The Impact of Ca2+ on Intracellular Distribution of Hemoglobin in Human Erythrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Samples
2.2. Buffers and Chemicals
2.3. Hb Variant Analysis
2.4. RBC Membrane Preparation
2.5. Morphological Characterization Using Cell Flow-Properties Analyzer (CFA)
2.6. Glucose Consumption, Lactate Release, and K+ Leakage Studies
2.7. Labeling Experiments
2.8. Separation on a Percoll Density Gradient
2.9. Statistics
3. Results
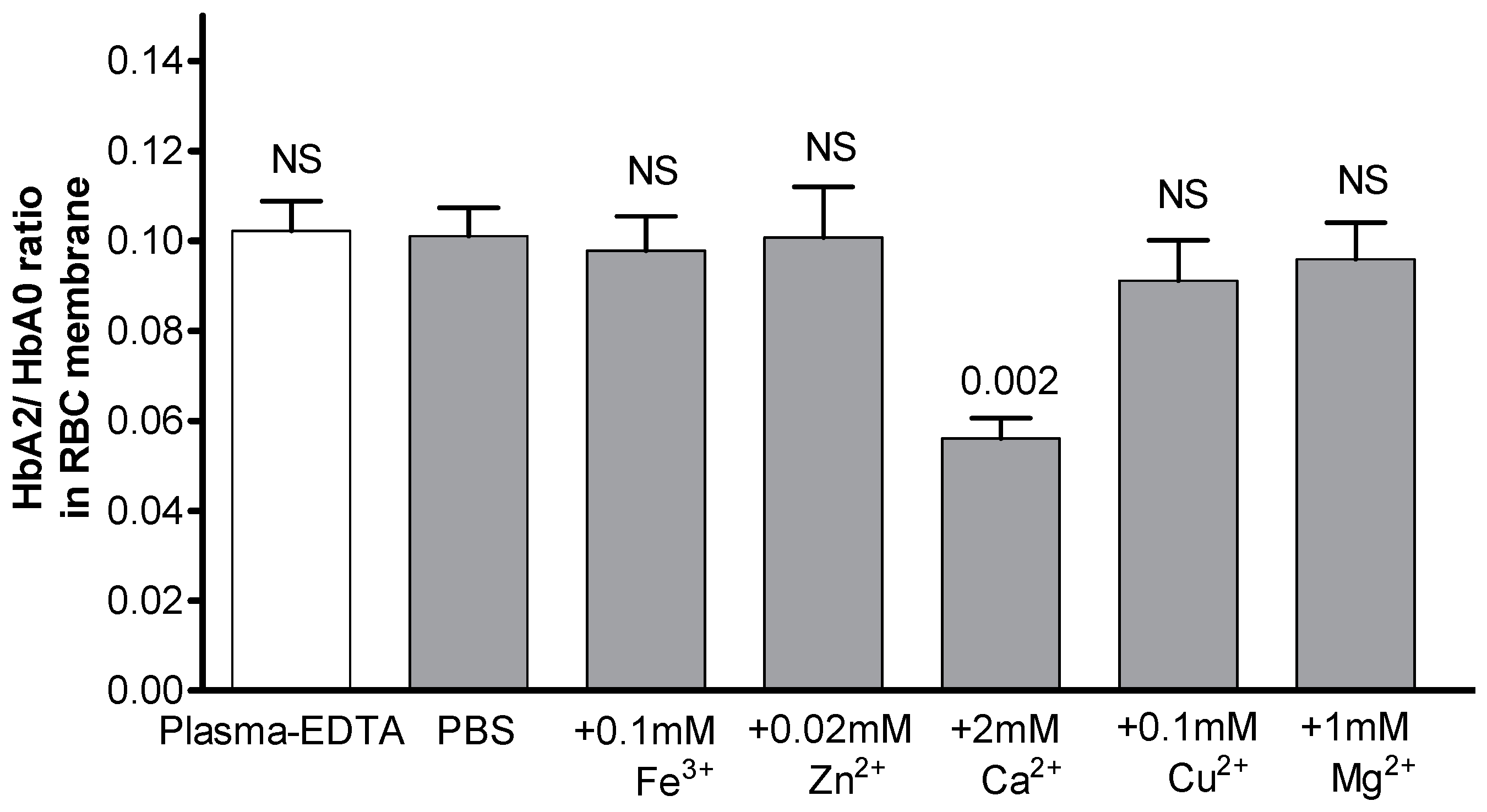
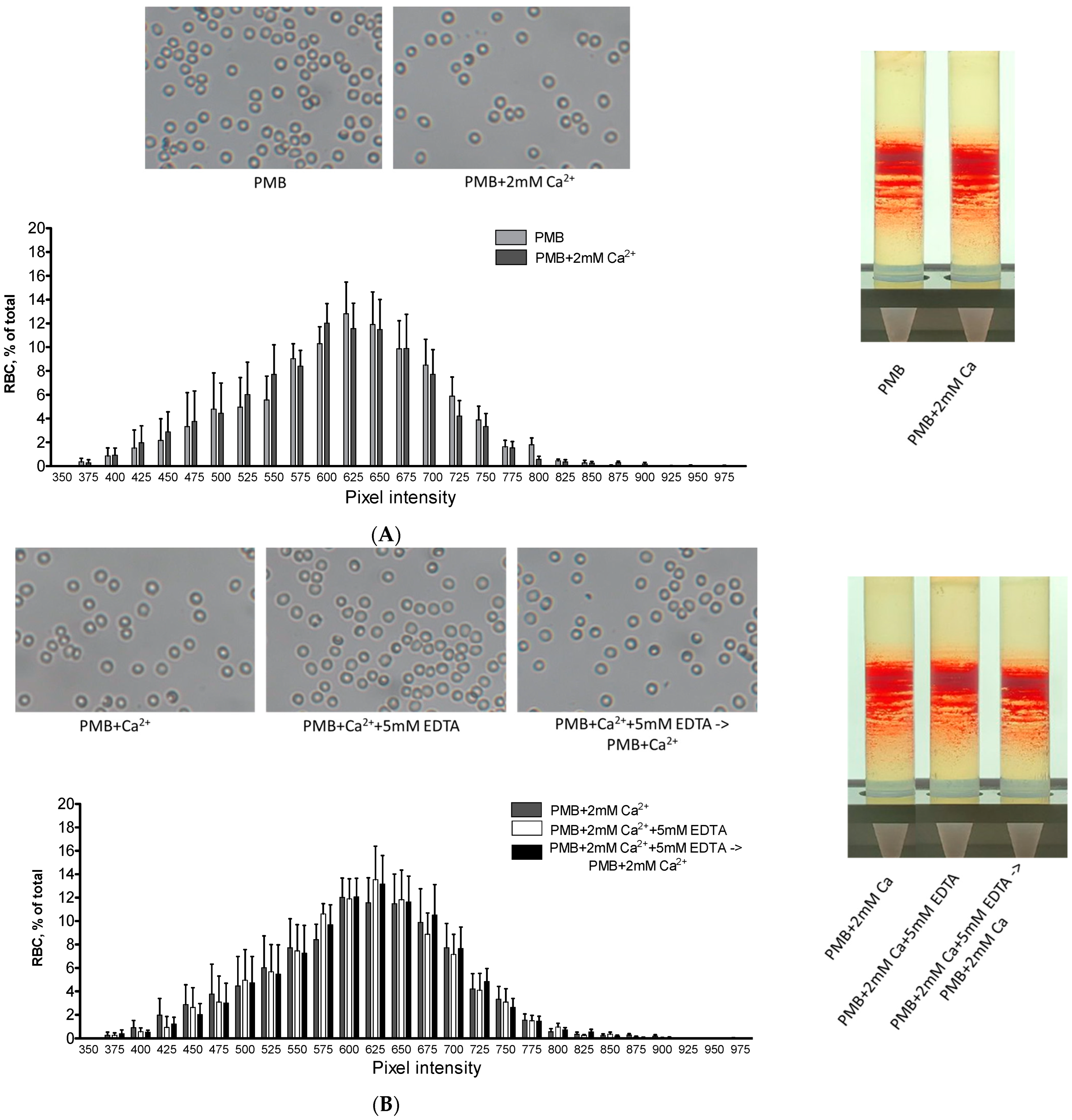
3.1. Effect of Extracellular Constituents on Hb Distribution in the Membrane
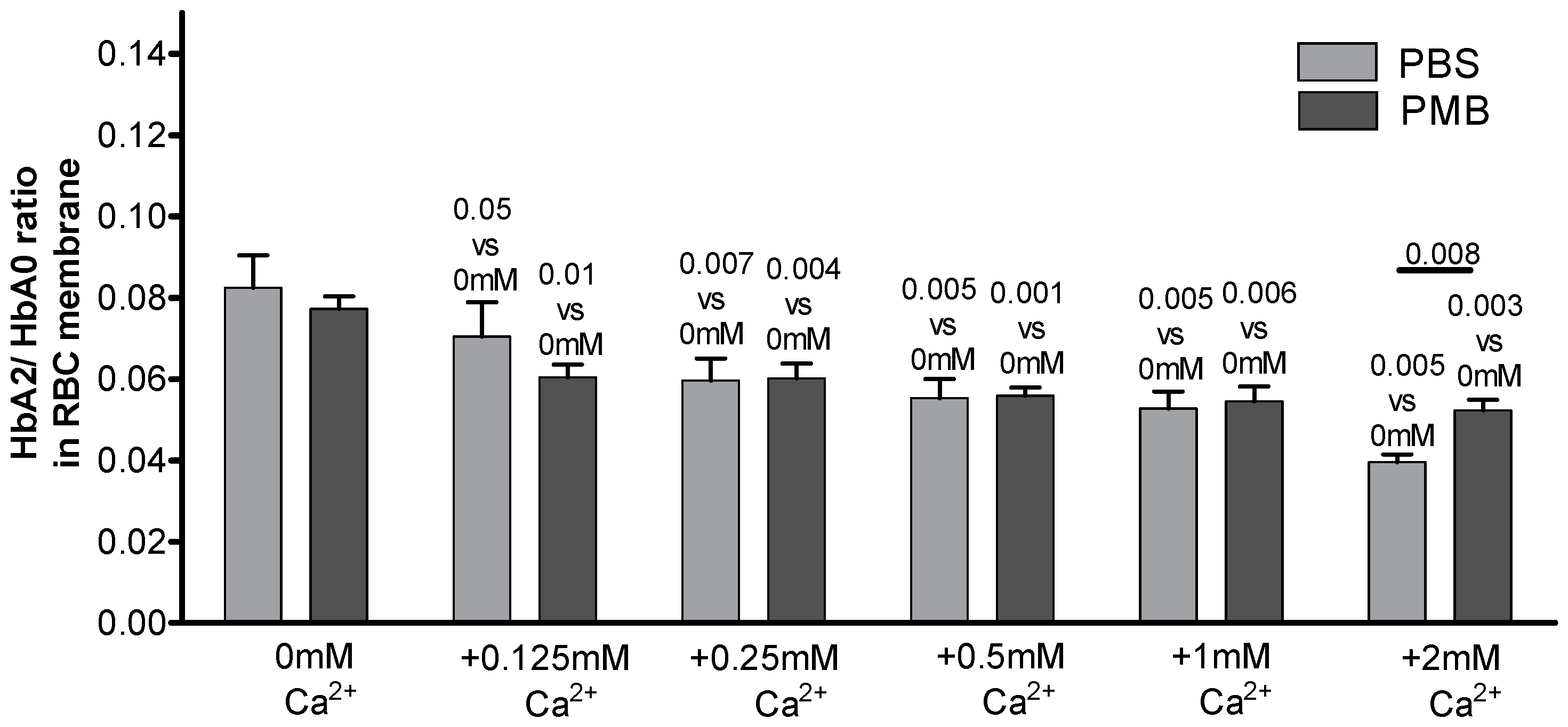
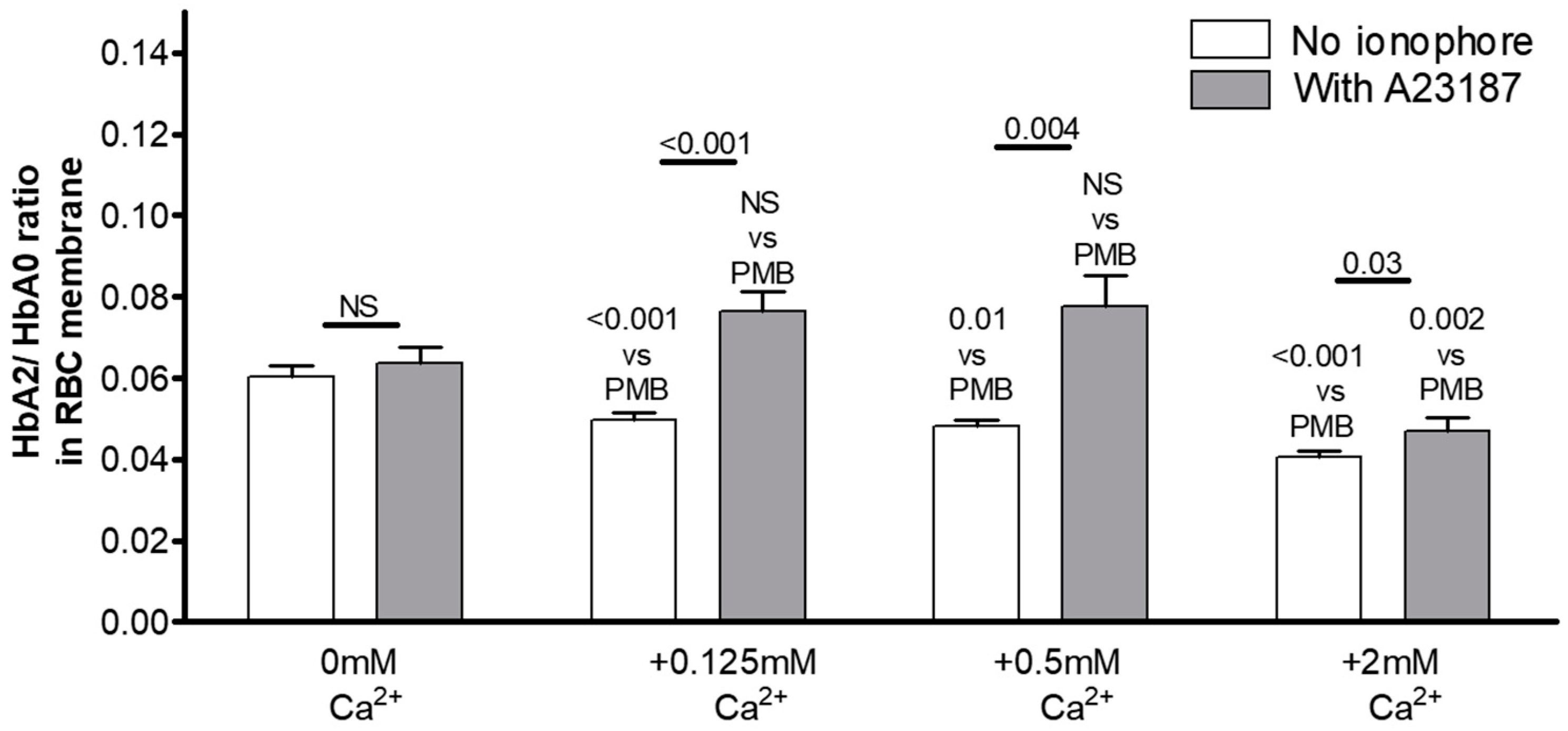
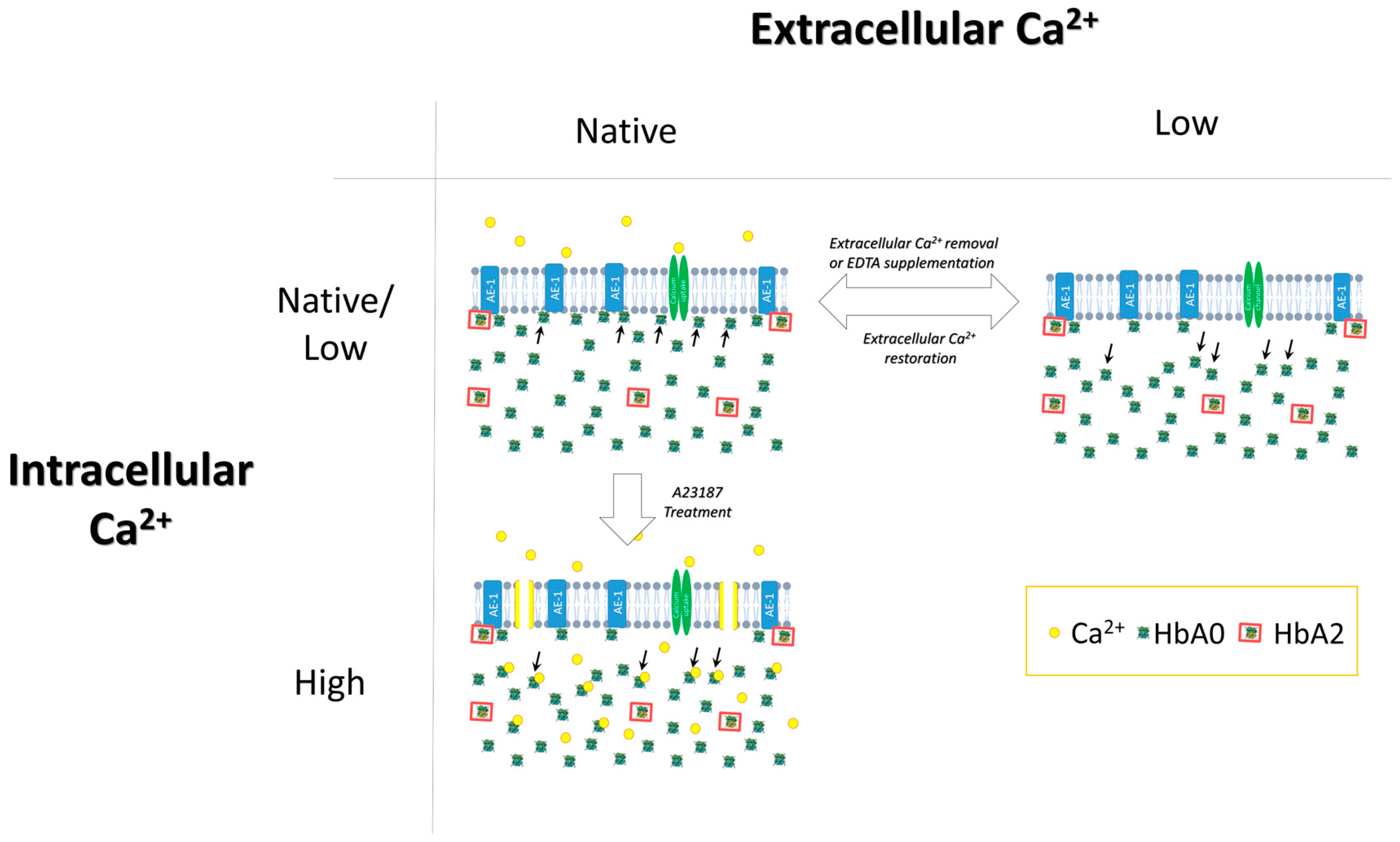
3.2. The Key Role of Extracellular Ca2+ in HbA2 Enrichment of the Membrane-Bound Hb Pool
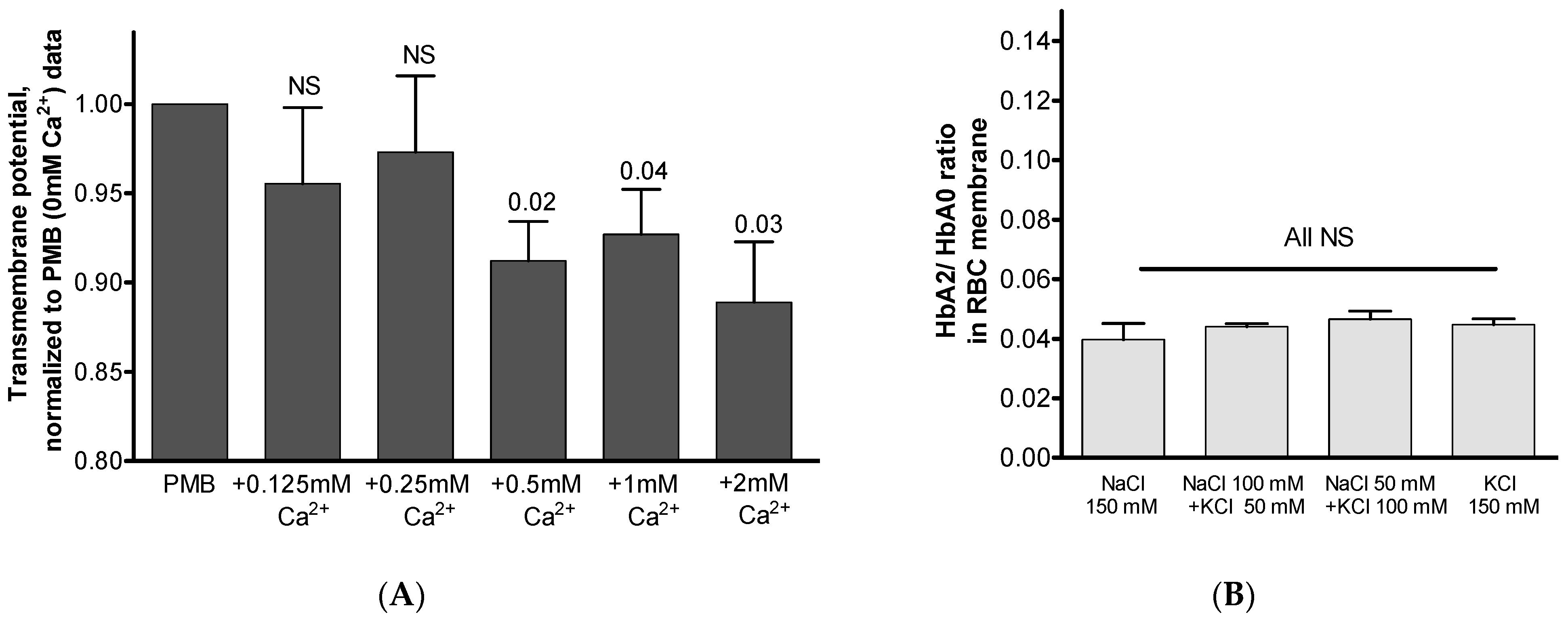
3.3. Possible Interrelated Effect of Hypocalcemia on Hb Distribution and RBC Structural and Metabolic Features
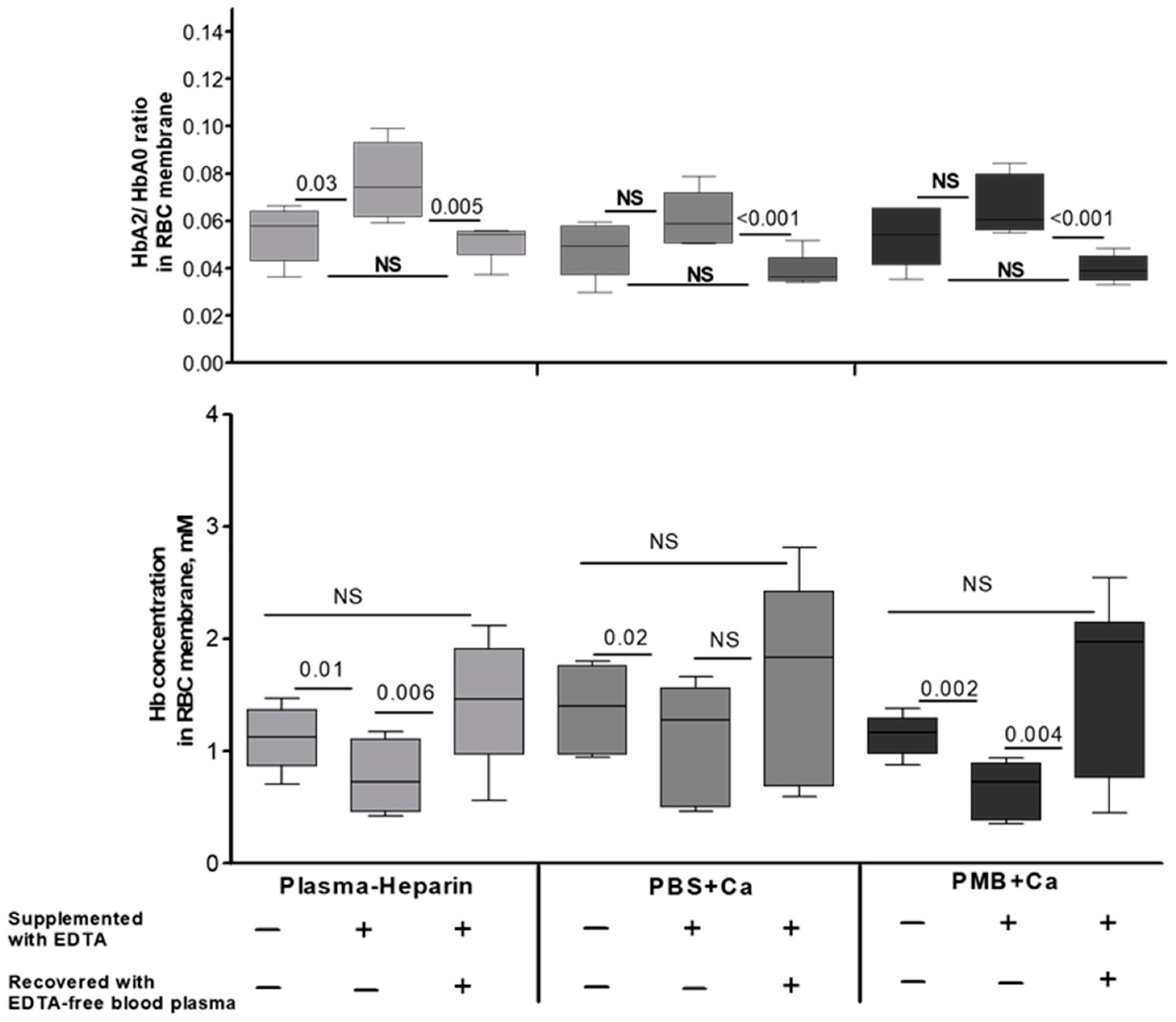
3.4. Possible Intracellular Mechanism(s) Governing Ca2+-Mediated Hb Translocation
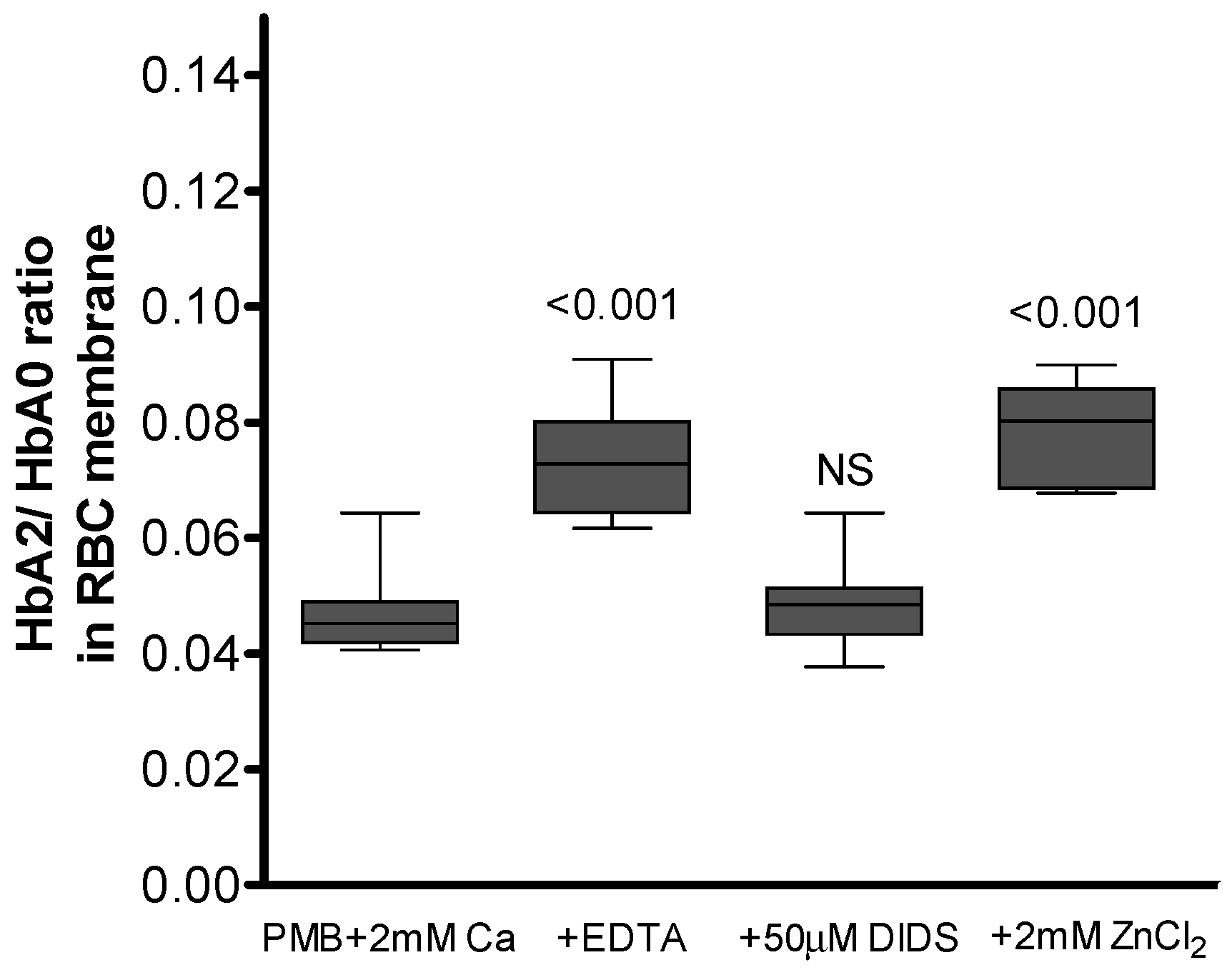
3.5. AE-1 as a Membrane Target for Ca2+-Induced Hb Binding
4. Discussion
4.1. Possible Membrane Targets for Ca2+-Regulated Presence of Hb
4.2. Suggestions for Laboratory and Clinical Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barvitenko, N.; Adragna, N.; Weber, R. Erythrocyte Signal Transduction Pathways, Their Oxygenation Dependence and Functional Significance. Cell. Physiol. Biochem. 2005, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kosmachevskaya, O.V.; Topunov, A.F. Alternate and Additional Functions of Erythrocyte Hemoglobin. Biochemistry 2018, 83, 1575–1593. [Google Scholar] [CrossRef] [PubMed]
- Issaian, A.; Hay, A.; Dzieciatkowska, M.; Roberti, D.; Perrotta, S.; Darula, Z.; Redzic, J.; Busch, M.P.; Page, G.P.; Rogers, S.C.; et al. The Interactome of the N-Terminus of Band 3 Regulates Red Blood Cell Metabolism and Storage Quality. Haematologica 2021, 106, 2971–2985. