MicroRNA-375 Is Induced during Astrocyte-to-Neuron Reprogramming and Promotes Survival of Reprogrammed Neurons when Overexpressed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Use
2.2. Virus Production
2.3. Human Astrocyte Culture, Virus Infection, and Cisplatin Treatment
2.4. RNA-seq and Bioinformatics Analyses
2.5. Laminectomy, Spinal Cord Injury, and Stereotaxic Viral Injection
2.6. Mouse Astrocyte Isolation and Culturing
2.7. Fluorescence Activated Cell Sorting (FACS)
2.8. Immunocytochemistry and Immunohistochemistry
2.9. Western Blot Analysis
2.10. Quantitative Reverse Transcriptase PCR (qRT-PCR)
2.11. Measurements and Statistical Analysis
3. Results
3.1. NeuroD1 Activates Downstream Neuronal Genes to Initiate AtN Reprogramming
3.2. Identification of NeuroD1-Induced “Core” Genes during Glia-to-Neuron Reprogramming
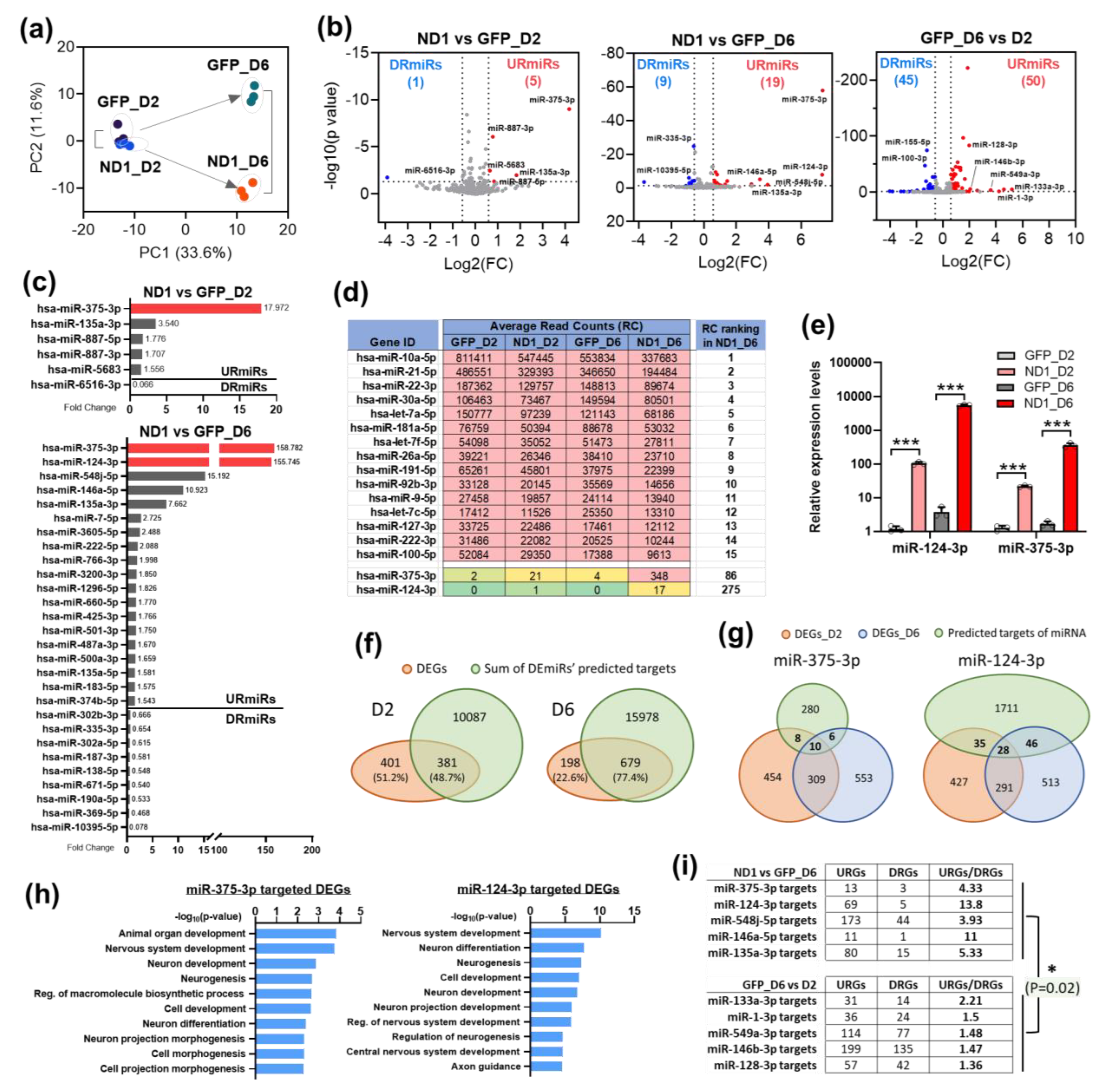
3.3. NeuroD1 Induces miRNA Expression Changes during AtN Reprogramming
3.4. MiR-375 Regulates nELAVLs’ Expression Level during NeuroD1-Mediated AtN Reprogramming
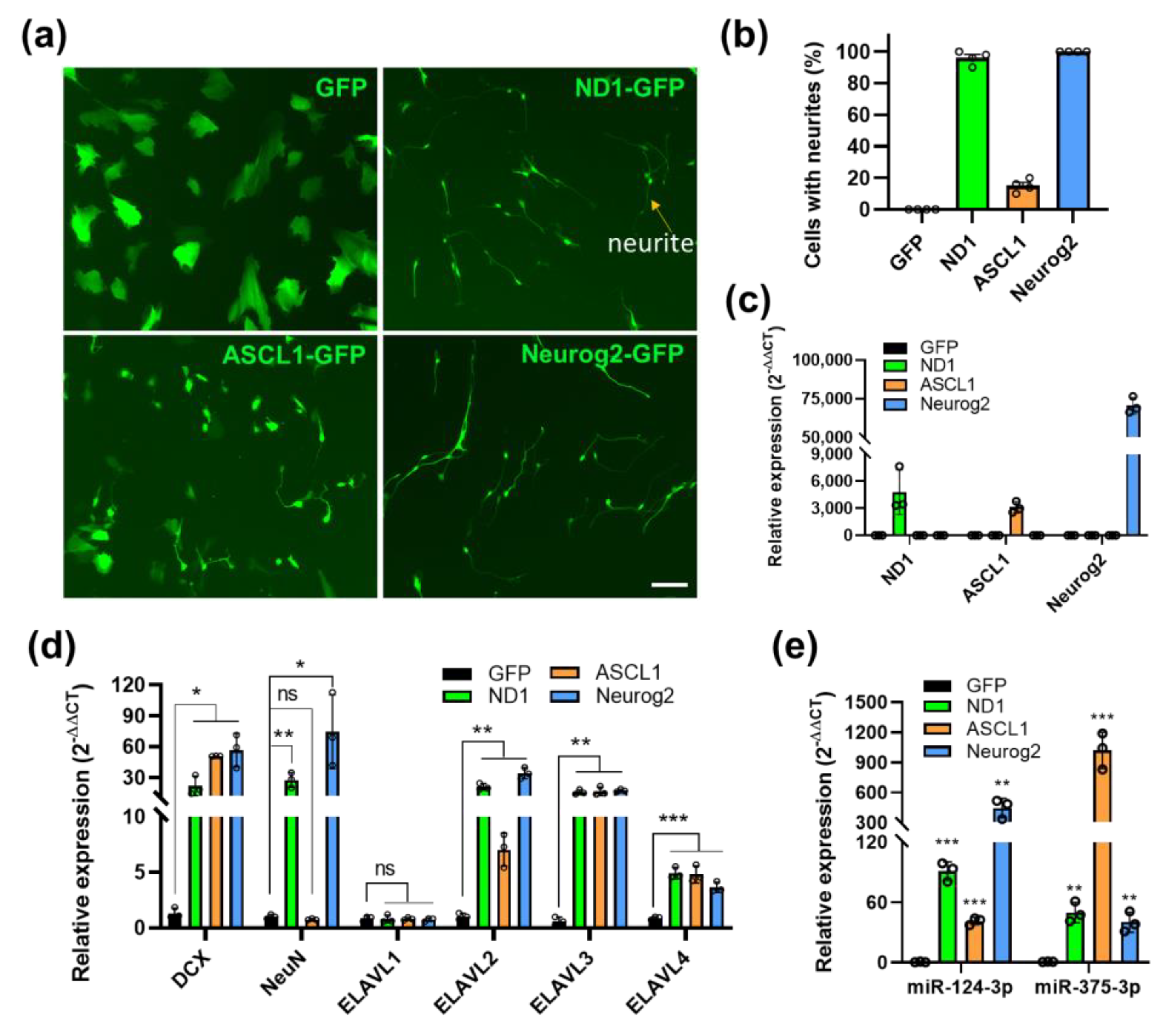
3.5. MiR-375/nELAVLs Are also Induced by Neuronal Reprogramming Factors ASCL1 and Neurog2
3.6. NeuroD1 Induces ELAVL4 Expression in the Injured Mouse Spinal Cord and miR-375/ELAVL4 Expression in Mouse Astrocyte Cultures during AtN Reprogramming
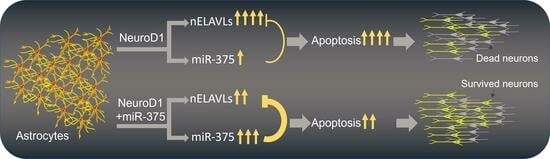
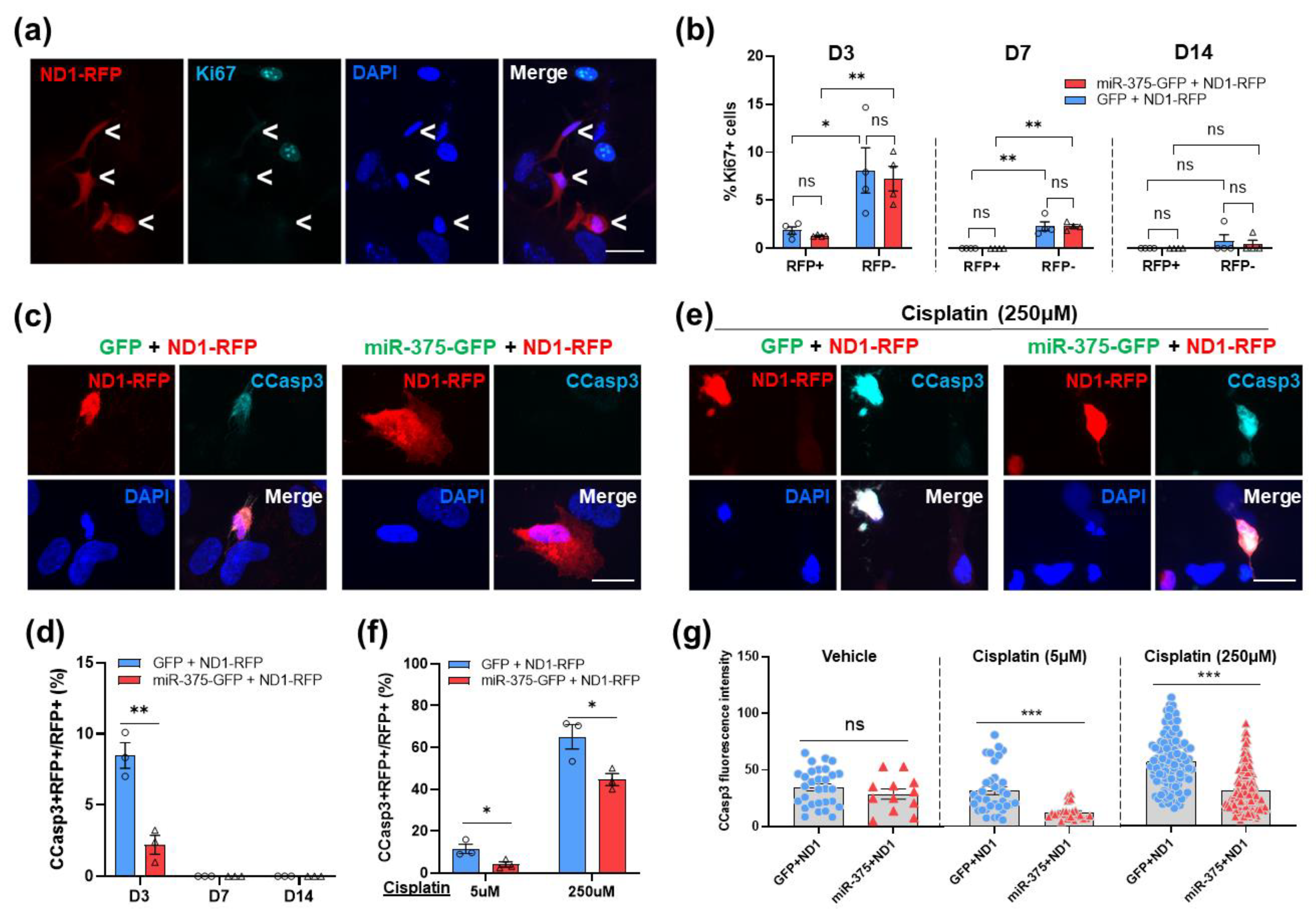
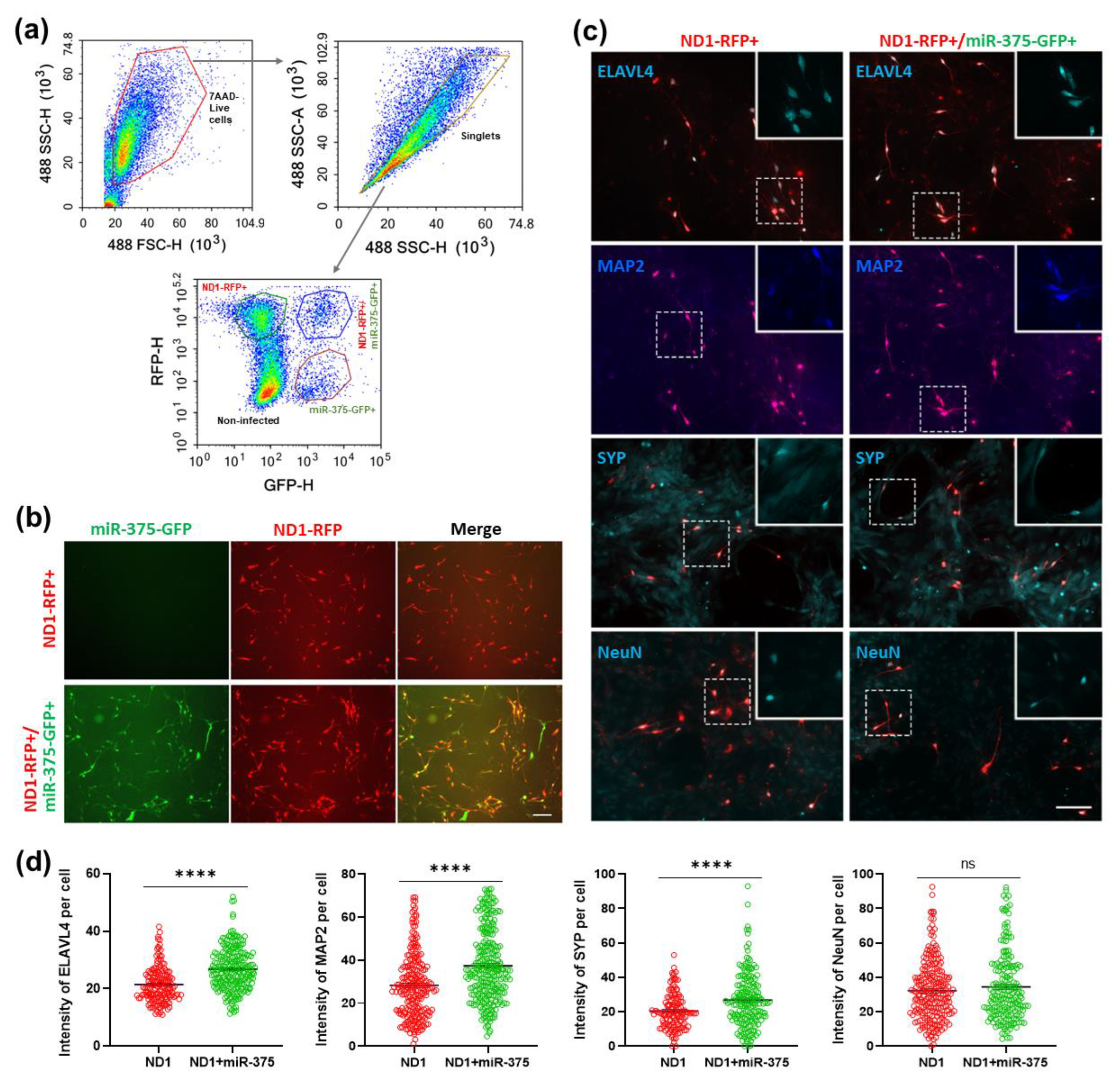
3.7. Overexpression of miR-375 Elicits a Protective Effect during NeuroD1-Mediated AtN Reprogramming by Reducing Apoptosis
3.8. Overexpression of ELAVL4 Triggers Apoptosis in HA and Reverses miR-375-Induced Survival-Promoting Effect during NeuroD1-Mediated AtN Reprogramming
4. Discussion
4.1. MiR-375 Is a Transcriptional Target of NeuroD1
4.2. MiR-375 Regulates Levels of nELAVLs during AtN Reprogramming
4.3. MiR-375 Is Neuroprotective during AtN Reprogramming
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.-L.; Serrano, C.; Zhong, X.; Ma, S.; Zou, Y.; Zhang, C.-L. Revisiting astrocyte to neuron conversion with lineage tracing in vivo. Cell 2021, 184, 5465–5481.e16. [Google Scholar] [CrossRef]
- Wang, Q.; Li, W.; Lei, W.; Chen, G.; Xiang, Z.; Xu, L.; Liu, M. Lineage tracing of direct astrocyte-to-neuron conversion in the mouse cortex. Neural Regen. Res. 2021, 16, 750–756. [Google Scholar] [CrossRef]
