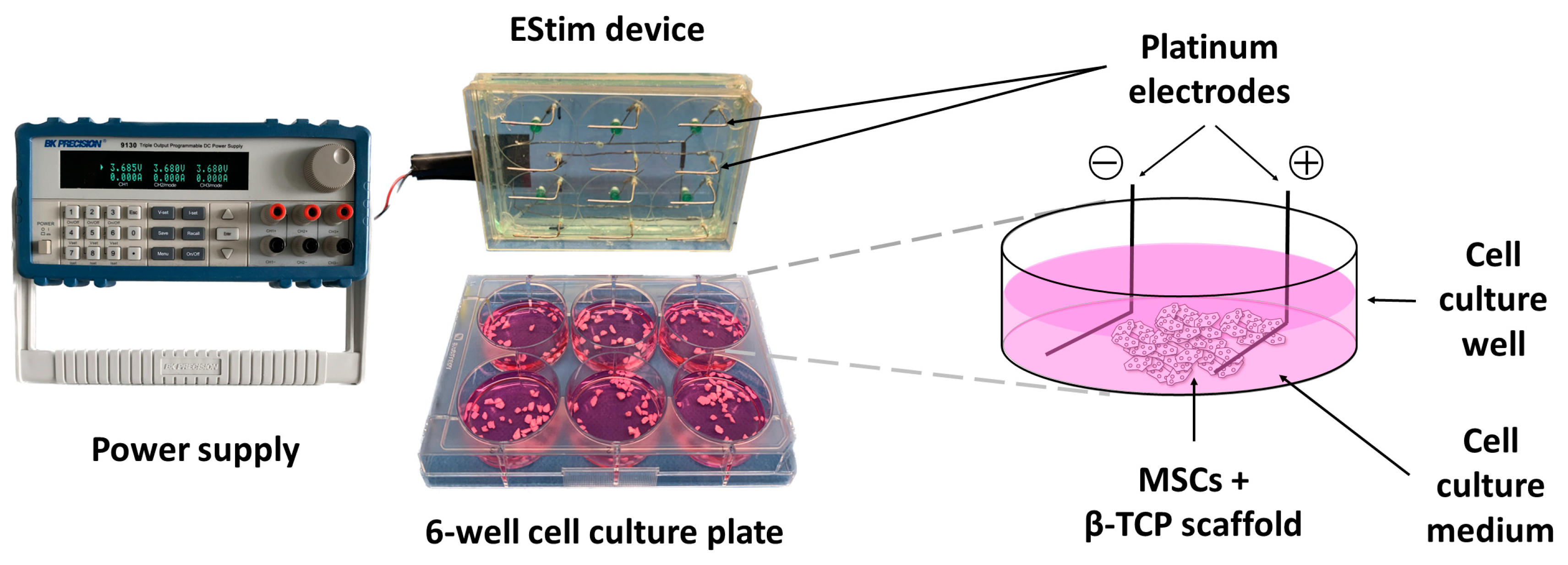
2.3. EStim Pretreatment of MSCs in 2D or 3D Conditions
2.4. Seeding Efficiency
2.5. Cell Metabolic Activity
2.6. Cell Distribution on 3D Constructs
2.7. Rat Critical Size Femur Defect Creation
2.8. Bone Healing Assessment
2.9. Micro-Computed Tomography Analysis
2.10. Bone Mechanical Strength Assessment
2.11. Histological Analysis
2.12. Detection of Transplanted Donor Cells: Y-Chromosome In Situ Hybridization
2.13. New Bone Formation Assessment
2.14. Immunohistochemistry
2.15. In Vivo Gene Expression of Osteogenic and Inflammation Markers
2.16. Statistical Analysis
3. Results
3.1. Seeding Efficiency
3.2. Metabolic Activity
3.3. Cell Distribution on 3D Constructs
3.4. Donor Cell Detection in Defect Tissues
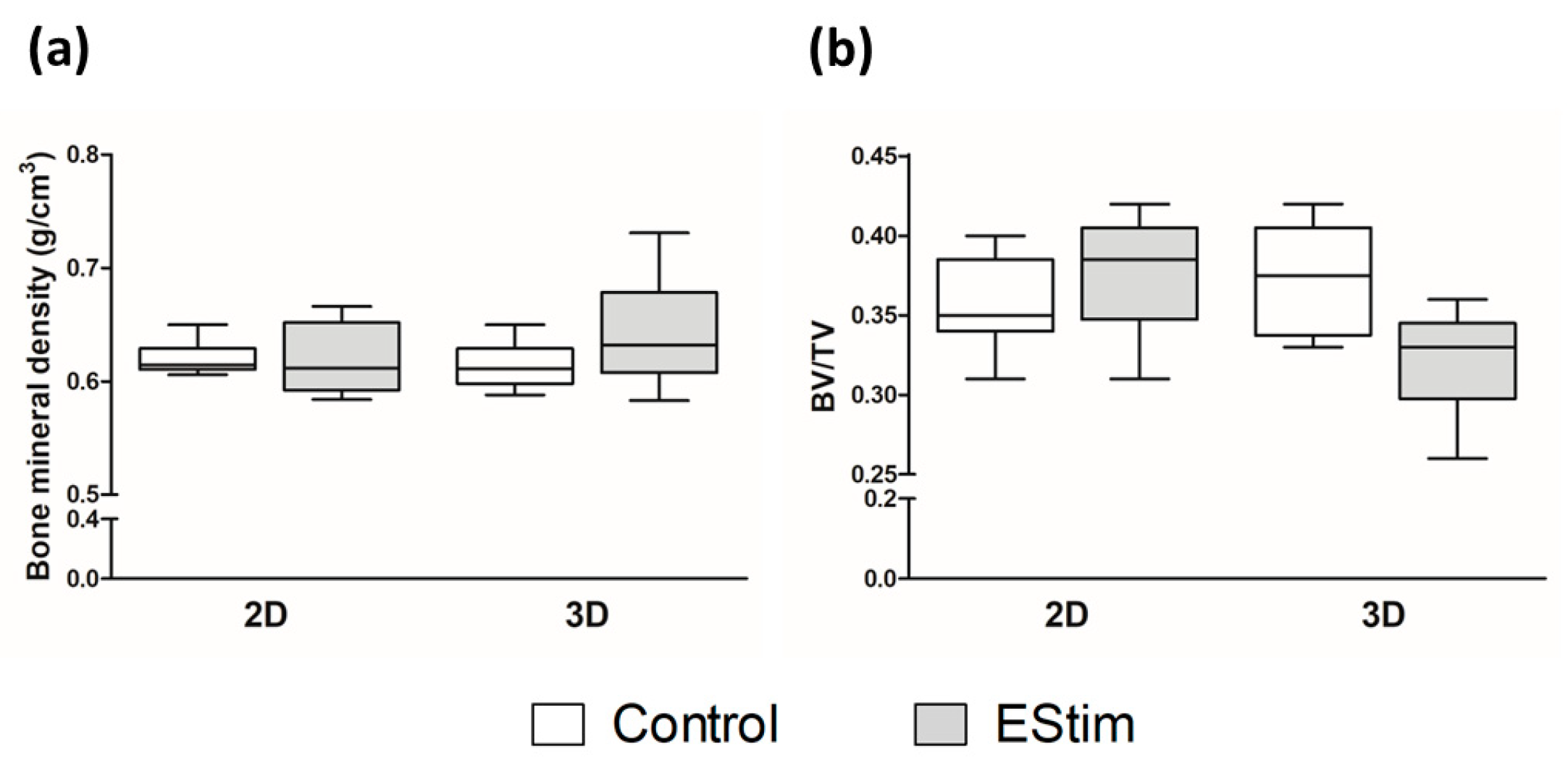
3.5. µCT Analysis
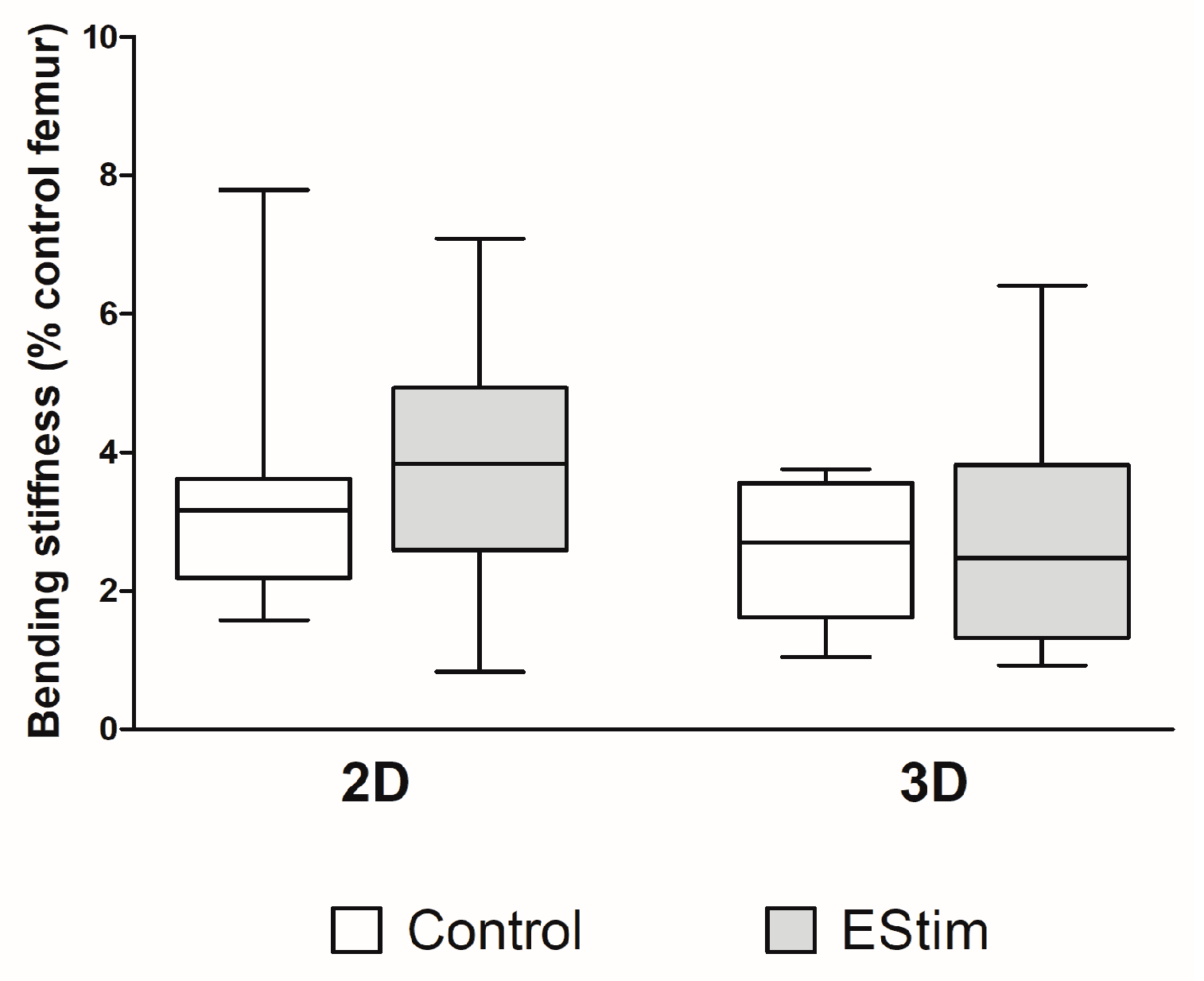
3.6. Bone Mechanical Strength
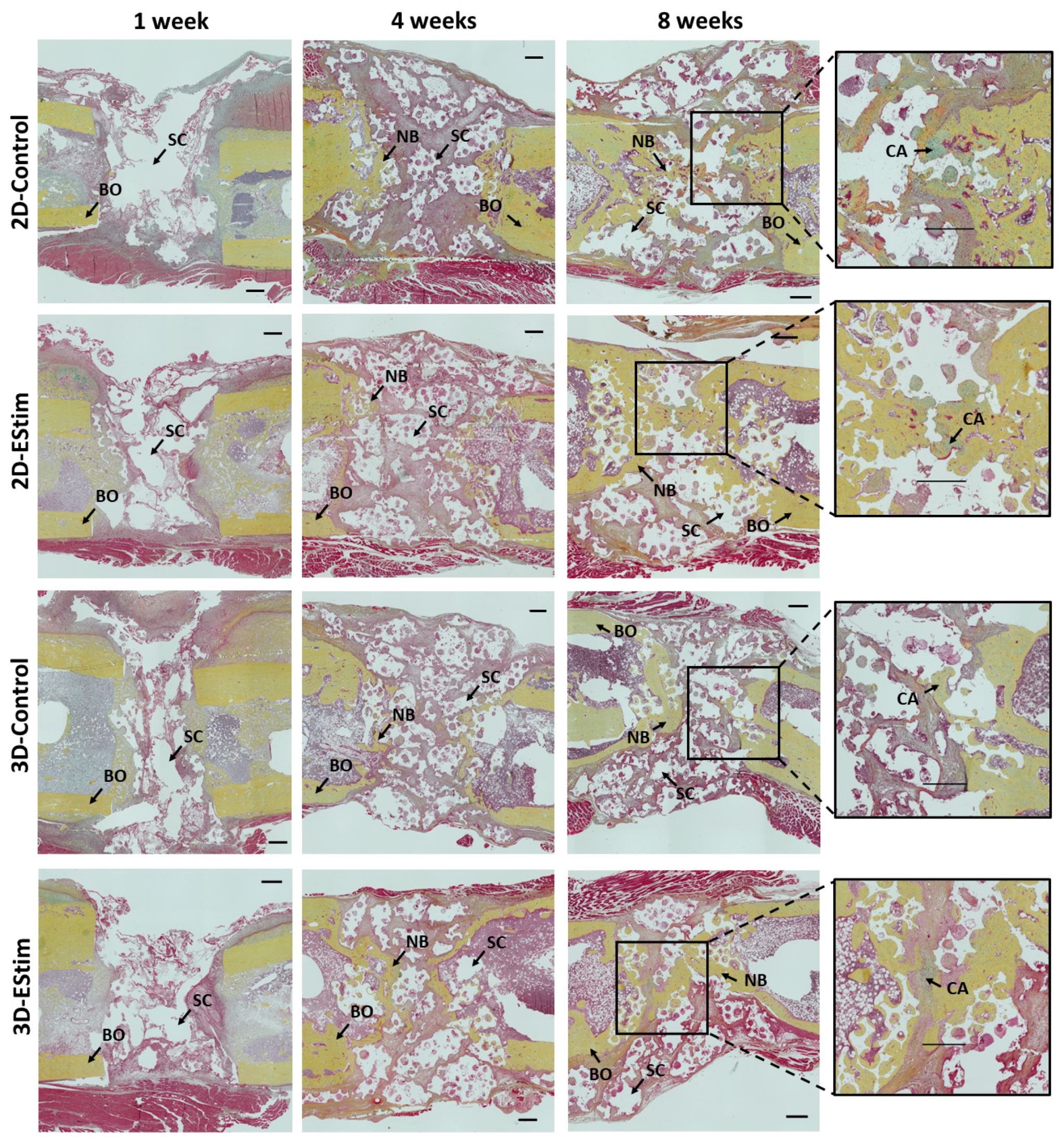
3.7. Histological and Immunohistological Assessment of Bone Healing
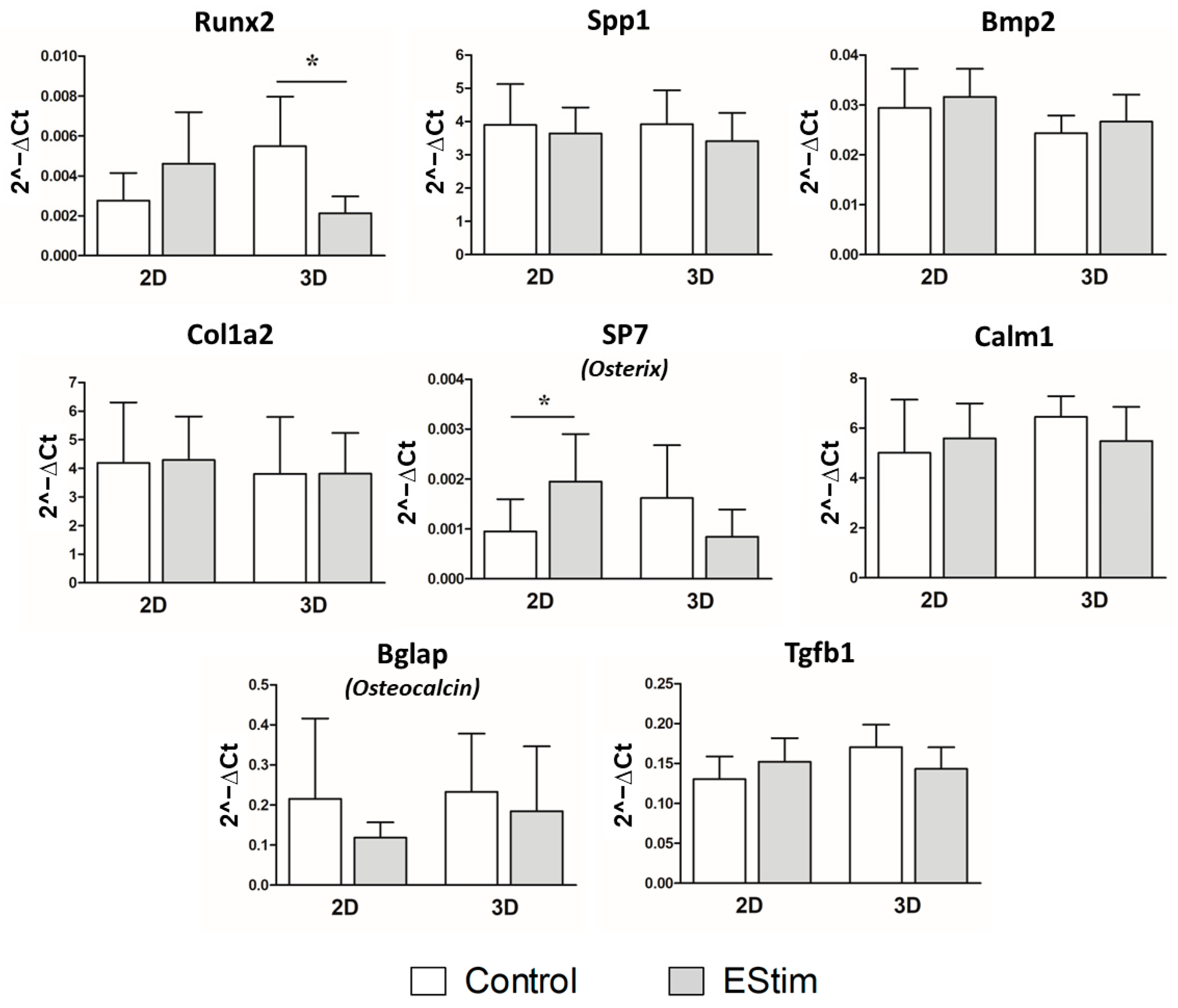
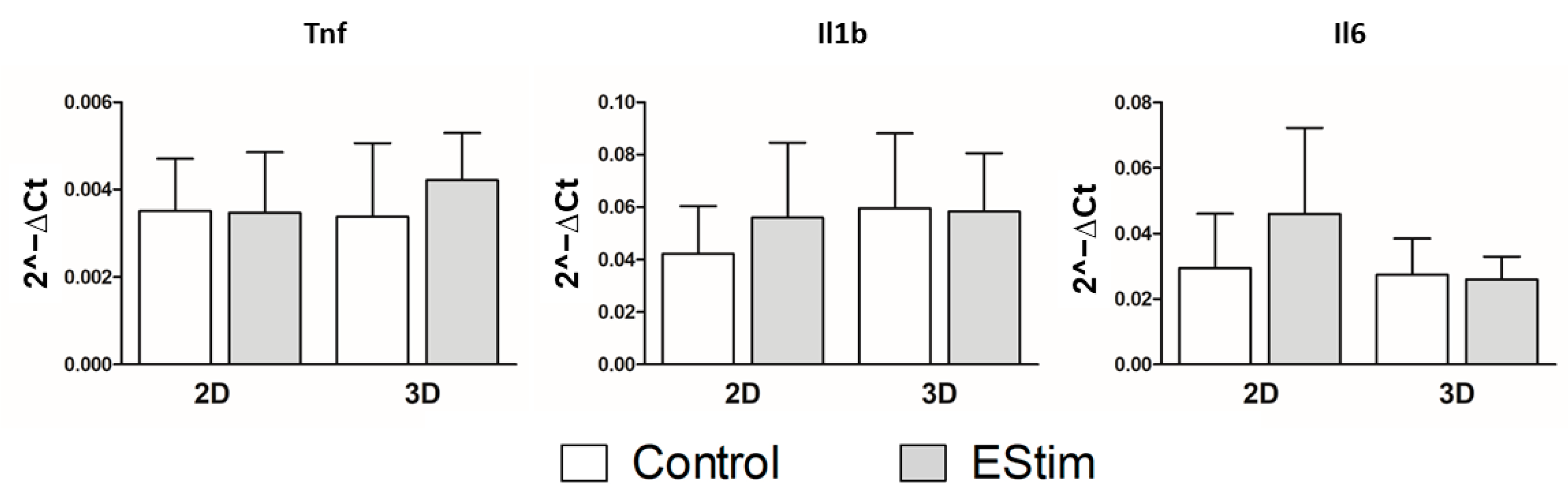
3.8. In Vivo Gene Expression of Osteogenic and Inflammation Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elsalanty, M.E.; Genecov, D.G. Bone Grafts in Craniofacial Surgery. Craniomaxillofac. Trauma Reconstr. 2009, 2, 125–134. [Google Scholar] [CrossRef]
- Bastami, F.; Nazeman, P.; Moslemi, H.; Rezai Rad, M.; Sharifi, K.; Khojasteh, A. Induced pluripotent stem cells as a new getaway for bone tissue engineering: A systematic review. Cell Prolif. 2017, 50, e12321. [Google Scholar] [CrossRef] [PubMed]
- Melek, L.N. Tissue engineering in oral and maxillofacial reconstruction. Tanta Dent. J. 2015, 12, 211–223. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Olivares-Navarrete, R.; Rettew, R.; Butera, R.J.; Alamgir, F.M.; Boyan, B.D.; Schwartz, Z. Electrical polarization of titanium surfaces for the enhancement of osteoblast differentiation. Bioelectromagnetics 2013, 34, 599–612. [Google Scholar] [CrossRef]