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.P. Biochemical Aspects of the Reaction of Hemoglobin and NO: Implications for Hb-Based Blood Substitutes. Free Radic. Biol. Med. 2000, 28, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Puchulu-Campanella, E.; Chu, H.; Anstee, D.J.; Galan, J.A.; Tao, W.A.; Low, P.S. Identification of the Components of a Glycolytic Enzyme Metabolon on the Human Red Blood Cell Membrane. J. Biol. Chem. 2013, 288, 848–858. [Google Scholar] [CrossRef]
- Gell, D.A. Structure and Function of Haemoglobins. Blood Cells Mol. Dis. 2018, 70, 13–42. [Google Scholar] [CrossRef]
- McMahon, T.J. Red Blood Cell Deformability, Vasoactive Mediators, and Adhesion. Front. Physiol. 2019, 10, 1417. [Google Scholar] [CrossRef]
- Welbourn, E.M.; Wilson, M.T.; Yusof, A.; Metodiev, M.v.; Cooper, C.E. The Mechanism of Formation, Structure and Physiological Relevance of Covalent Hemoglobin Attachment to the Erythrocyte Membrane. Free Radic. Biol. Med. 2017, 103, 95–106. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.v.; Nasybullina, E.I.; Blindar, V.N.; Topunov, A.F. Binding of Erythrocyte Hemoglobin to the Membrane to Realize Signal-Regulatory Function (Review). Appl. Biochem. Microbiol. 2019, 55, 83–98. [Google Scholar] [CrossRef]
- Morabito, R.; Romano, O.; la Spada, G.; Marino, A. H2O2-Induced Oxidative Stress Affects SO4= Transport in Human Erythrocytes. PLoS ONE 2016, 11, e0146485. [Google Scholar] [CrossRef][Green Version]
- Arashiki, N.; Kimata, N.; Manno, S.; Mohandas, N.; Takakuwa, Y. Membrane Peroxidation and Methemoglobin Formation Are Both Necessary for Band 3 Clustering: Mechanistic Insights into Human Erythrocyte Senescence. Biochemistry 2013, 52, 5760–5769. [Google Scholar] [CrossRef] [PubMed]
- Signorini, C.; Ferrali, M.; Ciccoli, L.; Sugherini, L.; Magnani, A.; Comporti, M. Iron Release, Membrane Protein Oxidation and Erythrocyte Ageing. FEBS Lett. 1995, 362, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Spinelli, S.; Basile, N.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Oxidation Stress as a Mechanism of Aging in Human Erythrocytes: Protective Effect of Quercetin. Int. J. Mol. Sci. 2022, 23, 7781. [Google Scholar] [CrossRef]