- Hoang, T.; Kim, D.W.; Appel, H.; Ozawa, M.; Zheng, S.; Kim, J.; Blackshaw, S. Ptbp1 deletion does not induce astrocyte-to-neuron conversion. Nature 2023, 618, E1–E7. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, L.; Wu, Z.; Chen, Y.; Wang, F.; Chen, G. In Vivo Direct Reprogramming of Reactive Glial Cells into Functional Neurons after Brain Injury and in an Alzheimer’s Disease Model. Cell Stem Cell 2013, 14, 188–202. [Google Scholar] [CrossRef]
- Su, Z.; Niu, W.; Liu, M.-L.; Zou, Y.; Zhang, C.-L. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 2014, 5, 3338. [Google Scholar] [CrossRef]
- Heinrich, C.; Bergami, M.; Gascón, S.; Lepier, A.; Viganò, F.; Dimou, L.; Sutor, B.; Berninger, B.; Götz, M. Sox2-Mediated Conversion of NG2 Glia into Induced Neurons in the Injured Adult Cerebral Cortex. Stem Cell Rep. 2014, 3, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Kang, X.; Hu, J.; Zhang, D.; Liang, Z.; Meng, F.; Zhang, X.; Xue, Y.; Maimon, R.; Dowdy, S.F.; et al. Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature 2020, 582, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, X. Neuronal reprogramming in treating spinal cord injury. Neural. Regen. Res. 2022, 17, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; Xu, X.-M.; Zhang, C.-L. Regeneration Through in vivo Cell Fate Reprogramming for Neural Repair. Front. Cell. Neurosci. 2020, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, G. In Vivo Reprogramming for CNS Repair: Regenerating Neurons from Endogenous Glial Cells. Neuron 2016, 91, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Puls, B.; Ding, Y.; Zhang, F.; Pan, M.; Lei, Z.; Pei, Z.; Jiang, M.; Bai, Y.; Forsyth, C.; Metzger, M.; et al. Regeneration of Functional Neurons After Spinal Cord Injury via in situ NeuroD1-Mediated Astrocyte-to-Neuron Conversion. Front. Cell. Dev. Biol. 2020, 8, 1595. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Irie, T.; Katsurabayashi, S.; Hayashi, Y.; Nagai, T.; Hamazaki, N.; Adefuin, A.M.D.; Miura, F.; Ito, T.; Kimura, H.; et al. Pioneer Factor NeuroD1 Rearranges Transcriptional and Epigenetic Profiles to Execute Microglia-Neuron Conversion. Neuron 2019, 101, 472–485.e7. [Google Scholar] [CrossRef] [PubMed]
- Brulet, R.; Matsuda, T.; Zhang, L.; Miranda, C.; Giacca, M.; Kaspar, B.K.; Nakashima, K.; Hsieh, J. NEUROD1 Instructs Neuronal Conversion in Non-Reactive Astrocytes. Stem Cell. Rep. 2017, 8, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Maeda, T.; Lee, J.E. NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes Dev. 1999, 13, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Iwafuchi-Doi, M.; Zaret, K.S. Pioneer transcription factors in cell reprogramming. Genes Dev. 2014, 28, 2679–2692. [Google Scholar] [CrossRef] [PubMed]