- Hu, W.W.; Hsu, Y.T.; Cheng, Y.C.; Li, C.; Ruaan, R.C.; Chien, C.C.; Chung, C.A.; Tsao, C.W. Electrical stimulation to promote osteogenesis using conductive polypyrrole films. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 37, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Mobini, S.; Leppik, L.; Barker, J.H. Direct current electrical stimulation chamber for treating cells in vitro. Biotechniques 2016, 60, 95–98. [Google Scholar] [CrossRef]
- Kwon, H.J.; Lee, G.S.; Chun, H. Electrical stimulation drives chondrogenesis of mesenchymal stem cells in the absence of exogenous growth factors. Sci. Rep. 2016, 6, 39302. [Google Scholar] [CrossRef]
- Mobini, S.; Leppik, L.; Thottakkattumana Parameswaran, V.; Barker, J.H. In vitro effect of direct current electrical stimulation on rat mesenchymal stem cells. PeerJ 2017, 5, e2821. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Kang, E.T.; Neoh, K.G. Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels. Acta Biomater. 2016, 32, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Leppik, L.P.; Froemel, D.; Slavici, A.; Ovadia, Z.N.; Hudak, L.; Henrich, D.; Marzi, I.; Barker, J.H. Effects of electrical stimulation on rat limb regeneration, a new look at an old model. Sci. Rep. 2015, 5, 18353. [Google Scholar] [CrossRef] [PubMed]
- Leppik, L.; Zhihua, H.; Mobini, S.; Thottakkattumana Parameswaran, V.; Eischen-Loges, M.; Slavici, A.; Helbing, J.; Pindur, L.; Oliveira, K.M.C.; Bhavsar, M.B.; et al. Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci. Rep. 2018, 8, 6307. [Google Scholar] [CrossRef]
- Eischen-Loges, M.; Oliveira, K.M.C.; Bhavsar, M.B.; Barker, J.H.; Leppik, L. Pretreating mesenchymal stem cells with electrical stimulation causes sustained long-lasting pro-osteogenic effects. PeerJ 2018, 6, e4959. [Google Scholar] [CrossRef]
- Leppik, L.; Sielatycka, K.; Henrich, D.; Han, Z.; Wang, H.; Eischen-Loges, M.J.; Oliveira, K.M.C.; Bhavsar, M.B.; Ratajczak, M.Z.; Barker, J.H. Role of Adult Tissue-Derived Pluripotent Stem Cells in Bone Regeneration. Stem Cell Rev. Rep. 2020, 16, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Leiblein, M.; Koch, E.; Winkenbach, A.; Schaible, A.; Nau, C.; Büchner, H.; Schröder, K.; Marzi, I.; Henrich, D. Size matters: Effect of granule size of the bone graft substitute (Herafill®) on bone healing using Masquelet’s induced membrane in a critical size defect model in the rat’s femur. J. Biomed. Mater. Res. Part. B App. Biomater. 2020, 108, 1469–1482. [Google Scholar] [CrossRef] [PubMed]