- Rauenbuehler, P.B.; Cordes, K.A.; Salhany, J.M. Identification of the Hemoglobin Binding Sites on the Inner Surface of the Erythrocyte Membrane. Biochim. Biophys. Acta (BBA)-Biomembr. 1982, 692, 361–370. [Google Scholar] [CrossRef]
- Sega, M.F.; Chu, H.; Christian, J.A.; Low, P.S. Fluorescence Assay of the Interaction between Hemoglobin and the Cytoplasmic Domain of Erythrocyte Membrane Band 3. Blood Cells Mol. Dis. 2015, 55, 266–271. [Google Scholar] [CrossRef]
- Datta, P.; Chakrabarty, S.; Chakrabarty, A.; Chakrabarti, A. Membrane Interactions of Hemoglobin Variants, HbA, HbE, HbF and Globin Subunits of HbA: Effects of Aminophospholipids and Cholesterol. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shaklai, N.; Ranney, H.R. Interaction of Hemoglobin with Membrane Lipids: A Source of Pathological Phenomena. Isr. J. Med. Sci. 1978, 14, 1152–1156. [Google Scholar]
- Giambona, A.; Passarello, C.; Renda, D.; Maggio, A. The Significance of the Hemoglobin A2 Value in Screening for Hemoglobinopathies. Clin. Biochem. 2009, 42, 1786–1796. [Google Scholar] [CrossRef]
- Steinberg, M.H.; Rodgers, G.P. HbA2: Biology, Clinical Relevance and a Possible Target for Ameliorating Sickle Cell Disease. Br. J. Haematol. 2015, 170, 781–786. [Google Scholar] [CrossRef]
- Fischer, S.; Nagel, R.L.; Bookchin, R.M.; Roth, E.F.; Tellez-Nagel, I. The Binding of Hemoglobin to Membranes of Normal and Sickle Erythrocytes. Biochim. Biophys. Acta (BBA)-Biomembr. 1975, 375, 422–433. [Google Scholar] [CrossRef]
- Wong, P. A Hypothesis on the Role of the Electrical Charge of Haemoglobin in Regulating the Erythrocyte Shape. Med. Hypotheses 2004, 62, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Ghashghaeinia, M.; Cluitmans, J.C.; Toulany, M.; Saki, M.; Köberle, M.; Lang, E.; Dreischer, P.; Biedermann, T.; Duszenko, M.; Lang, F.; et al. Age Sensitivity of NFκB Abundance and Programmed Cell Death in Erythrocytes Induced by NFκB Inhibitors. Cell. Physiol. Biochem. 2013, 32, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Gural, A.; Zelig, O.; Arbell, D.; Yedgar, S. Unit-to-Unit Variability in the Deformability of Red Blood Cells. Transfus. Apher. Sci. 2020, 59, 102876. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Rasmusen, T.L.; Zelig, O.; Arbell, D.; Yedgar, S. Inter-donor Variability in Deformability of Red Blood Cells in Blood Units. Transfus. Med. 2020, 30, 492–496. [Google Scholar] [CrossRef]