- Pataskar, A.; Jung, J.; Smialowski, P.; Noack, F.; Calegari, F.; Straub, T.; Tiwari, V.K. NeuroD1 reprograms chromatin and transcription factor landscapes to induce the neuronal program. EMBO J. 2016, 35, 24–45. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004. 116, 281–297.
- Rajman, M.; Schratt, G. MicroRNAs in neural development: From master regulators to fine-tuners. Development 2017, 144, 2310–2322. [Google Scholar] [CrossRef]
- Zhang, C.; Ge, X.; Liu, Q.; Jiang, M.; Li, M.W.; Li, H. MicroRNA-mediated non-cell-autonomous regulation of cortical radial glial transformation revealed by a Dicer1 knockout mouse model. Glia 2015, 63, 860–876. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.H.; Cuellar, T.L.; Koch, S.M.; Barker, A.J.; Harfe, B.D.; McManus, M.T.; Ullian, E.M. Conditional Loss of Dicer Disrupts Cellular and Tissue Morphogenesis in the Cortex and Hippocampus. J. Neurosci. 2008, 28, 4322–4330. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, H.; Fineberg, S.; Ghosh, L.; Tecedor, L.; Davidson, B. Dicer is required for proliferation, viability, migration and differentiation in corticoneurogenesis. Neuroscience 2012, 223, 285–295. [Google Scholar] [CrossRef]
- Nowakowski, T.J.; Mysiak, K.S.; Pratt, T.; Price, D.J. Functional Dicer Is Necessary for Appropriate Specification of Radial Glia during Early Development of Mouse Telencephalon. PLoS ONE 2011, 6, e23013. [Google Scholar] [CrossRef]
- Kuang, Y.; Liu, Q.; Shu, X.; Zhang, C.; Huang, N.; Li, J.; Jiang, M.; Li, H. Dicer1 and MiR-9 are required for proper Notch1 signaling and the Bergmann glial phenotype in the developing mouse cerebellum. Glia 2012, 60, 1734–1746. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jiang, M.; Kuang, Y.; Shu, X.; Li, J.; Li, M.W.; Li, H. Dicer1 Ablation Impairs Responsiveness of Cerebellar Granule Neuron Precursors to Sonic Hedgehog and Disrupts Expression of Distinct Cell Cycle Regulator Genes. Cerebellum 2016, 16, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Wu, H.; Lin, Q.; Wei, W.; Lu, X.-H.; Cantle, J.P.; Ao, Y.; Olsen, R.W.; Yang, X.W.; Mody, I.; et al. Deletion of Astroglial Dicer Causes Non-Cell-Autonomous Neuronal Dysfunction and Degeneration. J. Neurosci. 2011, 31, 8306–8319. [Google Scholar] [CrossRef] [PubMed]