- Nau, C.; Henrich, D.; Seebach, C.; Schröder, K.; Fitzsimmons, S.J.; Hankel, S.; Barker, J.H.; Marzi, I.; Frank, J. Treatment of Large Bone Defects with a Vascularized Periosteal Flap in Combination with Biodegradable Scaffold Seeded with Bone Marrow-Derived Mononuclear Cells: An Experimental Study in Rats. Tissue Eng. Part A 2016, 22, 133–141. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Schaible, A.; Relja, B.; Jugold, M.; Bönig, H.; Marzi, I. Cell-based therapy by implanted human bone marrow-derived mononuclear cells improved bone healing of large bone defects in rats. Tissue Eng. Part A 2015, 21, 1565–1578. [Google Scholar] [CrossRef]
- Söhling, N.; Heilani, M.; Fremdling, C.; Schaible, A.; Schröder, K.; Brune, J.C.; Eras, V.; Nau, C.; Marzi, I.; Henrich, D.; et al. One Stage Masquelets Technique: Evaluation of Different Forms of Membrane Filling with and without Bone Marrow Mononuclear Cells (BMC) in Large Femoral Bone Defects in Rats. Cells 2023, 12, 1289. [Google Scholar] [CrossRef]
- Verboket, R.D.; Irrle, T.; Busche, Y.; Schaible, A.; Schröder, K.; Brune, J.C.; Marzi, I.; Nau, C.; Henrich, D. Fibrous Demineralized Bone Matrix (DBM) Improves Bone Marrow Mononuclear Cell (BMC)-Supported Bone Healing in Large Femoral Bone Defects in Rats. Cells 2021, 10, 1249. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Seebach, C.; Henrich, D.; Kähling, C.; Wilhelm, K.; Tami, A.E.; Alini, M.; Marzi, I. Endothelial progenitor cells and mesenchymal stem cells seeded onto beta-TCP granules enhance early vascularization and bone healing in a critical-sized bone defect in rats. Tissue Eng. Part A 2010, 16, 1961–1970. [Google Scholar] [CrossRef]