- Barshtein, G.; Pries, A.R.; Goldschmidt, N.; Zukerman, A.; Orbach, A.; Zelig, O.; Arbell, D.; Yedgar, S. Deformability of Transfused Red Blood Cells Is a Potent Determinant of Transfusion-Induced Change in Recipient’s Blood Flow. Microcirculation 2016, 23, 479–486. [Google Scholar] [CrossRef]
- Peretz, S.; Livshits, L.; Pretorius, E.; Makhro, A.; Bogdanova, A.; Gassmann, M.; Koren, A.; Levin, C. The Protective Effect of the Spleen in Sickle Cell Patients. A Comparative Study between Patients with Asplenia/Hyposplenism and Hypersplenism. Front. Physiol. 2022, 13, 796837. [Google Scholar] [CrossRef]
- Hildrum, J.M.; Fjeld, B.; Risahagen, S.M.; Bernatek, B.J.; Klingenberg, O. Assessment of Hemoglobin A2 Stability at Room Temperature during 24 or 25 Days as Measured by High Pressure Liquid Chromatography and Capillary Electrophoresis. Int. J. Lab. Hematol. 2021, 43, e266–e270. [Google Scholar] [CrossRef]
- Balach, M.M.; Casale, C.H.; Campetelli, A.N. Erythrocyte Plasma Membrane Potential: Past and Current Methods for Its Measurement. Biophys. Rev. 2019, 11, 995–1005. [Google Scholar] [CrossRef]
- Moersdorf, D.; Egee, S.; Hahn, C.; Hanf, B.; Ellory, C.; Thomas, S.; Bernhardt, I. Transmembrane Potential of Red Blood Cells Under Low Ionic Strength Conditions. Cell. Physiol. Biochem. 2013, 31, 875–882. [Google Scholar] [CrossRef]
- Hoffman, J.F.; Laris, P.C. Determination of Membrane Potentials in Human and Amphiuma Red Blood Cells by Means of a Fluorescent Probe. J. Physiol. 1974, 239, 519–552. [Google Scholar] [CrossRef]
- Epps, D.E.; Wolfe, M.L.; Groppi, V. Characterization of the Steady-State and Dynamic Fluorescence Properties of the Potential-Sensitive Dye Bis-(1,3-Dibutylbarbituric Acid)Trimethine Oxonol (Dibac4(3)) in Model Systems and Cells. Chem. Phys. Lipids 1994, 69, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Koshkaryev, A.; Livshits, L.; Pajic-Lijakovic, I.; Gural, A.; Barshtein, G.; Yedgar, S. Non-Oxidative Band-3 Clustering Agents Cause the Externalization of Phosphatidylserine on Erythrocyte Surfaces by a Calcium-Independent Mechanism. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183231. [Google Scholar] [CrossRef] [PubMed]
