C. elegans Hemidesmosomes Sense Collagen Damage to Trigger Innate Immune Response in the Epidermis
Abstract
:1. Introduction
2. Materials and Methods
2.1. C. elegans Strains and Maintenance
2.2. CRISPR/Cas9-Mediated Mutagenesis of the dpy-10 Gene
2.3. Molecular Biology and Transgenesis
2.4. RNAi
2.5. Immunostaining and Fluorescence Microscopy
2.6. Quantitative RT-PCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Only a Small Group of Collagens Induces AMP Expression upon Damage
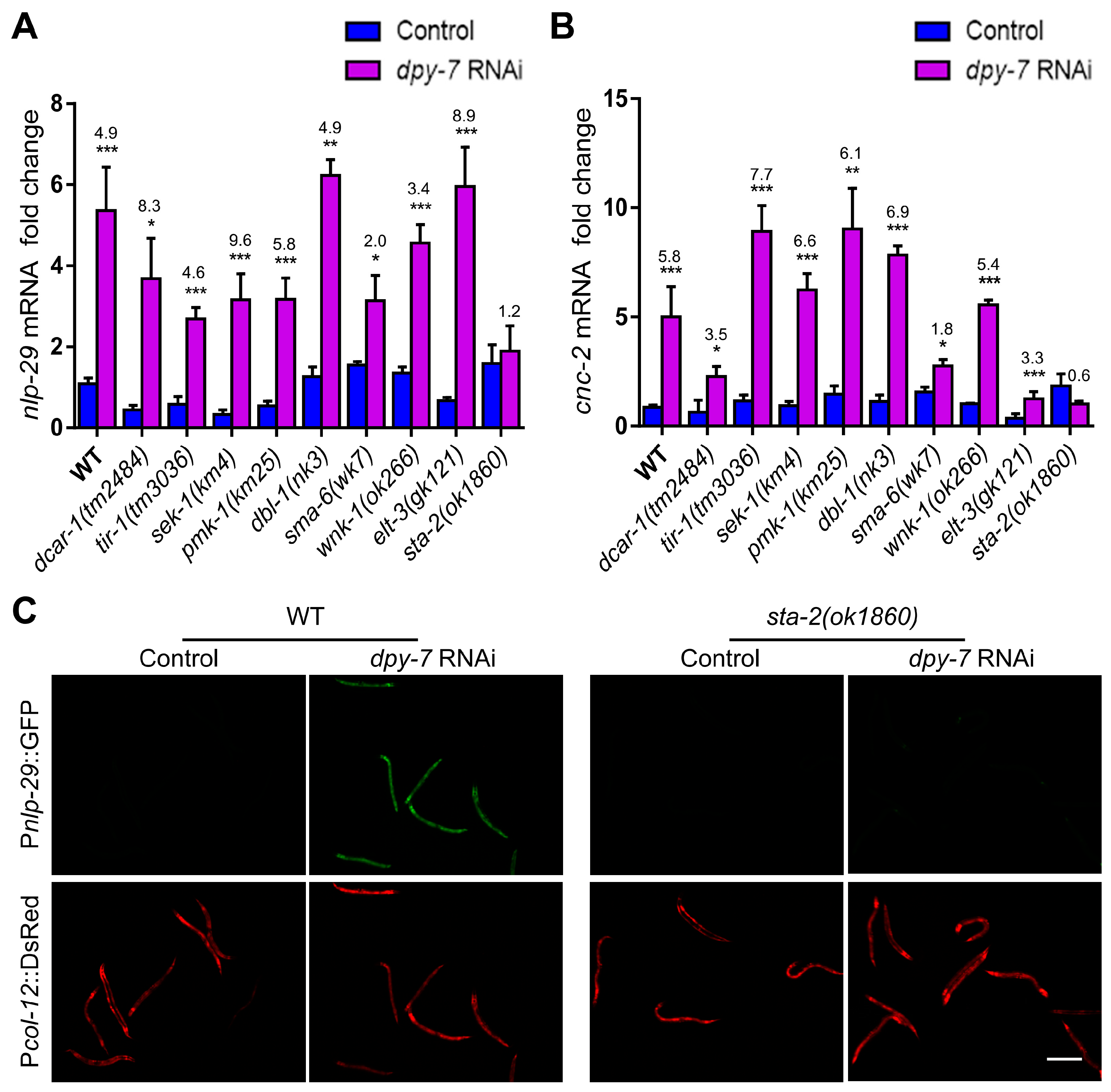
3.2. The Immune Response upon Collagen Loss Requires the Activation of STA-2 by Non-Classical Signaling Pathways
3.3. Specific DPY Collagens Regulate the Innate Immune Response through the MUP-4/STA-2 Complex
3.4. The Immunomodulatory Function of DPY Collagens Correlates with Their Expression Time but Not Their Localization
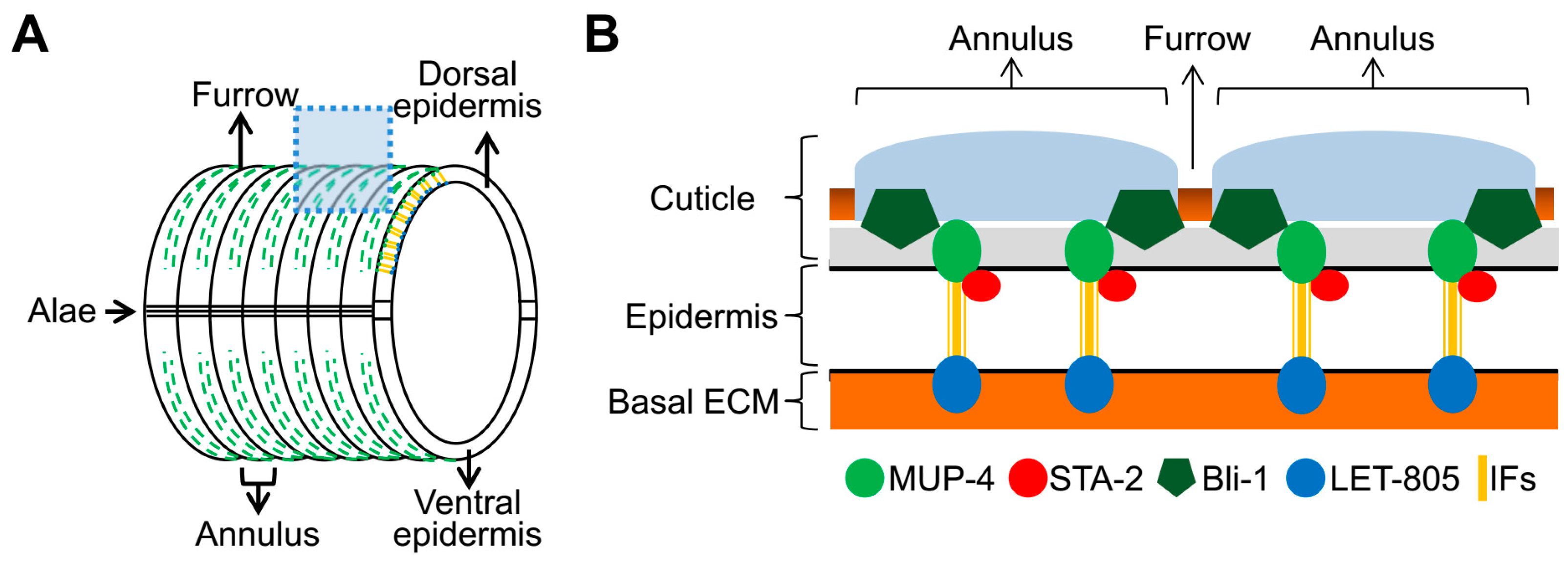
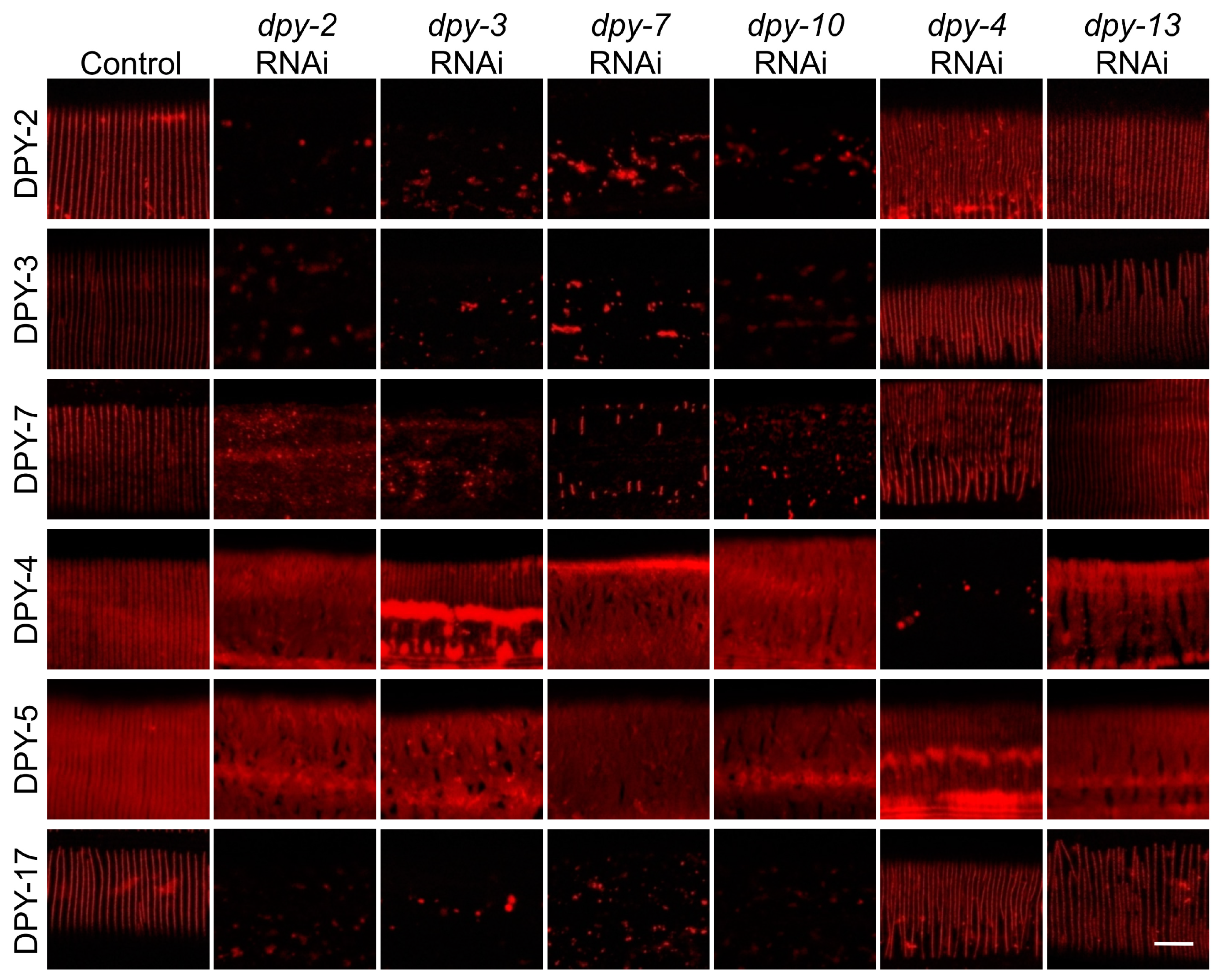
3.5. The Six DPY Collagens form a Substructure to Conjointly Control Cuticle Assemly and Immune Response
3.6. BLI-1/MUP-4, a Presumptive Collagen-Receptor Complex, Perceives the Damage Signal of DPY Collagens to Activate Immune Response
4. Conclusions and Discussion
Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myllyharju, J.; Kivirikko, K.I. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004, 20, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Leask, A.; Abraham, D.J. TGF-beta signaling and the fibrotic response. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004, 18, 816–827. [Google Scholar] [CrossRef]
- Hu, X.; Ivashkiv, L.B. Cross-regulation of signaling pathways by interferon-gamma: Implications for immune responses and autoimmune diseases. Immunity 2009, 31, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 2010, 10, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Weathington, N.M.; van Houwelingen, A.H.; Noerager, B.D.; Jackson, P.L.; Kraneveld, A.D.; Galin, F.S.; Folkerts, G.; Nijkamp, F.P.; Blalock, J.E. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat. Med. 2006, 12, 317–323. [Google Scholar] [CrossRef]