- Garvey, W.; Fathi, A.; Bigelow, F.; Carpenter, B.; Jimenez, C. Improved Movat pentachrome stain. Stain. Technol. 1986, 61, 60–62. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Schell, H.; Lienau, J.; Schulz, N.; Hoff, P.; Pfaff, M.; Schmidt, G.; Martin, C.; Perka, C.; Buttgereit, F.; et al. Initial immune reaction and angiogenesis in bone healing. J. Tissue Eng. Regen. Med. 2014, 8, 120–130. [Google Scholar] [CrossRef]
- Curtis, K.M.; Gomez, L.A.; Rios, C.; Garbayo, E.; Raval, A.P.; Perez-Pinzon, M.A.; Schiller, P.C. EF1alpha and RPL13a represent normalization genes suitable for RT-qPCR analysis of bone marrow derived mesenchymal stem cells. BMC Mol. Biol. 2010, 11, 61. [Google Scholar] [CrossRef]
- Morse, D.L.; Carroll, D.; Weberg, L.; Borgstrom, M.C.; Ranger-Moore, J.; Gillies, R.J. Determining suitable internal standards for mRNA quantification of increasing cancer progression in human breast cells by real-time reverse transcriptase polymerase chain reaction. Anal. Biochem. 2005, 342, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Shiyun, M.; Mahmoud, R.; Ze, Z. Electrical Stimulation in Tissue Regeneration. In Applied Biomedical Engineering; Gaetano, D.G., Alistair, M., Eds.; IntechOpen: Rijeka, Croatia, 2011; Chapter 3. [Google Scholar] [CrossRef]
- Staehlke, S.; Bielfeldt, M.; Zimmermann, J.; Gruening, M.; Barke, I.; Freitag, T.; Speller, S.; Van Rienen, U.; Nebe, B. Pulsed Electrical Stimulation Affects Osteoblast Adhesion and Calcium Ion Signaling. Cells 2022, 11, 2650. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Titushkin, I.; Cho, M. Regulation of mesenchymal stem cell adhesion and orientation in 3D collagen scaffold by electrical stimulus. Bioelectrochemistry 2006, 69, 133–141. [Google Scholar] [CrossRef]
- Samal, J.R.K.; Rangasami, V.K.; Samanta, S.; Varghese, O.P.; Oommen, O.P. Discrepancies on the Role of Oxygen Gradient and Culture Condition on Mesenchymal Stem Cell Fate. Adv. Health Mater. 2021, 10, e2002058. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.Y.; Sagun, J.E.; Leach, J.K. Reduced serum and hypoxic culture conditions enhance the osteogenic potential of human mesenchymal stem cells. Stem Cell Rev. Rep. 2015, 11, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Boyette, L.B.; Creasey, O.A.; Guzik, L.; Lozito, T.; Tuan, R.S. Human bone marrow-derived mesenchymal stem cells display enhanced clonogenicity but impaired differentiation with hypoxic preconditioning. Stem Cells Transl. Med. 2014, 3, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.; Hung, B.P.; Heyrani, N.; Lee, M.A.; Leach, J.K. Hypoxic Preconditioning of Mesenchymal Stem Cells with Subsequent Spheroid Formation Accelerates Repair of Segmental Bone Defects. Stem Cells 2018, 36, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Seebach, C.; Schultheiss, J.; Wilhelm, K.; Frank, J.; Henrich, D. Comparison of six bone-graft substitutes regarding to cell seeding efficiency, metabolism and growth behaviour of human mesenchymal stem cells (MSC) in vitro. Injury 2010, 41, 731–738. [Google Scholar] [CrossRef]
- Henrich, D.; Seebach, C.; Kaehling, C.; Scherzed, A.; Wilhelm, K.; Tewksbury, R.; Powerski, M.; Marzi, I. Simultaneous cultivation of human endothelial-like differentiated precursor cells and human marrow stromal cells on beta-tricalcium phosphate. Tissue Eng. Part C Methods 2009, 15, 551–560. [Google Scholar] [CrossRef]
- Störmann, P.; Kupsch, J.; Kontradowitz, K.; Leiblein, M.; Verboket, R.; Seebach, C.; Marzi, I.; Henrich, D.; Nau, C. Cultivation of EPC and co-cultivation with MSC on β-TCP granules in vitro is feasible without fibronectin coating but influenced by scaffolds’ design. Eur. J. Trauma Emerg. Surg. 2019, 45, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, Y.; Shi, H.; Liu, N.; Zhang, W.; Li, H. Influence of the intensity and loading time of direct current electric field on the directional migration of rat bone marrow mesenchymal stem cells. Front. Med. 2016, 10, 286–296. [Google Scholar] [CrossRef]
- Rhee, S.G. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Bodamyali, T.; Kanczler, J.M.; Simon, B.; Blake, D.R.; Stevens, C.R. Effect of faradic products on direct current-stimulated calvarial organ culture calcium levels. Biochem. Biophys. Res. Commun. 1999, 264, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical stimulation: A novel tool for tissue engineering. Tissue Eng. Part B Rev. 2013, 19, 48–57. [Google Scholar] [CrossRef]