- Makhro, A.; Hänggi, P.; Goede, J.S.; Wang, J.; Brüggemann, A.; Gassmann, M.; Schmugge, M.; Kaestner, L.; Speer, O.; Bogdanova, A. N-Methyl-d-Aspartate Receptors in Human Erythroid Precursor Cells and in Circulating Red Blood Cells Contribute to the Intracellular Calcium Regulation. Am. J. Physiol.-Cell Physiol. 2013, 305, C1123–C1138. [Google Scholar] [CrossRef] [PubMed]
- Makhro, A.; Haider, T.; Wang, J.; Bogdanov, N.; Steffen, P.; Wagner, C.; Meyer, T.; Gassmann, M.; Hecksteden, A.; Kaestner, L.; et al. Comparing the Impact of an Acute Exercise Bout on Plasma Amino Acid Composition, Intraerythrocytic Ca2+ Handling, and Red Cell Function in Athletes and Untrained Subjects. Cell Calcium 2016, 60, 235–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Low, P.S.; Allen, D.P.; Zioncheck, T.F.; Chari, P.; Willardson, B.M.; Geahlen, R.L.; Harrison, M.L. Tyrosine Phosphorylation of Band 3 Inhibits Peripheral Protein Binding. J. Biol. Chem. 1987, 262, 4592–4596. [Google Scholar] [CrossRef]
- Harrison, M.L.; Rathinavelu, P.; Arese, P.; Geahlen, R.L.; Low, P.S. Role of Band 3 Tyrosine Phosphorylation in the Regulation of Erythrocyte Glycolysis. J. Biol. Chem. 1991, 266, 4106–4111. [Google Scholar] [CrossRef]
- Passing, R.; Schubert, D. The Binding of Ca2⊕ to Solubilized Band 3 Protein of the Human Erythrocyte Membrane. Hoppe Seylers Z. Physiol. Chem. 1983, 364, 873–878. [Google Scholar] [CrossRef]
- Datta, P.; Chakrabarty, S.B.; Chakrabarty, A.; Chakrabarti, A. Interaction of Erythroid Spectrin with Hemoglobin Variants: Implications in β-Thalassemia. Blood Cells Mol. Dis. 2003, 30, 248–253. [Google Scholar] [CrossRef]
- Wallis, C.J.; Babitch, J.A.; Wenegieme, E.F. Divalent Cation Binding to Erythrocyte Spectrin. Biochemistry 1993, 32, 5045–5050. [Google Scholar] [CrossRef]
- Hall, T.G.; Bennett, V. Regulatory Domains of Erythrocyte Ankyrin. J. Biol. Chem. 1987, 262, 10537–10545. [Google Scholar] [CrossRef]