- Dustin, M.L.; de Fougerolles, A.R. Reprogramming T cells: The role of extracellular matrix in coordination of T cell activation and migration. Curr. Opin. Immunol. 2001, 13, 286–290. [Google Scholar] [CrossRef]
- Jacob, S.S.; Shastry, P.; Sudhakaran, P.R. Monocyte-macrophage differentiation in vitro: Modulation by extracellular matrix protein substratum. Mol. Cell Biochem. 2002, 233, 9–17. [Google Scholar] [CrossRef]
- Peng, D.H.; Rodriguez, B.L.; Diao, L.; Chen, L.; Wang, J.; Byers, L.A.; Wei, Y.; Chapman, H.A.; Yamauchi, M.; Behrens, C.; et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8(+) T cell exhaustion. Nat. Commun. 2020, 11, 4520. [Google Scholar] [CrossRef]
- Chisholm, A.D.; Xu, S. The Caenorhabditis elegans epidermis as a model skin. II: Differentiation and physiological roles. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 879–902. [Google Scholar] [CrossRef]
- Zhang, H.; Labouesse, M. The making of hemidesmosome structures in vivo. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2010, 239, 1465–1476. [Google Scholar] [CrossRef]
- Hong, L.; Elbl, T.; Ward, J.; Franzini-Armstrong, C.; Rybicka, K.K.; Gatewood, B.K.; Baillie, D.L.; Bucher, E.A. MUP-4 is a novel transmembrane protein with functions in epithelial cell adhesion in Caenorhabditis elegans. J. Cell Biol. 2001, 154, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Hresko, M.C.; Schriefer, L.A.; Shrimankar, P.; Waterston, R.H. Myotactin, a novel hypodermal protein involved in muscle-cell adhesion in Caenorhabditis elegans. J. Cell Biol. 1999, 146, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Pujol, N.; Zugasti, O.; Wong, D.; Couillault, C.; Kurz, C.L.; Schulenburg, H.; Ewbank, J.J. Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog. 2008, 4, e1000105. [Google Scholar] [CrossRef] [PubMed]