- Baht, G.S.; Vi, L.; Alman, B.A. The Role of the Immune Cells in Fracture Healing. Curr. Osteoporos. Rep. 2018, 16, 138–145. [Google Scholar] [CrossRef]
- Shiu, H.T.; Leung, P.C.; Ko, C.H. The roles of cellular and molecular components of a hematoma at early stage of bone healing. J. Tissue Eng. Regen. Med. 2018, 12, e1911–e1925. [Google Scholar] [CrossRef] [PubMed]
- Schlundt, C.; Fischer, H.; Bucher, C.H.; Rendenbach, C.; Duda, G.N.; Schmidt-Bleek, K. The multifaceted roles of macrophages in bone regeneration: A story of polarization, activation and time. Acta Biomater. 2021, 133, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Janko, M.; Pöllinger, S.; Schaible, A.; Bellen, M.; Schröder, K.; Heilani, M.; Fremdling, C.; Marzi, I.; Nau, C.; Henrich, D.; et al. Determination of the effective dose of bone marrow mononuclear cell therapy for bone healing in vivo. Eur. J. Trauma Emerg. Surg. 2020, 46, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.M.C.; Barker, J.H.; Berezikov, E.; Pindur, L.; Kynigopoulos, S.; Eischen-Loges, M.; Han, Z.; Bhavsar, M.B.; Henrich, D.; Leppik, L. Electrical stimulation shifts healing/scarring towards regeneration in a rat limb amputation model. Sci. Rep. 2019, 9, 11433. [Google Scholar] [CrossRef]
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89. [Google Scholar] [CrossRef]
- Lacey, D.C.; Simmons, P.J.; Graves, S.E.; Hamilton, J.A. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: Implications for bone repair during inflammation. Osteoarthr. Cartil. 2009, 17, 735–742. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, N.; Xu, X.; Xu, Y.; Li, S.; Zhang, J.; Yang, P. Dose-specific effects of tumor necrosis factor alpha on osteogenic differentiation of mesenchymal stem cells. Cell Prolif. 2011, 44, 420–427. [Google Scholar] [CrossRef]
- Wang, H.; Ni, Z.; Yang, J.; Li, M.; Liu, L.; Pan, X.; Xu, L.; Wang, X.; Fang, S. IL-1β promotes osteogenic differentiation of mouse bone marrow mesenchymal stem cells via the BMP/Smad pathway within a certain concentration range. Exp. Ther. Med. 2020, 20, 3001–3008. [Google Scholar] [CrossRef]