- Korsgren, C.; Peters, L.L.; Lux, S.E. Protein 4.2 Binds to the Carboxyl-Terminal EF-Hands of Erythroid α-Spectrin in a Calcium- and Calmodulin-Dependent Manner. J. Biol. Chem. 2010, 285, 4757–4770. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, W. Regulation of Protein 4.1R Interactions with Membrane Proteins by Ca2+ and Calmodulin. Front. Biosci. 2006, 11, 1522–1539. [Google Scholar] [CrossRef] [PubMed]
- Slatinskaya, O.; Maksimov, G. In the Erythrocyte Hemoglobin Conformation and Distribution during RBC Volume Changes. Russ. J. Biol. Phys. Chemisrty 2022, 7, 297–302. [Google Scholar] [CrossRef]
- Shviro, Y.; Zilber, I.; Shaklai, N. The Interaction of Hemoglobin with Phosphatidylserine Vesicles. Biochim. Biophys. Acta (BBA)-Biomembr. 1982, 687, 63–70. [Google Scholar] [CrossRef]
- Bucki, R.; Bachelot-Loza, C.; Zachowski, A.; Giraud, F.; Sulpice, J.-C. Calcium Induces Phospholipid Redistribution and Microvesicle Release in Human Erythrocyte Membranes by Independent Pathways. Biochemistry 1998, 37, 15383–15391. [Google Scholar] [CrossRef]
- Sears, M.E. Chelation: Harnessing and Enhancing Heavy Metal Detoxification—A Review. Sci. World J. 2013, 2013, 219840. [Google Scholar] [CrossRef]
- Keowmaneechai, E.; McClements, D.J. Influence of EDTA and Citrate on Physicochemical Properties of Whey Protein-Stabilized Oil-in-Water Emulsions Containing CaCl2. J. Agric. Food Chem. 2002, 50, 7145–7153. [Google Scholar] [CrossRef]
- Harrison, D.G.; Long, C. The Calcium Content of Human Erythrocytes. J. Physiol. 1968, 199, 367–381. [Google Scholar] [CrossRef]
- Pinteric, L.; Manery, J.; Chaudry, I.; Madapallimattam, G. The Effect of EDTA, Cations, and Various Buffers on the Morphology of Erythrocyte Membranes: An Electron-Microscopic Study. Blood 1975, 45, 709–724. [Google Scholar] [CrossRef]
- Hall, C.; Nagengast, A.K.; Knapp, C.; Behrens, B.; Dewey, E.N.; Goodman, A.; Bommiasamy, A.; Schreiber, M. Massive Transfusions and Severe Hypocalcemia: An Opportunity for Monitoring and Supplementation Guidelines. Transfusion 2021, 61, S188–S194. [Google Scholar] [CrossRef]
- Diaz, D.; Fonseca, V.; Aude, Y.W.; Lamas, G.A. Chelation Therapy to Prevent Diabetes-Associated Cardiovascular Events. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 258–266. [Google Scholar] [CrossRef] [PubMed]