- Zindy, F.; Lee, Y.; Kawauchi, D.; Ayrault, O.; Ben Merzoug, L.; Li, Y.; McKinnon, P.J.; Roussel, M.F. Dicer Is Required for Normal Cerebellar Development and to Restrain Medulloblastoma Formation. PLoS ONE 2015, 10, e0129642. [Google Scholar] [CrossRef]
- Hong, P.; Jiang, M.; Li, H. Functional requirement of dicer1 and miR-17-5p in reactive astrocyte proliferation after spinal cord injury in the mouse. Glia 2014, 62, 2044–2060. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, C.; Blum, R.; Gascón, S.; Masserdotti, G.; Tripathi, P.; Sánchez, R.; Tiedt, S.; Schroeder, T.; Götz, M.; Berninger, B. Directing Astroglia from the Cerebral Cortex into Subtype Specific Functional Neurons. PLOS Biol. 2010, 8, e1000373. [Google Scholar] [CrossRef]
- Liu, Y.P.; Vink, M.A.; Westerink, J.-T.; de Arellano, E.R.; Konstantinova, P.; Ter Brake, O.; Berkhout, B. Titers of lentiviral vectors encoding shRNAs and miRNAs are reduced by different mechanisms that require distinct repair strategies. RNA 2010, 16, 1328–1339. [Google Scholar] [CrossRef]
- Chang, K.; Marran, K.; Valentine, A.; Hannon, G.J. Packaging shRNA Retroviruses. Cold Spring Harb. Protoc. 2013, 2013, 734–737. [Google Scholar] [CrossRef]
- Challis, R.C.; Kumar, S.R.; Chan, K.Y.; Challis, C.; Beadle, K.; Jang, M.J.; Kim, H.M.; Rajendran, P.S.; Tompkins, J.D.; Shivkumar, K.; et al. Systemic AAV vectors for widespread and targeted gene delivery in rodents. Nat. Protoc. 2019, 14, 379–414. [Google Scholar] [CrossRef]
- Aurnhammer, C.; Haase, M.; Muether, N.; Hausl, M.; Rauschhuber, C.; Huber, I.; Nitschko, H.; Busch, U.; Sing, A.; Ehrhardt, A.; et al. Universal Real-Time PCR for the Detection and Quantification of Adeno-Associated Virus Serotype 2-Derived Inverted Terminal Repeat Sequences. Hum. Gene Ther. Methods 2012, 23, 18–28. [Google Scholar] [CrossRef]
- Lu, Y.-L.; Liu, Y.; McCoy, M.J.; Yoo, A.S. MiR-124 synergism with ELAVL3 enhances target gene expression to promote neuronal maturity. Proc. Natl. Acad. Sci. USA 2021, 118, e2015454118. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pei, Z.; Hossain, A.; Bai, Y.; Chen, G. Transcription factor-based gene therapy to treat glioblastoma through direct neuronal conversion. Cancer Biol. Med. 2021, 18, 860–874. [Google Scholar] [CrossRef]