- Taffoni, C.; Pujol, N. Mechanisms of innate immunity in C. elegans epidermis. Tissue Barriers 2015, 3, e1078432. [Google Scholar] [CrossRef]
- Martineau, C.N.; Kirienko, N.V.; Pujol, N. Innate immunity in C. elegans. Curr. Top. Dev. Biol. 2021, 144, 309–351. [Google Scholar] [CrossRef]
- Kim, D.H.; Ewbank, J.J. Signaling in the innate immune response. In WormBook: The Online Review of C. elegans Biology; WormBook: Pasadena, CA, USA, 2018; Volume 2018, pp. 1–35. [Google Scholar] [CrossRef]
- Zugasti, O.; Bose, N.; Squiban, B.; Belougne, J.; Kurz, C.L.; Schroeder, F.C.; Pujol, N.; Ewbank, J.J. Activation of a G protein-coupled receptor by its endogenous ligand triggers the innate immune response of Caenorhabditis elegans. Nat. Immunol. 2014, 15, 833–838. [Google Scholar] [CrossRef]
- Pujol, N.; Cypowyj, S.; Ziegler, K.; Millet, A.; Astrain, A.; Goncharov, A.; Jin, Y.; Chisholm, A.D.; Ewbank, J.J. Distinct innate immune responses to infection and wounding in the C. elegans epidermis. Curr. Biol. 2008, 18, 481–489. [Google Scholar] [CrossRef]
- Dierking, K.; Polanowska, J.; Omi, S.; Engelmann, I.; Gut, M.; Lembo, F.; Ewbank, J.J.; Pujol, N. Unusual regulation of a STAT protein by an SLC6 family transporter in C. elegans epidermal innate immunity. Cell Host Microbe 2011, 9, 425–435. [Google Scholar] [CrossRef]
- Zugasti, O.; Ewbank, J.J. Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-beta signaling pathway in Caenorhabditis elegans epidermis. Nat. Immunol. 2009, 10, 249–256. [Google Scholar] [CrossRef]
- Lee, K.Z.; Kniazeva, M.; Han, M.; Pujol, N.; Ewbank, J.J. The fatty acid synthase fasn-1 acts upstream of WNK and Ste20/GCK-VI kinases to modulate antimicrobial peptide expression in C. elegans epidermis. Virulence 2010, 1, 113–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Li, L.; Li, Y.; Fu, R.; Zhu, Y.; Li, J.; Zhou, Y.; Xiong, S.; Zhang, H. Structural