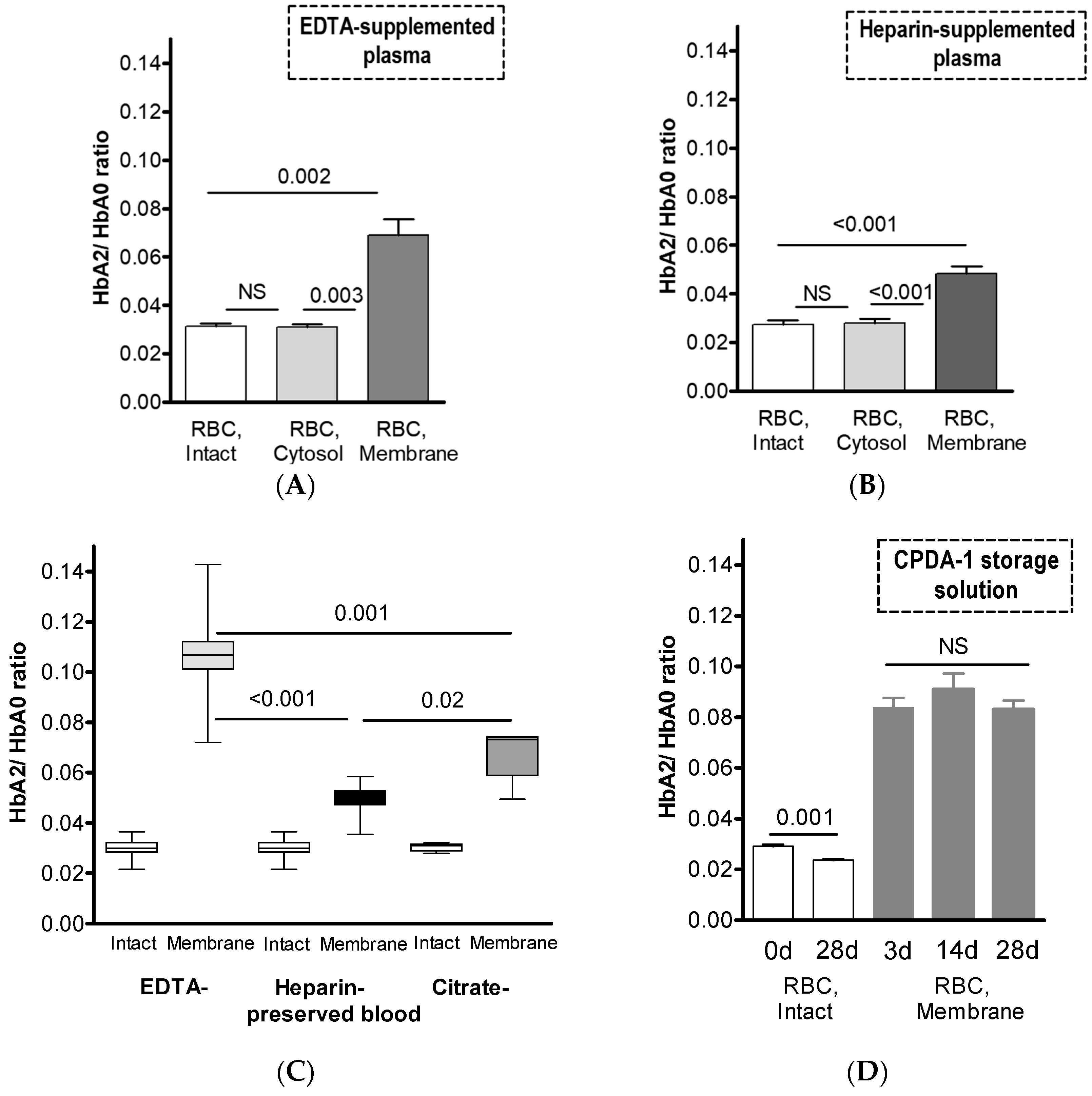

| EDTA-Supplemented Plasma (n = 6) | Heparin-Supplemented Plasma (n = 8) | |||||
|---|---|---|---|---|---|---|
| Hb Isoforms | RBC | RBC | ||||
| Intact | Membrane | Cytosol | Intact | Membrane | Cytosol | |
| HbF, % of total Hb | 0.30 ± 0.09 0.04 | 0.17 ± 0.14 | 0.27 ± 0.08 NS | 0.36 ± 0.13 NS | 0.38 ± 0.16 | 0.36 ± 0.12 NS |
| HbA2, % of total Hb | 3.02 ± 0.28 0.002 | 6.42 ± 1.46 | 2.98 ± 0.26 0.003 | 2.65 ± 0.52 <0.001 | 4.41 ± 0.72 | 2.60 ± 0.44 <0.001 |
| HbA0, % of total Hb | 96.7 ± 0.26 0.001 | 93.4 ± 1.33 | 96.8 ± 0.23 0.002 | 97.0 ± 0.49 <0.001 | 95.2 ± 0.67 | 97.0 ± 0.40 <0.001 |
| HbA2/HbA0 | 0.031 ± 0.003 0.002 | 0.069 ± 0.017 | 0.031 ± 0.003 0.003 | 0.027 ± 0.005 <0.001 | 0.046 ± 0.008 | 0.027 ± 0.005 <0.001 |
| Na+/K+/Cl−, mM | Phosphates, mM | Mg2+/Ca2+/Zn2+, mM | Glucose, mM | Amino Acids | HEPES, mM | Trisodium Citrate, mM | Citrate, mM | Adenine, mM | Albumin, % | pH | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma (EDTA) | Native | Native | Minor (chelated) | Native | Native | – | – | – | Native | Native | 7–7.4 |
| Plasma (Citrate) | Native | Native | Minor (chelated) | Native | Native | – | – | – | Native | Native | 7–7.4 |
| Plasma (Heparin) | Native | Native | Native | Native | Native | – | – | – | Native | Native | 7–7.4 |
| CPDA-1 | 16/0/0 | 16 | 0 | 161 | – | – | 89.4 | 15.5 | 2 | – | 5.5 |
| DPBS | 146/4.1/141 | 1.5 | 0.5/0.9/0 | – | – | – | – | – | – | – | 7–7.4 |
| PBS | 146/4.1/141 | 1.5 | 0 | – | – | – | – | – | – | – | 7–7.4 |
| PMB | 140/4/144 | – | 0.75/2/0.015 | 10 | Native | 20 | – | – | – | 0.1 | 7–7.4 |
| HbA2/HbA0 Ratio | |||
|---|---|---|---|
| Number | Intact | Membrane | |
| EDTA-plasma | 4 | 0.031 ± 0.003 | 0.101 ± 0.017 |
| EDTA-plasma, heated at 56 °C | 0.099 ± 0.014 NS | ||
| EDTA-plasma | 7 | 0.023 ± 0.002 | 0.072 ± 0.012 <0.001 |
| PMB | 0.040 ± 0.006 | ||
| PMB, supplemented with 5% BSA | 0.040 ± 0.008 NS | ||
| Number | K+ Release, mM Normalized to tHb | Glucose Consumption, mM Normalized to tHb | Lactate Efflux, mM Normalized to tHb | |