- Balcells, I.; Cirera, S.; Busk, P.K. Specific and sensitive quantitative RT-PCR of miRNAs with DNA primers. BMC Biotechnol. 2011, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Puls, B.; Chen, G. Transcriptomic analyses of NeuroD1-mediated astrocyte-to-neuron conversion. Dev. Neurobiol. 2022, 82, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.-X.; Yin, J.-C.; Chen, G. Transcriptome Analysis of Small Molecule–Mediated Astrocyte-to-Neuron Reprogramming. Front. Cell Dev. Biol. 2019, 7, 82. [Google Scholar] [CrossRef]
- Bhalala, O.G.; Pan, L.; Sahni, V.; McGuire, T.L.; Gruner, K.; Tourtellotte, W.G.; Kessler, J.A. microRNA-21 Regulates Astrocytic Response Following Spinal Cord Injury. J. Neurosci. 2012, 32, 17935–17947. [Google Scholar] [CrossRef]
- Liu, R.; Wang, W.; Wang, S.; Xie, W.; Li, H.; Ning, B. microRNA-21 regulates astrocytic reaction post-acute phase of spinal cord injury through modulating TGF-β signaling. Aging 2018, 10, 1474–1488. [Google Scholar] [CrossRef] [PubMed]
- Sanuki, R.; Onishi, A.; Koike, C.; Muramatsu, R.; Watanabe, S.; Muranishi, Y.; Irie, S.; Uneo, S.; Koyasu, T.; Matsui, R.; et al. miR-124a is required for hippocampal axogenesis and retinal cone survival through Lhx2 suppression. Nat. Neurosci. 2011, 14, 1125–1134. [Google Scholar] [CrossRef]
- Papagiannakopoulos, T.; Kosik, K.S. MicroRNA-124: Micromanager of Neurogenesis. Cell Stem Cell 2009, 4, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.-C.; Pastrana, E.; Tavazoie, M.; Doetsch, F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat. Neurosci. 2009, 12, 399–408. [Google Scholar] [CrossRef]
- Wohl, S.G.; Hooper, M.J.; Reh, T.A. MicroRNAs miR-25, let-7 and miR-124 regulate the neurogenic potential of Müller glia in mice. Development 2019, 146, dev179556. [Google Scholar] [CrossRef] [PubMed]
- Bronicki, L.M.; Jasmin, B.J. Emerging complexity of the HuD/ELAVl4 gene; implications for neuronal development, function, and dysfunction. RNA 2013, 19, 1019–1037. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Hutchison, E.R.; Lee, E.K.; Kuwano, Y.; Kim, M.M.; Masuda, K.; Srikantan, S.; Subaran, S.S.; Marasa, B.S.; Mattson, M.P.; et al. miR-375 Inhibits Differentiation of Neurites by Lowering HuD Levels. Mol. Cell. Biol. 2010, 30, 4197–4210. [Google Scholar] [CrossRef]