damage in the C. elegans epidermis causes release of STA-2 and induction of an innate immune response. Immunity 2015, 42, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Irazoqui, J.E. Why worms watch their hemidesmosomes and why you should, too. Immunity 2015, 42, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Page, A.P.; Johnstone, I.L. The cuticle. In WormBook: The Online Review of C. elegans Biology; WormBook: Pasadena, CA, USA, 2007; pp. 1–15. [Google Scholar] [CrossRef]
- Sandhu, A.; Badal, D.; Sheokand, R.; Tyagi, S.; Singh, V. Specific collagens maintain the cuticle permeability barrier in Caenorhabditis elegans. Genetics 2021, 217, iyaa047. [Google Scholar] [CrossRef]
- Levy, A.D.; Yang, J.; Kramer, J.M. Molecular and genetic analyses of the Caenorhabditis elegans dpy-2 and dpy-10 collagen genes: A variety of molecular alterations affect organismal morphology. Mol. Biol. Cell 1993, 4, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, I.L.; Shafi, Y.; Barry, J.D. Molecular analysis of mutations in the Caenorhabditis elegans collagen gene dpy-7. EMBO J. 1992, 11, 3857–3863. [Google Scholar] [CrossRef]
- Cohen, J.D.; Sundaram, M.V. C. elegans Apical Extracellular Matrices Shape Epithelia. J. Dev. Biol. 2020, 8, 23. [Google Scholar] [CrossRef]
- von Mende, N.; Bird, D.M.; Albert, P.S.; Riddle, D.L. dpy-13: A nematode collagen gene that affects body shape. Cell 1988, 55, 567–576. [Google Scholar] [CrossRef]
- Kramer, J.M.; Johnson, J.J.; Edgar, R.S.; Basch, C.; Roberts, S. The sqt-1 gene of C. elegans encodes a collagen critical for organismal morphogenesis. Cell 1988, 55, 555–565. [Google Scholar] [CrossRef]
- Dodd, W.; Tang, L.; Lone, J.C.; Wimberly, K.; Wu, C.W.; Consalvo, C.; Wright, J.E.; Pujol, N.; Choe, K.P. A Damage Sensor Associated with the Cuticle Coordinates Three Core Environmental Stress Responses in Caenorhabditis elegans. Genetics 2018, 208, 1467–1482. [Google Scholar] [CrossRef]