|---|---|---|---|---|
| PMB + 2 mM Ca2+ PMB | 10 | 0.061 ± 0.017 0.082 ± 0.035 0.03 | 1.866 ± 0.796 1.814 ± 0.649 NS | 0.184 ± 0.049 0.185 ± 0.038 NS |
| PMB + 2 mM Ca2+ | 8 | 0.098 ± 0.036 | 1.614 ± 0.517 | 0.171 ± 0.021 |
| PMB + 2 mM Ca2+ + 5 mM EDTA | 0.188 ± 0.036 <0.001 | 0.745 ± 0.806 0.02 | 0.098 ± 0.032 <0.001 | |
| PMB + 2 mM Ca2+ + 5 mM EDTA -> | ||||
| PMB + 2 mM Ca2+ | 0.099 ± 0.017 | 1.410 ± 0.657 | 0.137 ± 0.019 0.01 |
| Number | Fluo-4, Normalized to PMB Result | |
|---|---|---|
| PMB | 5 | 1 |
| PMB + 0.125 mM Ca2+ | 1.136 ± 0.085 0.02 | |
| PMB + 0.5 mM Ca2+ | 1.178 ± 0.091 0.01 | |
| PMB + 2 mM Ca2+ | 1.174 ± 0.098 0.02 | |
| PMB + 10 µM A23187 | 0.946 ± 0.088 NS | |
| PMB + 10 µM A23187 + 0.125 mM Ca2+ | 0.878 ± 0.105 NS | |
| PMB + 10 µM A23187 + 0.5 mM Ca2+ | 5.223 ± 0.427 <0.001 | |
| PMB + 10 µM A23187 + 2 mM Ca2+ | 6.63 ± 0.731 <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Livshits, L.; Peretz, S.; Bogdanova, A.; Zoabi, H.; Eitam, H.; Barshtein, G.; Galindo, C.; Feldman, Y.; Pajić-Lijaković, I.; Koren, A.; et al. The Impact of Ca2+ on Intracellular Distribution of Hemoglobin in Human Erythrocytes. Cells 2023, 12, 2280. https://doi.org/10.3390/cells12182280
Livshits L, Peretz S, Bogdanova A, Zoabi H, Eitam H, Barshtein G, Galindo C, Feldman Y, Pajić-Lijaković I, Koren A, et al. The Impact of Ca2+ on Intracellular Distribution of Hemoglobin in Human Erythrocytes. Cells. 2023; 12(18):2280. https://doi.org/10.3390/cells12182280
Chicago/Turabian StyleLivshits, Leonid, Sari Peretz, Anna Bogdanova, Hiba Zoabi, Harel Eitam, Gregory Barshtein, Cindy Galindo, Yuri Feldman, Ivana Pajić-Lijaković, Ariel Koren, and et al. 2023. "The Impact of Ca2+ on Intracellular Distribution of Hemoglobin in Human Erythrocytes" Cells 12, no. 18: 2280. https://doi.org/10.3390/cells12182280
APA StyleLivshits, L., Peretz, S., Bogdanova, A., Zoabi, H., Eitam, H., Barshtein, G., Galindo, C., Feldman, Y., Pajić-Lijaković, I., Koren, A., Gassmann, M., & Levin, C. (2023). The Impact of Ca2+ on Intracellular Distribution of Hemoglobin in Human Erythrocytes. Cells, 12(18), 2280. https://doi.org/10.3390/cells12182280