- Samaraweera, L.; Grandinetti, K.B.; Huang, R.; A Spengler, B.; A Ross, R. MicroRNAs define distinct human neuroblastoma cell phenotypes and regulate their differentiation and tumorigenicity. BMC Cancer 2014, 14, 309. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Sengupta, J.; Morin, M.; Neve, R.; Valenzuela, C.; Perrone-Bizzozero, N. Overexpression of HuD Accelerates Neurite Outgrowth and Increases GAP-43 mRNA Expression in Cortical Neurons and Retinoic Acid-Induced Embryonic Stem Cells in Vitro. Exp. Neurol. 2001, 168, 250–258. [Google Scholar] [CrossRef]
- Mullokandov, G.; Baccarini, A.; Ruzo, A.; Jayaprakash, A.D.; Tung, N.; Israelow, B.; Evans, M.J.; Sachidanandam, R.; Brown, B.D. High-throughput assessment of microRNA activity and function using microRNA sensor and decoy libraries. Nat. Methods 2012, 9, 840–846. [Google Scholar] [CrossRef]
- Yu, W.; Chen, Y.; Dubrulle, J.; Stossi, F.; Putluri, V.; Sreekumar, A.; Putluri, N.; Baluya, D.; Lai, S.Y.; Sandulache, V.C. Cisplatin generates oxidative stress which is accompanied by rapid shifts in central carbon metabolism. Sci. Rep. 2018, 8, 4306. [Google Scholar] [CrossRef]
- Keller, D.M.; McWeeney, S.; Arsenlis, A.; Drouin, J.; Wright, C.V.E.; Wang, H.; Wollheim, C.B.; White, P.; Kaestner, K.H.; Goodman, R.H. Characterization of Pancreatic Transcription Factor Pdx-1 Binding Sites Using Promoter Microarray and Serial Analysis of Chromatin Occupancy. J. Biol. Chem. 2007, 282, 32084–32092. [Google Scholar] [CrossRef]
- Poy, M.N.; Eliasson, L.; Krutzfeldt, J.; Kuwajima, S.; Ma, X.; MacDonald, P.E.; Pfeffer, S.; Tuschl, T.; Rajewsky, N.; Rorsman, P.; et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 2004, 432, 226–230. [Google Scholar] [CrossRef]
- Poy, M.N.; Hausser, J.; Trajkovski, M.; Braun, M.; Collins, S.; Rorsman, P.; Zavolan, M.; Stoffel, M. miR-375 maintains normal pancreatic α- and β-cell mass. Proc. Natl. Acad. Sci. USA 2009, 106, 5813–5818. [Google Scholar] [CrossRef]
- Kloosterman, W.P.; Lagendijk, A.K.; Ketting, R.F.; Moulton, J.D.; A Plasterk, R.H. Targeted Inhibition of miRNA Maturation with Morpholinos Reveals a Role for miR-375 in Pancreatic Islet Development. PLOS Biol. 2007, 5, e203. [Google Scholar] [CrossRef] [PubMed]
- Kapsimali, M.; Kloosterman, W.P.; de Bruijn, E.; Rosa, F.; Plasterk, R.H.; Wilson, S.W. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol. 2007, 8, R173. [Google Scholar] [CrossRef]
- Bhinge, A.; Namboori, S.C.; Bithell, A.; Soldati, C.; Buckley, N.J.; Stanton, L.W. MiR-375 is Essential for Human Spinal Motor Neuron Development and May Be Involved in Motor Neuron Degeneration. STEM CELLS 2015, 34, 124–134. [Google Scholar] [CrossRef]