- Zugasti, O.; Thakur, N.; Belougne, J.; Squiban, B.; Kurz, C.L.; Soulé, J.; Omi, S.; Tichit, L.; Pujol, N.; Ewbank, J.J. A quantitative genome-wide RNAi screen in C. elegans for antifungal innate immunity genes. BMC Biol. 2016, 14, 35. [Google Scholar] [CrossRef]
- Miao, R.; Li, M.; Zhang, Q.; Yang, C.; Wang, X. An ECM-to-Nucleus Signaling Pathway Activates Lysosomes for C. elegans Larval Development. Dev. Cell 2020, 52, 21–37.e25. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Kamath, R.S.; Fraser, A.G.; Dong, Y.; Poulin, G.; Durbin, R.; Gotta, M.; Kanapin, A.; Le Bot, N.; Moreno, S.; Sohrmann, M.; et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 2003, 421, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Finney, M.; Ruvkun, G. The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell 1990, 63, 895–905. [Google Scholar] [CrossRef]
- Lamitina, T.; Huang, C.G.; Strange, K. Genome-wide RNAi screening identifies protein damage as a regulator of osmoprotective gene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 12173–12178. [Google Scholar] [CrossRef]
- Wheeler, J.M.; Thomas, J.H. Identification of a novel gene family involved in osmotic stress response in Caenorhabditis elegans. Genetics 2006, 174, 1327–1336. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Fu, R.; Zhu, Y.; Zhang, H. Periodic subcellular structures undergo long-range synchronized reorganization during C. elegans epidermal development. J. Cell Sci. 2020, 133, jcs246793. [Google Scholar] [CrossRef]
- McMahon, L.; Muriel, J.M.; Roberts, B.; Quinn, M.; Johnstone, I.L. Two sets of interacting collagens form functionally distinct substructures within a Caenorhabditis elegans extracellular matrix. Mol. Biol. Cell 2003, 14, 1366–1378. [Google Scholar] [CrossRef]