- Wang, M.K.; Sun, H.Q.; Xiang, Y.C.; Jiang, F.; Su, Y.P.; Zou, Z.M. Different roles of TGF-β in the multi-lineage differentiation of stem cells. World J. Stem Cells 2012, 4, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Pricola, K.L.; Kuhn, N.Z.; Haleem-Smith, H.; Song, Y.; Tuan, R.S. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J. Cell. Biochem. 2009, 108, 577–588. [Google Scholar] [CrossRef]
- Xie, Z.; Tang, S.; Ye, G.; Wang, P.; Li, J.; Liu, W.; Li, M.; Wang, S.; Wu, X.; Cen, S.; et al. Interleukin-6/interleukin-6 receptor complex promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Stem Cell. Res. Ther. 2018, 9, 13. [Google Scholar] [CrossRef]
- Marupanthorn, K.; Tantrawatpan, C.; Kheolamai, P.; Tantikanlayaporn, D.; Manochantr, S. Bone morphogenetic protein-2 enhances the osteogenic differentiation capacity of mesenchymal stromal cells derived from human bone marrow and umbilical cord. Int. J. Mol. Med. 2017, 39, 654–662. [Google Scholar] [CrossRef]
- Elsafadi, M.; Manikandan, M.; Almalki, S.; Mobarak, M.; Atteya, M.; Iqbal, Z.; Hashmi, J.A.; Shaheen, S.; Alajez, N.; Alfayez, M.; et al. TGFβ1-Induced Differentiation of Human Bone Marrow-Derived MSCs Is Mediated by Changes to the Actin Cytoskeleton. Stem Cells Int. 2018, 2018, 6913594. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current Insights into Collagen Type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Ge, C.; Xiao, G.; Roca, H.; Jiang, D. Transcriptional Regulation of Osteoblasts. Ann. N. Y. Acad. Sci. 2007, 1116, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Janeczek Portalska, K.; Leferink, A.; Groen, N.; Fernandes, H.; Moroni, L.; van Blitterswijk, C.; de Boer, J. Endothelial differentiation of mesenchymal stromal cells. PLoS ONE 2012, 7, e46842. [Google Scholar] [CrossRef] [PubMed]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Wilhelm, K.; Barker, J.H.; Marzi, I. Endothelial progenitor cells improve directly and indirectly early vascularization of mesenchymal stem cell-driven bone regeneration in a critical bone defect in rats. Cell Transplant. 2012, 21, 1667–1677. [Google Scholar] [CrossRef]