- Baroukh, N.N.; Van Obberghen, E. Function of microRNA-375 and microRNA-124a in pancreas and brain. FEBS J. 2009, 276, 6509–6521. [Google Scholar] [CrossRef]
- Juan-Mateu, J.; Rech, T.H.; Villate, O.; Lizarraga-Mollinedo, E.; Wendt, A.; Turatsinze, J.-V.; Brondani, L.A.; Nardelli, T.R.; Nogueira, T.C.; Esguerra, J.L.S.; et al. Neuron-enriched RNA-binding Proteins Regulate Pancreatic Beta Cell Function and Survival. J. Biol. Chem. 2017, 292, 3466–3480. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kakumoto, K.; Yoshida, T.; Kuwako, K.-I.; Miyazaki, T.; Yamaguchi, J.; Konno, A.; Hata, J.; Uchiyama, Y.; Hirai, H.; et al. Elavl3 is essential for the maintenance of Purkinje neuron axons. Sci. Rep. 2018, 8, 2722. [Google Scholar] [CrossRef]
- DeBoer, E.M.; Azevedo, R.; Vega, T.A.; Brodkin, J.; Akamatsu, W.; Okano, H.; Wagner, G.C.; Rasin, M.-R. Prenatal Deletion of the RNA-Binding Protein HuD Disrupts Postnatal Cortical Circuit Maturation and Behavior. J. Neurosci. 2014, 34, 3674–3686. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tidei, J.J.; Polich, E.D.; Gao, Y.; Zhao, H.; Perrone-Bizzozero, N.I.; Guo, W.; Zhao, X. Positive feedback between RNA-binding protein HuD and transcription factor SATB1 promotes neurogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, E4995–E5004. [Google Scholar] [CrossRef] [PubMed]
- Tiruchinapalli, D.M.; Ehlers, M.D.; Keene, J.D. Activity-dependent expression of RNA binding protein HuD and its association with mRNAs in neurons. RNA Biol. 2008, 5, 157–168. [Google Scholar] [CrossRef]
- Bronicki, L.M.; Bélanger, G.; Jasmin, B.J. Characterization of Multiple Exon 1 Variants in Mammalian HuD mRNA and Neuron-Specific Transcriptional Control via Neurogenin 2. J. Neurosci. 2012, 32, 11164–11175. [Google Scholar] [CrossRef]
- Bolognani, F.; Qiu, S.; Tanner, D.C.; Paik, J.; Perrone-Bizzozero, N.I.; Weeber, E.J. Associative and spatial learning and memory deficits in transgenic mice overexpressing the RNA-binding protein HuD. Neurobiol. Learn. Mem. 2007, 87, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Shkumatava, A.; Stark, A.; Sive, H.; Bartel, D.P. Coherent but overlapping expression of microRNAs and their targets during vertebrate development. Genes Dev. 2009, 23, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Gascón, S.; Murenu, E.; Masserdotti, G.; Ortega, F.; Russo, G.L.; Petrik, D.; Deshpande, A.; Heinrich, C.; Karow, M.; Robertson, S.P.; et al. Identification and Successful Negotiation of a Metabolic Checkpoint in Direct Neuronal Reprogramming. Cell Stem Cell 2015, 18, 396–409. [Google Scholar] [CrossRef]