- Thein, M.C.; McCormack, G.; Winter, A.D.; Johnstone, I.L.; Shoemaker, C.B.; Page, A.P. Caenorhabditis elegans exoskeleton collagen COL-19: An adult-specific marker for collagen modification and assembly, and the analysis of organismal morphology. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2003, 226, 523–539. [Google Scholar] [CrossRef]
- Aggad, D.; Brouilly, N.; Omi, S.; Essmann, C.L.; Dehapiot, B.; Savage-Dunn, C.; Richard, F.; Cazevieille, C.; Politi, K.A.; Hall, D.H.; et al. Meisosomes, folded membrane microdomains between the apical extracellular matrix and epidermis. eLife 2023, 12, e75906. [Google Scholar] [CrossRef]
- Zhang, H.; Landmann, F.; Zahreddine, H.; Rodriguez, D.; Koch, M.; Labouesse, M. A tension-induced mechanotransduction pathway promotes epithelial morphogenesis. Nature 2011, 471, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Jiang, X.; Yang, Y.; Wang, C.; Zhang, Y.; Zhu, Y.; Zhang, H. Bidirectional regulation of structural damage on autophagy in the C. elegans epidermis. Autophagy 2022, 18, 2731–2745. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Li, W.; Dong, Y.; Xia, C.; Fu, R. C. elegans Hemidesmosomes Sense Collagen Damage to Trigger Innate Immune Response in the Epidermis. Cells 2023, 12, 2223. https://doi.org/10.3390/cells12182223
Zhu Y, Li W, Dong Y, Xia C, Fu R. C. elegans Hemidesmosomes Sense Collagen Damage to Trigger Innate Immune Response in the Epidermis. Cells. 2023; 12(18):2223. https://doi.org/10.3390/cells12182223
Chicago/Turabian StyleZhu, Yi, Wenna Li, Yifang Dong, Chujie Xia, and Rong Fu. 2023. "C. elegans Hemidesmosomes Sense Collagen Damage to Trigger Innate Immune Response in the Epidermis" Cells 12, no. 18: 2223. https://doi.org/10.3390/cells12182223
APA StyleZhu, Y., Li, W., Dong, Y., Xia, C., & Fu, R. (2023). C. elegans Hemidesmosomes Sense Collagen Damage to Trigger Innate Immune Response in the Epidermis. Cells, 12(18), 2223. https://doi.org/10.3390/cells12182223