- Mostafavi, E.; Medina-Cruz, D.; Kalantari, K.; Taymoori, A.; Soltantabar, P.; Webster, T.J. Electroconductive Nanobiomaterials for Tissue Engineering and Regenerative Medicine. Bioelectricity 2020, 2, 120–149. [Google Scholar] [CrossRef]
- Dixon, D.T.; Gomillion, C.T. Conductive Scaffolds for Bone Tissue Engineering: Current State and Future Outlook. J. Funct. Biomater. 2021, 13, 1. [Google Scholar] [CrossRef]
- Asri, N.A.N.; Mahat, M.M.; Zakaria, A.; Safian, M.F.; Abd Hamid, U.M. Fabrication Methods of Electroactive Scaffold-Based Conducting Polymers for Tissue Engineering Application: A Review. Front. Bioeng. Biotechnol. 2022, 10, 876696. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianconi, S.; Oliveira, K.M.C.; Klein, K.-L.; Wolf, J.; Schaible, A.; Schröder, K.; Barker, J.; Marzi, I.; Leppik, L.; Henrich, D. Pretreatment of Mesenchymal Stem Cells with Electrical Stimulation as a Strategy to Improve Bone Tissue Engineering Outcomes. Cells 2023, 12, 2151. https://doi.org/10.3390/cells12172151
Bianconi S, Oliveira KMC, Klein K-L, Wolf J, Schaible A, Schröder K, Barker J, Marzi I, Leppik L, Henrich D. Pretreatment of Mesenchymal Stem Cells with Electrical Stimulation as a Strategy to Improve Bone Tissue Engineering Outcomes. Cells. 2023; 12(17):2151. https://doi.org/10.3390/cells12172151
Chicago/Turabian StyleBianconi, Santiago, Karla M. C. Oliveira, Kari-Leticia Klein, Jakob Wolf, Alexander Schaible, Katrin Schröder, John Barker, Ingo Marzi, Liudmila Leppik, and Dirk Henrich. 2023. "Pretreatment of Mesenchymal Stem Cells with Electrical Stimulation as a Strategy to Improve Bone Tissue Engineering Outcomes" Cells 12, no. 17: 2151. https://doi.org/10.3390/cells12172151
APA StyleBianconi, S., Oliveira, K. M. C., Klein, K.-L., Wolf, J., Schaible, A., Schröder, K., Barker, J., Marzi, I., Leppik, L., & Henrich, D. (2023). Pretreatment of Mesenchymal Stem Cells with Electrical Stimulation as a Strategy to Improve Bone Tissue Engineering Outcomes. Cells, 12(17), 2151. https://doi.org/10.3390/cells12172151