- De Santis, R.; Santini, L.; Colantoni, A.; Peruzzi, G.; de Turris, V.; Alfano, V.; Bozzoni, I.; Rosa, A. FUS Mutant Human Motoneurons Display Altered Transcriptome and microRNA Pathways with Implications for ALS Pathogenesis. Stem Cell Rep. 2017, 9, 1450–1462. [Google Scholar] [CrossRef] [PubMed]






| GenBank Accession | Gene Name | Forward | Reverse | Size (bp) |
|---|---|---|---|---|
| MIMAT0000728 | miR-375-3p | gtttgttcgttcggctc | cagtttttttttttttttcacgc | 40 * |
| MIMAT0000422 | miR-124-3p | taaggcacgcggtga | ccagtttttttttttttttggcat | 40 * |
| NR_138085 | U6 | gcttcggcagcacatatactaaaat | cgcttcacgaatttgcgtgtcat | 89 |
| NM_001419 | ELAVL1 | gatcctctggcagatgtttgg | cgcggatcactttcacattg | 59 |
| NM_001171195 | ELAVL2 | acaagcgaattgaggcagaag | tggcaccgggaggtttc | 63 |
| NM_001420 | ELAVL3 | gctgagcccatcacagtcaa | gcctgccccgtcttctg | 56 |
| NM_021952 | ELAVL4 | catggagcctcaggtgtcaa | ctggagggtccattgcttgt | 57 |
| NM_010894 | NeuroD1 | gaggaacacgaggcagacaag | tgcattcatggcttcaagctc | 51 # |
| NM_000555 | DCX | tgcttgggcctcacactagc | catataccgcaatcaaggaaatactc | 51 |
| NM_001082575 | NeuN | tcgtagagggacggaaaattga | gccgttggtgtaggggttc | 85 |
| NM_001256799 | GAPDH | ggtgaaggtcggagtcaacgg | gaggtcaatgaaggggtcattg | 112 |
| NM_008085 | GAPDH | aggtcggtgtgaacggatttg | tgtagaccatgtagttgaggtca | 123 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Sokirniy, I.; Wang, X.; Jiang, M.; Mseis-Jackson, N.; Williams, C.; Mayes, K.; Jiang, N.; Puls, B.; Du, Q.; et al. MicroRNA-375 Is Induced during Astrocyte-to-Neuron Reprogramming and Promotes Survival of Reprogrammed Neurons when Overexpressed. Cells 2023, 12, 2202. https://doi.org/10.3390/cells12172202
Chen X, Sokirniy I, Wang X, Jiang M, Mseis-Jackson N, Williams C, Mayes K, Jiang N, Puls B, Du Q, et al. MicroRNA-375 Is Induced during Astrocyte-to-Neuron Reprogramming and Promotes Survival of Reprogrammed Neurons when Overexpressed. Cells. 2023; 12(17):2202. https://doi.org/10.3390/cells12172202
Chicago/Turabian StyleChen, Xuanyu, Ivan Sokirniy, Xin Wang, Mei Jiang, Natalie Mseis-Jackson, Christine Williams, Kristopher Mayes, Na Jiang, Brendan Puls, Quansheng Du, and et al. 2023. "MicroRNA-375 Is Induced during Astrocyte-to-Neuron Reprogramming and Promotes Survival of Reprogrammed Neurons when Overexpressed" Cells 12, no. 17: 2202. https://doi.org/10.3390/cells12172202